Abstract
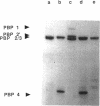
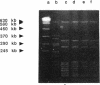
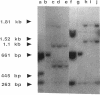
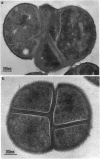
The inactivation of FemB by insertion of Tn551 in the central part of the femB open reading frame was shown to increase susceptibility of methicillin-resistant Staphylococcus aureus strains toward beta-lactam antibiotics to the same extent as did inactivation of femA. Strains carrying the methicillin resistance determinant (mec) and expressing PBP 2' were affected to the same extent as were strains selected for in vitro resistance, which did not express PBP 2'. Both femA and femB, which form an operon, are involved in a yet unknown manner in the glycine interpeptide bridge formation of the S. aureus peptidoglycan. FemB inactivation was shown to reduce the glycine content of peptidoglycan by approximately 40%, depending on the S. aureus strain. The reduction of the interpeptide bridge glycine content led to significant reduction in peptidoglycan cross-linking, as measured by gel permeation high-pressure liquid chromatography of muramidase-digested cell walls. Maximum peptide chain length was reduced by approximately 40%. It is shown that the complete pentaglycine interpeptide bridge is important for the sensitivity against beta-lactam antibiotics and for the undisturbed activity of the staphylococcal cell wall-synthesizing and hydrolyzing enzymes, as was also apparent from electron microscopic examinations, which revealed aberrant placement of cross walls and retarded cell separation, leading to a pseudomulticellular phenotype of the cells for both femA and femB mutants.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger-Bächi B., Barberis-Maino L., Strässle A., Kayser F. H. FemA, a host-mediated factor essential for methicillin resistance in Staphylococcus aureus: molecular cloning and characterization. Mol Gen Genet. 1989 Oct;219(1-2):263–269. doi: 10.1007/BF00261186. [DOI] [PubMed] [Google Scholar]
- Berger-Bächi B. Genetics of methicillin resistance in Staphylococcus aureus. J Antimicrob Chemother. 1989 May;23(5):671–673. doi: 10.1093/jac/23.5.671. [DOI] [PubMed] [Google Scholar]
- Berger-Bächi B. Insertional inactivation of staphylococcal methicillin resistance by Tn551. J Bacteriol. 1983 Apr;154(1):479–487. doi: 10.1128/jb.154.1.479-487.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger-Bächi B., Strässle A., Gustafson J. E., Kayser F. H. Mapping and characterization of multiple chromosomal factors involved in methicillin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1992 Jul;36(7):1367–1373. doi: 10.1128/aac.36.7.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger-Bächi B., Strässle A., Kayser F. H. Characterization of an isogenic set of methicillin-resistant and susceptible mutants of Staphylococcus aureus. Eur J Clin Microbiol. 1986 Dec;5(6):697–701. doi: 10.1007/BF02013308. [DOI] [PubMed] [Google Scholar]
- Berger-Bächi B., Strässle A., Kayser F. H. Natural methicillin resistance in comparison with that selected by in-vitro drug exposure in Staphylococcus aureus. J Antimicrob Chemother. 1989 Feb;23(2):179–188. doi: 10.1093/jac/23.2.179. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Fontana R. Penicillin-binding proteins and the intrinsic resistance to beta-lactams in gram-positive cocci. J Antimicrob Chemother. 1985 Oct;16(4):412–416. doi: 10.1093/jac/16.4.412. [DOI] [PubMed] [Google Scholar]
- Giesbrecht P., Wecke J., Reinicke B. On the morphogenesis of the cell wall of staphylococci. Int Rev Cytol. 1976;44:225–318. doi: 10.1016/s0074-7696(08)61651-4. [DOI] [PubMed] [Google Scholar]
- Goering R. V., Duensing T. D. Rapid field inversion gel electrophoresis in combination with an rRNA gene probe in the epidemiological evaluation of staphylococci. J Clin Microbiol. 1990 Mar;28(3):426–429. doi: 10.1128/jcm.28.3.426-429.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goering R. V., Winters M. A. Rapid method for epidemiological evaluation of gram-positive cocci by field inversion gel electrophoresis. J Clin Microbiol. 1992 Mar;30(3):577–580. doi: 10.1128/jcm.30.3.577-580.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman B. J., Tomasz A. Expression of methicillin resistance in heterogeneous strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Jan;29(1):85–92. doi: 10.1128/aac.29.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman B., Tomasz A. Altered penicillin-binding proteins in methicillin-resistant strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1981 May;19(5):726–735. doi: 10.1128/aac.19.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hash J. H., Rothlauf M. V. The N,O-diacetylmuramidase of Chalaropsis species. I. Purification and crystallization. J Biol Chem. 1967 Dec 10;242(23):5586–5590. [PubMed] [Google Scholar]
- Hächler H., Berger-Bächi B., Kayser F. H. Genetic characterization of a Clostridium difficile erythromycin-clindamycin resistance determinant that is transferable to Staphylococcus aureus. Antimicrob Agents Chemother. 1987 Jul;31(7):1039–1045. doi: 10.1128/aac.31.7.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayser F. H., Benner E. J., Troy R., Hoeprich P. D. Experimental and clinical aspects of resistance determinants. Mode of resistance against beta-lactam antibiotics in staphylococci. Ann N Y Acad Sci. 1971 Jun 11;182:106–117. doi: 10.1111/j.1749-6632.1971.tb30649.x. [DOI] [PubMed] [Google Scholar]
- Kornblum J., Hartman B. J., Novick R. P., Tomasz A. Conversion of a homogeneously methicillin-resistant strain of Staphylococcus aureus to heterogeneous resistance by Tn551-mediated insertional inactivation. Eur J Clin Microbiol. 1986 Dec;5(6):714–718. doi: 10.1007/BF02013311. [DOI] [PubMed] [Google Scholar]
- Kuhl S. A., Pattee P. A., Baldwin J. N. Chromosomal map location of the methicillin resistance determinant in Staphylococcus aureus. J Bacteriol. 1978 Aug;135(2):460–465. doi: 10.1128/jb.135.2.460-465.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labischinski H., Barnickel G., Naumann D., Keller P. Conformational and topological aspects of the three-dimensional architecture of bacterial peptidoglycan. Ann Inst Pasteur Microbiol. 1985 Jan-Feb;136A(1):45–50. doi: 10.1016/s0769-2609(85)80020-x. [DOI] [PubMed] [Google Scholar]
- Maidhof H., Reinicke B., Blümel P., Berger-Bächi B., Labischinski H. femA, which encodes a factor essential for expression of methicillin resistance, affects glycine content of peptidoglycan in methicillin-resistant and methicillin-susceptible Staphylococcus aureus strains. J Bacteriol. 1991 Jun;173(11):3507–3513. doi: 10.1128/jb.173.11.3507-3513.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuhashi M., Song M. D., Ishino F., Wachi M., Doi M., Inoue M., Ubukata K., Yamashita N., Konno M. Molecular cloning of the gene of a penicillin-binding protein supposed to cause high resistance to beta-lactam antibiotics in Staphylococcus aureus. J Bacteriol. 1986 Sep;167(3):975–980. doi: 10.1128/jb.167.3.975-980.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews P. R., Stewart P. R. Amplification of a section of chromosomal DNA in methicillin-resistant Staphylococcus aureus following growth in high concentrations of methicillin. J Gen Microbiol. 1988 Jun;134(6):1455–1464. doi: 10.1099/00221287-134-6-1455. [DOI] [PubMed] [Google Scholar]
- Sidow T., Johannsen L., Labischinski H. Penicillin-induced changes in the cell wall composition of Staphylococcus aureus before the onset of bacteriolysis. Arch Microbiol. 1990;154(1):73–81. doi: 10.1007/BF00249181. [DOI] [PubMed] [Google Scholar]
- Snowden M. A., Perkins H. R. Peptidoglycan cross-linking in Staphylococcus aureus. An apparent random polymerisation process. Eur J Biochem. 1990 Jul 31;191(2):373–377. doi: 10.1111/j.1432-1033.1990.tb19132.x. [DOI] [PubMed] [Google Scholar]
- Stewart G. C., Rosenblum E. D. Genetic behavior of the methicillin resistance determinant in Staphylococcus aureus. J Bacteriol. 1980 Dec;144(3):1200–1202. doi: 10.1128/jb.144.3.1200-1202.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonin E., Tomasz A. Beta-lactam-specific resistant mutants of Staphylococcus aureus. Antimicrob Agents Chemother. 1986 Oct;30(4):577–583. doi: 10.1128/aac.30.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubukata K., Nonoguchi R., Matsuhashi M., Konno M. Expression and inducibility in Staphylococcus aureus of the mecA gene, which encodes a methicillin-resistant S. aureus-specific penicillin-binding protein. J Bacteriol. 1989 May;171(5):2882–2885. doi: 10.1128/jb.171.5.2882-2885.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wecke J., Lahav M., Ginsburg I., Kwa E., Giesbrecht P. Inhibition of wall autolysis of staphylococci by sodium polyanethole sulfonate "liquoid". Arch Microbiol. 1986 Mar;144(2):110–115. doi: 10.1007/BF00414719. [DOI] [PubMed] [Google Scholar]
- de Jonge B. L., Chang Y. S., Gage D., Tomasz A. Peptidoglycan composition in heterogeneous Tn551 mutants of a methicillin-resistant Staphylococcus aureus strain. J Biol Chem. 1992 Jun 5;267(16):11255–11259. [PubMed] [Google Scholar]
- de Jonge B. L., Chang Y. S., Gage D., Tomasz A. Peptidoglycan composition of a highly methicillin-resistant Staphylococcus aureus strain. The role of penicillin binding protein 2A. J Biol Chem. 1992 Jun 5;267(16):11248–11254. [PubMed] [Google Scholar]