Abstract
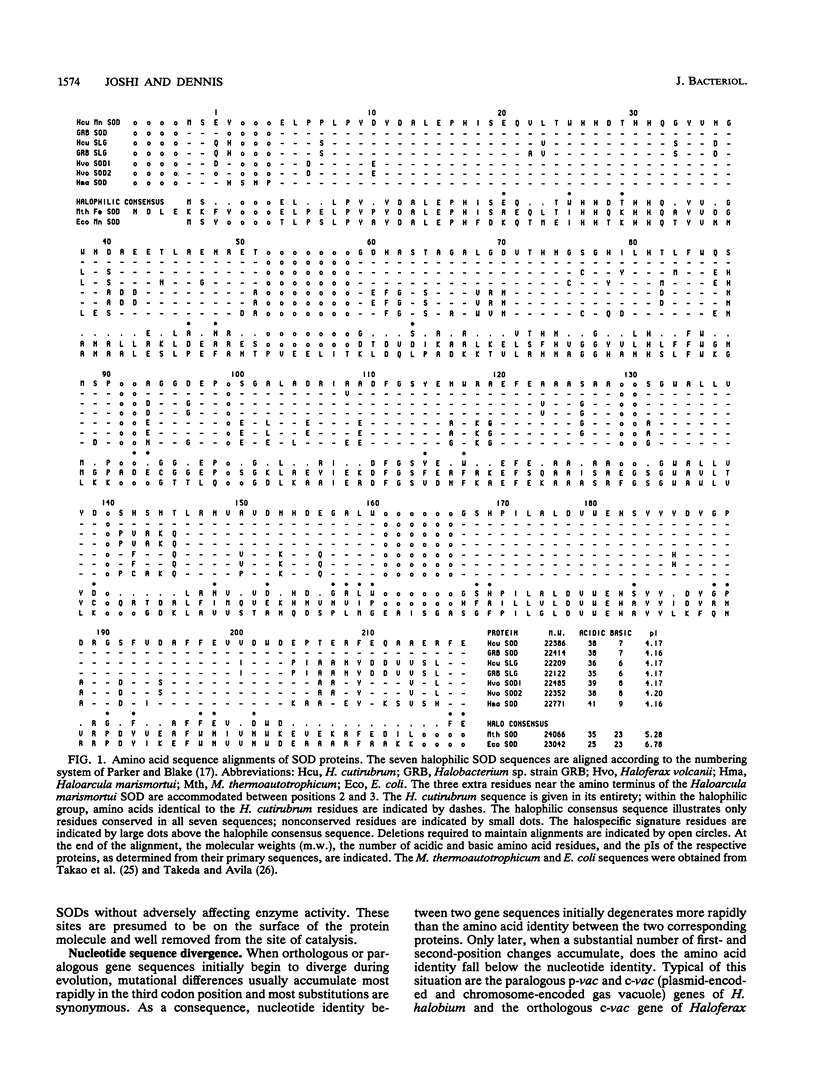
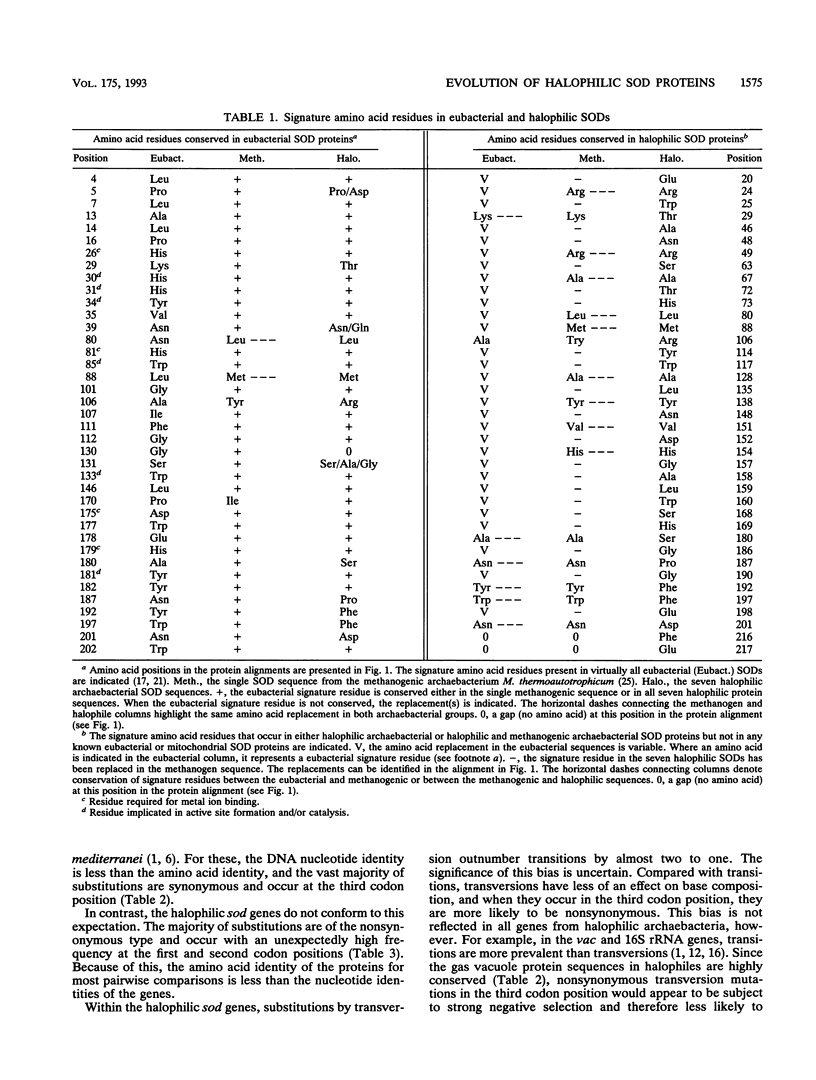
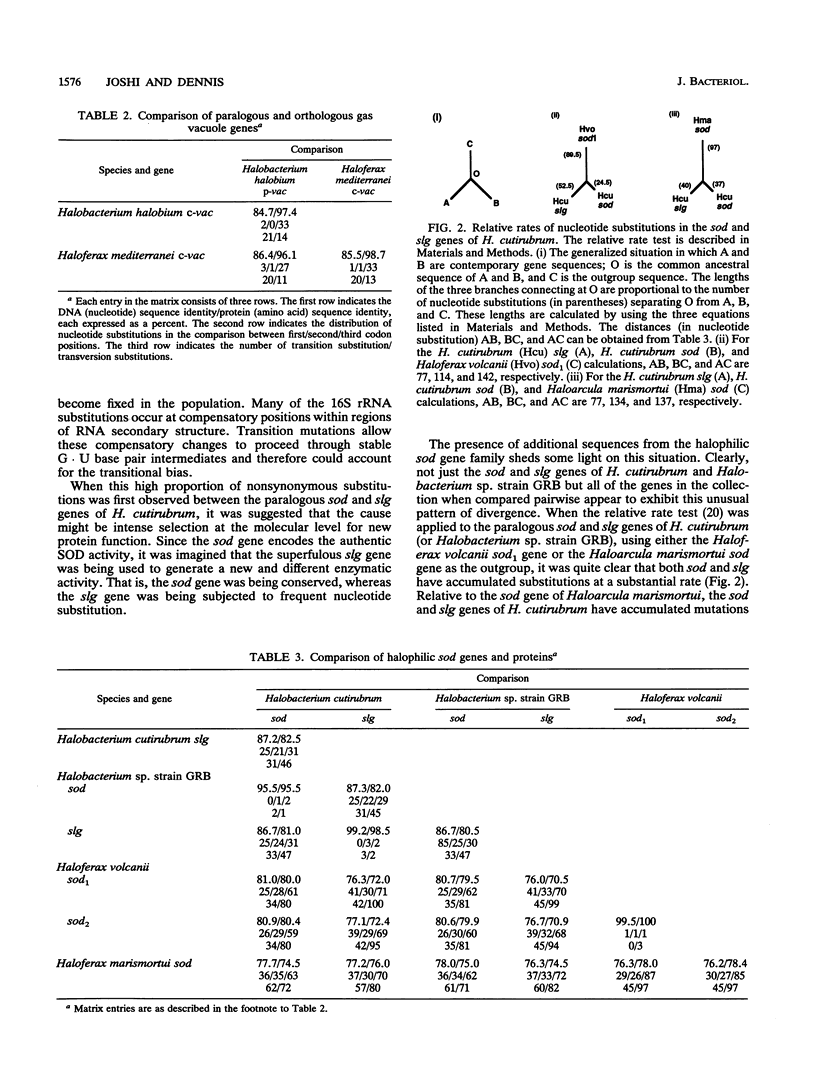
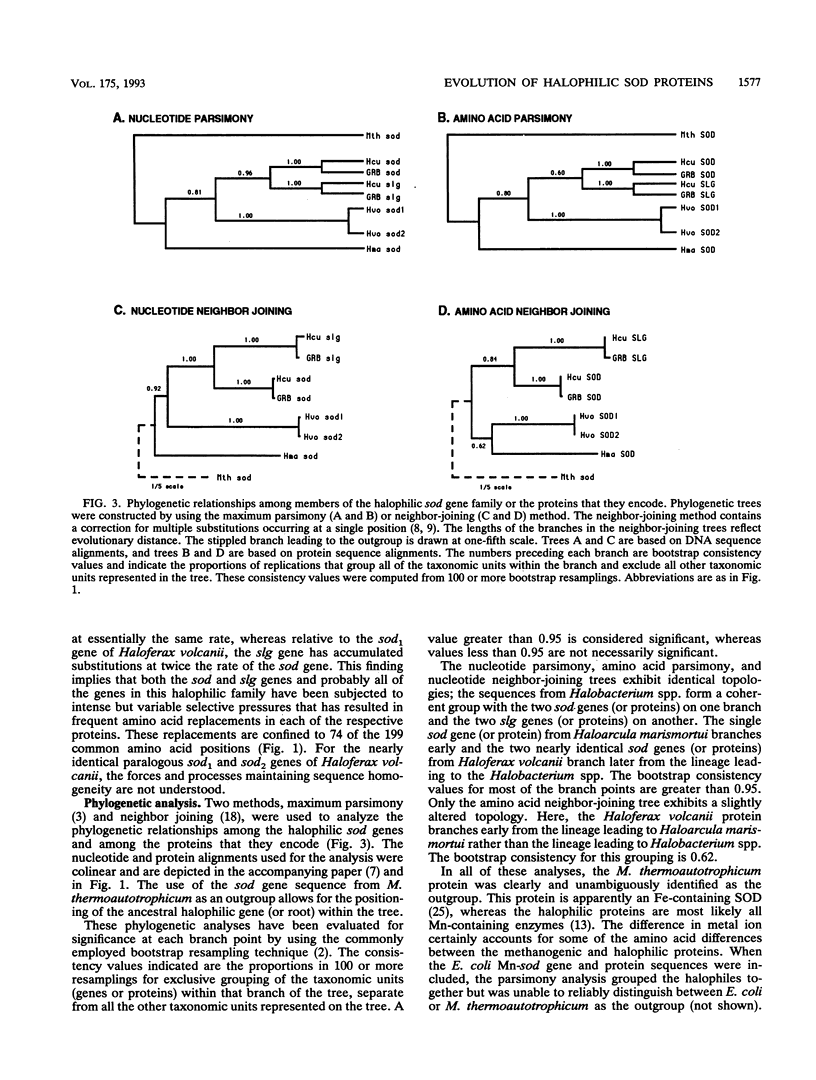
The protein sequences of seven members of the superoxide dismutase (SOD) family from halophilic archaebacteria have been aligned and compared with each other and with the homologous Mn and Fe SOD sequences from eubacteria and the methanogenic archaebacterium Methanobacterium thermoautotrophicum. Of 199 common residues in the SOD proteins from halophilic archaebacteria, 125 are conserved in all seven sequences, and 64 of these are encoded by single unique triplets. The 74 remaining positions exhibit a high degree of variability, and for almost half of these, the encoding triplets are connected by at least two nonsynonymous nucleotide substitutions. The majority of nucleotide substitutions within the seven genes are nonsynonymous and result in amino acid replacement in the respective protein; silent third-codon-position (synonymous) substitutions are unexpectedly rare. Halophilic SODs contain 30 specific residues that are not found at the corresponding positions of the methanogenic or eubacterial SOD proteins. Seven of these are replacements of highly conserved amino acids in eubacterial SODs that are believed to play an important role in the three-dimensional structure of the protein. Residues implicated in formation of the active site, catalysis, and metal ion binding are conserved in all Mn and Fe SODs. Molecular phylogenies based on parsimony and neighbor-joining methods coherently group the halophile sequences but surprisingly fail to distinguish between the Mn SOD of Escherichia coli and the Fe SOD of M. thermoautotrophicum as the outgroup. These comparisons indicate that as a group, the SODs of halophilic archaebacteria have many unique and characteristic features. At the same time, the patterns of nucleotide substitution and amino acid replacement indicate that these genes and the proteins that they encode continue to be subject to strong and changing selection. This selection may be related to the presence of oxygen radicals and the inter- and intracellular composition and concentration of metal cations.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Englert C., Horne M., Pfeifer F. Expression of the major gas vesicle protein gene in the halophilic archaebacterium Haloferax mediterranei is modulated by salt. Mol Gen Genet. 1990 Jul;222(2-3):225–232. doi: 10.1007/BF00633822. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Biological effects of the superoxide radical. Arch Biochem Biophys. 1986 May 15;247(1):1–11. doi: 10.1016/0003-9861(86)90526-6. [DOI] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Horne M., Englert C., Pfeifer F. Two genes encoding gas vacuole proteins in Halobacterium halobium. Mol Gen Genet. 1988 Aug;213(2-3):459–464. doi: 10.1007/BF00339616. [DOI] [PubMed] [Google Scholar]
- Joshi P., Dennis P. P. Characterization of paralogous and orthologous members of the superoxide dismutase gene family from genera of the halophilic archaebacteria. J Bacteriol. 1993 Mar;175(6):1561–1571. doi: 10.1128/jb.175.6.1561-1571.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980 Dec;16(2):111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- Kirby T. W., Lancaster J. R., Jr, Fridovich I. Isolation and characterization of the iron-containing superoxide dismutase of Methanobacterium bryantii. Arch Biochem Biophys. 1981 Aug;210(1):140–148. doi: 10.1016/0003-9861(81)90174-0. [DOI] [PubMed] [Google Scholar]
- Lanyi J. K. Salt-dependent properties of proteins from extremely halophilic bacteria. Bacteriol Rev. 1974 Sep;38(3):272–290. doi: 10.1128/br.38.3.272-290.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May B. P., Dennis P. P. Evolution and regulation of the gene encoding superoxide dismutase from the archaebacterium Halobacterium cutirubrum. J Biol Chem. 1989 Jul 25;264(21):12253–12258. [PubMed] [Google Scholar]
- May B. P., Dennis P. P. Superoxide dismutase from the extremely halophilic archaebacterium Halobacterium cutirubrum. J Bacteriol. 1987 Apr;169(4):1417–1422. doi: 10.1128/jb.169.4.1417-1422.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May B. P., Dennis P. P. Unusual evolution of a superoxide dismutase-like gene from the extremely halophilic archaebacterium Halobacterium cutirubrum. J Bacteriol. 1990 Jul;172(7):3725–3729. doi: 10.1128/jb.172.7.3725-3729.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May B. P., Tam P., Dennis P. P. The expression of the superoxide dismutase gene in Halobacterium cutirubrum and Halobacterium volcanii. Can J Microbiol. 1989 Jan;35(1):171–175. doi: 10.1139/m89-026. [DOI] [PubMed] [Google Scholar]
- Mylvaganam S., Dennis P. P. Sequence heterogeneity between the two genes encoding 16S rRNA from the halophilic archaebacterium Haloarcula marismortui. Genetics. 1992 Mar;130(3):399–410. doi: 10.1093/genetics/130.3.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. W., Blake C. C. Iron- and manganese-containing superoxide dismutases can be distinguished by analysis of their primary structures. FEBS Lett. 1988 Mar 14;229(2):377–382. doi: 10.1016/0014-5793(88)81160-8. [DOI] [PubMed] [Google Scholar]
- Saitou N., Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987 Jul;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- Salin M. L., Oesterhelt D. Purification of a manganese-containing superoxide dismutase from Halobacterium halobium. Arch Biochem Biophys. 1988 Feb 1;260(2):806–810. doi: 10.1016/0003-9861(88)90511-5. [DOI] [PubMed] [Google Scholar]
- Sarich V. M., Wilson A. C. Generation time and genomic evolution in primates. Science. 1973 Mar 16;179(4078):1144–1147. doi: 10.1126/science.179.4078.1144. [DOI] [PubMed] [Google Scholar]
- Smith M. W., Doolittle R. F. A comparison of evolutionary rates of the two major kinds of superoxide dismutase. J Mol Evol. 1992 Feb;34(2):175–184. doi: 10.1007/BF00182394. [DOI] [PubMed] [Google Scholar]
- Stallings W. C., Pattridge K. A., Strong R. K., Ludwig M. L. Manganese and iron superoxide dismutases are structural homologs. J Biol Chem. 1984 Sep 10;259(17):10695–10699. [PubMed] [Google Scholar]
- Stallings W. C., Pattridge K. A., Strong R. K., Ludwig M. L. The structure of manganese superoxide dismutase from Thermus thermophilus HB8 at 2.4-A resolution. J Biol Chem. 1985 Dec 25;260(30):16424–16432. [PubMed] [Google Scholar]
- Takao M., Yasui A., Oikawa A. Unique characteristics of superoxide dismutase of a strictly anaerobic archaebacterium Methanobacterium thermoautotrophicum. J Biol Chem. 1991 Aug 5;266(22):14151–14154. [PubMed] [Google Scholar]
- Takeda Y., Avila H. Structure and gene expression of the E. coli Mn-superoxide dismutase gene. Nucleic Acids Res. 1986 Jun 11;14(11):4577–4589. doi: 10.1093/nar/14.11.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R. Bacterial evolution. Microbiol Rev. 1987 Jun;51(2):221–271. doi: 10.1128/mr.51.2.221-271.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


