Abstract
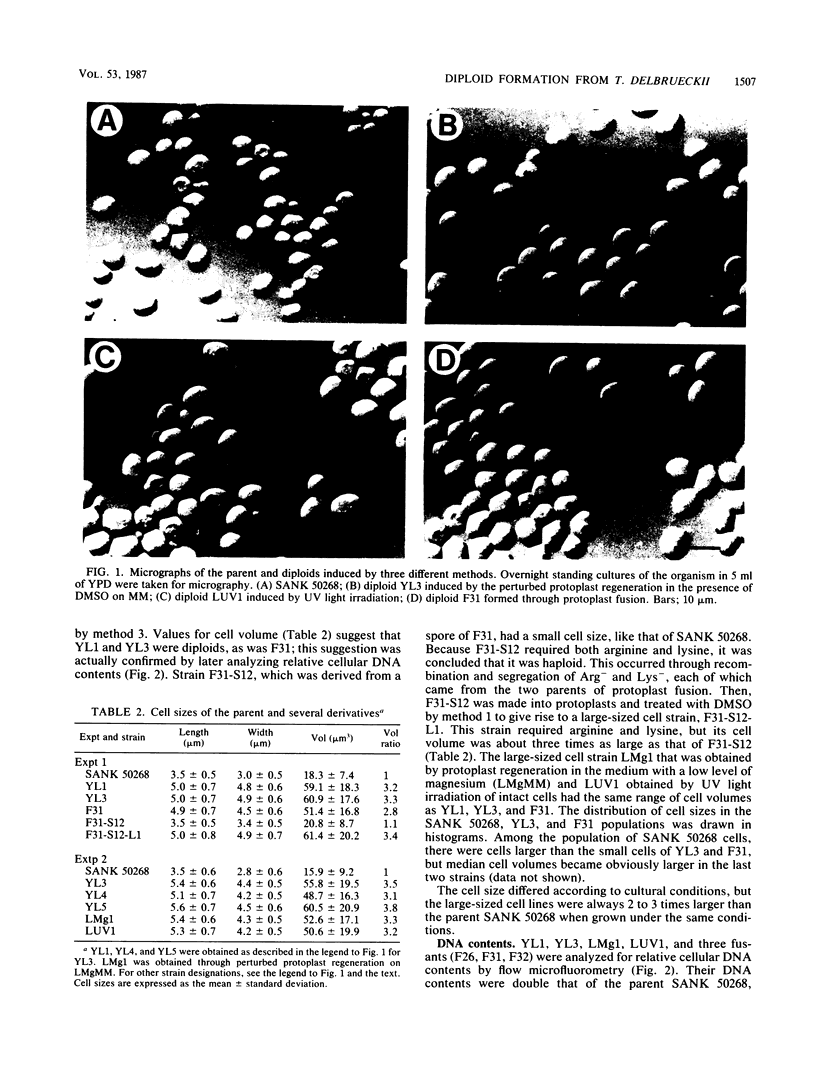
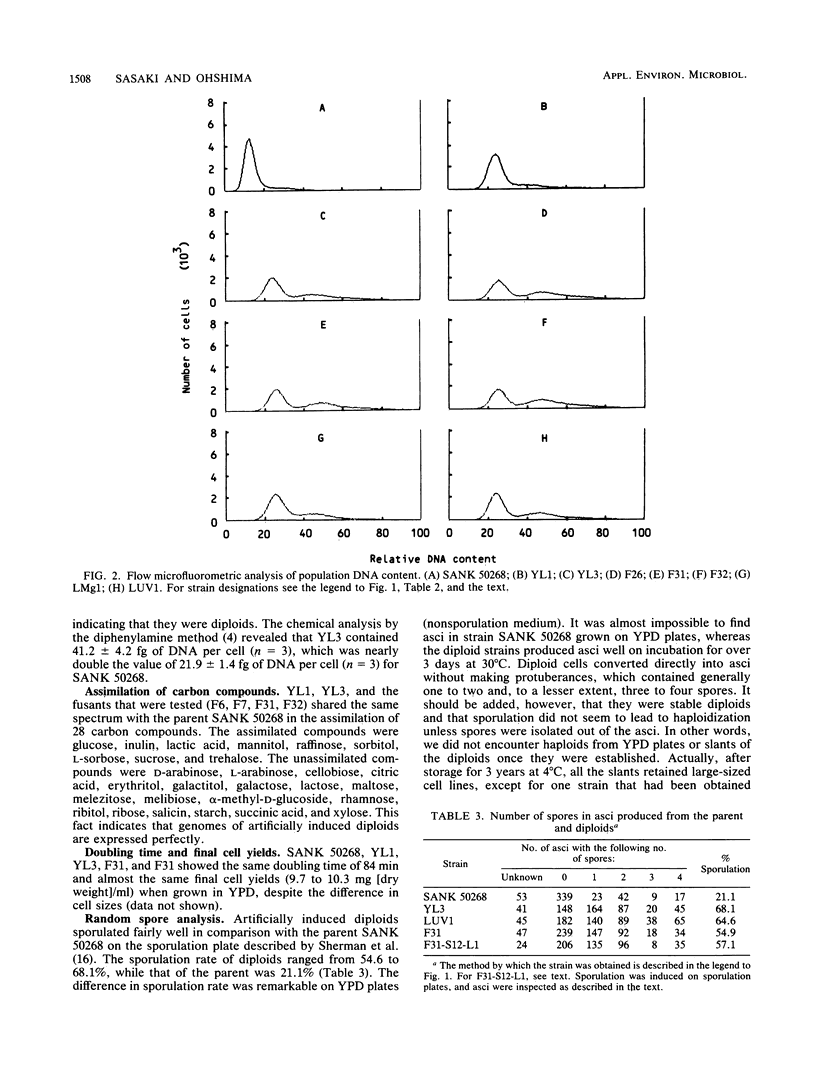
The yeast Torulaspora delbrueckii, which propagates as a haploid, was made into a diploid by treatment with dimethyl sulfoxide (DMSO) on the regeneration of protoplasts. The diploid state was stably inherited; the cell volume was three times that of the parent strain and the cellular DNA content was two times that of the parental strain. No essential difference was found between diploids induced by DMSO and those formed through intraspecific protoplast fusion. The diploid strains sporulated fairly well, with their cells converting directly into asci. Random spore analysis revealed that diploids induced through protoplast fusion gave rise to auxotrophic segregants (haploids) with the parental genetic marker or to segregants formed by recombination, while diploids induced by DMSO from a doubly auxotrophic parent gave rise to no recombinant, indicating that it was chromosomally homoallelic in nature. The magnesium level in the protoplast regeneration medium was found to be an important factor for inducing diploid formation. At 0.2 mM magnesium diploids appeared even in the absence of DMSO, while at 2 mM magnesium diploids never appeared unless DMSO was added to the regeneration medium. Evidence is provided that the diploids induced by DMSO or a low magnesium level are due to direct diploidization but not protoplast fusion. UV light irradiation of intact cells (without protoplasts), 10% of which survived, also produced diploids among this surviving population. From these results we conclude that the perturbation of protoplast regeneration or of cell division by the treatments mentioned above somehow induced direct diploidization of T. delbrueckii.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahkong Q. F., Fisher D., Tampion W., Lucy J. A. Mechanisms of cell fusion. Nature. 1975 Jan 17;253(5488):194–195. doi: 10.1038/253194a0. [DOI] [PubMed] [Google Scholar]
- Bostock C. J. DNA synthesis in the fission yeast Schizosaccharomyces pombe. Exp Cell Res. 1970 Apr;60(1):16–26. doi: 10.1016/0014-4827(70)90484-2. [DOI] [PubMed] [Google Scholar]
- Finkle B. J., Appleman D. The Effect of Magnesium Concentration on Growth of Chlorella. Plant Physiol. 1953 Oct;28(4):664–673. doi: 10.1104/pp.28.4.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., Katsumaru H. Dynamics of nuclear actin bundle induction by dimethyl sulfoxide and factors affecting its development. J Cell Biol. 1980 Jan;84(1):131–140. doi: 10.1083/jcb.84.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fulton A. M., Bond D. J. Dimethylsulfoxide induces aneuploidy in a fungal test system. Mol Gen Genet. 1984;197(2):347–349. doi: 10.1007/BF00330985. [DOI] [PubMed] [Google Scholar]
- ISHITANI C. A high frequency of heterozygous diploids and somatic recombination produced by ultra-violet light in imperfect fungi. Nature. 1956 Sep 29;178(4535):706–706. doi: 10.1038/178706a0. [DOI] [PubMed] [Google Scholar]
- Ohshima Y., Sugaura T., Horita M., Sasaki T. Industrial Application of Artificially Induced Diploid Strains of Torulaspora delbrueckii. Appl Environ Microbiol. 1987 Jul;53(7):1512–1514. doi: 10.1128/aem.53.7.1512-1514.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parry J. M., Sharp D., Tippins R. S., Parry E. M. Radiation-induced mitotic and meiotic aneuploidy in the yeast Saccharomyces cerevisiae. Mutat Res. 1979 Jun;61(1):37–55. doi: 10.1016/0027-5107(79)90005-8. [DOI] [PubMed] [Google Scholar]
- Slater M. L., Sharrow S. O., Gart J. J. Cell cycle of Saccharomycescerevisiae in populations growing at different rates. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3850–3854. doi: 10.1073/pnas.74.9.3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobey R. A., Crissman H. A. Unique techniques for cell analysis utilizing mithramycin and flow microfluorometry. Exp Cell Res. 1975 Jun;93(1):235–239. doi: 10.1016/0014-4827(75)90445-0. [DOI] [PubMed] [Google Scholar]
- Walker G. M., Duffus J. H. Magnesium ions and the control of the cell cycle in yeast. J Cell Sci. 1980 Apr;42:329–356. doi: 10.1242/jcs.42.1.329. [DOI] [PubMed] [Google Scholar]
- Walker G. M. Magnesium and cell cycle control: an update. Magnesium. 1986;5(1):9–23. [PubMed] [Google Scholar]
- Yee B., Tsuyumu S., Adams B. G. Biological effects of dimethyl sulfoxide on yeast. Biochem Biophys Res Commun. 1972 Dec 4;49(5):1336–1342. doi: 10.1016/0006-291x(72)90613-4. [DOI] [PubMed] [Google Scholar]
- de Bruijne A. W., van Steveninck J. Lysis of yeast cells and erythrocytes by dimethylsulfoxide. Biochem Pharmacol. 1972 Jan 15;21(2):153–162. doi: 10.1016/0006-2952(72)90265-1. [DOI] [PubMed] [Google Scholar]



