Abstract
We examined relations between brain volumes assessed by MRI and cognitive function in subjects in whom we have previously reported associations of cumulative lead dose with: 1) longitudinal declines in cognitive function; 2) smaller volumes of several regions of interest (ROIs) in the brain; and 3) increased prevalence and severity of white matter lesions. We used two complementary methods (ROI- [evaluating 20 ROIs] and voxel-wise) to examine associations between brain volumes and cognitive function using multiple linear regression. MRIs and cognitive testing were obtained from 532 former organolead workers with a mean (SD) age of 56.1 (7.7) years and a mean of 18.0 (11.0) years since last occupational exposure to lead at the time of MRI acquisition. Cognitive testing was grouped into six domains of function (visuo-construction, verbal memory and learning, visual memory, executive functioning, eye-hand coordination, processing speed). Results indicated that larger ROI volumes were associated with better cognitive function in five of six cognitive domains, with significant associations observed for visuo-construction (15 of 20 p ≤ 0.05), processing speed (12 p ≤ 0.05), visual memory (11 p ≤ 0.05), executive functioning (11 p ≤ 0.05), and eye-hand coordination (11 p ≤ 0.05). Significant structure-function relations were also identified in the voxel-wise analysis with low false discovery rates (all less than 2.2%). Thus, larger volumes were associated with better cognitive function using both ROI- and voxel-based methods. In this cohort, an interesting group in which to examine structure-function relations, this finding provides a necessary condition to support the hypothesis that lead may influence cognitive function by its effect on brain volumes.
We have previously reported on relations of lead dose with cognitive function and brain structure in a well-characterized cohort of older former workers with past exposure to organic and inorganic lead. Past cumulative lead dose (estimated by measurement of the concentration of lead in tibia bone) was associated with: 1) cognitive test scores (Stewart et al. 1999) and progressive declines in cognitive function decades since last occupational lead exposure (Links et al. 2001); 2) smaller volumes of a number of regions of interest (ROIs) in the brain (Stewart et al. 2006); and 3) increased prevalence and severity of white matter lesions (Stewart et al. 2006). Furthermore, the association of tibia lead with decrements in cognitive function was stronger in subjects with the apolipoprotein E ε4 allele (Stewart et al. 2002). In this manuscript we report on relations of brain volumes with cognitive function. Understanding structure-function relations in this cohort is part of a broader goal (summarized in Figure 1) to evaluate whether cognitive decline in relation to past lead exposure could be related to structural lesions that are at least persistent. One necessary condition for the hypothesis that lead influences cognitive function through its influence on brain volumes is that brain volumes must be associated with cognitive function (Figure 1). The analysis presented herein addresses this condition, and is also of more general interest because few large-scale studies have examined structure-function relations in younger (i.e., less then 65 years of age) and disease-free (i.e., no neurodegenerative or other CNS conditions) populations, with extensive measures of cognitive function, using two distinct but complementary methods to explore structure-function relations.
Figure 1.
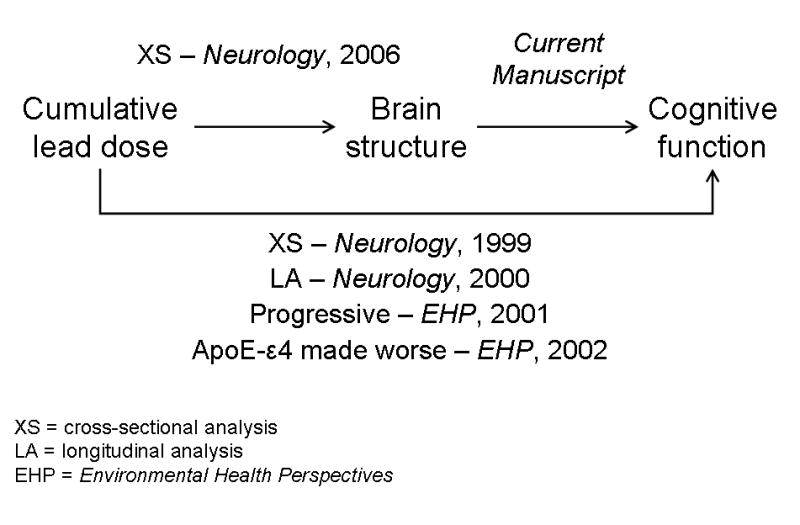
Schematic summary of relations of lead dose, brain structural volumes, and cognitive function. Citations refer to published manuscripts that have reported various parts of these relations. The current manuscript reports relations of brain volumes with cognitive function using two separate but complementary methods.
The former lead workers are an interesting population in which to evaluate structure-function relations in the aging brain because the aforementioned observations suggest that lead may cause accelerated aging in the brain. To our knowledge, no prior studies have used voxel-based morphometry to compare relations of brain structure with a continuous range of cognitive function in a broad set of cognitive domains (although one study evaluated function in three domains in 44 Huntington’s disease patients (Kassubek et al. 2005)). This approach has the advantage of not requiring that volumes of interest be specified a priori; allows exploration of structure-function relations not constrained by arbitrary anatomical definitions of structures; and does not require arbitrary categorization of “normal” or “abnormal” cognitive function using neurobehavioral tests. Herein, we compare relations of cognitive function measures with local tissue volumes using two distinct but complementary methods, one based on regions-of-interest (ROIs) and the other using voxel-based morphometry, in 532 former lead workers in whom we have already described important lead-related influences on function and structure.
METHODS
Study Design and Overview
Data were derived from individuals recruited during two study phases between 1994 and 2003, as previously described (Stewart et al. 2006). In phase I (1994-1997), former employees of a chemical manufacturing plant in the eastern United States were identified and recruited. Annual measures of cognitive function were obtained. In phase II (2001-2003), we enrolled additional study participants and obtained MRI data. The detailed methods for phase I (Schwartz et al. 2000; Stewart et al. 1999) and phase II (Stewart et al. 2006) are described elsewhere and summarized herein. The study was reviewed and approved by the Johns Hopkins Bloomberg School of Public Health Committee on Human Research and written informed consent was obtained from all participants.
Selection and Recruitment of Study Subjects
Study subjects were former workers in a facility that manufactured organolead gasoline additives (Schwartz et al. 1993), but not occupationally exposed to lead at the time of enrollment, and all were male and between the ages of 40 and 70 years in 1995. In phase I, a total of 703 former lead workers were enrolled and completed one to four visits. In phase II, another 276 former lead workers were enrolled and completed one or two visits. During phase II, MRIs were completed on 589 of the 979 former lead workers. All participants in earlier phases of the study were eligible for MRI measurement; reasons for not completing the MRI include subject death, illness, safety (e.g., metal objects in head or eye), lack of interest, moved out of state, and claustrophobia. Tibia lead, an estimate of lifetime cumulative lead dose, was measured with 109Cd-induced K-shell X-ray fluorescence (in μg lead per gram bone mineral) on 532 of the 589 individuals who completed MRI acquisition. Tibia lead was back-extrapolated to peak tibia lead, the estimated level at the end of employment in the factory, using years since last occupational exposure to lead and assuming first-order (mono-exponential) clearance from tibia (Stewart et al. 1999). The primary analysis was limited to the 514 former lead workers with MRIs adequate for voxel-wise analysis, but we compare associations in former lead workers and non-exposed controls using cross-product models described below. We also had MRIs from 116 population-based controls without a history of occupational lead exposure (Stewart et al. 1999) and compared structure-function relations in non-exposed controls to those in former lead workers. To evaluate whether possible selection bias among lead workers with MRIs and bone lead measurements could account for study results, we determined that average cognitive function did not differ by MRI status and the relations of tibia lead with neurobehavioral test scores did not differ in those with and without MRIs (Stewart et al. 2006). We concluded that there was unlikely to be meaningful selection bias among those who completed the MRI that could influence study results (Stewart et al. 2006).
Data Collection
Detailed data collection methods have been previously described (Stewart et al. 2006). After obtaining consent, blood pressure, height, weight, questionnaire interview, and cognitive testing data were obtained as well as two 10-ml blood specimens by venipuncture. The remaining description is confined to measures specifically used for the analysis presented herein.
Neurobehavioral Assessment
The neurobehavioral test battery has been previously described (Schwartz et al. 2000; Stewart et al. 1999; Stewart et al. 2002). Cognitive test scores were first summarized by six domain-specific scores to minimize multiple comparisons and improve the measurement properties of the functional outcomes. All cognitive tests were z-transformed, standardized for direction, and then averaged within domain to derive summary scores for each domain. Tests were grouped based on neuropsychological theory (Lezak et al. 2004) and supported by examination of correlation matrices (Spearman and Pearson), the results of exploratory factor analysis (data not shown), and the results of confirmatory factor analysis from another study (Shih et al. 2006) to group tests in a consistent way across the two studies. The domains included visuo-construction (consisting of Rey complex figure, copy task, and Block design from the Wechsler Adult Intelligence Scale [WAIS]), verbal memory and learning (Rey auditory verbal learning test immediate recall, delayed recall, and recognition, and serial digit learning), visual memory (Rey complex figure delayed recall and symbol digit), executive functioning (consisting of the mean of three z-transformed difference scores: Purdue pegboard assembly minus both hands, Stroop C form minus A form, and trail-making test B minus A), eye-hand coordination (Purdue pegboard dominant hand, non-dominant hand, and both hands, and trail-making test A), and processing speed (simple reaction time).
MRI Acquisition
All subjects were imaged at the same location on the same General Electric 1.5 T Signa model. A set of 1.5mm T1-weighted images through the entire brain were acquired, using contiguous coronal MRI images. Only the T1-weighted images were used for volumetric analysis, acquired using spoiled gradient recalled acquisition in steady state sequence (echo time [TE] = 5 msec, repetition time [TR] = 35msec, flip angle = 45°, one excitation, voxel size = 0.9375 mm by 0.9375 mm by 1.5 mm, field of view 24 cm, matrix size 256 × 256). Proton density and T2-weighted dual-echo axial images were also acquired parallel to the anterior-posterior commissure line, using an interleaved technique with no gap to provide maximal data for subsequent segmentation and ratings of white matter disease. These images were used to exclude unsuspected pathology in the brain that could obscure the analysis. Eighteen of the MRIs were not suitable for volumetric analysis due to image quality.
Image Analysis
Quantitative analysis of MR volumes was completed using previously published methods (Goldszal et al. 1998). In particular, extracranial tissue, the cerebellum, and brainstem structures inferior to the mamillary bodies were removed by a single experienced, image processing technician using a semi-automated procedure (Goldszal et al. 1998). The remaining tissue was classified, using an adaptive Bayesian segmentation algorithm (Yan and Karp 1995), into gray matter, white matter, and cerebrospinal fluid (CSF). All remaining image processing was fully automated and operator-independent.
The segmented images provide quantitative volumetric measures of total gray, white, and brain (gray plus white) matter. To obtain the volumes of predefined ROIs, regional analysis was performed via computerized template matching techniques previously reported and validated (Stewart et al. 2006). A digital atlas bearing anatomical definitions of several brain regions – all major lobar subdivisions and a number of smaller regions – was used as reference (Kabani et al. 1998). A computerized image analysis algorithm based on pattern matching was then used to warp this reference atlas to each participant’s MRI, resulting in volumes of 20 ROIs for further analysis (Stewart et al. 2006).
For the voxel-wise approach, regional analysis of volumes examined in normalized space (RAVENS) was used for quantification of regional volumes and investigation of local brain changes (Goldszal et al. 1998). The segmented images were first transformed to the Talairach stereotaxic coordinate space (Talairach and Tournoux 1988), using an elastic deformation algorithm (Davatzikos 1996; Shen and Davatzikos 2003). This approach allows quantification of absolute volumes within the standard reference space and applies a boundary constraint for the ventricles. Gray and white matter volumes of frontal, parietal, temporal, and occipital brain regions are determined automatically within the Talairach coordinate space (Andreasen et al. 1996; Goldszal et al. 1998; Resnick et al. 2000). In addition, the RAVENS approach yields brain maps for voxel-based analysis of local volumetric differences. In particular, the image warping that registers each individual scan to the atlas is a tissue-preserving spatial transformation. This means that if a particular brain region is compressed (or expanded) to fit a smaller (or larger) atlas, its tissue density increases (decreases) accordingly, so that its total amount of tissue is unaffected by the image transformation. This procedure yields three tissue density (RAVENS) maps, one for gray matter, one for white matter, and one for cerebrospinal fluid. The value of the RAVENS maps in each brain region reflects the amount of brain tissue measured from the respective scan in that brain region. Regional volumetric measurements are performed via voxel-based analysis of the RAVENS maps. Relations among the RAVENS maps and cognitive function variables reflect regional relations between brain volume and the respective functions.
Statistical Analysis
Multiple linear regression was used to evaluate the cross-sectional associations of brain volumes with cognitive domain scores using both ROI-based and voxel-wise approaches. Cognitive data from the visit closest to the time of the MRI acquisition were used in the analysis. In both methods regression models were adjusted for age, visit number (to adjust for practice effects, because of the longitudinal nature of the study, cognitive function testing could represent any of the first to the sixth time such testing was performed), height (cm), tobacco use (never, previous, current), alcohol use (never, previous, current), hypertension, diabetes, tibia lead concentration (μg/g), and education (five ordinal categories). One individual was excluded from the analysis based on MRI evidence of hydrocephalus and large outlying values for many volumes. Model diagnostics were used to evaluate influence and normality for the ROI-based analysis (e.g., normal quantile plots were examined for all of the ROI fits). Univariate analyses showed that all cognitive domains, ROI volumes, and voxel volumes were approximately normally distributed and there were no significant outliers or influential points except for those subjects identified with clinical or registration abnormalities.
ROI-based approach
To be consistent with the results of our prior published report, associations of the six cognitive domains were examined with 20 previously selected ROI volumes (volumes of total brain, total gray matter, total white matter, four lobar gray matters, four lobar white matters, cerebellum, medial structures, cingulate gyrus, insula, corpus callosum, internal capsule, hippocampus, entorhinal cortex, and amygdala) (Stewart et al. 2006). For bilateral structures, the volume represented the sum of right and left structures to minimize multiplicity concerns, but analyses were also performed separately for left- and right-sided ROI volumes and summarized in the results. To facilitate interpretation, each of the 120 associations was represented by a “clock plot” that provided a visual summary of the strength, direction, and statistical significance of the association. Details on the interpretation of the symbols on the plot are in the caption to Figure 2. The “clock plots” were created in the R statistical programming language (version 2.2.1) (R Development Core Team 2006). Multiplicity was not addressed in this plot, as it was used primarily as an exploratory tool for comparison with the voxel-wise results. However, to address the possibility of chance associations, we report the level of false discovery rate control for the ROI associations (Benjamini and Hochberg 1995; Storey 2002). To evaluate whether relations of ROI volumes with cognitive domain scores were different in lead workers and controls, we evaluated regression models with cross-product terms between lead worker vs. control status and ROI volume.
Figure 2.
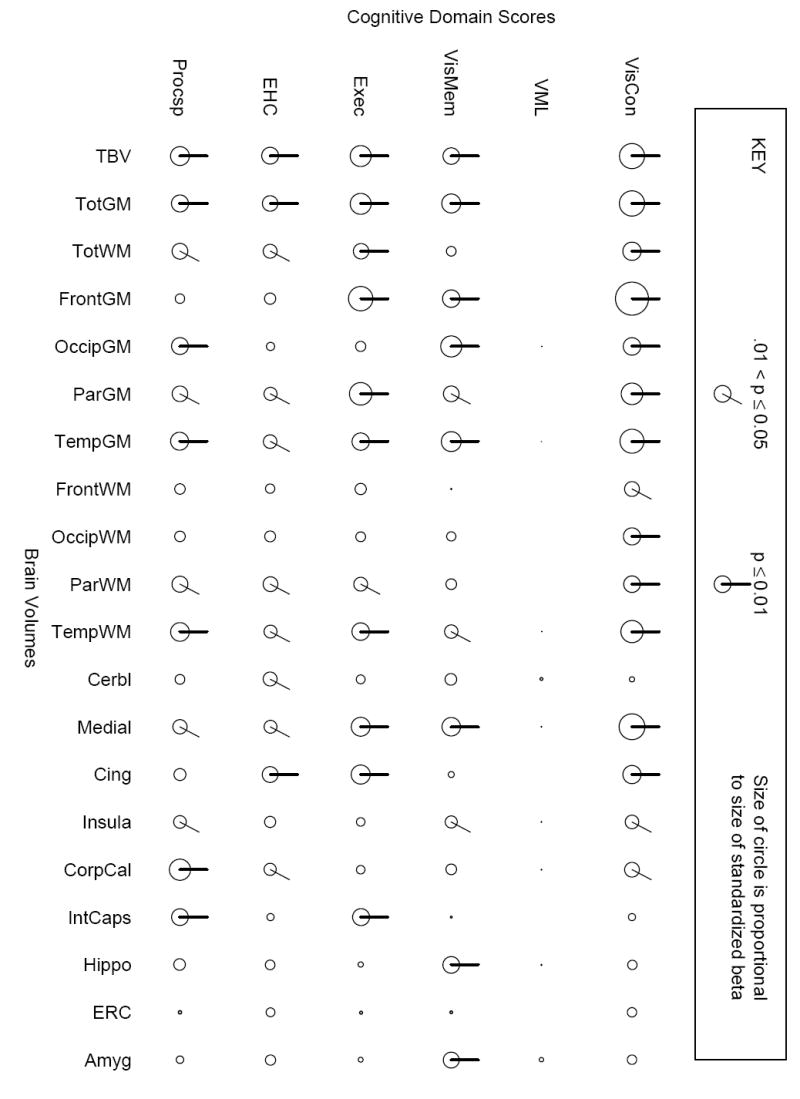
“Clock plots” representing associations of cognitive domain scores with brain volumes using an ROI-based approach, adjusting for age, visit number, height, tobacco, alcohol, education, diabetes, and hypertension. All cognitive domain scores and ROIs were z-transformed so that the magnitudes are directly comparable. The rows consist of the six cognitive domain scores and the columns of the 20 ROIs, which are (roughly) arranged from largest to smallest volume from left to right. The diameter of the circle is proportional to the magnitude of the association between the specific domain score and structure volume. If a line is on the circle, this indicates the p-value was less than or equal to 0.05. Lines on the top of the circle indicate positive betas (i.e., increasing volume is associated with better cognitive domain function), and where the line appears on the circle corresponds to the size of the p-value (the bold line at 1:00 o’clock corresponds to a p-value ≤ 0.01, the line at 12:00 o’clock to > 0.01 to ≤ 0.05). This is summarized in a key at the top of the graphical display. Abbreviations are as follows: VisCon = visuo-construction, VML = verbal memory and learning, VisMem = visual memory, Exec = executive functioning, EHC = eye-hand coordination, Procsp = processing speed, TBV = total brain volume, VolGM = total gray matter volume, VolWM = total white matter volume, Front = frontal, Occip = occipital, Par = parietal, Temp = temporal, Cerbl = cerebellar, Medial = medical structures (bilateral amygdala, cuneus, entorhinal cortex, hippocampal formation, lingual gyrus, medial front-orbital gyrus, medial frontal gyrus, medial occipito-temporal gyrus, parahippocampal gyrus, perirhinal cortex, precuneus, and uncus), Cing = cingulate gyrus, CorpCal = corpus callosum, IntCaps = internal capsule, Hippo = hippocampus, ERC = entorhinal cortex, and Amyg = amygdala.
Voxel-wise approach
Like the ROI-based analysis, cognitive domain scores were regressed on voxel volumes, on a voxel-by-voxel basis, controlling for the aforementioned covariates using linear regression in the R statistical programming language. The SPM2 package (Statistical Parametric Software, Functional Imaging Laboratory, Wellcome Department of Imaging Neuroscience, University College London, 2003) was used to perform its standard smoothing procedure but was not used for regression because it treats the voxel volume as the regressor and cognitive domain scores as the independent variables. To be exactly analogous to the ROI-based analysis, we used the programs of R so that domain scores were the dependent variables in each approach. Also like in the ROI-based analysis, to evaluate whether relations of voxel volumes with cognitive domain scores were different in lead workers and controls, we evaluated regression models with cross-product terms between lead worker vs. control status and voxel volume.
We used MRIcro (Rorden and Brett 2000) for glass brain projections of the association maps of t-statistics with each of the six cognitive domain scores in three views (coronal, sagittal, transverse), represented by thresholding the t-statistics at two significance levels (red color, 3.09 < t ≤ 4.265, uncorrected p-value of 0.00002 ≤ p < 0.001 and orange color, t > 4.265, uncorrected p-value of p < 0.00002). We did not correct for multiplicity in this analysis because random field theory-based correction can over-control for type I error at the expense of sensitivity. To illustrate the dependence of the results on the threshold level, associations for two levels are displayed. Finally, to address the possibility of chance associations, we report the associated false discovery rates for the voxel-by-voxel comparisons (Benjamini and Hochberg 1995; Storey 2002).
To assist in the interpretation of the domain-associated volumes, we determined the volume of the domain-associated areas within individuals and provide summary statistics for each domain across individuals. Recall that voxels in the RAVENs maps contain volumetric measurements for each subject. Therefore, by using the thresholded (p < 0.001), between-subject, domain-associated results as a mask and then applying it to individual RAVENs maps, we produced a collection of individual-specific volumes. These volumes contained in the mask were then added to create a volume summary for the associated areas derived from the voxel-wise analysis. The result is a volumetric summary for each subject of the area where structure and function are most strongly correlated between subjects.
RESULTS
Descriptive Summary of Study Subjects
The 514 former workers in this analysis were, on average, 56.1 years of age and had a mean duration of occupational lead exposure of 8.7 years. The average time interval between last occupational exposure to lead and when the MRI was obtained was 18 years. Individuals were predominantly white, more than 90% completed high school, and approximately 35% had some college (Table 1). Summary statistics for the 20 ROIs have been previously reported (Stewart et al. 2006). As expected, ROI volumes were correlated; for example, the highest correlations were observed between total brain volume and the 19 other ROIs (Pearson’s r ranging from 0.43 for entorhinal cortex to 0.89 for total gray matter volume, with a median for all ROIs of 0.70). Occipital white matter had the lowest correlations with other ROIs (ranging from 0.16 for parietal gray matter to 0.71 for total white matter, median of 0.28). Cognitive domain scores were also correlated, ranging from a low of Pearson’s r = 0.36 (processing speed and visuo-construction) to a high of 0.60 (executive functioning and eye-hand coordination).
Table 1.
Summary statistics for 532 former lead workers included in the analysis, Former Lead Workers Study, 2001-2003.
| Variable | Value |
|---|---|
| Age, years, mean (SD) | 56.1 (7.7) |
|
| |
| White race/ethnicity, % | 92.3 |
|
| |
| Educational level, % | |
| Less than high school | 6.2 |
| High school graduate | 58.8 |
| Some college | 30.5 |
| College graduate | 4.5 |
|
| |
| Tobacco consumption, % | |
| Never | 29.9 |
| Current | 20.2 |
| Previous | 49.9 |
|
| |
| Alcohol consumption, % | |
| Never | 4.0 |
| Current | 73.5 |
| Previous | 22.6 |
|
| |
| Hypertension*, % | |
| Yes | 38.0 |
| No | 62.0 |
|
| |
| Diabetes, % | |
| Yes | 8.0 |
| No | 92.0 |
|
| |
| Year enrolled, % | |
| 1994 | 20.7 |
| 1995 | 39.4 |
| 1996-97 | 7.2 |
| 2001-03 | 32.8 |
|
| |
| Cognitive domain scores, mean (SD) | |
| Visuo-construction | -0.354 (1.028) |
| Verbal memory and learning | 0.261 (0.795) |
| Visual memory | 0.149 (0.918) |
| Executive functioning | -0.161 (0.763) |
| Eye-hand coordination | -0.100 (0.878) |
| Processing speed | -0.159 (0.769) |
Defined as SBP > 140 or DBP > 90 or self-reported use of anti-hypertensive medications
** Means show departures from expected value of ‘0’ and standard deviations from expected value of ‘1’ because z-transformation was performed on all subjects with neurobehavioral data, not just those with MRIs
ROI-based Method
After adjustment for covariates, brain volumes were selectively associated with cognitive domain scores (Figure 2). At the p ≤ 0.05 level of significance, 15 ROIs were associated with performance in visuo-construction, 12 with processing speed, 11 with visual memory, executive functioning, and eye-hand coordination, and none with verbal memory and learning. In terms of the magnitude of the associations, on average, visuo-construction had the largest regression coefficients. Entorhinal cortex volume was not significantly associated with performance in any domain and four ROIs (frontal white matter, occipital white matter, hippocampus, amygdala) were associated with performance in only one domain; in contrast, six ROIs (total brain, total gray matter, parietal gray matter, temporal gray matter, temporal white matter, and medial structures) were associated with performance in five domains. At the p < 0.05 threshold, the false discovery rate is controlled at 0.053, while at the p < 0.01 threshold it is controlled at 0.012 (Benjamini and Hochberg 1995; Storey 2002); these are very close to the nominal levels and suggest that our associations are not likely to be false positive results.
In the separate left- and right-sided ROI-based analysis, associations were substantively similar with the exception that more left-sided than right-sided ROIs were associated (p ≤ 0.05) with performance in eye-hand coordination (15 on left, 9 on right) and processing speed (13 on left, 10 on right), consistent with the fact that most subjects were right-handed. In the cross-product models, there was no evidence that relations of ROI volumes with cognitive domain scores differed in former lead workers and non-exposed controls (i.e., in the 120 models, fewer p-values for cross-products terms of former lead worker vs. non-exposed control status and ROI volume were ≤ 0.05 than expected due to chance).
Voxel-wise Based Method
There was substantial variation in the total volume and location of the areas of domain-associated volumes identified in the voxel-wise analysis. The largest associated volumes for gray matter and white matter (at p < 0.001 threshold, mean [SD]), were with visuo-construction (53.6 [6.0] cm3) and executive functioning (45.5 [4.8] cm3), respectively (Figures 3A and 3B). This was followed by executive functioning and processing speed for the magnitude of the gray matter association volumes and processing speed and visuo-construction for white matter association volumes. Performance in verbal memory and learning was not associated with either gray matter or white matter volumes. Notably, and consistent with the ROI-based analysis, there was no evidence that relations of cognitive domain scores with voxel volumes differed in former lead workers and non-exposed controls (data not shown).
Figure 3.
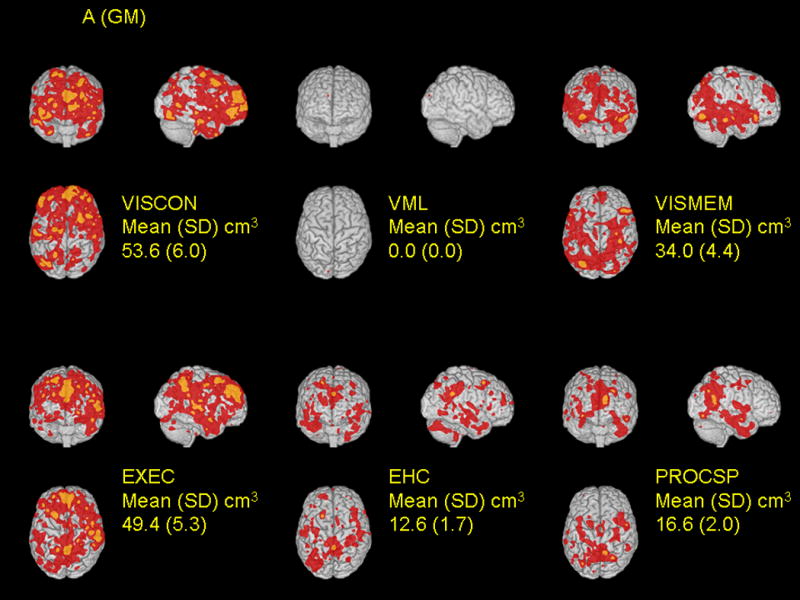
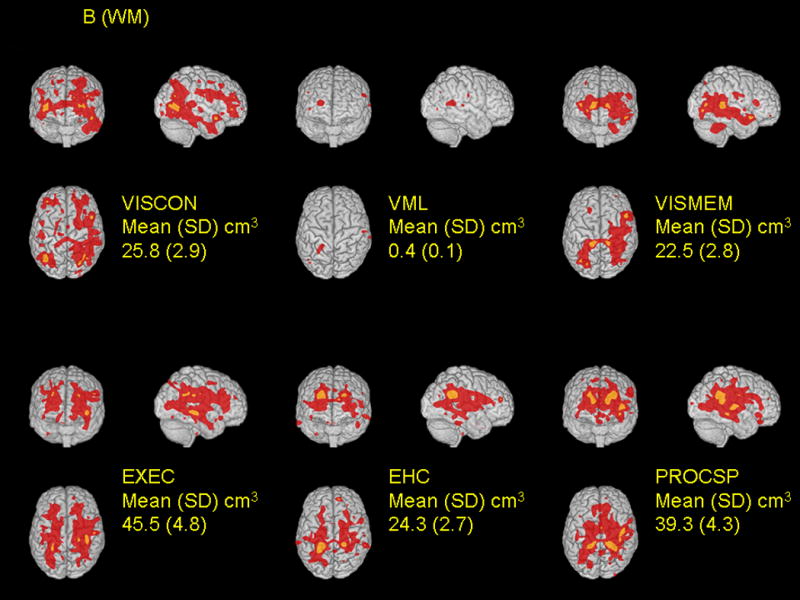
Spatial distribution of relations between local tissue volumes and performance in six cognitive domains are shown for gray matter (A) and white matter (B). Cognitive domain scores were separately regressed on voxel volumes on a voxel-by-voxel basis, adjusting for covariates (see Methods). The t-statistics for the cognitive function terms were thresholded at t > 3.09 (corresponding to a p-value 0.00002 ≤ p < 0.001, red color) and t > 4.265 (corresponding to a p-value < 0.00002, orange color). For each domain, associations are shown for coronal, sagittal, and transverse glass brain projections. The mean (SD) values are a summary for each subject of the area where structure and function are most strongly correlated between subjects, at the p < 0.001 level of significance.
Voxel associations were somewhat specific to each domain (Table 2). We expressed specificity by the extent to which associated voxels were common across domains (i.e., defined as percent overlap – the voxel volume common to two domains divided by the union of the volumes for the two domains). Using the lower threshold (t > 3.09, red areas, Figure 3), the calculated percent overlap was higher on average for white matter than for gray matter associations. For gray matter, the percent overlap was highest (16%) for visuo-construction and executive functioning, followed by eye-hand coordination and processing speed (15%). For white matter, the percent overlap was highest for processing speed with both executive functioning and eye-hand coordination (30%), followed by eye-hand coordination with executive functioning (29%).
Table 2.
Percent overlap* among areas of associations between voxel volumes and cognitive domain scores in voxel-wise analysis for gray matter and white matter
| Percent Overlap, at t > 3.09 and t > 4.265** |
|||||
|---|---|---|---|---|---|
| Domain
|
Matter
|
VISMEM
|
EXEC
|
EHC
|
PROCSP
|
| VISCON | Gray | 7, 3 | 16, 7 | 3, 0 | 3, 0 |
| White | 17, 4 | 12, 0 | 5, 0 | 8, 0 | |
| VISMEM | Gray | 8, 0 | 4, 0 | 10, 2 | |
| White | 16, 0 | 12, 0 | 19, 2 | ||
| EXEC | Gray | 7, 1 | 10, 0 | ||
| White | 29, 5 | 30, 1 | |||
| EHC | Gray | 15, 0 | |||
| White | 30, 13 | ||||
Calculated as the intersection of the areas comparing two domains divided by the union of the areas in those two domains. As there were no associations with verbal memory and learning in the voxel-wise analysis, overlap with this domain is not shown in the table.
The first number is the percent overlap of the voxels associated with domain scores at t > 3.09 and the second number the percent overlap at t > 4.265.
Because the thresholding scheme that we used for the voxel-wise analysis may appear too susceptible to chance associations, we investigated multiplicity by estimating the associated false discovery rates for the association volumes (Benjamini and Hochberg 1995; Storey 2002). For example, for gray matter, the false discovery rates were all less then 0.6%, a very low rate, for the lower (t > 3.09) threshold (Storey and Tibshirani 2003); for white matter and the lower threshold, false discovery rates were all less than 2.2%. Based on these rates, and the relative symmetry and spatial contiguity of the results (Figures 3), we are confident that conclusions are not driven by chance associations.
DISCUSSION
In a group of former organolead manufacturing workers, in whom we have previously reported what we term “accelerated aging” associated with cumulative lead dose, using two different but complementary methods while controlling for covariates, we found the two methods were generally consistent with each other in the observed associations of cognitive domain scores with brain volumes. In this cohort of 45 to 75 year old men with past occupational exposure to organic and inorganic lead, we had previously observed that peak tibia lead concentration (an estimate of past cumulative lead dose) was associated with neurobehavioral test scores at cross-section (Stewart et al. 1999), longitudinal decline in cognitive function (Schwartz et al. 2000), the prevalence and severity of white matter lesions, and with decreased volumes in both larger (e.g., total brain, lobar gray and white matter) and smaller (e.g., cingulate gyrus, insula, corpus callosum) ROIs (Stewart et al. 2006), almost two decades after occupational exposure had ended. In part, Figure 1 indicates that a relation between structure and function is a necessary condition in supporting the hypothesis that the observed influence of lead dose on cognitive function is mediated through a persistent change in brain volumes. We have now demonstrated that brain volumes are associated with cognitive domain scores and there is some specificity in these relations by domain.
The ROI-based approach provides a standard way to measure volumes of anatomically (and to a large extent functionally) distinct areas and, compared to total tissue volumes over the entire brain, allows us to examine the spatial heterogeneity of relations of volumes with cognitive function. However, the ROI approach is non-specific in two ways. First, ROIs encompass complete but somewhat complex and heterogeneous structures, where only part of the structure may be relevant to a specific function. Second, cognitive functions are not generally defined by anatomic structures, but rather a process that simultaneously and sequentially engages parts of numerous structures. The voxel-wise approach overcomes these two limitations, but is itself faced with inferential challenges. Some of this is due to statistical multiplicity issues, and there is no uniformly accepted method to define the overall statistical significance of the observed relations. The voxel-wise method does not rely on a priori ROI definitions, and examines the entire brain in an unbiased region-by-region manner, thereby allowing us to find new ROIs that might be specific to certain brain pathologies, and form new hypotheses. Therefore, ROI-based approaches are more “informed,” but constrained by the specific anatomical definitions, while voxel-based analysis is completely unconstrained but also uninformed. These two approaches are therefore complementary.
In our previously published analyses of lead and cognitive decline, because tibia lead was associated with declines in function in a broad range of cognitive domains (but with the strongest effects on verbal memory and learning, visual memory, and executive function) (Schwartz et al. 2000), we predicted that it would be associated with smaller volumes in several structures ranging from large to small, evidence of both global and more specific effects on brain structure (Stewart et al. 2006). In our analysis of the relations of peak tibia lead with brain volumes (Stewart et al. 2006), we confirmed this in observing at least borderline (p ≤ 0.07) associations of lead with 10 ROIs (total brain, total gray matter, total white matter, frontal parietal gray matter, temporal and parietal white matter, cingulate gyrus, insula, and corpus callosum). Of the domains with the strongest evidence of longitudinal declines associated with lead, two – visual memory and executive function – had consistent associations with ROI volumes. Although cross-sectional associations of these types cannot provide unequivocal evidence that the effect of lead on cognitive function is mediated through an effect on brain structures, these findings are a necessary, but not sufficient, condition.
Prior studies have evaluated relations between brain structure and cognitive function using a large number of approaches to study populations, imaging methods and acquisition protocols, volumetric methods, cognitive assessment, and statistical analysis (Coffey et al. 2001). Most prior authors have used one of three common methods: 1) determined volumes of a small set of brain regions of interest (ROI) and used linear regression to evaluate associations with neurobehavioral test scores on a continuous scale (Coffey et al. 2001; MacLullich et al. 2004; MacLullich et al. 2002; Tisserand et al. 2000; Van Petten et al. 2004); 2) used voxel-based lesion-symptom mapping in patients with strokes or other central nervous system lesions (Bates et al. 2003; Dronkers et al. 2004; Schoch et al. 2005); or 3) used neurobehavioral testing to categorize study subjects into groups with poor or declining versus normal cognitive function (or compared patients with CNS disease, e.g., Huntington’s disease, to controls) then used voxel-based morphometry to compare voxel-wise associations in the two groups (Chetelat et al. 2005; Kassubek et al. 2005; Peinemann et al. 2005; Tisserand et al. 2004).
Cumulative knowledge based on the neuropsychological literature (Lezak et al. 2004), often from patients with dementing illnesses or specific lesions, and on the more recent functional and structural neuroimaging literature suggests that, especially for complex cognitive tasks, large neural networks encompassing a number of discrete anatomical areas are involved in task completion. However, the extant literature is also clear that specific tasks are mediated by certain areas of the brain and allows predictions to be made regarding expected associations between brain volumes and cognitive processes. In the current study, we found a number of expected structure-function associations. For example, visuo-construction function, associated with the integrity of the frontal, parietal, and occipital lobes (Antonova et al. 2005; de Jong et al. 1999), was associated with decreased volume in frontal white matter (ROI-based) and frontal-parietal-occipital regions (voxel-wise). In the ROI analysis, visual memory, associated with right temporal-occipital lobe function (Lezak et al. 2004), was found to be related, but not exclusively so, to volumes of the temporal and parietal lobe. Classically, executive functioning relies heavily on the pre-frontal cortex (cingulate cortex and the dorsolateral prefrontal cortex [DLFPC]), and our ROI analysis found relations with volumes in the frontal lobe. However, other ROI associations were also observed (occipital lobe, insula, corpus callosum), and the voxel-wise analysis identified associations in the frontal lobe and cingulate, as well as the occipital and parietal lobes.
There is little information available on the anatomical sites involved in eye-hand coordination with the exception of one study that reported that Trails A performance was associated with white matter hyperintensities without regard to a specific anatomical location (van der Flier et al. 2005). Processing speed, measured by a simple reaction time task, has been linked to functioning of the DLPFC and ventral pre-motor brain regions. Our ROI-based analysis found that processing speed was associated with reduced volumes in many regions (frontal, parietal, occipital, medial, cingulate, insula, hippocampus), while the voxel-wise analysis identified mainly reduced parietal-occipital volumes. The insula and corpus callosum were associated with visual memory, executive functioning, eye-hand coordination, and processing speed performance. These anatomical regions are involved in somatosensory function (Robinson and Burton 1980) and body awareness and all four of these cognitive domains required these processes (Karnath et al. 2005). Verbal memory and learning has been historically linked to the pre-frontal cortex, posterior cingulate cortex, precuneus, thalamus, left temporal lobe and hippocampus (Grasby et al. 1993), but surprisingly, both our methods failed to observe any relations of this domain with volumes.
It may be difficult to compare the results of our study to others for a number of reasons. First, several of the cognitive domains were comprised of multiple individual test scores. Most prior studies, especially with functional MRI, report structure-function relations with single tests. It is likely that optimal performance on each test involves different neural networks so that combining different tests would logically involve multiple brain regions because of the involvement of different neural networks involved in task performance. Also, each task required a number of different processes. Finally, it is also accepted that many of the tasks utilized in our study rely more heavily on one side of brain more that the other. To reduce the number of comparisons, in our ROI analysis, right and left brain structures were combined into a single volume, resulting in larger brain regions than would result in studies examining functional brain laterality. However, it should be noted that our voxel-wise analysis allows for laterality, and is not constrained by this point.
In conclusion, we have shown that brain volumes are associated with cognitive function in both ROI-based and voxel-wise analyses. In this group of older subjects, who are experiencing declines in cognitive function due to aging and past lead exposure, it is likely the variation in net loss that accounts for the structure-function relations. The findings suggest that absolute brain volumes are associated with cognitive function.
Acknowledgments
This research was supported by grant R01 AG10785 from the National Institute on Aging (NIA). Its content is solely the responsibility of the authors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen NC, Rajarethinam R, Cizadlo T, Arndt S, Swayze VWI, Flashman LA, et al. Automatic atlas-based volume estimation of human brain regions from MR images. Journal of Computer Assisted Tomography. 1996;20(1):98–106. doi: 10.1097/00004728-199601000-00018. [DOI] [PubMed] [Google Scholar]
- Antonova E, Kumari V, Morris R, Halari R, Anilkumar A, Mehrotra R, et al. The relationship of structural alterations to cognitive deficits in schizophrenia: a voxel-based morphometry study. Biol Psychiatry. 2005;58(6):457–467. doi: 10.1016/j.biopsych.2005.04.036. [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, et al. Voxel-based lesion-symptom mapping. Nat Neurosci. 2003;6(5):448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Royal Statist Soc B. 1995;57:289–300. [Google Scholar]
- Chetelat G, Landeau B, Eustache F, Mezenge F, Viader F, de la Sayette V, et al. Using voxel-based morphometry to map the structural changes associated with rapid conversion in MCI: a longitudinal MRI study. Neuroimage. 2005;27(4):934–946. doi: 10.1016/j.neuroimage.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Ratcliff G, Saxton JA, Bryan RN, Fried LP, Lucke JF. Cognitive correlates of human brain aging: a quantitative magnetic resonance imaging investigation. J Neuropsychiatry Clin Neurosci. 2001;13(4):471–485. doi: 10.1176/jnp.13.4.471. [DOI] [PubMed] [Google Scholar]
- Davatzikos C. Spatial normalization of 3D brain images using deformable models. Journal of Computer Assisted Tomography. 1996;20(4):656–665. doi: 10.1097/00004728-199607000-00031. [DOI] [PubMed] [Google Scholar]
- de Jong BM, Willemsen AT, Paans AM. Brain activation related to the change between bimanual motor programs. Neuroimage. 1999;9(3):290–297. doi: 10.1006/nimg.1998.0410. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Jr, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92(12):145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Goldszal AF, Davatzikos C, Pham DL, Yan MX, Bryan RN, Resnick SM. An image-processing system for qualitative and quantitative volumetric analysis of brain images. J Comput Assist Tomogr. 1998;22(5):827–837. doi: 10.1097/00004728-199809000-00030. [DOI] [PubMed] [Google Scholar]
- Grasby PM, Frith CD, Friston KJ, Bench C, Frackowiak RS, Dolan RJ. Functional mapping of brain areas implicated in auditory--verbal memory function. Brain. 1993;116(Pt 1):1–20. doi: 10.1093/brain/116.1.1. [DOI] [PubMed] [Google Scholar]
- Kabani N, MacDonald JD, Holmes CJ, Evans AC. A 3D atlas of the human brain. NeuroImage. 1998;7(4):S717. [Google Scholar]
- Karnath HO, Baier B, Nagele T. Awareness of the functioning of one’s own limbs mediated by the insular cortex? J Neurosci. 2005;25(31):7134–7138. doi: 10.1523/JNEUROSCI.1590-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassubek J, Juengling FD, Ecker D, Landwehrmeyer GB. Thalamic atrophy in Huntington’s disease co-varies with cognitive performance: a morphometric MRI analysis. Cereb Cortex. 2005;15(6):846–853. doi: 10.1093/cercor/bhh185. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological Assessment. 4. Oxford: Oxford University Press; 2004. [Google Scholar]
- Links JM, Schwartz BS, Simon D, Bandeen-Roche K, Stewart WF. Characterization of toxicokinetics and toxicodynamics with linear systems theory: application to lead-associated cognitive decline. Environ Health Perspect. 2001;109(4):361–368. doi: 10.1289/ehp.01109361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLullich AM, Edmond CL, Ferguson KJ, Wardlaw JM, Starr JM, Seckl JR, et al. Size of the neocerebellar vermis is associated with cognition in healthy elderly men. Brain Cogn. 2004;56(3):344–348. doi: 10.1016/j.bandc.2004.08.001. [DOI] [PubMed] [Google Scholar]
- MacLullich AM, Ferguson KJ, Deary IJ, Seckl JR, Starr JM, Wardlaw JM. Intracranial capacity and brain volumes are associated with cognition in healthy elderly men. Neurology. 2002;59(2):169–174. doi: 10.1212/wnl.59.2.169. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Schuller S, Pohl C, Jahn T, Weindl A, Kassubek J. Executive dysfunction in early stages of Huntington’s disease is associated with striatal and insular atrophy: a neuropsychological and voxel-based morphometric study. J Neurol Sci. 2005;239(1):11–19. doi: 10.1016/j.jns.2005.07.007. [DOI] [PubMed] [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2006. [Google Scholar]
- Resnick SM, Goldszal AF, Davatzikos C, Golski S, Kraut MA, Metter EJ, et al. One-year age changes in MRI brain volumes in older adults. Cereb Cortex. 2000;10(5):464–472. doi: 10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- Robinson CJ, Burton H. Organization of somatosensory receptive fields in cortical areas 7b, retroinsula, postauditory and granular insula of M. fascicularis. J Comp Neurol. 1980;192(1):69–92. doi: 10.1002/cne.901920105. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions. Behav Neurol. 2000;12(4):191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Schoch B, Dimitrova A, Gizewski ER, Timmann D. Functional localization in the human cerebellum based on voxelwise statistical analysis: A study of 90 patients. Neuroimage. 2005 doi: 10.1016/j.neuroimage.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Schwartz BS, Bolla KI, Stewart W, Ford DP, Agnew J, Frumkin H. Decrements in neurobehavioral performance associated with mixed exposure to organic and inorganic lead. Am J Epidemiol. 1993;137(9):1006–1021. doi: 10.1093/oxfordjournals.aje.a116757. [DOI] [PubMed] [Google Scholar]
- Schwartz BS, Stewart WF, Bolla KI, Simon PD, Bandeen-Roche K, Gordon PB, et al. Past adult lead exposure is associated with longitudinal decline in cognitive function. Neurology. 2000;55(8):1144–1150. doi: 10.1212/wnl.55.8.1144. [DOI] [PubMed] [Google Scholar]
- Shen D, Davatzikos C. Very high-resolution morphometry using mass-preserving deformations and HAMMER elastic registration. Neuroimage. 2003;18(1):28–41. doi: 10.1006/nimg.2002.1301. [DOI] [PubMed] [Google Scholar]
- Shih RA, Glass TA, Bandeen-Roche K, Carlson MC, Bolla KI, Todd AC, et al. Environmental lead exposure and cognitive function in community-dwelling older adults. Neurology. 2006;67(9):1556–1562. doi: 10.1212/01.wnl.0000239836.26142.c5. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Schwartz BS, Davatzikos C, Shen D, Liu D, Wu X, et al. Past adult lead exposure is linked to neurodegeneration measured by brain MRI. Neurology. 2006;66(10):1476–1484. doi: 10.1212/01.wnl.0000216138.69777.15. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Schwartz BS, Simon D, Bolla KI, Todd AC, Links J. Neurobehavioral function and tibial and chelatable lead levels in 543 former organolead workers. Neurology. 1999;52(8):1610–1617. doi: 10.1212/wnl.52.8.1610. [DOI] [PubMed] [Google Scholar]
- Stewart WF, Schwartz BS, Simon D, Kelsey K, Todd AC. ApoE genotype, past adult lead exposure, and neurobehavioral function. Environ Health Perspect. 2002;110(5):501–505. doi: 10.1289/ehp.02110501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storey JD. A direct approach to false discovery rates. J Royal Stat Soc B. 2002;64:479–498. [Google Scholar]
- Storey JD, Tibshirani R. Statistical significance for genomewide studies. Proc Natl Acad Sci U S A. 2003;100(16):9440–9445. doi: 10.1073/pnas.1530509100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme Medical; 1988. [Google Scholar]
- Tisserand DJ, van Boxtel MP, Pruessner JC, Hofman P, Evans AC, Jolles J. A voxel-based morphometric study to determine individual differences in gray matter density associated with age and cognitive change over time. Cereb Cortex. 2004;14(9):966–973. doi: 10.1093/cercor/bhh057. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Visser PJ, van Boxtel MP, Jolles J. The relation between global and limbic brain volumes on MRI and cognitive performance in healthy individuals across the age range. Neurobiol Aging. 2000;21(4):569–576. doi: 10.1016/s0197-4580(00)00133-0. [DOI] [PubMed] [Google Scholar]
- van der Flier WM, Middelkoop HA, Weverling-Rijnsburger AW, Admiraal-Behloul F, Bollen EL, Westendorp RG, et al. Neuropsychological correlates of MRI measures in the continuum of cognitive decline at old age. Dement Geriatr Cogn Disord. 2005;20(23):82–88. doi: 10.1159/000086072. [DOI] [PubMed] [Google Scholar]
- Van Petten C, Plante E, Davidson PS, Kuo TY, Bajuscak L, Glisky EL. Memory and executive function in older adults: relationships with temporal and prefrontal gray matter volumes and white matter hyperintensities. Neuropsychologia. 2004;42(10):1313–1335. doi: 10.1016/j.neuropsychologia.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Yan MXH, Karp JN. An adaptive Bayesian approach to three-dimensional MR brain segmentation. Proceedings of XIVth International Conference on Information Processing in Medical Imaging; 1995. pp. 201–213. [Google Scholar]


