Abstract
We have shown that polyamide nucleic acids (PNAs) targeted to the PBS (PNAPBS) and A-loop (PNAA-loop) sequences, when transfected into cells, inhibit HIV-1 replication by blocking the initiation of reverse transcription via destabilizing tRNA3Lys primer from the viral genome. Here we demonstrate that both PNAPBS and PNAA-loop conjugated with the membrane-transducing peptide (MTD) vectors penetratin and Tat are rapidly taken up by cells and inhibit HIV-1 replication. Moreover, MTD peptide conjugates of PNAPBS and PNAA-loop displayed potent virucidal activity against HIV-1. Brief exposure of HIV-1 virions to these conjugates rendered them noninfectious. The IC50 values for virucidal activity were in the range of ~50 nM; IC50 values for inhibition of HIV-1 replication/infection were 0.5 μM–0.7 μM. The virucidal property of these conjugates suggests that a cocktail of anti-HIV-1 PNA-MTD peptide conjugates targeting critical regions of the HIV-1 genome could serve as a prophylactic agent for inactivating HIV-1 virions after exposure to HIV-1.
Keywords: HIV-1, polyamide nucleic acid, membrane transducing peptides, PNA-peptide conjugate, primer binding site, virucidal activity, A-loop
INTRODUCTION
Despite the best efforts of the research community and pharmaceutical companies all over the world, the AIDS pandemic continues to grow. Developing vaccines for human immunodeficiency virus type-1 (HIV-1), the etiological agent of AIDS, is predicted to be the best means of preventing the spread of this virus. However, the recent failure of an experimental vaccine based on recombinant gp-120 protein in phase III trials (Cohen, 2003) and the slow progress of other vaccine trials (Letvin, 2005) portends that development of an effective vaccine will take longer than anticipated. While highly active antiretroviral therapy (HAART) has demonstrated success in treating AIDS patients, it is not curative. Most patients eventually develop resistance to HAART because of the selection of genetic mutations in the virus results in resistance to drugs. HAART also does not help prevent the spread of HIV-1 infections, a goal that is essential to for control the AIDS pandemic.
Because the majority of new infections occur through sexual contacts (http://www.cdc.gov/hiv/stats/2003SurveillanceReport/table3.htm), new strategies, including the widespread use of topical formulations of virucidal agents, need to be rapidly deployed. Enfuvirtide (T-20) is the only FDA-approved inhibitor of HIV-1 entry in clinical use (Kilby et al., 1998). This drug is reported to have synergistic efficacy in the presence of PRO 542, another potent entry inhibitor (Jacobson et al., 2004, Nagashima et al., 2001). Recently, it has been suggested that the N-terminal 33-amino-acid long sequence of gp120 also acts as a fusion inhibitor (Gerber et al., 2004). It also has been suggested that various surfactants have anti-HIV-1 virucidal activity (Bestman-Smith et al., 2001). Nonoxynol-9, a well-known spermicide, has been reported to inactivate HIV-1 in vitro (Bourinbaiar and Lee-Huang, 1994). Unfortunately, studies have revealed that after prolonged and frequent use of Nonoxynol-9, lesions and perforations may develop in the vaginal surface, rendering women who use this ointment more susceptible to HIV-1 infection (Van Damme et al., 2002).
Synthesis of proviral DNA, an essential step in the life cycle of HIV-1, occurs exclusively in the multi-step process of reverse transcription. The 5′(R-U5-PBS) untranslated region of HIV-1 viral genome (1–333 nt) is a highly conserved region containing several immutable stretches. In the 5′-untranslated region, the 18-mer stretch from 183–201 nucleotides is known as a primer binding site (PBS). Because the 3′ terminals 18-nucleotides of the cellular tRNA3Lys are complementary to the PBS, they prime onto the PBS and initiate the reverse-transcriptase-catalyzed synthesis of proviral DNA. This unique feature of immutable PBS makes it as a potentially useful therapeutic target.
Another important site is the A-loop region, which is located upstream to the PBS-region from 168–173 nt. This region is responsible for the selection of and, thereafter, the exclusive interaction with the cellular tRNA3Lys. Although several cellular tRNAs are packaged along tRNA3Lys, HIV-1 is quite conservative in using tRNA3Lys as primer for the initiation of reverse transcription (Mak and Kleiman, 1997, Isel et al., 1995). Therefore, sequestering the A-loop region and making it unavailable for interaction with tRNA3Lys will ultimately interfere with the selection and binding of the initiating primer.
Polyamide nucleic acids (PNAs), a novel class of DNA analogs, were first synthesized as potent antisense agents; these analogs are comprised of a 2-aminoethyl glycine backbone with the purine and pyrimidine bases attached via an ethylene linker (Nielsen et al., 1991). Being a structural, but not chemical mimic of the sugar phosphate backbone of DNA/RNA, PNAs are not recognized as substrates for cellular nucleases and proteases (Demidov et al., 1994). We have demonstrated that the PBS sequence is a unique target that can be used to halt viral replication by sequestering this region and blocking the initiation of reverse transcription using complementary naked PNA (Lee et al., 1998; Kaushik and Pandey 2002) We have also shown that a 15-mer PNA targeted to the A-loop sequence can specifically sequester the target sequence and inhibit the initiation of reverse transcription (Kaushik et al., 2001). The major drawback associated with PNAs as antisense agents is their low level of uptake by cells. The peptide backbone confers a hydrophobic character and a net neutral charge to PNAs, making them unlikely candidates for cellular uptake. Many different approaches, including microinjection (Taylor et al., 1997), PNA-DNA co-transfection (Nulf and Corey, 2004), and electroporation (Shammas et al., 2004), have been taken in attempts to overcome this obstacle. In the present study, we have addressed the bio-delivery issue by conjugating 16-mer PNAs complementary to the primer-binding site and the A-loop region of the viral genome with two different peptide sequences: penetratin, a 16-amino acid antennapedia peptide derived from the third helix of the homeodomain and Tat peptide, a 13-amino acid peptide comprising 48–60 residues of the human immunodeficiency virus-1 Tat protein. Both penetratin (Derossi et al., 1998) and Tat peptides (Vives et al., 1997) have been shown to be potent membrane-transducing peptides (MTD).
RESULTS
Binding affinity of the PNAs remains unaltered when conjugated with MTD peptides
PNAs form more stable PNA: RNA and PNA: DNA hybrids, as confirmed by the finding of relatively much higher Tms than those of their DNA: RNA and DNA: DNA homologues (Nielsen et al., 1991, Lee et al., 1998). To examine the influence of conjugated MTD peptides on the binding affinity of PNAs for their target sequence, we performed a gel mobility shift assay by incubating U5-PBS RNA with increasing concentrations of PNAPBS-Penetratin and PNAPBS-Tat. At a 1:4 molar ratio of U5-PBS to PNA-peptide conjugate, the shift was visible; at an equimolar ratio, a >90% shift was obtained (Fig. 2). Similar results were obtained with PNAA-loop-MTD peptide conjugates (results not shown). There is no apparent difference between the binding pattern of PNA alone and that of the PNA-MTD peptide conjugates, confirming that the affinity of the PNA remains unaltered even after conjugation with the MTD peptides.
Figure 2. Binding affinity of PNAPBS-MTD peptide conjugates to their target sequences.
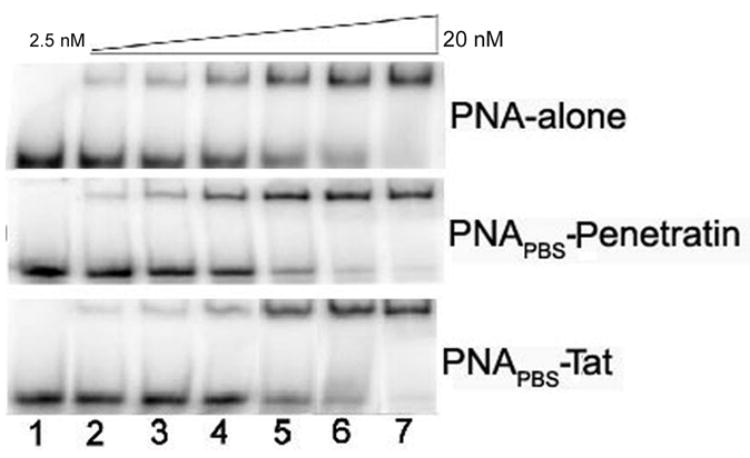
The gel mobility shift assay was performed to assess the binding affinity of the PNAPBS-MTD peptide conjugates in comparison to that of unconjugated naked PNAPBS control. PNAPBS-Penetratin, PNAPBS-Tat, and unconjugated naked PNAPBS were incubated at varying concentrations with 10 nM of internally 32P-labeled U5-PBS RNA transcript in the binding buffer for 30 min at room temperature. The incubated samples were then loaded on a pre-run 8% native polyacrylamide gel. The RNA: PNA complex was resolved and separated from free RNA by running at 150 V for 3 h. Lane 1 represents U5-PBS RNA alone; lanes 2 to 7 represent increasing amounts of PNAPBS or its MTD conjugates, ranging through 2.5, 5.0, 7.5, 10, 15, and 20 nM. (A) PNAPBS alone; (B) PNAPBS-Penetratin; (C) PNAPBS-Tat.
PNA-MTD peptide conjugates are efficiently taken up by the cell
It is expected that the extent of cellular uptake of PNA cargo mediated by MTD peptides may depend on the solubility of PNA, which is mainly governed by its sequence and composition. A pyrimidine base content of >40% enhances the solubility of PNAs, while a composition of purine bases >60% decreases their solubility. Pyrimidine bases in PNAPBS constitute 50%, while those of PNAA-loop constitute 60% of total bases, rendering them highly soluble in buffer solution under physiological conditions. The conjugation of PNAs with highly polar MTD peptides may also enhance the solubility of even those PNAs that are less soluble in polar solvents. We examined the cellular uptake of these PNAs conjugated to either Penetratin or Tat peptide in CEM cells, using flow cytometry. The cells were incubated for 1 min with increasing concentrations of the fluorescein-labeled PNAPBS-peptide conjugates at 4°C and 37°C. After washing the cells with phosphate-buffered saline and resuspending them in RPMI medium with 2% fetal calf serum, flow cytometry analysis was performed. Figure 3 shows a representative result for the experiments obtained with PNAPBS-MTD peptide conjugates. The uptake pattern of PNAPBS-Penetratin was found to be rapid and, at a 300-nM concentration, almost 100% of the cells were positive for fluorescein. While the uptake pattern of PNAPBS-Tat was found to increase linearly, only 80% of the cells were fluorescence-positive at 500 nM (Fig. 3, Panel A & B). Similar results were obtained with PNAA-loop-MTD peptide conjugates (data not shown). To rule out any artifacts due to nonspecific adherence of the fluorescein-tagged PNA-MTD peptide conjugates to the outer surface of the cell membrane, the cells were further treated with 0.01% trypsin for 10 min, washed, and examined by flow cytometry. It was observed that trypsin treatment resulted in only a marginal 5%–8% reduction in the fluorescence of cells, while the overall uptake pattern remained unchanged (Fig. 3, Panel C & D). For visual demonstration of the uptake and cellular localization of PNA-peptide conjugates, the cells incubated with the flu-PNAPBS-peptide conjugate were examined by fluorescence microscopy. As shown in the figure (Fig. 4) the PNA-penetratin conjugate is efficiently internalized and localized in the cytoplasm, the site for reverse transcriptase process.
Figure 3. Flow cytometry analysis of uptake of anti-HIV-1 PNAPBS-MTD peptide conjugates.
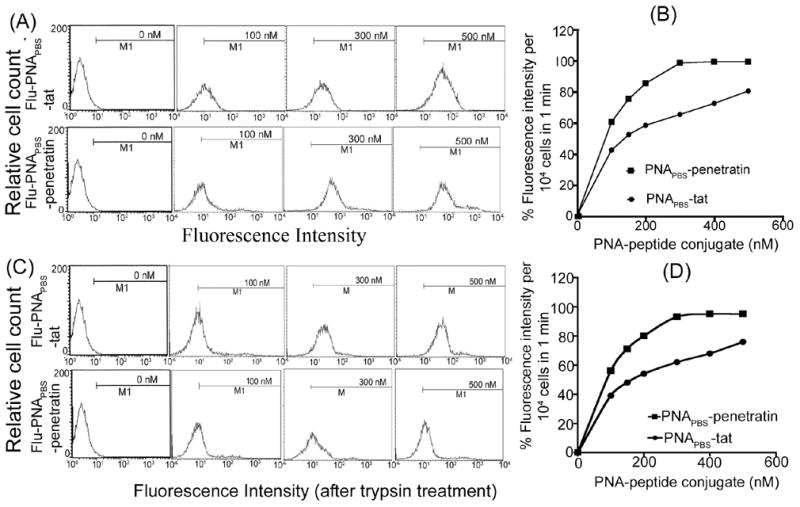
CEM cells (0.5 × 106) were incubated with increasing concentrations of PNAPBS-Tat and PNAPBS-Penetratin for 1 min at 4°C and 37°C. After washing thoroughly with phosphate buffered saline solution, the cells were resuspended in RPMI media with 2% FCS. (A) Flow cytometry data at 37°C in the presence of propidium iodide (B) The percent of FITC-uptake per 104 cells as a function of the concentration of PNAPBS-MTD peptide conjugates; (C) The flow cytometry data after trypsin treatment for 10 min; (D) The percent uptake per 104 cells after trypsin treatment.
Figure 4.
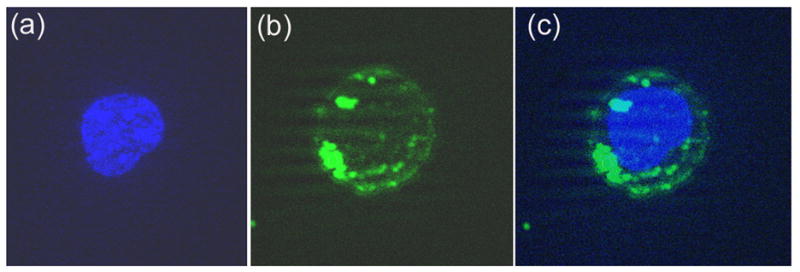
Fluorescence microscopy of the uptake of fluorescein-labeled PNAPBS-penetratin in CEM cells. The cells were incubated with fluorescein-labeled PNAPBS-penetratin conjugate for 30 min and then fixed and stained as described in the Materials and Methods. An aliquot of the cells was examined using the ZEISS Axiovert 200M microscope. Panel (a)-nucleus stained with DAPI; Panel (b)- cell cytoplasm showing bright green fluorescence of the fluorescent PNA; Panel (c)-nuclei merged with cell cytoplasm.
Uptake kinetic studies suggest cooperative interaction
The mechanism by which Penetratin and Tat peptide enter the mammalian cells is still not entirely clear. The uptake mechanism for Penetratin and Tat peptides has been shown to occur via an energy-independent nonendocytotic process, as these peptides are efficiently taken up by cells even at 4°C (Derossi et al., 1998, Vives et al., 1997). Neither sequence-specific inversion nor synthesis with D-amino acids alters the cellular uptake of Antennapedia (Penetratin) or Tat, indicating that interaction with chiral receptors is not the major pathway of entry into cells (Derossi et al., 1996). Recent reports have contradicted earlier findings regarding the uptake pattern of Tat peptide, suggesting that a specialized form of endocytosis has a major function in this process (Brooks et al., 2005; Kaplan et al., 2005). Flow cytometry revealed that a similar pattern of uptake was exhibited by PNA-MTD peptide conjugates. Because flow cytometry of uptake provides only qualitative data, we also used I125-labeled PNA-MTD peptide conjugates to determine the uptake kinetics of PNA-MTD peptide conjugates. The PNA moiety of the conjugate was tagged with radioiodine via a lone Tyr residue at its C-terminus, while MTD peptide devoid of Tyr residue remained attached at the N-terminus of PNA. The CEM cells were incubated with varying concentrations of individual radiolabeled PNA-MTD peptide conjugates for 30 s at room temperature followed by filtration and washing of the glass-fiber (GF-B, Schleicher and Schuell, Keene, NH) filters. Based on the specific radioactivity of radioiodinated conjugates, the actual conjugate concentration internalized by the cells was determined by measuring the radioactivity in the cells by gamma counting. We observed that cellular uptake of individual anti-HIV-1 PNA-MTD peptide conjugates displayed a sigmoidal curve, suggesting cooperative interaction between the conjugates and the cell membrane. The cooperative index was obtained from a Hill plot of the uptake data (Fig. 5). This index is the ratio of substrate concentration ([S]0.9/[S]0.1) at 0.9 Vmax and 0.1 Vmax of uptake, and is a measure of the amount of conjugate that would be required to increase the uptake velocity from 0.1 Vmax to 0.9 Vmax. The cooperative indices for PNAPBS-Penetratin and PNAPBS-Tat were 6.85 and 8.0, respectively, suggesting that an increase in their Vmax from 0.1 to 0.9 can be easily achieved by increasing their respective concentrations by 6.85- and 8.0-fold, respectively.
Figure 5. Uptake kinetics of PNAPBS-MTD peptide conjugates.
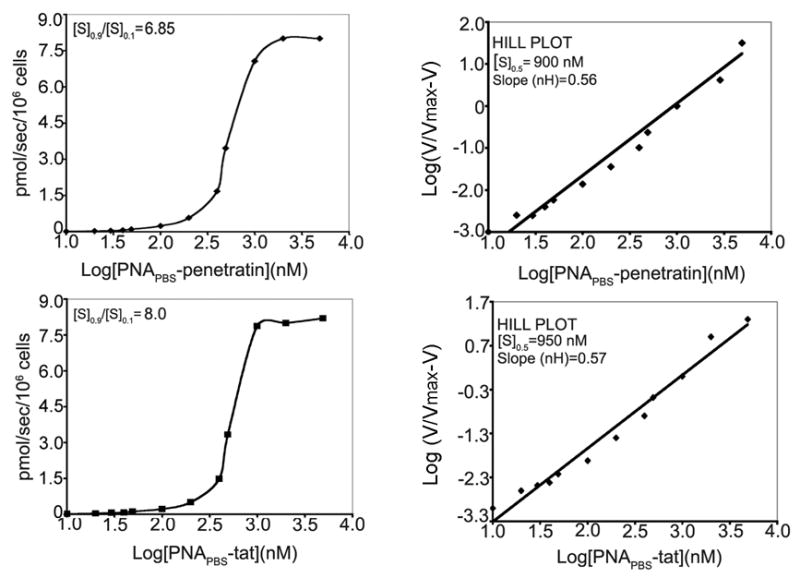
CEM cells (0.5 ×106) were incubated with either I125-labeled PNAPBS-Penetratin or PNAPBS-Tat conjugate for 30 sec at room temperature. The cells were filtered on glass fiber filters (GF-B) and washed with phosphate buffered saline. The incorporated radioactivity was counted in a gamma counter. The cooperative index ([S]0.9/[S]0.1) was determined by plotting the uptake of conjugate against the log of conjugate concentration as shown in the left panel. In the right panel, the log of the conjugate concentration was plotted against log V/Vmax-V to obtain a Hill plot. The value for Hill coefficient (nH), as well as [S] 0.5, denoting the concentration of conjugate at which uptake velocity is 0.5 Vmax, was determined from the slope of the Hill plot.
A Hill plot of the uptake was calculated to determine the nH values for individual PNA-MTD peptide conjugates. The [S]0.5 and nH values for individual PNA-MTD peptide conjugates were determined from this plot, where [S]0.5 value indicates the concentration of the conjugate at which uptake velocity is 0.5 of Vmax. The [S]0.5 values were, respectively, 900 nM and 950 nM for PNAPBS-Penetratin and PNAPBS-Tat. The values of the Hill coefficient (nH) were less than 1 for both of the PNA-MTD peptides, suggesting that the cooperativity of uptake is not due to multiple interaction sites on the cell membrane. In an uptake system following sigmoidal kinetics, the velocity of uptake can be significantly enhanced by a small increase in substrate; in contrast, an uptake system following hyperbolic kinetics requires a large amount of substrate to achieve a similar increase in velocity. The sigmoidal kinetics we observed by the PNA-MTD conjugates can be viewed as favorable criteria for their qualification as potential virucidal agents.
PNA-MTD peptide conjugates show efficient anti-HIV-1 activity
We have shown that anti-HIV-1 PNAs targeting the PBS region, as well as the A-loop region upstream to the PBS region in the 5′-UTR of the HIV-1 genome, inhibit HIV-1 replication when transfected into cells (Kaushik and Pandey, 2002; Kaushik et al., 2001). Exploring means of improving the bio-delivery of anti-HIV PNAs, we conjugated them with membrane-transducing peptides via a disulfide bond. We conjugated Penetratin, a 16-amino-acid homeodomain protein, and Tat, a 13-amino-acid protein comprising 48–60 amino acids of the HIV-1 Tat peptide, with both the anti-PBS and anti-A-loop PNAs. We then examined the efficiency of PNA-peptide conjugates in inhibiting the replication of HIV-1. For this purpose activated PBMC as well as CEM T-lymphocyte cells infected with pseudo HIV-1 virions carrying firefly luciferase reporter gene were grown in the presence of increasing concentrations of PNA-peptide conjugates. The CEM cells were harvested after 48 hrs while PBMC cells were harvested after 7 days. The cells were washed once with phosphate-buffered saline, then lysed. After normalization of the protein content, an aliquot of the extract was analyzed for quantitative levels of luciferase expression to evaluate the level of HIV-1 infection. Median dose values (IC50) were calculated by plotting concentration versus percentage inhibition of luciferase expression. The median dose effect values (IC50) of PNAPBS–MTD conjugates were in the range of 0.28–0.32μM with PBMC were while 0.53 to 0.55 μM with CEM cells. Similar results were also obtained with PNAA-loop-MTD peptide conjugates (Fig. 6, Table 1A).
Figure 6. The antiviral efficacy of PNAPBS- and PNAA-loop-MTD peptide conjugates.
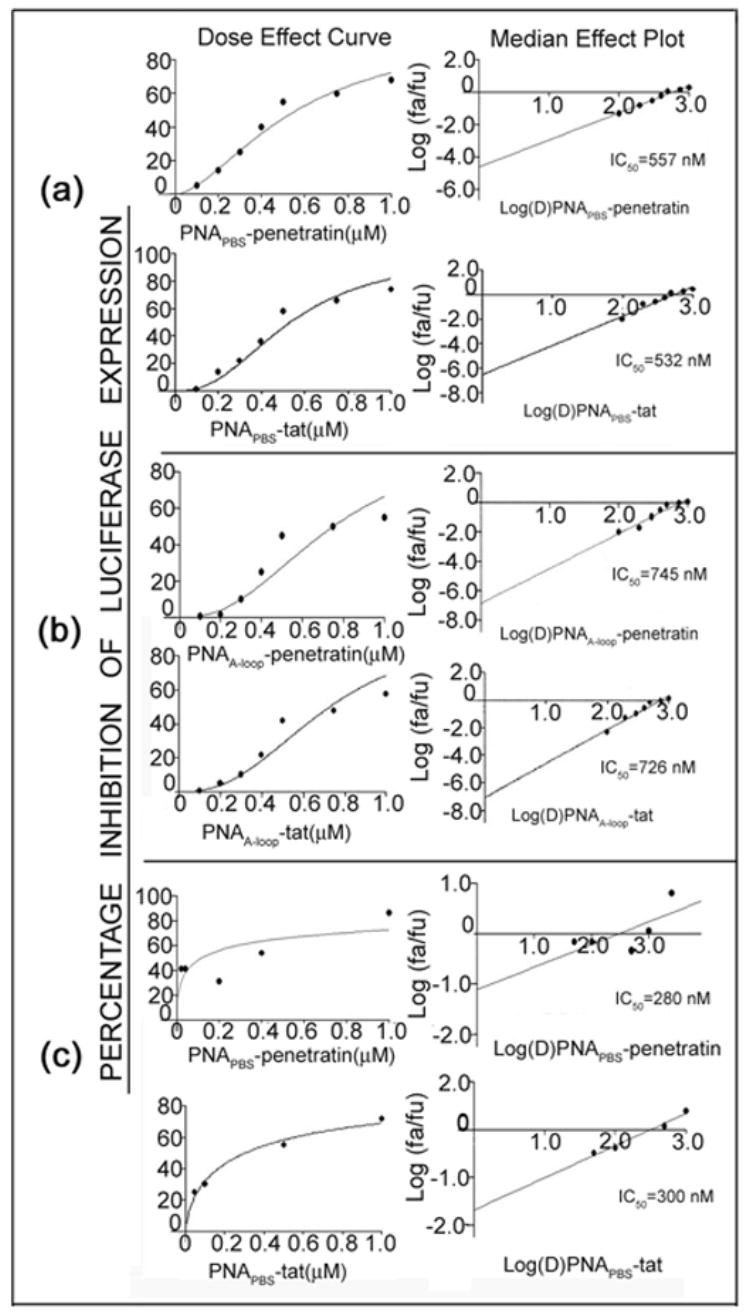
T lymphocyte cells were first infected with pseudo-HIV-1 virions for 2 h in the presence of varying concentrations of either PNAPBS-MTD peptide conjugates (a) or PNAA-loop-MTD conjugates (b). Unconjugated PNA and peptides alone were included as control. The infected cells were further grown in the presence of increasing amounts of respective PNA-MTD conjugates for 48 h. The cells were harvested, washed, and lysed. An aliquot of the cell lysate was examined for luciferase activity and the percentage of inhibition of luciferase expression in treated cells was determined with respect to that in untreated control. Median-effect plots and dose-effect curves were calculated. The dose median values (IC50) for individual PNAPBS- and PNAA-Loop-MTD peptide conjugates are indicated. Antiviral efficacy was also examined in PHA stimulated peripheral blood mononuclear lymphocyte (PBMC) cells using PNAPBS-peptides (c) and the infected cells were grown for seven days before harvesting. The cell lysate was examined for luciferase activity as described above. Naked PNA and peptide alone had no effect on antiviral efficacy as naked PNA are found to be incompetent of cellular uptake and presence of peptide had no effect on viral infectivity. In both these cases the levels of luciferase expression was found to be similar to the untreated virions, therefore no values can be generated to put in the CalcuSyn software.
Table 1.
Table 1A: Antiviral and virucidal activity of PNA-peptide conjugates targeting PBS and A-loop sequences in the U5-PBS region of the HIV-1 genome. The antiviral and virucidal activities of the conjugates were assessed in complete RPMI medium containing 10% fetal calf serum as described in the Materials and Methods, The median effective doses (IC50) of these conjugates were calculated from data shown in Figs. 5 and 6AB. Table 1B. Virucidal activity of the PNA-peptide conjugates in the presence of different concentration of serum. The infectious HIV-1 virions were first preincubated with different concentration of PNAPBS-peptide conjugates in the presence of 20–100% fetal calf serum and their infectivity was then assessed in CEM cells as described in the Materials and Methods. The IC50 for virucidal activity at different serum concentrations were calculated from dose effect curves and median-effect plots as described in the Materials and Methods. Representative plots at 60% and 100% serum concentrations are shown in Fig. 6 C and D.
| PNA-peptide conjugate | Virucidal activity IC50[nM] | Inhibitory effect on HIV replication IC50 [nM] | |||
| CEM cells | PBMC | ||||
| PNAA-loop-pen | 56 | 745 | ND | ||
| PNAA-loop-tat | 47 | 726 | ND | ||
| PNAPBS-pen | 49 | 557 | 280 | ||
| PNAPBS-tat | 50 | 532 | 320 | ||
| PNA-peptide conjugate | % of Fetal Calf Serum | ||||
| 20 | 40 | 60 | 80 | 100 | |
| Virucidal activity (IC50 in nM) | |||||
| PNAPBS-pen | 50 | 58 | 54 | 58 | 50 |
| PNAPBS-tat | 52 | 58 | 58 | 59 | 60 |
PNA-MTD peptide conjugates as potent virucidal agents
We found that PNA-MTD peptides targeting the PBS and A-loop sequences were efficient in inhibiting the HIV-1 replication/infection. We then examined whether these conjugate are able to block HIV-1 infection by inactivating the virion particles (virucidal) or whether they have an antiviral effect only after their entry into the cell. We evaluated the virucidal activity of these conjugates by a brief exposure of purified HIV-1 virions to increasing concentrations of individual PNA-MTD conjugates. We separated the virion particles from the free conjugates by ultracentrifugation and used them to infect lymphocytes. After growing the infected lymphocytes for 48 h, we examined the cell lysates for luciferase activity. The extent of luciferase activity was inhibited with the increasing concentrations of PNA-MTD conjugates with which virion particles were incubated. The median effective doses (IC50) were ≈ 50 nM for both PNAPBS-Tat and PNAPBS-Penetratin; those for PNAA-loop-Tat and PNAA-loop-Penetratin were 47nM and 56 nM, respectively (Fig. 7A, B, Table 1A).
Figure 7. Dose-effect curve (IC50) of virucidal activity of individual PNAPBS- and PNAA-loop-MTD peptide conjugates.
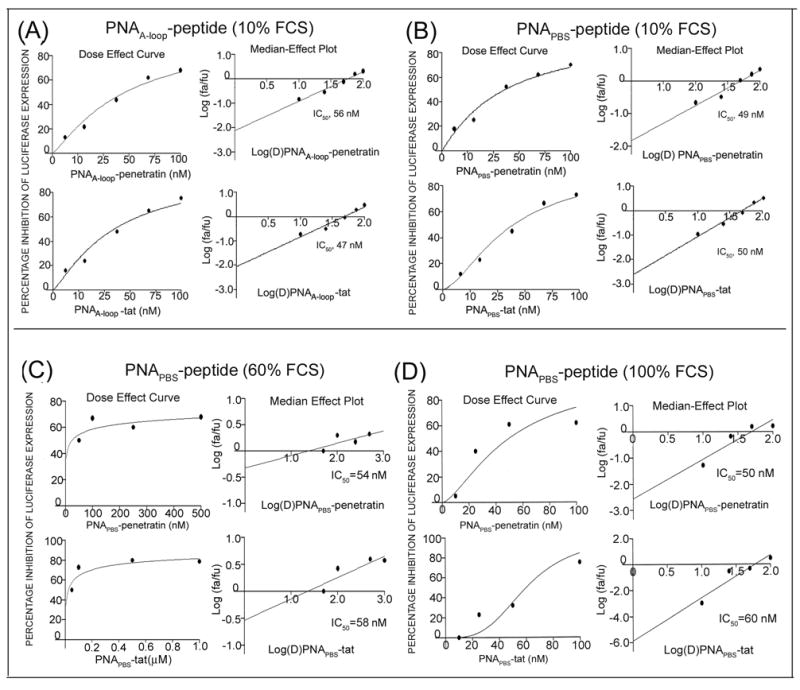
The HIV-1 virions (equivalent to 100 ng of p24) were first incubated with increasing concentrations of individual PNA-MTD peptide conjugates for 2 h in complete RPMI medium containing 10% FCS. Naked PNA and peptides alone were included as control. The pretreated virions were then used to infect the CEM cells. The infected cells were grown for 48 h and the extent of infection was determined by measuring the levels of luciferase reporter enzyme in cell lysate. Dose-effect curves and median-effect plots (IC50) were calculated. Dose-effect curves for virucidal activity of individual PNAA-Loop-MTD peptide conjugate (A), and individual PNAPBS-MTD peptide conjugates in the presence of 10% FCS (B), 60% FCS (C) and 100% FCS (D). No values could be generated for naked PNA and peptide alone.
Effect of increasing serum concentration on the virucidal activity of the conjugate: The observed virucidal activity of anti-HIV-1 PNA-peptide conjugate shown as above was noted in the complete RPMI medium containing 10% fetal calf serum. Since infectious HIV-1 virions circulate and survive in the blood serum, we also examined virucidal activity at higher serum concentration ranging from 20–100% serum. It was observed that increasing serum concentration has no significant effect on the virucidal activity of the conjugate (Fig. 7C, D, and Table 1B).
Inhibition of endogenous reverse transcription in HIV-1 virions pretreated with PNA-peptide conjugates
Among retroviruses, the synthesis of proviral DNA occurs by a multistep reverse transcription process. HIV-1 exclusively uses tRNA3Lys as the initiating primer as it anneals among the naturally occurring cellular tRNAs at the PBS and the upstream A-loop region in the 5′-UTR (Wakefield et al., 1995). We have shown that both unconjugated PNAPBS and PNAA-loop efficiently displace tRNA3Lys-U5-PBS complex and inhibit the initiation of reverse transcription (Kaushik et al., 2001, Kaushik and Pandey, 2002). Since HIV-1 virions pretreated with PNAPBS and PNAA-loop-MTD peptide conjugates were rendered noninfectious in the present study, we postulated that these conjugates might be readily internalized into the virion particles and bind to their target sequences on the viral genome. To test this hypothesis, we ultracentrifuged virion particles pretreated with varying concentrations of individual conjugates through a 20% sucrose cushion, then disrupted the virion pellets and examined them for endogenous reverse transcription. As shown in Figure 8, the initiation of endogenous reverse transcription was completely blocked in virion particles pretreated with both PNAPBS-peptide and PNAA-loop-peptide conjugates. The virions untreated or treated with scrambled PNA-MTD conjugates (CGG-ACT-AAG-TCC-ATT-GC) were also included as controls. In both the cases the reverse transcription products were larger than 490 nucleotides, indicating that strand transfer had taken place after synthesis of (-) strand strong-stop DNA, suggesting that the conjugates permeated the virus envelope and bound to their cognate sequences on the viral RNA, thus preventing the initiation of the reverse transcription. It can be argued that the conjugates may have tightly attached to the external surface of the viral envelope and bound to the target only after the virions were disrupted. However, this possibility is ruled out by the fact that virions envelope derived from cell membrane, permeation of conjugates into virion particles would likely occur by a mechanism similar to that of cellular uptake, which was not affected by trypsin treatment after incubation with fluorescein-labeled conjugate (data not shown). Further, the naked PNA alone when incubated with virion particles had no influence on endogenous RT activity (data not shown). The use of I125 labeled PNATAR-Transportan conjugate showed that, after incubation with virions, the radioactive PNA conjugate co-sedimented with the virion particles (Chaubey et al., 2005). That finding indicates that virions are highly vulnerable to treatment with targeted anti-HIV-1 PNA-MTD peptide conjugates as judged by loss of their infectivity upon exposure to individual conjugates, indicating the potential of this class of compounds as potent virucidal agents against HIV-1.
Figure 8. Endogenous RT activity of HIV-1 virions after brief exposure to individual PNAPBS- and PNAA-loop-MTD peptide conjugates.
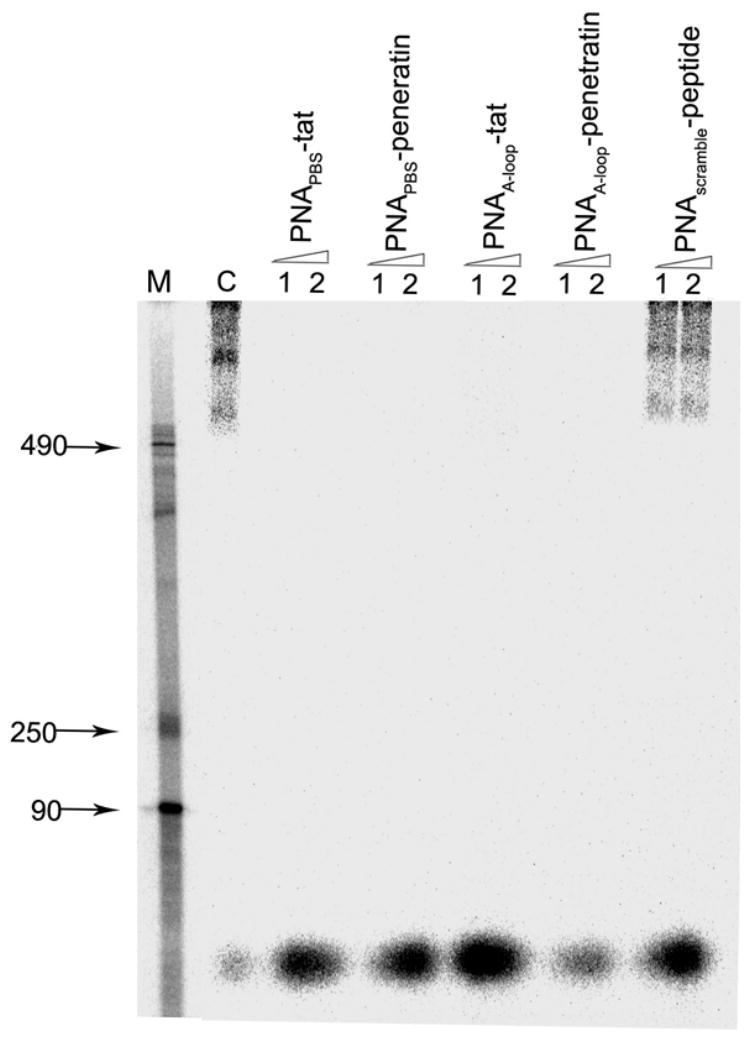
The virion particles pretreated with individual PNA-MTD peptide conjugates at 250 nM (lane 1) and 500 nM (lane 2) concentrations were centrifuged at 100,000 g through a 20% sucrose cushion to separate them from free PNA-MTD peptide conjugates. The scrambled PNA-MTD-peptide conjugate was also used separately as the negative control. The virion pellets were disrupted in disruption buffer. Aliquots of the disrupted virions were examined for endogenous RT activity. The reaction products, after phenol-chloroform extraction and ethanol precipitation, were resolved on 6% denaturing polyacrylamide gel. The control (C) represents the endogenous RT activity in untreated disrupted HIV-1 virions showing products resulting from the strand transfer step. The lane marked M represents the DNA markers corresponding to 90, 250, and 490 bases.
PNA-peptide conjugates have no toxic effect on cellular proliferation: Incorporation of [3H] thymidine was studied to examine the effect of PNA-Speptide conjugates on cellular proliferation. The cells were grown in presence of varying concentrations of PNA-peptide conjugates (1–10 μM) and assayed for the incorporation of [3H] thymidine in the cellular DNA. The results shown in Fig. 9 suggest that PNA-peptide conjugates are well tolerated and therefore not toxic to the cells at the indicated concentrations.
Figure 9.
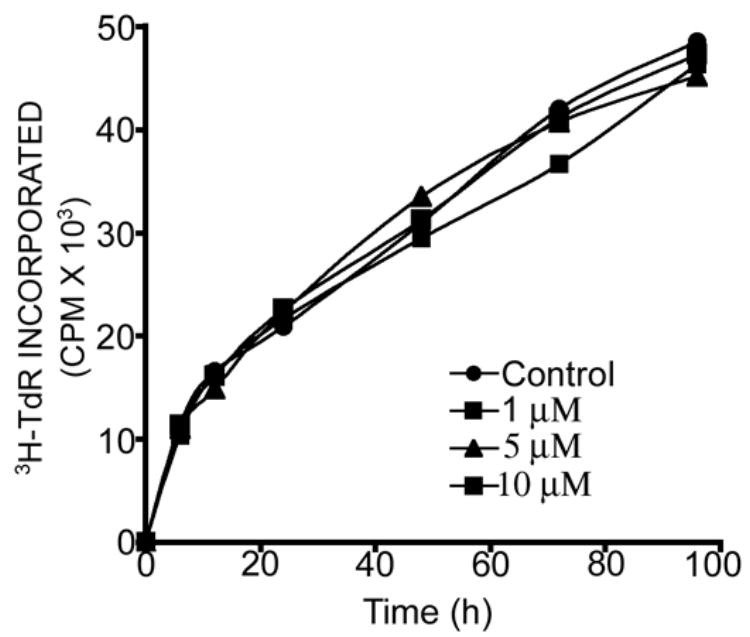
Effect of PNA-peptide conjugate on [3H] thymidine incorporation into cellular DNA in CEM cells: CEM cells were grown in the absence and presence of PNAPBS-penetratin (1 μM, 5 μM and 10 μM). The culture media was supplemented with 10 μCi of [methyl-3H] Thymidine/mL. The cells were harvested at 6, 12, 18, 24, 48, 72 and 96 h. The cells were assayed for the amount of [3H]-Thymidine (TdR) incorporated into DNA by using TCA precipitation assay.
DISCUSSION
In pursuit of a new microbicidal/virucidal agent for use against HIV-1, we have targeted various conserved regions in the U5-PBS region of viral RNA. In the life cycle of retroviruses, a family to which HIV-1 belongs, reverse transcription has a pivotal function. HIV-1 uses tRNA3Lys, one of several cellular tRNAs packaged during virion assembly, exclusively as primer for the initiation of the reverse transcription of minus-strand strong-stop DNA (Mak & Kleiman et al., 1997). By virtue of complementarity, 18-nucleotides of the 3′-terminal of tRNA3Lys anneal to the primer binding site, located downstream from the U5 region of the 5′-LTR from 183–201 of the viral RNA genome. Inducing mutations in the primer binding site to make it complementary to tRNAHis transiently results in the production of infectious virus that reverts back to use tRNA3Lys in in-vitro cell culture (Wakefield & Morrow, 1996; Zhang et al., 1998). This set of experiments demonstrates that HIV-1 PBS, given appropriate mutational changes, can use tRNAIle and tRNAHis as primers. However, the appearance of viruses may be delayed as compared to that of the wild type one produced while using tRNA3Lys (Wakefield et al. 1995). When non-tRNA3Lys primers are used for reverse transcription, defective replication patterns are seen. Also, the rates of virus production using tRNAHis and tRNAMet are 1/10 and 1/100, respectively, as compared to viruses produced using tRNA3Lys (Wei et al., 2005). The viruses produced in this way show varied capacities for” infectivity, as the virions using tRNAMet are produced at 1/100 that of wild type, while virions made using tRNAHis do not produce virions at all. In U5-PBS, another region also reportedly has a crucial function in the selection and stabilization of tRNA3Lys. That region on viral genome is the A-loop region (nucleotides 168–173), so named because it comprises four consecutive “A” located upstream to the U5 PBS region. This A-rich loop anneals with the U-rich region of tRNA3Lys, thus providing further selectivity and stability of the tRNA3Lys primer (Isel et al. 1995).
These observations suggest that highly conserved and immutable PBS and A-loop sequences in the U5-PBS region may be ideal targets for intervention by HIV-1 replication. Han et al. (2004) have reported that transfection of a 21-nucleotide siRNA targeted to the PBS site exerts an inhibitory effect on HIV-1 production after HIV-1 infection. In contrast, targeting gag mRNA using siRNA has a transient effect on virus production. After a few days, virus production overcomes the effect of siRNA and reverts to normal. However, similarly targeting the A-loop region with siRNA showed no consistent decrease in virus production (Han et al., 2004). In contrast, targeting the A-loop region with PNAA-loop-MTD peptide conjugates resulted in decreased HIV-1 replication/infection. It is possible that once the PNA sequence recognizes and binds to its target, the secondary structure of RNA is lost, resulting in inability of the A-loop to provide an annealing site to tRNA3Lys. Besides these points, we found that the PNA bound upstream of the PBS sequences resulted in complete blockage of reverse transcription (Fig. 8). It has been reported that PNA is able to invade double-stranded DNA and bind to its target, displacing the second strand into a D-loop structure (Nielsen et al., 1991).
Earlier, we demonstrated that a 16-mer polyamide nucleic acid complementary to the 5 nucleotides of the PBS and the 11 upstream sequences spanning to the stem region of the A-loop inhibits virus replication in cell culture (Kaushik and Pandey 2002). Although we noted strong antiviral efficacy when naked PNAPBS was transfected into cells, issues about bio-delivery needed to be solved to achieve the practical potential of this approach. In the present study, we have demonstrated that both PNAPBS and PNAA-loop, after conjugation with membrane-transducing peptides, are rapidly taken up by cells when added into the culture medium. Further, we found that cellular uptake of these PNA-MTD peptide conjugates was neither due to endocytosis nor receptor-mediated, since identical uptake patterns were observed at both physiological and reduced temperatures (Fig. 3). This finding was consistent with our earlier observations (Tripathi et al., 2005; Chaubey et al., 2005) that uptake of another anti-HIV-1 PNA-MTD peptide conjugate targeted to HIV-1 LTR was not affected after the treatment of cells with phenylarsine oxide, which selectively modifies and modulates their receptor functions.
The FACS studies using PNA-penetratin conjugates showed rapid and saturating cellular uptake, with 98% fluorescence-positive cells at subnanomolar concentration after 1 min of exposure to the fluorescence-labeled PNA-peptide conjugates. Our similar studies with PNA-Tat peptide were equally promising, showing almost 80% fluorescence-positive cells. Although the mode of entry of cell penetrating peptides (CPPs) remains unclear, since conflicting description of internalization process vary from energy independent cell penetration of membrane to endocytotic uptake. Some recent reports do suggest their entry via endosomal pathway (El-Andaloussi et al., 2005). More recent reports further suggest that mode of internalization of CPP is influenced by cargo attached to it (Tunnemann et al., 2006). The unconjugated Tat peptide has been shown to be taken up by endocytosis while in presence of a counteranion such as pyrenebutyrate it is taken up by direct membrane translocation resulting in diffuse cytosolic distribution (Takeuchi et al., 2006). We have earlier shown that uptake of PNATAR-Transportan conjugate is temperature independent and not affected by phenylarsine treatment of the cells suggesting non-involvement of endocytosis or receptor mediated internalization of this conjugate (Chaubey et al., 2005). In the present studies, we noted similar uptake pattern for both PNA-Penetratin and PNA-Tat conjugates. These observations for the uptake of PNA-peptide conjugates are in disagreement with the uptake of unconjugated Tat peptide which is ATP and temperature dependent indicating the involvement of endocytosis mechanism (Richard et al., 2003, 2005). Therefore it is possible that uptake of CPP alone and in conjugated form may widely differ and may be influenced by the nature of the cargo it is carrying. The uptake kinetics data for PNA-peptide conjugates suggest a cooperative binding model, as the conjugates follow a sigmoidal pathway. The cooperative indices ([S]0.9/[S]0.1) for PNAPBS-Penetratin and PNAPBS-Tat were 6.85 and 8.0, respectively, indicating that to achieve the uptake velocity from 0.1Vmax to 0.9Vmax, only an 6.85-fold increase in the concentration of PNAPBS-penetratin or an 8.0-fold increase in the concentration of PNAPBS-Tat would be required. For a compound following a hyperbolic uptake pathway, a 90-fold increase in the substrate concentration would be required to achieve a similar increase in Vmax from 0.1 to 0.9. Therefore, low values for cooperative indices are extremely beneficial for a compound to qualify as a drug.
All of the preceding observations suggest that PNA-peptide conjugates targeted toward conserved sequences on the regulatory U5-PBS region of viral RNA may be potential agents for drug development. The IC50 values of these conjugates for antiviral efficacy were in the range of 500–750 nM. The most exciting observation was that these conjugates, besides displaying antiviral efficacy in cell culture, were potently virucidal against HIV-1. Brief exposure of HIV-1 virions to these conjugates rendered them noninfectious. The median effective dose (IC50) values for virucidal activity of these PNA-peptide conjugates was ≈50 nM. The observed virucidal effect could be the result of either disruption of the virion envelope by the conjugates or internalization of the conjugates into the virion particles (Chaubey et al., 2005). We demonstrated that brief exposure of cell-free HIV-1 virions to these conjugates, followed by the separation of virions from the free conjugate by ultracentrifugation, resulted in complete blockage of endogenous reverse transcription in the disrupted virion particles (Fig 8). Similar inactivation of virion particles and inhibition of endogenous reverse transcription has been demonstrated by pretreatment of HIV-1 virions with AZT-TP, a nucleoside reverse transcriptase inhibitor and nevirapine in the presence of polyamines (Argyris et al., 2006). We believe that PNA-MTD peptide conjugates represent a promising new class of compounds and plan to pursue further investigation into their value as topical antiviral/microbicidal agents.
MATERIALS AND METHODS
Design of PNA and peptide sequences
The PNAs were custom-synthesized by Applied Biosystems, Bedford, MA. A 16-mer anti-PBS PNA sequence, CGCCACTGCTAGAGAT, was designed targeting five nucleotides of the PBS sequences and 11 nucleotides upstream to PBS in the 5′-nontranslated region of the viral genome. At the N-terminal of the PNA sequences, a cysteine was covalently linked for further conjugation with the peptide sequence using disulphide linkage. For uptake kinetic studies, the sequence synthesis was started with a tyrosine unit at the C-terminal, which was used for labeling the sequence with I125 (Fig. 1). For cellular uptake studies, we used fluorescein-labeled PNA. For targeting the A-rich region upstream of PBS sequence (nucleotides 168–173), a 16-mer anti-A loop PNA sequence, ATTTTCCACACTGACA, was synthesized.
Figure 1.
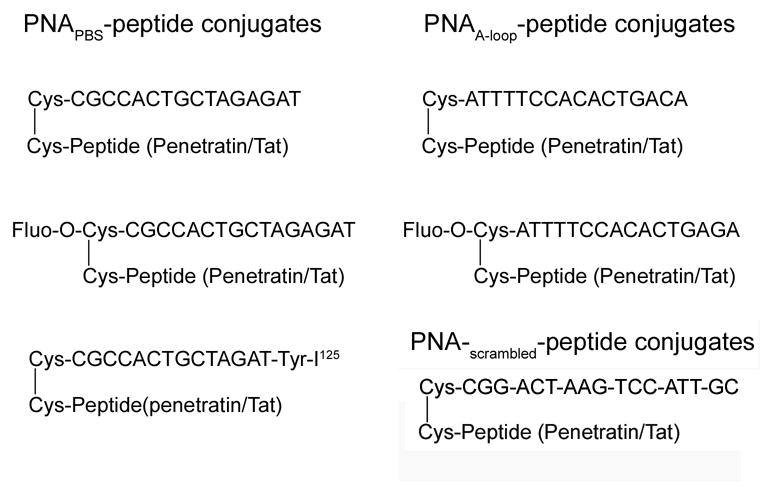
The sequences of different PNA-peptide conjugates used.
We obtained the 16-mer penetratin (RQIKIWFQNRRMKWKK), and 13-mer Tat (GRKKRRQRRRPPQ) peptides from Bachem Biosciences Inc., King of Prussia, PA. The peptide sequences bear a cysteine at the N-terminal and the -SH group of cysteine was activated using a 3-nitro pyridine sulphenyl (NPYS) group. We used this cysteine for conjugation with PNA sequences.
Synthesis of PNAPBS-Tat, PNAPBS-Penetratin, PNAA-loop-Tat, and PNAA-loop-Penetratin
The synthesis and purification of all PNA MTD peptide conjugates were carried out as described before (Tripathi et al., 2005). We determined the concentration of the conjugates by dividing OD at 260 nm by molar extinction coefficient [160.4 ml/(μmol × cm)] for PBS PNA and [142.5 ml/(μmol × cm)] for A-loop PNA. We characterized the PNA-peptide conjugates by matrix-assisted laser desorption ionization time-of-flight (MALDI-TOF).
Synthesis of fluorescein-labeled PNA
For this purpose, we synthesized individual PNA on solid support having a cysteine at the N-terminal. An “egl” linker (8-amino-3, 6-dioxaoctanoic acid) was also attached to the amino group of N-terminal cysteine to which fluorescein was attached as described before (Tripathi et al., 2005). The fluorescein-tagged PNA was kept protected from light and further conjugated with MTD peptides.
Preparation of 32P-labeled U5-PBS RNA template
We linearized the pU5-PBS plasmid using Xho-1, then transcribed it to generate a 250-base-long run-off RNA transcript of the U5-PBS region, using a T7 RNA polymerase transcription kit (Roche Biochemicals, Indianapolis, IN). The RNA transcript was internally labeled using α32P-CTP (3,000 Ci/mmol; Perkin-Elmer Life and Analytical Sciences (Boston, MA). We purified the run-off transcript by phenol: chloroform: isoamyl alcohol extraction followed by ethanol precipitation. We air-dried the RNA and resuspended it in water pretreated with diethyl pyrocarbonate (DEPC), then stored it at −70°C.
Gel mobility shift assay
We incubated an aliquot of internally 32P-labeled U5-PBS RNA transcript (10 nM) with varying concentrations of PNA or PNA-peptide conjugates in a binding buffer containing 50 mM Tris-HCl, pH 8.0, 75 mM KCl, 5 mM MgCl2, 1 mM DTT, 0.01% NP-40, and 500 ng poly r(I-C) in a final volume of 10 μl. After incubation at room temperature for 30 min, we added 5 μl of gel loading dye (0.2% bromophenol blue and 12% glycerol) to each sample. The samples were run (150 V) on an 8% native polyacrylamide gel using Tris-borate EDTA buffer at room temperature for 4 h. The gel was subjected to PhosphorImager analysis (Molecular Dynamics, Sunnyvale, CA).
Evaluation of Cellular uptake of fluo-O-PNA-MTD peptide conjugate by flow cytometry
We used CEM T-cell lymphocytes to study the uptake of PNA-MTD peptide conjugates. We grew the cells in log phase, harvested them, washed them with phosphate-buffered saline and resuspended them in RPMI medium with 2% FCS. We incubated approximately 0.5 ×106 cells with fluorescein-labeled PNA-peptide conjugates for 1 min at 4°C and 37°C. The cells were washed twice with phosphate-buffered saline and resuspended in RPMI medium with 2% FCS, then analyzed by flow cytometry on a Becton Dickinson, (San Jose, CA) flow cytometer. To exclude uptake by dead cells, we did the scanning in the presence of propidium iodide (1 μg/ml). We did a second set of experiments by incubating the fluorescein-positive cells in the presence of 0.01% trypsin in phosphate-buffered saline for 10 min. We washed the cells twice with phosphate-buffered saline and suspended them in RPMI medium containing 2% FCS and 1 μg/ml propidium iodide. We evaluated the fluorescence intensity in the trypsin-treated cells by flow cytometry. Cell Quest Pro software from Becton Dickinson was used to acquire and analyze 104 events detected by the FL1 detector (fluorescein) and excluded by the FL3 detector (propidium iodide).
Visualization of Cellular uptake of fluo-O-PNA-MTD peptide conjugate by fluorescence microscopy
CEM cells (1× 106) were incubated with 100 nM of flu-PNAPBS-penetratin for 30 min and then treated with 0.01% trypsin as described above. Cells were washed with phosphate buffer saline and resuspended in 250 μl of cell fixing reagent (BD Cytofix Buffer, BD Biosciences Pharmingen, San Diego, CA) for 15 min at room temperature. Subsequently cells were washed with 1× phosphate buffered saline containing 0.02 % Tween-20 and 0.5 % BSA and then treated with 100 μl (10 μg/mL) of 4′,6′-diamidino-2-phenylindole (DAPI, Sigma). Cells were washed again with the same buffer three times and 10 μl of the cell suspension was put on a glass-slide and covered with a cover slip. The ZEISS Axiovert 200M microscope fitted with an ApoTome imaging system was used for fluorescence microscopy. All images shown are from a representative axial plane.
Uptake kinetics of PNA-MTD peptide conjugate
For these experiments, we labeled the Tyr residue of the PNA moiety of the PNA-MTD peptide conjugate with (125I) using the chloramine-T labeling kit (MP Biomedical Inc, Irvine, CA) as described before (Tripathi et al., 2005). The final purified product was lyophilized, resuspended in deionized water, and quantified spectrophotometrically by measuring the OD at 260 nm. We adjusted the specific radioactivity by adding cold unlabeled PNA-MTD-peptide conjugates. We harvested CEM cells grown in log phase and used 0.5 × 106 cells for each experiment. We incubated the cells for 30 sec with increasing concentrations of I125-PNA-MTD peptide conjugates and filtered them on GF-B filters under mild negative pressure. We washed the cells repeatedly with phosphate-buffered saline to remove any traces of free unincorporated radiolabeled PNA-MTD peptide conjugates. The filters were air-dried and counted in a gamma counter. The [S]0.9/[S]0.1 ratio and cooperativity index of the substrate at 0.9 Vmax and 0.1 Vmax were determined by plotting the uptake versus substrate concentration. Hill coefficient (nH) was determined from the slope of the Hill plot.
Production and purification of HIV-1 pseudovirions
For production of highly infectious pseudotyped HIV-1 virions, we co-transfected 293T cells with pHIV-1JR-CSF-lucenv(-) and pVSV-G, using the calcium phosphate transfection system (Invtrogen Carlsbad, CA) as described before (Planelles et al., 1995). The culture supernatants saved at 24, 48, and 72 h after transfection were pooled and analyzed for p24 antigen using the ELISA p24 antigen kit (ZeptomMetrix, Buffalo, NY). We isolated the pseudotyped HIV-1 virions from the culture supernatant by filtration through a 0.45-μm pore size PVDF membrane (Millipore Bedford, MA) then ultracentrifuged them at 70,000 g for 45 min. The viral pellet was resuspended in complete Dulbecco’s medium and stored at −80°C.
Measurement of luciferase activity
Cells infected with infectious pseudo-HIV-1 virions carrying the luciferase reporter gene were harvested, washed with phosphate-buffered saline, and resuspended in 1 × passive lysis buffer (Promega, Madison, WI). The solutions were gently shaken on a rocker at 25°C for 15 min and centrifuged. We placed the lysate on a 96-well plate Fluotrac 200 (Greiner Labortechnik, Germany) and added 100 μl of luciferase assay reporter (Promega). We measured the firefly luciferase activity on a Packard Top Count Luminometer. The total light unit was normalized by total protein content in the cell lysate. Total protein was quantified using the DC protein assay kit (Bio-Rad Laboratories, Hercules, CA).
Endogenous reverse transcriptase activity of HIV-1 virions after treatment with PNA-peptide conjugates
We incubated HIV-1 virions (equivalent to 80 pg of p24) with individual PNA-MTD peptide conjugates at 250-nM or 500-nM concentrations for 30 min at 37°C, then ultracentrifuged the incubation mixture through 20% sucrose solution (Beckman Coulter Optima LE-80K ultra, Palo Alto, CA) at 100,000 g for 1 h at 4°C. We resuspended the virion pellets in disruption buffer containing 50 mM Tris-HCl, pH 7.8, 10 mM DTT, 66 mM KCl, 0.3 mM EDTA, 0.14% NP-40, and 1 U/μl RNAsin. We examined an aliquot of the disrupted virions for endogenous reverse transcription in reverse transcriptase buffer composed of 5 mM MgCl2, 50 mM KCl, 10 mM DTT, 30-μg actinomycin D/ml, 25 μg BSA/ml, 10 μM of dATP, dGTP and dTTP, and 1.25 μM of dCTP with 5 μCi of α32P-dCTP (3000 Ci/mmol). The reaction was carried out at 37°C for 10 min followed by the addition of more dNTPs (0.5 mM each), then resumed for another 30 min. The reaction was quenched by the addition of 50 mM of EDTA, followed by phenol-chloroform extraction and alcohol precipitation. We air-dried the pellet and dissolved it in formamide gel loading dye. The reverse transcription products were resolved on 6% polyacrylamide-urea denaturing gel.
Antiviral efficacy of PNA-MTD peptide conjugates
We grew CEM CD4+ lymphocytes 12D7 (Kashanchi et al., 1994) in RPMI-1640 medium supplemented with 10% fetal calf serum and 4 mM L-glutamine at 37°C in 5% CO2 containing humidified air. We harvested early-mid log phase cells and washed them with an equal volume of phosphate-buffered saline without Ca2+ and Mg2+. We incubated approximately 0.5 × 106 cells suspended in 1 ml of RPMI-1640 medium with pseudovirions (equivalent to 14 ng of p24) by gentle rocking for 2 h in presence of respective amounts of PNA-peptide conjugates. The cells were centrifuged, washed with phosphate-buffered saline and resuspended in complete RPMI media in a 12-well plate containing increasing amounts of conjugates (200 nM- 1 μM). After 48 h, the cells were harvested, washed with phosphate-buffered saline, and lysed in 1 × passive lysis buffer (Promega) with gentle shaking on a rocker for 15 min at room temperature. We centrifuged the lysed cells at 15,000 rpm for 15 min and assayed an aliquot of the clear lysate for reporter luciferase activity.
Antiviral efficacy experiments were also carried out using peripheral blood mononuclear (PBMC) cells. PHA-stimulated PBMC cells were suspended in complete medium (RPMI 1640, 15% FCS, 5 % Interleukin-2, 1%-pen-strep, 2mM L-glutamine, 2 mg/mL polybrene). For per assay 1 × 106 cells were used and incubated with pseudovirions (equivalent to 14 ng of p24) by gentle rocking for 2 h in presence of respective concentrations of PNA-peptide conjugates. The cells were then centrifuged and grown in presence of increasing concentrations of PNAPBS-penetratin and left for seven days (Liu et al, 1998). The assessment of luciferase activity in the cell lysate was carried out as discussed above.
Virucidal activity of individual PNA-MTD-peptide conjugates
We incubated pseudovirions equivalent to 100 ng of p24 in complete RPMI medium with increasing amounts of individual PNA-MTD conjugates, ranging from 0.01–1 μM, for 2 h at 37°C. We separated the treated virions from free conjugates by ultracentrifugation at 100,000 g for 1 h and used them to infect CEM T lymphocytes as described earlier. The infected cells were grown in fresh complete RPMI media for 48 h, harvested, lysed, and assayed for luciferase activity, also as described. These experiments were also performed in the presence of different concentrations (20–100%) of fetal calf serum. The virion particles were first centrifuged and then resuspended in different concentration of serum in RPMI medium. Each of the virion samples containing different serum concentration was treated with the conjugate and examined for their infectivity in CEM cells as above.
Calculation of IC50
Using CalcuSyn software, we determined median dose effects (IC50) for individual PNA-MTD peptide conjugates. The software, developed by BIOSOFT (Cambridge, UK), is based on equations developed by Chou (Chou, 1974 & 1977) correlating the “dose” and “effect.” The equation for calculating median dose, [fa/fu = (D/Dm)m], takes into account the fraction affected by dose (fa) and the fraction unaffected, fu = 1−fa where D is the dose of the drug and Dm is the median dose; m is an exponent signifying the sigmoidicity of the dose-effect curve. We include the IC50 values determined by CalcuSyn software corresponding to the median effective doses with 95% confidence limits in the results table [Table 1].
Evaluation of toxic effect of PNA-peptide conjugates on cellular proliferation. Cellular proliferation of CEM cells in the presence of PNAPBS-penetratin conjugate was determined by measuring the levels of [3H] thymidine incorporated in their nuclei. Briefly, the CEM cells in log phase were grown in the presence of varying concentrations (1, 5 and 10 μM) of PNAPBS-penetratin and supplemented with 10 μCi of [methyl-3H] Thymidine/mL (85 Ci/mmol). The cells were harvested at different time intervals up to 96h, harvested, washed with phosphate buffered saline and finally resuspended in lysis buffer comprising of 1% NP-40 in phosphate buffered saline solution. The nucleic acids were precipitated by adding cold 10% tricholoroacetic acid. The precipitated nucleic acids were collected on glass-fiber filter and washed extensively with cold 10% tricholoroacetic acid and once with 70% ethanol. The filters were dried, placed in scintillation vial and counted for radioactivity in a Liquid Scintillation Counter to assess the extent of radiolabel incorporation. Protein content in each assay was estimated by the Bio-Rad DC Protein assay and the results are expressed as counts per minute per gram of protein.
Acknowledgments
This research was supported by a grant from NIAID/NIH (AI42520). We are thankful to Dr. R. Stephens, and Antonio Fernández from the Department of Pediatrics, New Jersey Medical School for providing us activated PBMC cells.
Abbreviations used
- PBS
primer-binding site
- HIV-1
human immunodeficiency virus type-1
- RT
reverse transcriptase
- PNA
polyamide nucleic acid
- dNTP
deoxynucleoside triphosphate
- DMF
dimethylformamide
- TFA
trifluoroacetic acid
- MTD
membrane transducing
- NMP
N-methyl pyrrolidinone
- DCM
dichloromethane
- Tyr
Tyrosine
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Argyris EG, Dornadula G, Nunnari G, Acheampong E, Zhang C, Mehlman K, Pomerantz RJ, Zhang H. Inhibition of endogenous reverse transcription of human and nonhuman primate lentiviruses: Potential for development of lentivirucides. Virology. 2006 doi: 10.1016/j.viro.2006.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestman-Smith J, Piret J, Desormeaux A, Tremblay MJ, Omar RF, Bergeron MG. Sodium lauryl sulfate abrogates human immunodeficiency virus infectivity by affecting viral attachment. Antimicrob Agents Chemother. 2001;45:2229–2237. doi: 10.1128/AAC.45.8.2229-2237.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourinbaiar AS, Lee-Huang S. Comparative in vitro study of contraceptive agents with anti-HIV activity: gramicidin, nonoxynol-9, and gossypol. Contraception. 1994;49:131–137. doi: 10.1016/0010-7824(94)90088-4. [DOI] [PubMed] [Google Scholar]
- Brooks H, Lebleu B, Vives E. Tat peptide-mediated cellular delivery: back to basics. Adv Drug Deliv Rev. 2005;57:559–577. doi: 10.1016/j.addr.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Chaubey B, Tripathi S, Ganguly S, Harris D, Casale RA, Pandey VN. A PNA-transportan conjugate targeted to the TAR region of the HIV-1 genome exhibits both antiviral and virucidal properties. Virology. 2005;331:418–428. doi: 10.1016/j.virol.2004.10.032. [DOI] [PubMed] [Google Scholar]
- Chou TC. Relationships between inhibition constants and fractional inhibitions in enzyme-catalyzed reactions with different numbers of reactants, different reaction mechanisms, and different types of mechanisms of inhibition. Mol Pharmacol. 1974;39:235–247. [PubMed] [Google Scholar]
- Chou TC. Derivation and properties of Michaelis-Menten type and Hill equations for reference ligands. J Theoret Biol. 1977;39:345–356. doi: 10.1016/0022-5193(76)90169-7. [DOI] [PubMed] [Google Scholar]
- Cohen J. AIDS vaccine trial produces disappointment and confusion. Science. 2003;299:1290–1291. doi: 10.1126/science.299.5611.1290. [DOI] [PubMed] [Google Scholar]
- Demidov V, Potaman VN, Frank-Kamenetskii MD, Buchardt O, Egholm M, Nielsen PE. Stability of peptide nucleic acids in human serum and cellular extracts. Biochem Pharmacol. 1994;48:1309–1313. doi: 10.1016/0006-2952(94)90171-6. [DOI] [PubMed] [Google Scholar]
- Derossi D, Chassaing G, Prochiantz A. Trojan Peptides: the penetratin system for intracellular delivery. Trends Cell Biol. 1998;8:84–87. [PubMed] [Google Scholar]
- Derossi D, Calvet S, Trembleau A, Brunissen A, Chassaing G, Prochiantz A. Cell internalization of the third helix of the antennapedia homeodomain is receptor independent. J Biol Soc. 1996;271:18188–18193. doi: 10.1074/jbc.271.30.18188. [DOI] [PubMed] [Google Scholar]
- El-Andaloussi S, Holm T, Langel U. Cell-penetrating peptides: mechanisms and applications. Curr Pharm Des. 2005;11:3597–3611. doi: 10.2174/138161205774580796. [DOI] [PubMed] [Google Scholar]
- Gerber D, Pritsker M, Gunther-Ausborn S, Johnson B, Blumenthal R, Shai Y. Inhibition of HIV-1 Envelope Glycoprotein-mediated Cell Fusion by a DL-Amino Acid-containing Fusion Peptide. J Biol Chem. 2004;279:48224–48230. doi: 10.1074/jbc.M403436200. [DOI] [PubMed] [Google Scholar]
- Han W, Wind-Rotolo M, Kirkman RL, Morrow CD. Inhibition of human immunodeficiency virus type 1 replication by siRNA targeted to the highly conserved primer-binding site. Virology. 2004;330:221–232. doi: 10.1016/j.virol.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Isel C, Ehresmann C, Keith G, Ehresmann B, Marquet R. Initiation of reverse transcription of HIV-1: secondary structure of the HIV-1 RNA/tRNA (3Lys) (template/primer) J Mol Biol. 1995;247:236–250. doi: 10.1006/jmbi.1994.0136. [DOI] [PubMed] [Google Scholar]
- Jacobson JM, Israel RJ, Lowy I, Ostrow NA, Vassilatos LS, Barish M, Tran DNH, Sullivan BM, Ketas TJ, O’Neill TJ, Nagashima KA, Huang W, Petropoulos CJ, Moore JP, Maddon PJ, Olson WC. Treatment of Advanced Human Immunodeficiency Virus Type 1 Disease with the Viral Entry Inhibitor PRO 542. Antimicrob Agents Chemother. 2004;48:423–429. doi: 10.1128/AAC.48.2.423-429.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan IM, Wadia JS, Dowdy SF. Cationic TAT peptide transduction domain enters cells by macropinocytosis. J Control Release. 2005;102:247–253. doi: 10.1016/j.jconrel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Kashanchi F, Shibata R, Ross EK, Brady JN, Martin MA. Second-site long terminal repeat (LTR) revertants of replication-defective human immunodeficiency virus: effects of revertant TATA box motifs on virus infectivity, LTR-directed expression, in vitro RNA synthesis, and binding of basal transcription factors TFIID and TFIIA. J Virol. 1994;68:3298–3307. doi: 10.1128/jvi.68.5.3298-3307.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik N, Talele TT, Monel R, Palumbo P, Pandey VN. Destabilization of tRNA3Lys from the primer-binding site of HIV-1 genome by anti-A loop polyamide nucleotide analog. Nucleic Acids Res. 2001;29:5099–5106. doi: 10.1093/nar/29.24.5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik N, Basu A, Palumbo P, Myers RL, Pandey VN. Anti-TAR polyamide analog conjugated with a membrane-permeating peptide human immunodeficiency virus type 1 production. J Viro. 2002;76:3881–3891. doi: 10.1128/JVI.76.8.3881-3891.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik N, Pandey VN. PNA targeting the PBS and A-loop sequences of HIV-1 genome destabilizes packaged tRNA3Lys in the virions and inhibits HIV-1 replication. Virology. 2002;303:297–308. doi: 10.1006/viro.2002.1630. [DOI] [PubMed] [Google Scholar]
- Kilby JM, Hopkins S, Venetta TM, Dimassimo B, Cloud GA, Lee JY, Alldredge L, Hunter E, Lambert D, Bolognesi D, Matthews T, Johnson MR, Nowak MA, Shaw GM, Saag MS. Potent suppression of HIV-1 replication in humans by T-20, a peptide inhibitor of gp41-mediated virus entry. Nat Med. 1998;4:1302–1307. doi: 10.1038/3293. [DOI] [PubMed] [Google Scholar]
- Lee R, Kaushik N, Modak MJ, Vinayak R, Pandey VN. Polyamide Nucleic Acid targeted to the primer binding site of the HIV-1 RNA genome blocks in vitro HIV-1 reverse transcription. Biochemistry. 1998;37:900–910. doi: 10.1021/bi972197m. [DOI] [PubMed] [Google Scholar]
- Letvin NL. Progress toward an HIV vaccine. Annu Rev Med. 2005;56:213–223. doi: 10.1146/annurev.med.54.101601.152349. [DOI] [PubMed] [Google Scholar]
- Mak J, Kleiman L. Primer tRNAs for reverse transcription. J Viro. 1997;71:8087–8095. doi: 10.1128/jvi.71.11.8087-8095.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Chen B, Landau NR. Use of luciferase reporter viruses for studying HIV entry. In: Michael NL, Kim JH, editors. HIV Protocols, Methods in Molecular Medicine. Vol. 17. Humana Press; Totowa, NJ: 1998. pp. 35–40. [DOI] [PubMed] [Google Scholar]
- Nagashima KA, Thompson DAD, Rosenfield SI, Maddon PJ, Dragic T, Olson WC. Human immunodeficiency virus type 1-entry inhibitors PRO 542 and T-20 are potently synergistic in blocking virus-cell and cell-cell fusion. J Infect Dis. 2001;183:1121–1125. doi: 10.1086/319284. [DOI] [PubMed] [Google Scholar]
- Nielsen PE, Egholm M, Berg RH, Buchardt O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science. 1991;254:1497–1500. doi: 10.1126/science.1962210. [DOI] [PubMed] [Google Scholar]
- Nulf CJ, Corey DR. Intracellular inhibition of hepatitis C virus (HCV) internal ribosomal entry site (IRES)-dependent translation by peptide nucleic acids (PNAs) and locked nucleic acids (LNAs) Nucleic Acids Res. 2004;32:3792–3798. doi: 10.1093/nar/gkh706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planelles V, Bachelerie F, Jowett JB, Haislip A, Xie Y, Banooni P, Masuda T, Chen IS. Fate of the human immunodeficiency virus type 1 provirus in infected cells: a role for vpr. J Virol. 1995;69:5883–5889. doi: 10.1128/jvi.69.9.5883-5889.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard JP, Melikov K, Vives E, Ramos C, Verbeure B, Gait MJ, Chernomordik LV, Lebleu B. Cell-penetrating Peptides. J Biol Chem. 2003;278:585–590. doi: 10.1074/jbc.M209548200. [DOI] [PubMed] [Google Scholar]
- Richard JP, Melikov K, Brooks H, Prevot P, Lebleu B, Chernomordik LV. Cellular uptake of unconjugated TAT peptide involves clathrin dependent endocytosis and Heparan Sulfate Receptors. J Biol Chem. 2005;280:15300–15306. doi: 10.1074/jbc.M401604200. [DOI] [PubMed] [Google Scholar]
- Riguet E, Tripathi S, Chaubey B, Désiré J, Pandey VN, Décout JL. A Peptide Nucleic Acid-Neamine Conjugate That Targets and Cleaves HIV-1 TAR RNA Inhibits Viral Replication. J Med Chem. 2004;47:4806–4809. doi: 10.1021/jm049642d. [DOI] [PubMed] [Google Scholar]
- Shammas MA, Liu X, Gavory G, Raney KD, Balasubraminanian S, Shmookler Reis RJ. Targeting the single-strand G-rich overhang of telomeres with PNA inhibits cell growth and induces apoptosis of human immortal cells. Exp Cell Res. 2004;295:204–214. doi: 10.1016/j.yexcr.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Takeuchi T, Kosuge M, Tadokoro A, Sugiura Y, Nishi M, Kawata M, Sakai N, Matile S, Futaki S. Direct and rapid cytosolic delivery using cell-penetrating peptides mediated by pyrenebutyrate. ACS Chem Biol. 2006;1:299–303. doi: 10.1021/cb600127m. [DOI] [PubMed] [Google Scholar]
- Tunnemann G, Martin RM, Haupt S, Patsch C, Edenhofer F, Cardoso MC. Cargo-dependent mode of uptake and bioavailability of TAT-containing proteins and peptides in living cells. FASEB J. 2006;20:1775–1784. doi: 10.1096/fj.05-5523com. [DOI] [PubMed] [Google Scholar]
- Taylor RW, Chinnery PF, Turnbull DM, Lightowlers RN. Selective inhibition of mutant human mitochondrial DNA replication in vitro by peptide nucleic acids. Nat Genet. 1997;15:212–215. doi: 10.1038/ng0297-212. [DOI] [PubMed] [Google Scholar]
- Tripathi S, Chaubey B, Ganguly S, Harris D, Casale RA, Pandey VN. Anti-HIV-1 Activity of Anti-TAR Polyamide Nucleic Acid Conjugated with Various Membrane Transducing Peptides. Nucl Acids Res. 2005;33:4345–4356. doi: 10.1093/nar/gki743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme L, Ramjee G, Alary M, Vuylsteke B, Chandeying V, Rees H, Sirivongrangson P, Mukenge-Tshibaka L, Ettiegne-Traore V, Uaheowitchai C, Karim SS, Masse B, Perriens J, Laga M. COL-1492 Study Group, 2002. Effectiveness of COL-1492, a nonoxynol-9 vaginal gel, on HIV-1 transmission in female sex workers: a randomised controlled trial. Lancet. 2002;360:971–977. doi: 10.1016/s0140-6736(02)11079-8. [DOI] [PubMed] [Google Scholar]
- Vives E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272:16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- Wakefield JK, Wolf AG, Morrow CD. Human Immunodeficiency Virus Type 1 Can Use Different tRNAs as Primers for Reverse Transcription but Selectively Maintains a Primer Binding Site Complementary to tRNA3Lys. J Viro. 1995;69:6021–6029. doi: 10.1128/jvi.69.10.6021-6029.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield JK, Morrow CD. Mutations within the Primer Binding Site of the Human Immunodeficiency Virus Type 1 Define Sequence Requirements Essential for Reverse Transcription. Virology. 1996;220:290–298. doi: 10.1006/viro.1996.0317. [DOI] [PubMed] [Google Scholar]
- Wei M, Cen S, Niu M, Guo F, Kleiman L. Defective replication in human immunodeficiency virus type 1 when non-tRNA3Lys primers are used for reverse transcription. 2005;79:9081–9087. doi: 10.1128/JVI.79.14.9081-9087.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Kang S-M, Li Y, Morrow CD. Genetic analysis of the U5-PBS of a novel HIV-1 reveals multiple interactions between the tRNA and RNA genome required for initiation of reverse transcription. RNA. 1998;4:394–406. [PMC free article] [PubMed] [Google Scholar]


