Abstract
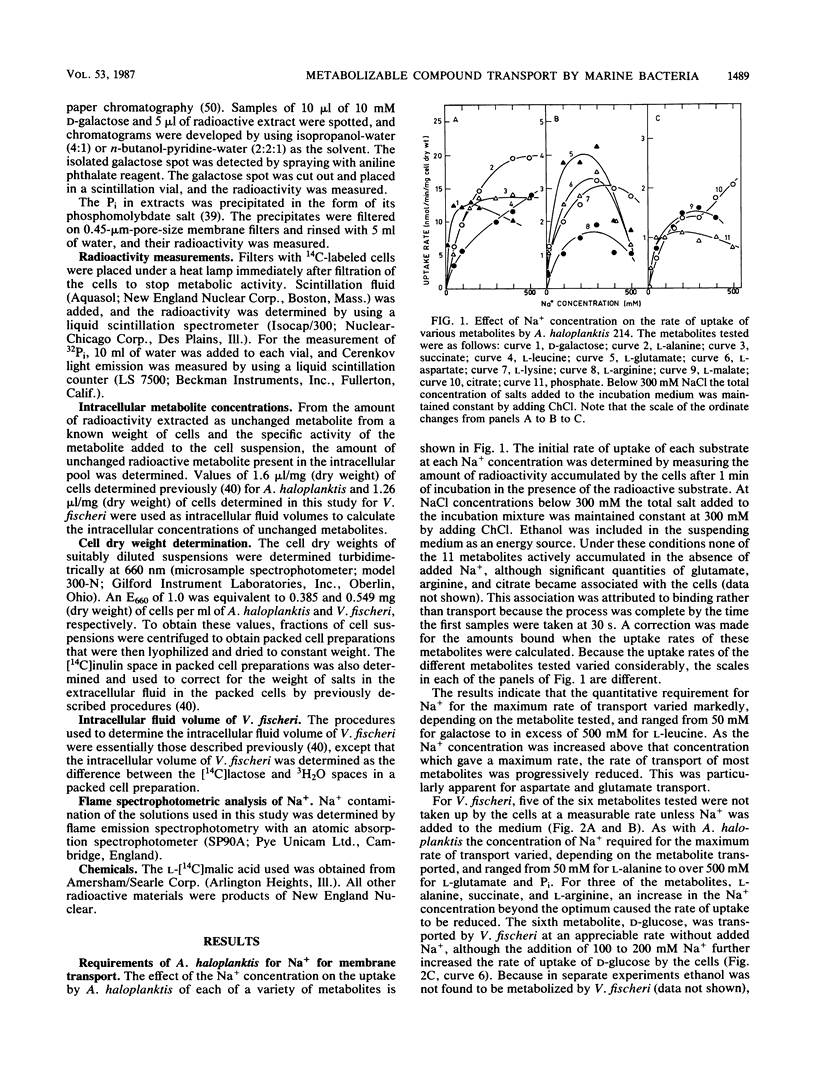
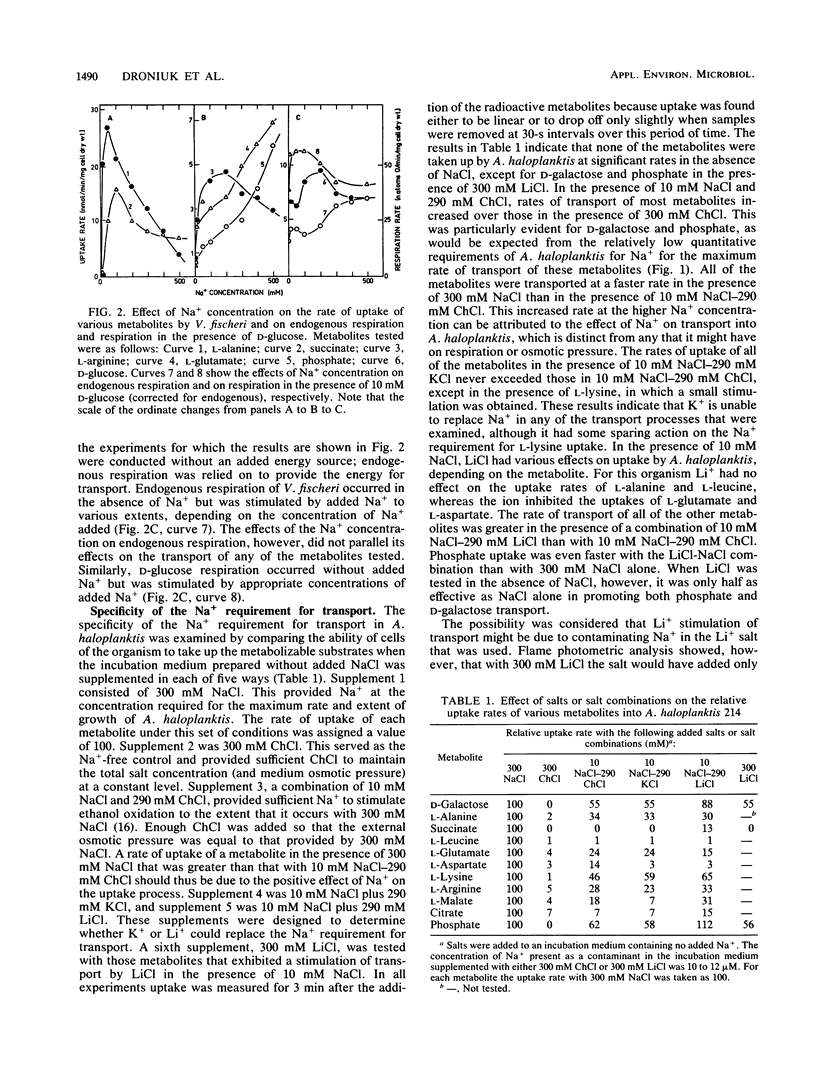
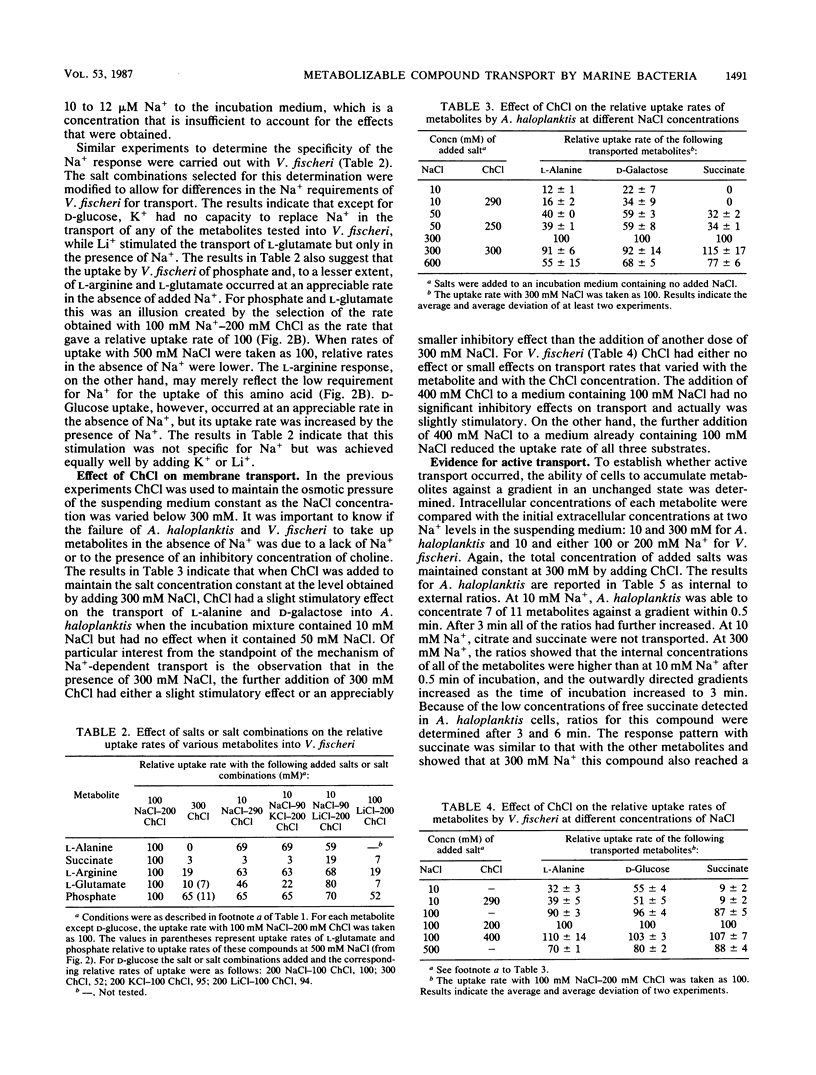
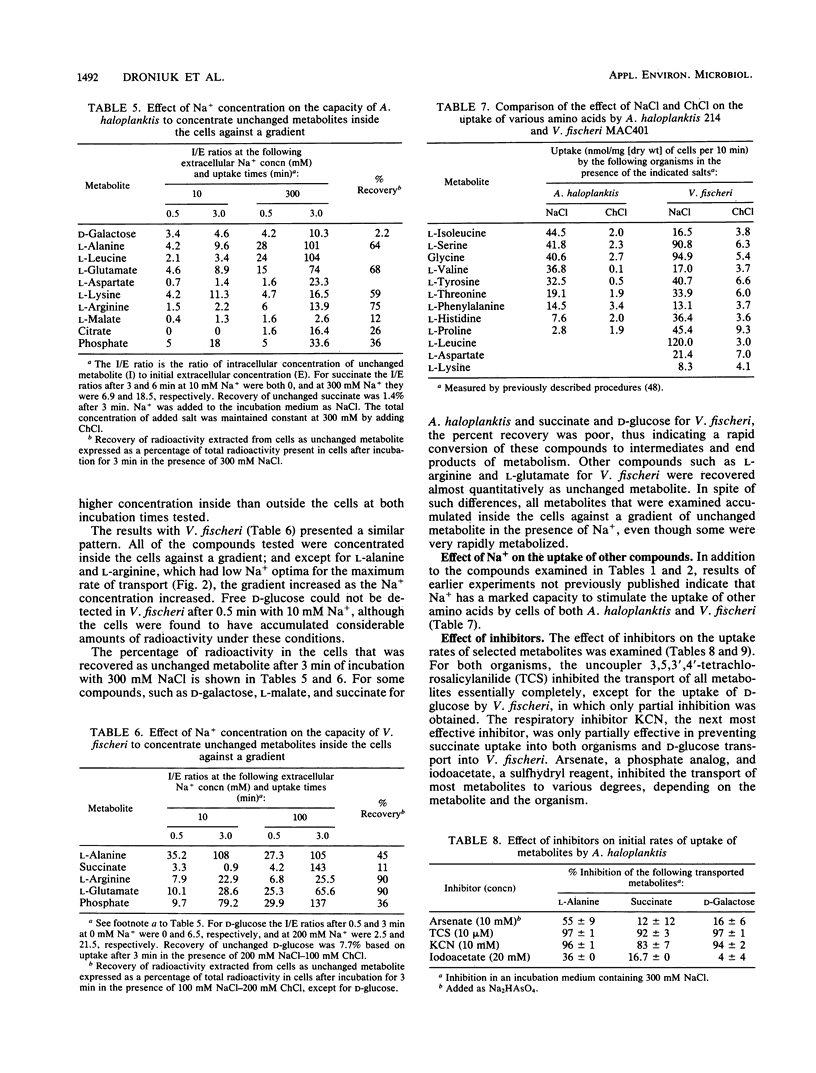
The rates of uptake by Alteromonas haloplanktis of 19 metabolizable compounds and by V. fischeri of 16 of 17 metabolizable compounds were negligible in the absence of added alkali-metal cations but rapid in the presence of Na+. Only d-glucose uptake by V. fischeri occurred at a reasonable rate in the absence of alkali-metal cations, although the rate was further increased by added Na+, K+, or Li+. Quantitative requirements for Na+ for the uptake of 11 metabolites by A. haloplanktis and of 6 metabolites by V. fischeri and the characteristics of the Na+ response at constant osmotic pressure varied with each metabolite and were different from the Na+ effects on the energy sources used. Li+ stimulated transport of some metabolites in the presence of suboptimal Na+ concentrations and for a few replaced Na+ for transport but functioned less effectively. K+ had a small capacity to stimulate lysine transport. The rate of transport of most of the compounds increased to a maximum at 50 to 300 mM Na+, depending on the metabolite, and then decreased as the Na+ concentration was further increased. For a few metabolites, the rate of transport continued to increase in a biphasic manner as the Na+ concentration was increased to 500 mM. Concentrations of choline chloride equimolar to inhibitory concentrations of NaCl were either not inhibitory or appreciably less inhibitory than those of NaCl. All metabolites examined accumulated inside the cells against a gradient of unchanged metabolite in the presence of Na+, even though some were very rapidly metabolized. The transport of l-alanine, succinate, and d-galactose into A. haloplanktis and of l-alanine and succinate into V. fischeri was inhibited essentially completely by the uncoupler 3,5,3′,4′-tetrachlorosalicylanilide. Glucose uptake by V. fischeri was inhibited partially by 3,5,3′,4′-tetrachlorosalicylanilide and also by arsenate and iodoacetate.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumann P., Baumann L. Catabolism of D-fructose and D-ribose by Pseudomonas doudoroffii. I. Physiological studies and mutant analysis. Arch Microbiol. 1975 Nov 7;105(3):225–240. doi: 10.1007/BF00447141. [DOI] [PubMed] [Google Scholar]
- Caldwell D. R., Hudson R. F. Sodium, an obligate growth requirement for predominant rumen bacteria. Appl Microbiol. 1974 Mar;27(3):549–552. doi: 10.1128/am.27.3.549-552.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C. C., Tsuchiya T., Yamane Y., Wood J. M., Wilson T. H. Na+ (Li+)-proline cotransport in Escherichia coli. J Membr Biol. 1985;84(2):157–164. doi: 10.1007/BF01872213. [DOI] [PubMed] [Google Scholar]
- Drapeau G. R., Matula T. I., MacLeod R. A. Nutrition and metabolism of marine bacteria. XV. Relation of Na+-activated transport to the Na+ requirement of a marine pseudomonad for growth. J Bacteriol. 1966 Jul;92(1):63–71. doi: 10.1128/jb.92.1.63-71.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fein J. E., MacLeod R. A. Characterization of neutral amino acid transport in a marine pseudomonad. J Bacteriol. 1975 Dec;124(3):1177–1190. doi: 10.1128/jb.124.3.1177-1190.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo J. F., Gibson J. Regulation of phosphate accumulation in the unicellular cyanobacterium Synechococcus. J Bacteriol. 1979 Nov;140(2):508–517. doi: 10.1128/jb.140.2.508-517.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern Y. S., Barash H., Dover S., Druck K. Sodium and potassium requirements for active transport of glutamate by Escherichia coli K-12. J Bacteriol. 1973 Apr;114(1):53–58. doi: 10.1128/jb.114.1.53-58.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassan H. M., MacLeod R. A. Kinetics of Na+-dependent K+ ion transport in a marine pseudomonad. J Bacteriol. 1975 Jan;121(1):160–164. doi: 10.1128/jb.121.1.160-164.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodson R. E., Azam F. Occurrence and Characterization of a Phosphoenolpyruvate: Glucose Phosphotransferase System in a Marine Bacterium, Serratia marinorubra. Appl Environ Microbiol. 1979 Dec;38(6):1086–1091. doi: 10.1128/aem.38.6.1086-1091.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones K., Heathcote J. G. The rapid resolution of naturally occurring amino acids by thin-layer chromatography. J Chromatogr. 1966 Sep;24(1):106–111. doi: 10.1016/s0021-9673(01)98107-5. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. Transport studies in bacterial membrane vesicles. Science. 1974 Dec 6;186(4167):882–892. doi: 10.1126/science.186.4167.882. [DOI] [PubMed] [Google Scholar]
- Kakinuma Y., Unemoto T. Sucrose uptake is driven by the Na+ electrochemical potential in the marine bacterium Vibrio alginolyticus. J Bacteriol. 1985 Sep;163(3):1293–1295. doi: 10.1128/jb.163.3.1293-1295.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanna G., DeVoe L., Brown L., Niven D. F., MacLeod R. A. Relationship between ion requirements for respiration and membrane transport in a marine bacterium. J Bacteriol. 1984 Jan;157(1):59–63. doi: 10.1128/jb.157.1.59-63.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada M., Horikoshi K. Sodium-ion stimulated amino acid uptake in membrane vesicles of alkalophilic Bacillus no. 8-1. J Biochem. 1980 Dec;88(6):1757–1764. doi: 10.1093/oxfordjournals.jbchem.a133150. [DOI] [PubMed] [Google Scholar]
- Krulwich T. A., Guffanti A. A. Physiology of acidophilic and alkalophilic bacteria. Adv Microb Physiol. 1983;24:173–214. doi: 10.1016/s0065-2911(08)60386-0. [DOI] [PubMed] [Google Scholar]
- Krulwich T. A. Na+/H+ antiporters. Biochim Biophys Acta. 1983 Dec 30;726(4):245–264. doi: 10.1016/0304-4173(83)90011-3. [DOI] [PubMed] [Google Scholar]
- Kushner D. J., Hamaide F., MacLeod R. A. Development of salt-resistant active transport in a moderately halophilic bacterium. J Bacteriol. 1983 Mar;153(3):1163–1171. doi: 10.1128/jb.153.3.1163-1171.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyi J. K. The role of Na+ in transport processes of bacterial membranes. Biochim Biophys Acta. 1979 Dec 20;559(4):377–397. doi: 10.1016/0304-4157(79)90011-x. [DOI] [PubMed] [Google Scholar]
- MACLEOD R. A., CLARIDGE C. A., HORI A., MURRAY J. F. Observations on the function of sodium in the metabolism of a marine bacterium. J Biol Chem. 1958 Jun;232(2):829–834. [PubMed] [Google Scholar]
- MACLEOD R. A., ONOFREY E. Nutrition and metabolism of marine bacteria. II. Observations on the relation of sea water to the growth of marine bacteria. J Bacteriol. 1956 Jun;71(6):661–667. doi: 10.1128/jb.71.6.661-667.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACLEOD R. A. THE QUESTION OF THE EXISTENCE OF SPECIFIC MARINE BACTERIA. Bacteriol Rev. 1965 Mar;29:9–24. [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. E., Greene R. V., Lanyi J. K. Light-activated amino acid transport systems in Halobacterium halobium envelope vesicles: role of chemical and electrical gradients. Biochemistry. 1977 Jul 12;16(14):3227–3235. doi: 10.1021/bi00633a029. [DOI] [PubMed] [Google Scholar]
- MacLeod R. A., Goodbody M., Thompson J. Osmotic effects of membrane permeability in a marine bacterium. J Bacteriol. 1978 Mar;133(3):1135–1143. doi: 10.1128/jb.133.3.1135-1143.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishita H., Takada H. Sparing effect of lithium ion on the specific requirement for sodium ion for growth of Vibrio parahaemolyticus. Can J Microbiol. 1976 Sep;22(9):1263–1268. doi: 10.1139/m76-187. [DOI] [PubMed] [Google Scholar]
- Niiya S., Yamasaki K., Wilson T. H., Tsuchiya T. Altered cation coupling to melibiose transport in mutants of Escherichia coli. J Biol Chem. 1982 Aug 10;257(15):8902–8906. [PubMed] [Google Scholar]
- Niven D. F., MacLeod R. A. Sodium ion-proton antiport in a marine bacterium. J Bacteriol. 1978 Jun;134(3):737–743. doi: 10.1128/jb.134.3.737-743.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niven D. F., MacLeod R. A. Sodium ion-substrate symport in a marine bacterium. J Bacteriol. 1980 May;142(2):603–607. doi: 10.1128/jb.142.2.603-607.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce S. M., Hildebrandt V. A., Lee T. Third system for neutral amino acid transport in a marine pseudomonad. J Bacteriol. 1977 Apr;130(1):37–47. doi: 10.1128/jb.130.1.37-47.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUGINO Y., MIYOSHI Y. THE SPECIFIC PRECIPITATION OF ORTHOPHOSPHATE AND SOME BIOCHEMICAL APPLICATIONS. J Biol Chem. 1964 Jul;239:2360–2364. [PubMed] [Google Scholar]
- Sorensen E. N., Rosen B. P. Effects of sodium and lithium ions on the potassium ion transport systems of Escherichia coli. Biochemistry. 1980 Apr 1;19(7):1458–1462. doi: 10.1021/bi00548a030. [DOI] [PubMed] [Google Scholar]
- Srivastava V. S., MacLeod R. A. Nutritional requirements of some marine luminous bacteria. Can J Microbiol. 1971 May;17(5):703–711. doi: 10.1139/m71-113. [DOI] [PubMed] [Google Scholar]
- Stock J., Roseman S. A sodium-dependent sugar co-transport system in bacteria. Biochem Biophys Res Commun. 1971 Jul 2;44(1):132–138. doi: 10.1016/s0006-291x(71)80168-7. [DOI] [PubMed] [Google Scholar]
- Thompson J., MacLeod R. A. Na+ and K+ gradients and alpha-aminoisobutyric acid transport in a marine pseudomonad. J Biol Chem. 1973 Oct 25;248(20):7106–7111. [PubMed] [Google Scholar]
- Tokuda H., Sugasawa M., Unemoto T. Roles of Na+ and K+ in alpha-aminoisobutyric acid transport by the marine bacterium Vibrio alginolyticus. J Biol Chem. 1982 Jan 25;257(2):788–794. [PubMed] [Google Scholar]
- Tokuda H., Unemoto T. Characterization of the respiration-dependent Na+ pump in the marine bacterium Vibrio alginolyticus. J Biol Chem. 1982 Sep 10;257(17):10007–10014. [PubMed] [Google Scholar]
- Tsuchiya T., Lopilato J., Wilson T. H. Effect of lithium ion on melibiose transport in Escherichia coli. J Membr Biol. 1978 Jul 21;42(1):45–59. doi: 10.1007/BF01870393. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T., Oho M., Shiota-Niiya S. Lithium ion-sugar cotransport via the melibiose transport system in Escherichia coli. Measurement of Li+ transport and specificity. J Biol Chem. 1983 Nov 10;258(21):12765–12767. [PubMed] [Google Scholar]
- Tsuchiya T., Wilson T. H. Cation-sugar cotransport in the melibiose transport system of Escherichia coli. Membr Biochem. 1978;2(1):63–79. doi: 10.3109/09687687809063858. [DOI] [PubMed] [Google Scholar]
- Unemoto T., Hayashi M., Hayashi M. Na+-dependent activation of NADH oxidase in membrane fractions from halophilic Vibrio alginolyticus and V. costicolus. J Biochem. 1977 Nov;82(5):1389–1395. doi: 10.1093/oxfordjournals.jbchem.a131826. [DOI] [PubMed] [Google Scholar]
- Unemoto T., Hayashi M. NADH: quinone oxidoreductase as a site of Na+-dependent activation in the respiratory chain of marine Vibrio alginolyticus. J Biochem. 1979 Jun;85(6):1461–1467. doi: 10.1093/oxfordjournals.jbchem.a132474. [DOI] [PubMed] [Google Scholar]
- Wong P. T., Thompson J., MacLeod R. A. Nutrition and metabolism of marine bacteria. XVII. Ion-dependent retention of alpha-aminoisobutyric acid and its relation to Na+ dependent transport in a marine pseudomonad. J Biol Chem. 1969 Feb 10;244(3):1016–1025. [PubMed] [Google Scholar]
- Yazyu H., Shiota S., Futai M., Tsuchiya T. Alteration in cation specificity of the melibiose transport carrier of Escherichia coli due to replacement of proline 122 with serine. J Bacteriol. 1985 Jun;162(3):933–937. doi: 10.1128/jb.162.3.933-937.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



