Abstract
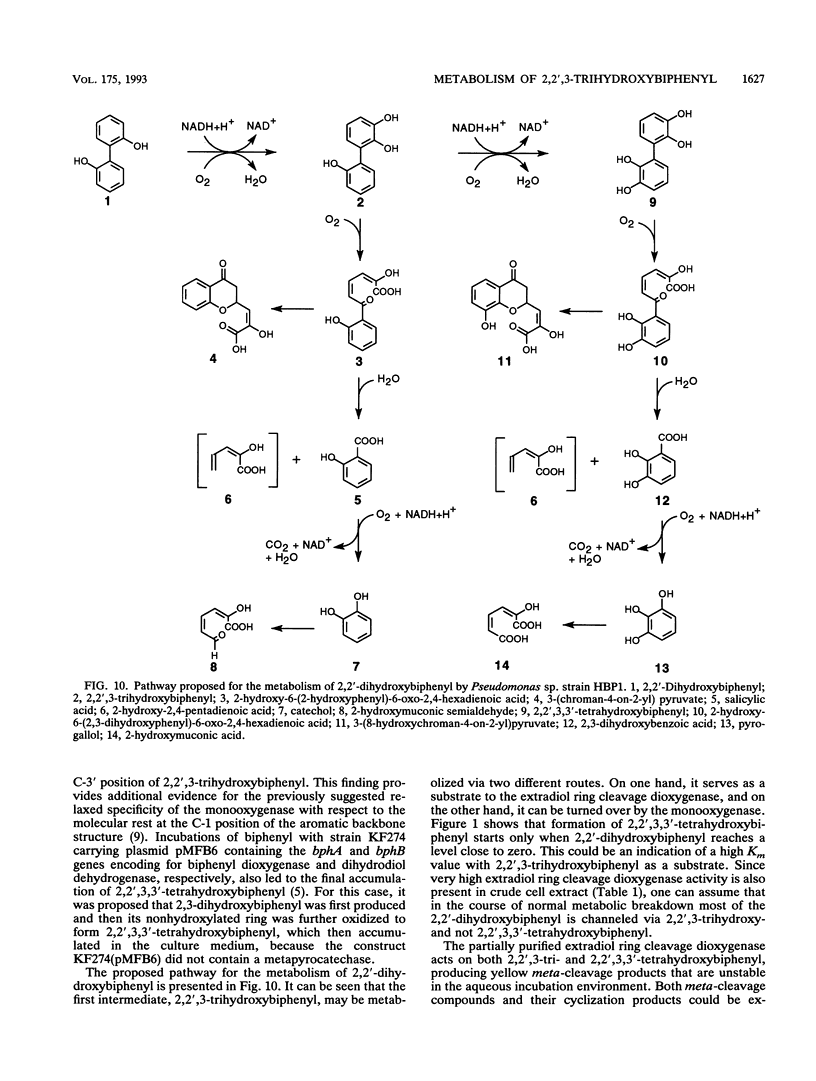
Cells of Pseudomonas sp. strain HBP1 grown on 2-hydroxy- or 2,2'-dihydroxybiphenyl contain NADH-dependent monooxygenase activity that hydroxylates 2,2'-dihydroxybiphenyl. The product of this reaction was identified as 2,2',3-trihydroxybiphenyl by 1H nuclear magnetic resonance and mass spectrometry. Furthermore, the monooxygenase activity also hydroxylates 2,2',3-trihydroxybiphenyl at the C-3' position, yielding 2,2',3,3'-tetrahydroxybiphenyl as a product. An estradiol ring cleavage dioxygenase activity that acts on both 2,2',3-tri- and 2,2',3,3'-tetrahydroxybiphenyl was partially purified. Both substrates yielded yellow meta-cleavage compounds that were identified as 2-hydroxy-6-(2-hydroxyphenyl)-6-oxo-2,4-hexadienoic acid and 2-hydroxy-6-(2,3-dihydroxyphenyl)-6-oxo-2,4-hexadienoic acid, respectively, by gas chromatography-mass spectrometry analysis of their respective trimethylsilyl derivatives. The meta-cleavage products were not stable in aqueous incubation mixtures but gave rise to their cyclization products, 3-(chroman-4-on-2-yl)pyruvate and 3-(8-hydroxychroman-4-on-2-yl)pyruvate, respectively. In contrast to the meta-cleavage compounds, which were turned over to salicylic acid and 2,3-dihydroxybenzoic acid, the cyclization products are not substrates to the meta-cleavage product hydrolase activity. NADH-dependent salicylate monooxygenase activity catalyzed the conversions of salicylic acid and 2,3-dihydroxybenzoic acid to catechol and pyrogallol, respectively. The partially purified estradiol ring cleavage dioxygenase activity that acted on the hydroxybiphenyls also produced 2-hydroxymuconic semialdehyde and 2-hydroxymuconic acid from catechol and pyrogallol, respectively.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Engesser K. H., Strubel V., Christoglou K., Fischer P., Rast H. G. Dioxygenolytic cleavage of aryl ether bonds: 1,10-dihydro-1,10-dihydroxyfluoren-9-one, a novel arene dihydrodiol as evidence for angular dioxygenation of dibenzofuran. FEMS Microbiol Lett. 1989 Nov;53(1-2):205–209. doi: 10.1016/0378-1097(89)90392-3. [DOI] [PubMed] [Google Scholar]
- Fortnagel P., Harms H., Wittich R. M., Francke W., Krohn S., Meyer H. Cleavage of dibenzofuran and dibenzodioxin ring systems by a Pseudomonas bacterium. Naturwissenschaften. 1989 May;76(5):222–223. doi: 10.1007/BF00627694. [DOI] [PubMed] [Google Scholar]
- Fortnagel P., Harms H., Wittich R. M., Krohn S., Meyer H., Sinnwell V., Wilkes H., Francke W. Metabolism of Dibenzofuran by Pseudomonas sp. Strain HH69 and the Mixed Culture HH27. Appl Environ Microbiol. 1990 Apr;56(4):1148–1156. doi: 10.1128/aem.56.4.1148-1156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa K., Tomizuka N., Kamibayashi A. Effect of chlorine substitution on the bacterial metabolism of various polychlorinated biphenyls. Appl Environ Microbiol. 1979 Aug;38(2):301–310. doi: 10.1128/aem.38.2.301-310.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higson F. K., Focht D. D. Bacterial metabolism of hydroxylated biphenyls. Appl Environ Microbiol. 1989 Apr;55(4):946–952. doi: 10.1128/aem.55.4.946-952.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler H. P., Kohler-Staub D., Focht D. D. Degradation of 2-hydroxybiphenyl and 2,2'-dihydroxybiphenyl by Pseudomonas sp. strain HBP1. Appl Environ Microbiol. 1988 Nov;54(11):2683–2688. doi: 10.1128/aem.54.11.2683-2688.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohler H. P., van der Maarel M. J., Kohler-Staub D. Selection of Pseudomonas sp. strain HBP1 Prp for metabolism of 2-propylphenol and elucidation of the degradative pathway. Appl Environ Microbiol. 1993 Mar;59(3):860–866. doi: 10.1128/aem.59.3.860-866.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saeki Y., Nozaki M., Senoh S. Cleavage of pyrogallol by non-heme iron-containing dioxygenases. J Biol Chem. 1980 Sep 25;255(18):8465–8471. [PubMed] [Google Scholar]
- Strubel V., Engesser K. H., Fischer P., Knackmuss H. J. 3-(2-hydroxyphenyl)catechol as substrate for proximal meta ring cleavage in dibenzofuran degradation by Brevibacterium sp. strain DPO 1361. J Bacteriol. 1991 Mar;173(6):1932–1937. doi: 10.1128/jb.173.6.1932-1937.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White-Stevens R. H., Kamin H. Studies of a flavoprotein, salicylate hydroxylase. I. Preparation, properties, and the uncoupling of oxygen reduction from hydroxylation. J Biol Chem. 1972 Apr 25;247(8):2358–2370. [PubMed] [Google Scholar]


