Abstract
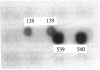
A specific ribonucleoside triphosphate reductase is induced in anaerobic Escherichia coli. This enzyme, as isolated, lacks activity in the test tube and can be activated anaerobically with S-adenosylmethionine, NADPH, and two previously uncharacterized E. coli fractions. The gene for one of these, previously named dA1, was cloned and sequenced. We found an open reading frame coding for a polypeptide of 248 amino acid residues, with a molecular weight of 27,645 and with an N-terminal segment identical to that determined by direct Edman degradation. In a Kohara library, the gene hybridized between positions 3590 and 3600 on the physical map of E. coli. The deduced amino acid sequence shows a high extent of sequence identity with that of various ferredoxin (flavodoxin) NADP+ reductases. We therefore conclude that dA1 is identical with E. coli ferredoxin (flavodoxin) NADP+ reductase. Biochemical evidence from a bacterial strain, now constructed and overproducing dA1 activity up to 100-fold, strongly supports this conclusion. The sequence of the gene shows an apparent overlap with the reported sequence of mvrA, previously suggested to be involved in the protection against superoxide (M. Morimyo, J. Bacteriol. 170:2136-2142, 1988). We suggest that a frameshift introduced during isolation or sequencing of mvrA caused an error in the determination of its sequence.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews S. C., Shipley D., Keen J. N., Findlay J. B., Harrison P. M., Guest J. R. The haemoglobin-like protein (HMP) of Escherichia coli has ferrisiderophore reductase activity and its C-terminal domain shares homology with ferredoxin NADP+ reductases. FEBS Lett. 1992 May 18;302(3):247–252. doi: 10.1016/0014-5793(92)80452-m. [DOI] [PubMed] [Google Scholar]
- Appleyard R K. Segregation of Lambda Lysogenicity during Bacterial Recombination in Escherichia Coli K12. Genetics. 1954 Jul;39(4):429–439. doi: 10.1093/genetics/39.4.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 8. Microbiol Rev. 1990 Jun;54(2):130–197. doi: 10.1128/mr.54.2.130-197.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee R. V., Harder S. R., Ragsdale S. W., Matthews R. G. Mechanism of reductive activation of cobalamin-dependent methionine synthase: an electron paramagnetic resonance spectroelectrochemical study. Biochemistry. 1990 Feb 6;29(5):1129–1135. doi: 10.1021/bi00457a005. [DOI] [PubMed] [Google Scholar]
- Blaschkowski H. P., Neuer G., Ludwig-Festl M., Knappe J. Routes of flavodoxin and ferredoxin reduction in Escherichia coli. CoA-acylating pyruvate: flavodoxin and NADPH: flavodoxin oxidoreductases participating in the activation of pyruvate formate-lyase. Eur J Biochem. 1982 Apr;123(3):563–569. [PubMed] [Google Scholar]
- Eliasson R., Fontecave M., Jörnvall H., Krook M., Pontis E., Reichard P. The anaerobic ribonucleoside triphosphate reductase from Escherichia coli requires S-adenosylmethionine as a cofactor. Proc Natl Acad Sci U S A. 1990 May;87(9):3314–3318. doi: 10.1073/pnas.87.9.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson R., Pontis E., Fontecave M., Gerez C., Harder J., Jörnvall H., Krook M., Reichard P. Characterization of components of the anaerobic ribonucleotide reductase system from Escherichia coli. J Biol Chem. 1992 Dec 15;267(35):25541–25547. [PubMed] [Google Scholar]
- Fontecave M., Eliasson R., Reichard P. Oxygen-sensitive ribonucleoside triphosphate reductase is present in anaerobic Escherichia coli. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2147–2151. doi: 10.1073/pnas.86.7.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K., Huennekens F. M. Activation of methionine synthetase by a reduced triphosphopyridine nucleotide-dependent flavoprotein system. J Biol Chem. 1974 Nov 10;249(21):6745–6753. [PubMed] [Google Scholar]
- Harder J., Eliasson R., Pontis E., Ballinger M. D., Reichard P. Activation of the anaerobic ribonucleotide reductase from Escherichia coli by S-adenosylmethionine. J Biol Chem. 1992 Dec 15;267(35):25548–25552. [PubMed] [Google Scholar]
- Karplus P. A., Daniels M. J., Herriott J. R. Atomic structure of ferredoxin-NADP+ reductase: prototype for a structurally novel flavoenzyme family. Science. 1991 Jan 4;251(4989):60–66. [PubMed] [Google Scholar]
- Karplus P. A., Walsh K. A., Herriott J. R. Amino acid sequence of spinach ferredoxin:NADP+ oxidoreductase. Biochemistry. 1984 Dec 18;23(26):6576–6583. doi: 10.1021/bi00321a046. [DOI] [PubMed] [Google Scholar]
- Kohara Y., Akiyama K., Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987 Jul 31;50(3):495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- Larsson A., Sjöberg B. M. Identification of the stable free radical tyrosine residue in ribonucleotide reductase. EMBO J. 1986 Aug;5(8):2037–2040. doi: 10.1002/j.1460-2075.1986.tb04461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungquist E., Bukhari A. I. State of prophage Mu DNA upon induction. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3143–3147. doi: 10.1073/pnas.74.8.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morimyo M. Isolation and characterization of methyl viologen-sensitive mutants of Escherichia coli K-12. J Bacteriol. 1988 May;170(5):2136–2142. doi: 10.1128/jb.170.5.2136-2142.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neidle E. L., Hartnett C., Ornston L. N., Bairoch A., Rekik M., Harayama S. Nucleotide sequences of the Acinetobacter calcoaceticus benABC genes for benzoate 1,2-dioxygenase reveal evolutionary relationships among multicomponent oxygenases. J Bacteriol. 1991 Sep;173(17):5385–5395. doi: 10.1128/jb.173.17.5385-5395.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raleigh E. A., Wilson G. Escherichia coli K-12 restricts DNA containing 5-methylcytosine. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9070–9074. doi: 10.1073/pnas.83.23.9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki I., Bertani G. Growth abnormalities in Hfr derivatives of Escherichia coli strain C. J Gen Microbiol. 1965 Sep;40(3):365–376. doi: 10.1099/00221287-40-3-365. [DOI] [PubMed] [Google Scholar]
- Steitz J. A., Jakes K. How ribosomes select initiator regions in mRNA: base pair formation between the 3' terminus of 16S rRNA and the mRNA during initiation of protein synthesis in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4734–4738. doi: 10.1073/pnas.72.12.4734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelander L., Reichard P. Reduction of ribonucleotides. Annu Rev Biochem. 1979;48:133–158. doi: 10.1146/annurev.bi.48.070179.001025. [DOI] [PubMed] [Google Scholar]
- Wagner A. F., Frey M., Neugebauer F. A., Schäfer W., Knappe J. The free radical in pyruvate formate-lyase is located on glycine-734. Proc Natl Acad Sci U S A. 1992 Feb 1;89(3):996–1000. doi: 10.1073/pnas.89.3.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubay G. In vitro synthesis of protein in microbial systems. Annu Rev Genet. 1973;7:267–287. doi: 10.1146/annurev.ge.07.120173.001411. [DOI] [PubMed] [Google Scholar]