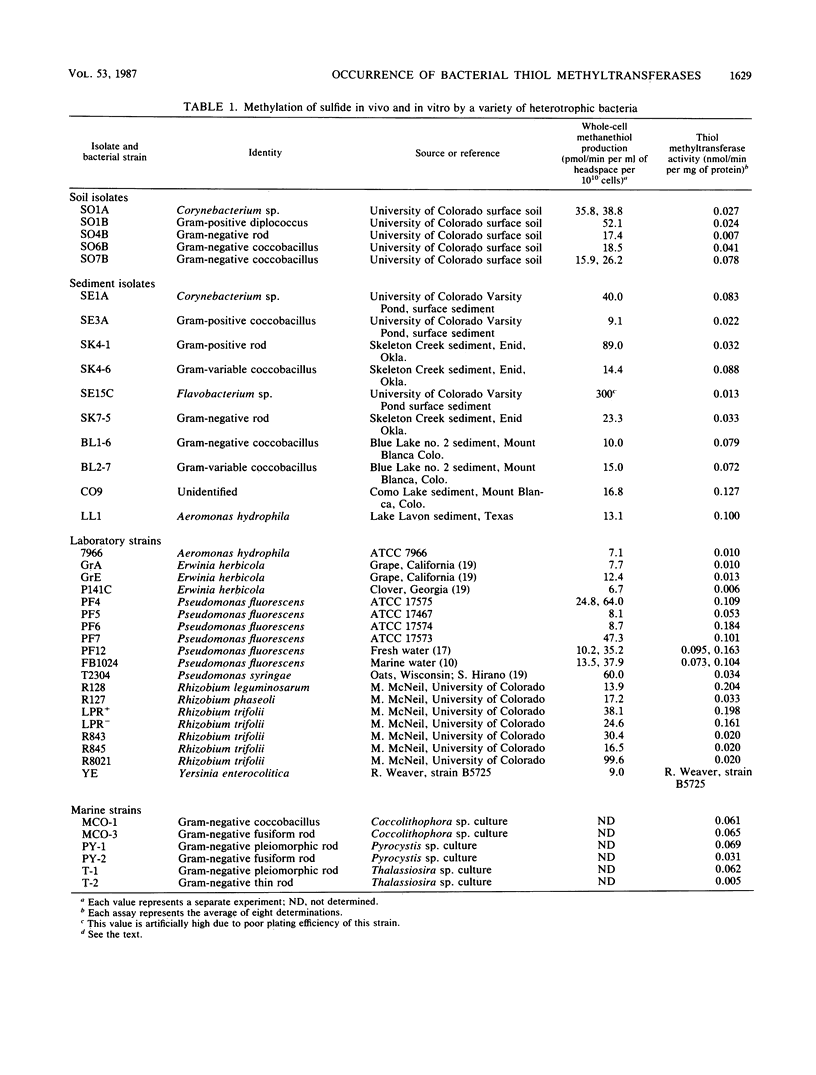
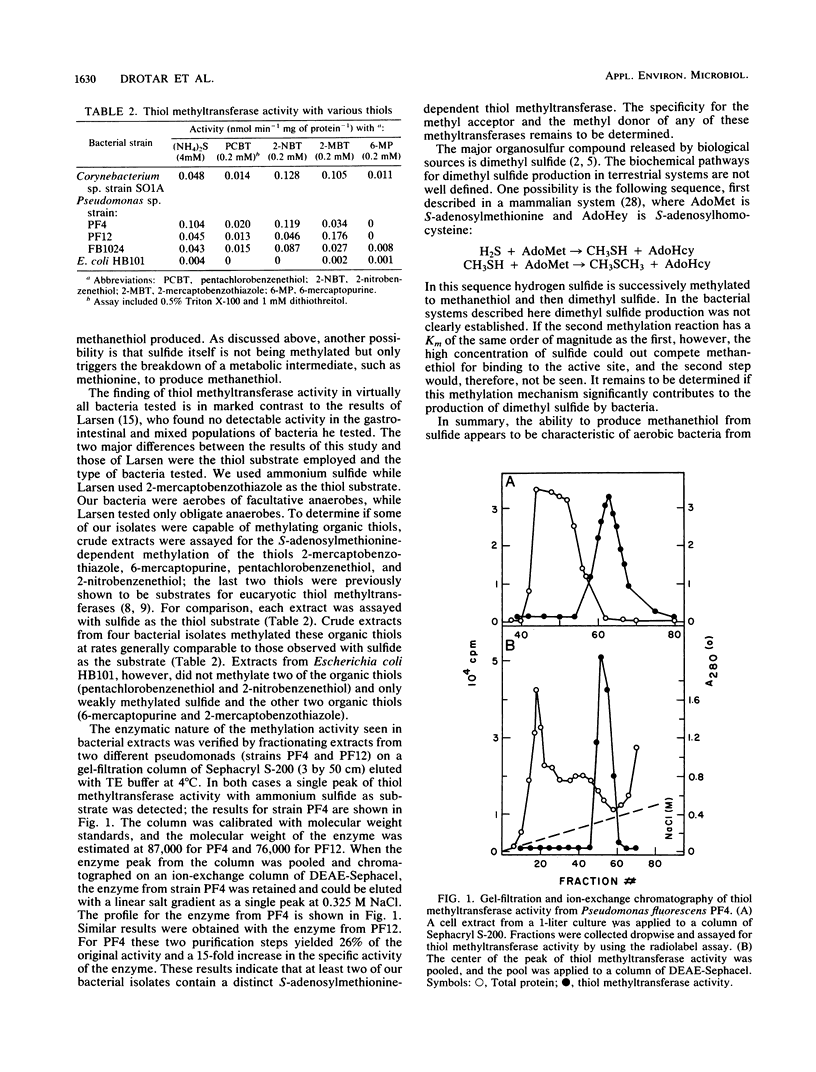
Abstract
A majority of heterotrophic bacteria isolated from soil, water, sediment, vegetation, and marine algae cultures methylated sulfide, producing methanethiol. This was demonstrated with intact cells by measuring the emission of methanethiol with a sulfur-selective chemiluminescence detector, and in cell extracts by detection of sulfide-dependent thiol methyltransferase activity. Extracts of two Pseudomonas isolates were fractionated by gel-filtration and ion-exchange chromatography, and with sulfide as the substrate a single peak of thiol methyltransferase activity was seen in each case. Extracts of several bacterial strains also contained thiol methyltransferase activity with organic thiols as substrates. Thus, S-adenosylmethionine-dependent thiol methyltransferase activities are widespread in bacteria and may contribute to biogenic emissions of methylated sulfur gases and to the production of methyl thioethers.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON D. G., CANTONI G. L. Enzymatic cleavage of dimethylpropiothetin by Polysiphonia lanosa. J Biol Chem. 1956 Sep;222(1):171–177. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Kadota H., Ishida Y. Production of volatile sulfur compounds by microorganisms. Annu Rev Microbiol. 1972;26:127–138. doi: 10.1146/annurev.mi.26.100172.001015. [DOI] [PubMed] [Google Scholar]
- Langheinrich W., Ring K. Regulation of amino acid transport in growing cells of Streptomyces hydrogenans. I. Modulation of transport capacity and amino acid pool composition during the growth cycle. Arch Microbiol. 1976 Sep 1;109(3):227–235. doi: 10.1007/BF00446633. [DOI] [PubMed] [Google Scholar]
- Larsen G. L. Distribution of cysteine conjugate beta-lyase in gastrointestinal bacteria and in the environment. Xenobiotica. 1985 Mar;15(3):199–209. doi: 10.3109/00498258509045350. [DOI] [PubMed] [Google Scholar]
- Phelps P., Giddings T. H., Prochoda M., Fall R. Release of cell-free ice nuclei by Erwinia herbicola. J Bacteriol. 1986 Aug;167(2):496–502. doi: 10.1128/jb.167.2.496-502.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal W., Starkey R. L. Microbial decomposition of methionine and identity of the resulting sulfur products. J Bacteriol. 1969 Jun;98(3):908–913. doi: 10.1128/jb.98.3.908-913.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tegtmeier F., Brunner G. Solubilization characteristics of pig liver S-methyltransferase. Enzyme. 1983;30(3):185–195. doi: 10.1159/000469573. [DOI] [PubMed] [Google Scholar]
- Weinshilboum R. M., Sladek S., Klumpp S. Human erythrocyte thiol methyltransferase: radiochemical microassay and biochemical properties. Clin Chim Acta. 1979 Sep 15;97(1):59–71. doi: 10.1016/0009-8981(79)90025-1. [DOI] [PubMed] [Google Scholar]
- Weisiger R. A., Jakoby W. B. Thiol S-methyltransferase from rat liver. Arch Biochem Biophys. 1979 Sep;196(2):631–637. doi: 10.1016/0003-9861(79)90317-5. [DOI] [PubMed] [Google Scholar]
- Weisiger R. A., Pinkus L. M., Jakoby W. B. Thiol S-methyltransferase: suggested role in detoxication of intestinal hydrogen sulfide. Biochem Pharmacol. 1980 Oct 15;29(20):2885–2887. doi: 10.1016/0006-2952(80)90029-5. [DOI] [PubMed] [Google Scholar]
- Zinder S. H., Doemel W. N., Brock T. D. Production of volatile sulfur compounds during the decomposition of algal mats. Appl Environ Microbiol. 1977 Dec;34(6):859–860. doi: 10.1128/aem.34.6.859-860.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


