Abstract
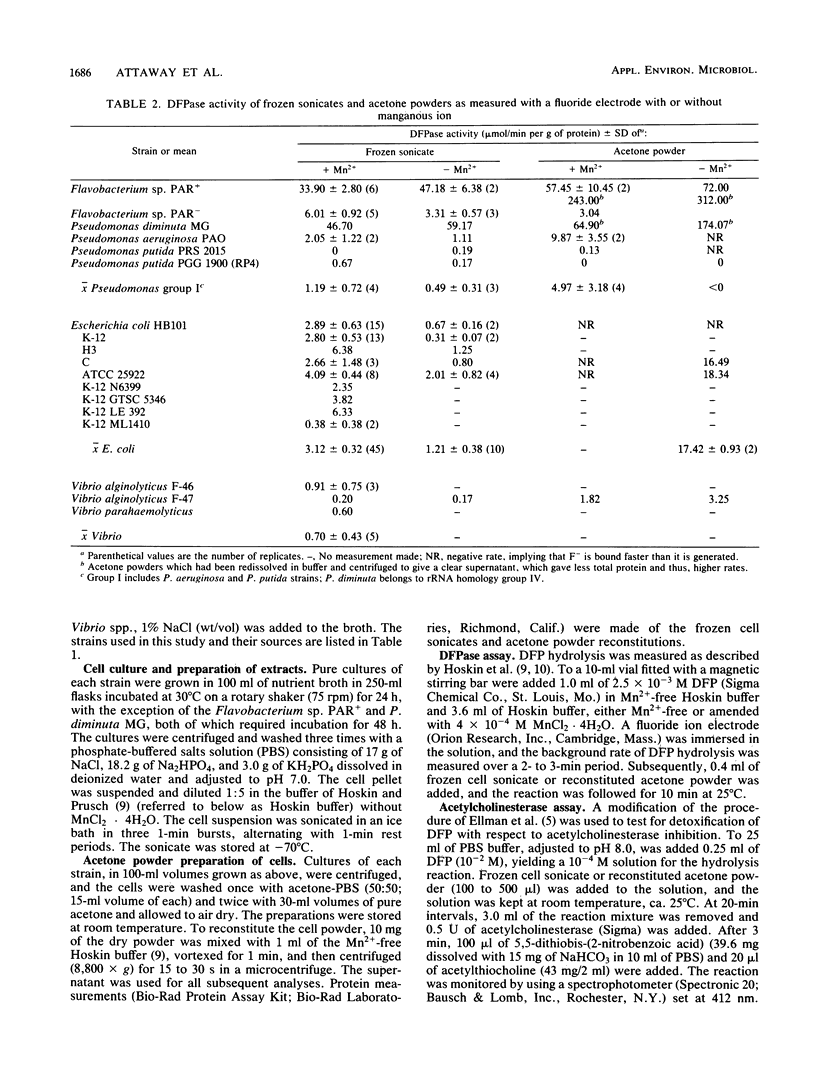
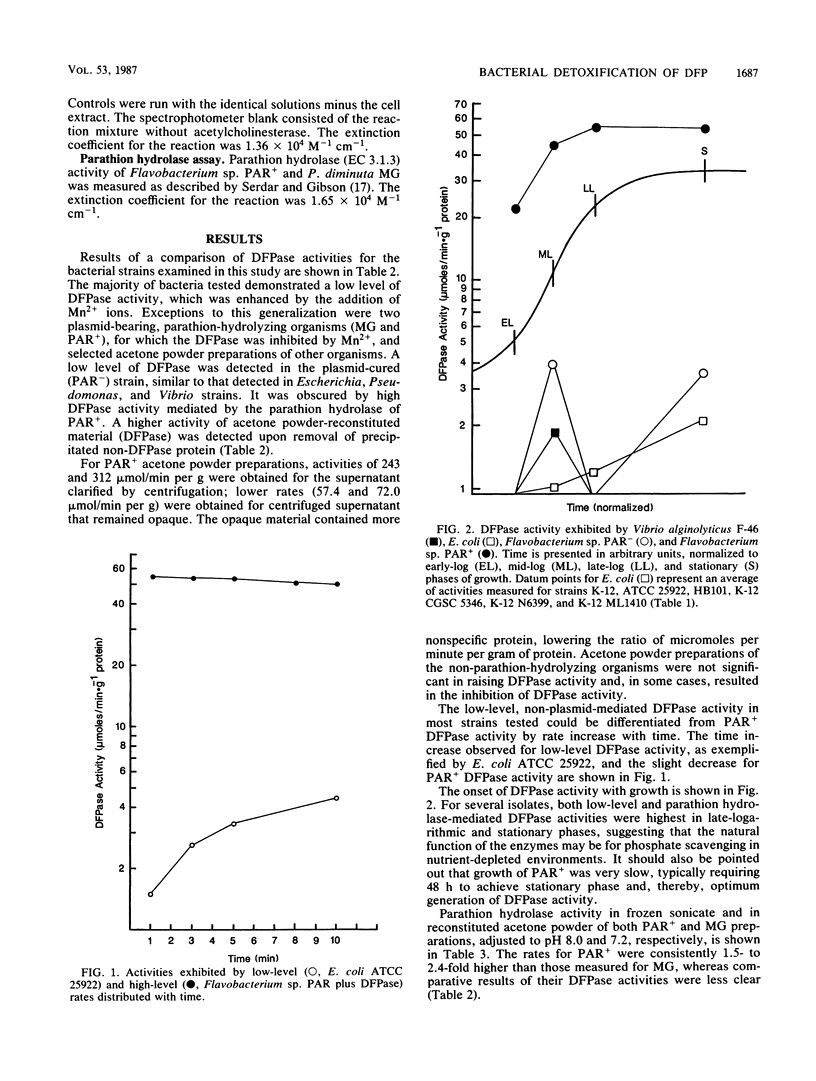
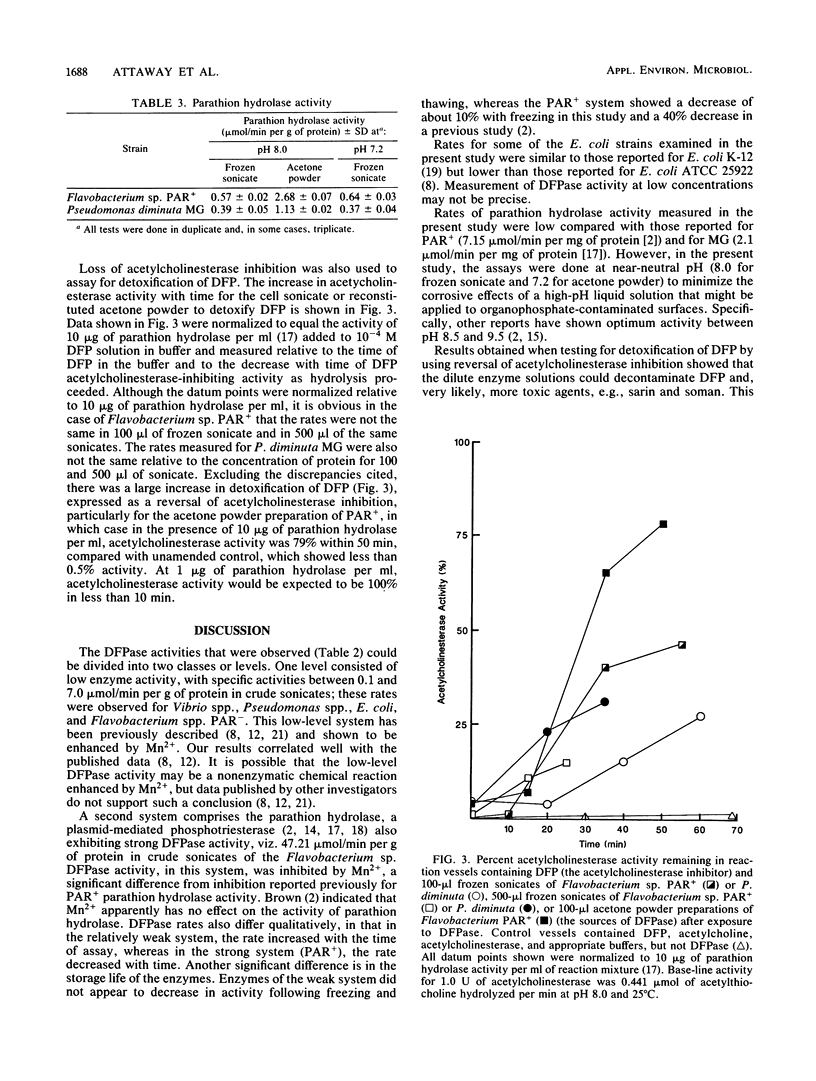
The ability of 18 gram-negative bacterial isolates to detoxify diisopropyl fluorophosphate, a structural analog of the agents soman and sarin, was investigated. Detoxification by both frozen cell sonicates and acetone powders was assayed by two methods, i.e., the hydrolytic release of fluoride, measured by a fluoride-specific ion electrode, and the disappearance of acetylcholinesterase inhibition in vitro. Frozen cell sonicates for all strains exhibited some activity (F- ion release). In general, acetone powder preparations produced higher activity than frozen cell sonicates did, and the highest activities were exhibited by strains with known parathion hydrolase activity. Two ranges in activity were observed, low level, ranging from 0.1 to 7.0 mumol/min per g of protein, and high level, detected only in parathion hydrolase-producing strains, from 47 to greater than 300 mumol/min per g of protein. Results indicate that parathion hydrolase was nonspecific in phosphoesterase activity. Also, it was an effective detoxicant at low concentrations and near-neutral pH.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cook A. M., Daughton C. G., Alexander M. Phosphonate utilization by bacteria. J Bacteriol. 1978 Jan;133(1):85–90. doi: 10.1128/jb.133.1.85-90.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook A. M., Daughton C. G., Alexander M. Phosphorus-containing pesticide breakdown products: quantitative utilization as phosphorus sources by bacteria. Appl Environ Microbiol. 1978 Nov;36(5):668–672. doi: 10.1128/aem.36.5.668-672.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Gay D. D., Hoskin F. C. Stereospecificity and active site requirements in a diisopropylphosphorofluoridate-hydrolyzing enzyme. Biochem Pharmacol. 1979 Apr 1;28(7):1259–1261. doi: 10.1016/0006-2952(79)90339-3. [DOI] [PubMed] [Google Scholar]
- Hoskin F. C. Distribution of diisopropylphosphorofluoridate-hydrolyzing enzyme between sheath and axoplasm of squid giant axon. J Neurochem. 1976 May;26(5):1043–1045. doi: 10.1111/j.1471-4159.1976.tb06491.x. [DOI] [PubMed] [Google Scholar]
- Hoskin F. C., Kirkish M. A., Steinmann K. E. Two enzymes for the detoxication of organophosphorus compounds--sources, similarities, and significance. Fundam Appl Toxicol. 1984 Apr;4(2 Pt 2):S165–S172. doi: 10.1016/0272-0590(84)90149-0. [DOI] [PubMed] [Google Scholar]
- Hoskin F. C., Prusch R. D. Characterization of a DFP-hydrolyzing enzyme in squid posterior salivary gland by use of Soman, DFP and manganous ion. Comp Biochem Physiol C. 1983;75(1):17–20. doi: 10.1016/0742-8413(83)90004-x. [DOI] [PubMed] [Google Scholar]
- Hoskin F. C., Roush A. H. Hydrolysis of nerve gas by squid-type diisopropyl phosphorofluoridate hydrolyzing enzyme on agarose resin. Science. 1982 Mar 5;215(4537):1255–1257. doi: 10.1126/science.7058344. [DOI] [PubMed] [Google Scholar]
- MOUNTER L. A., BAXTER R. F., CHANUTIN A. Dialkylfluorophosphatases of microorganisms. J Biol Chem. 1955 Aug;215(2):699–704. [PubMed] [Google Scholar]
- Moore J. K., Braymer H. D., Larson A. D. Isolation of a Pseudomonas sp. Which Utilizes the Phosphonate Herbicide Glyphosate. Appl Environ Microbiol. 1983 Aug;46(2):316–320. doi: 10.1128/aem.46.2.316-320.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulbry W. W., Karns J. S., Kearney P. C., Nelson J. O., McDaniel C. S., Wild J. R. Identification of a plasmid-borne parathion hydrolase gene from Flavobacterium sp. by southern hybridization with opd from Pseudomonas diminuta. Appl Environ Microbiol. 1986 May;51(5):926–930. doi: 10.1128/aem.51.5.926-930.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munnecke D. M. Enzymatic hydrolysis of organophosphate insecticides, a possible pesticide disposal method. Appl Environ Microbiol. 1976 Jul;32(1):7–13. doi: 10.1128/aem.32.1.7-13.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg A., Alexander M. Microbial cleavage of various organophosphorus insecticides. Appl Environ Microbiol. 1979 May;37(5):886–891. doi: 10.1128/aem.37.5.886-891.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serdar C. M., Gibson D. T., Munnecke D. M., Lancaster J. H. Plasmid Involvement in Parathion Hydrolysis by Pseudomonas diminuta. Appl Environ Microbiol. 1982 Jul;44(1):246–249. doi: 10.1128/aem.44.1.246-249.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T., Tsunoda M., Mitsuhashi S. Distribution of R factors among Shigella strains isolated in Japan (II). Jpn J Microbiol. 1973 Jul;17(4):291–295. doi: 10.1111/j.1348-0421.1973.tb00775.x. [DOI] [PubMed] [Google Scholar]
- Wheelis M. L., Ornston L. N. Genetic control of enzyme induction in the -ketoadipate pathway of Pseudomonas putida: deletion mapping of cat mutations. J Bacteriol. 1972 Feb;109(2):790–795. doi: 10.1128/jb.109.2.790-795.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zech R., Wigand K. D. Organophosphate-detoxicating enzymes in E. coli. Gelfiltration and isoelectric focusing of DFPase, paraoxonase and unspecific phosphohydrolases. Experientia. 1975 Feb 15;31(2):157–158. doi: 10.1007/BF01990678. [DOI] [PubMed] [Google Scholar]


