Abstract
The phenomenon of phase variation in the insect-pathogenic bacterium Xenorhabdus luminescens was investigated. Differential activity of the lipase enzyme (EC 3.1.1.3) was observed between the two phases of the bacteria. The enzyme was found to be secreted into the culture medium, and about five to six times greater specific activity was secreted by the primary phase than by the secondary form. The lipase gene (lip-1) was cloned and sequenced. The data imply that there is only a single Tween 80-utilizing lipase gene in X. luminescens K122. The sequence revealed a translation product of 645 amino acids, from which a hydrophobic leader sequence of 24 amino acids is removed during processing. The structure of the gene was shown to be the same in the primary and secondary forms of X. luminescens. In addition, transcription was found to start at the same position, 169 bp upstream of the translation initiation codon, in the two forms of the bacteria. Equal amounts of lipase RNA accumulated in the two forms, and at least as much lipase protein was secreted by the secondary form as by the primary. This suggests that the difference in specific activity between the enzymes secreted by the two phases probably arises from a posttranslational type of regulation.
Full text
PDF

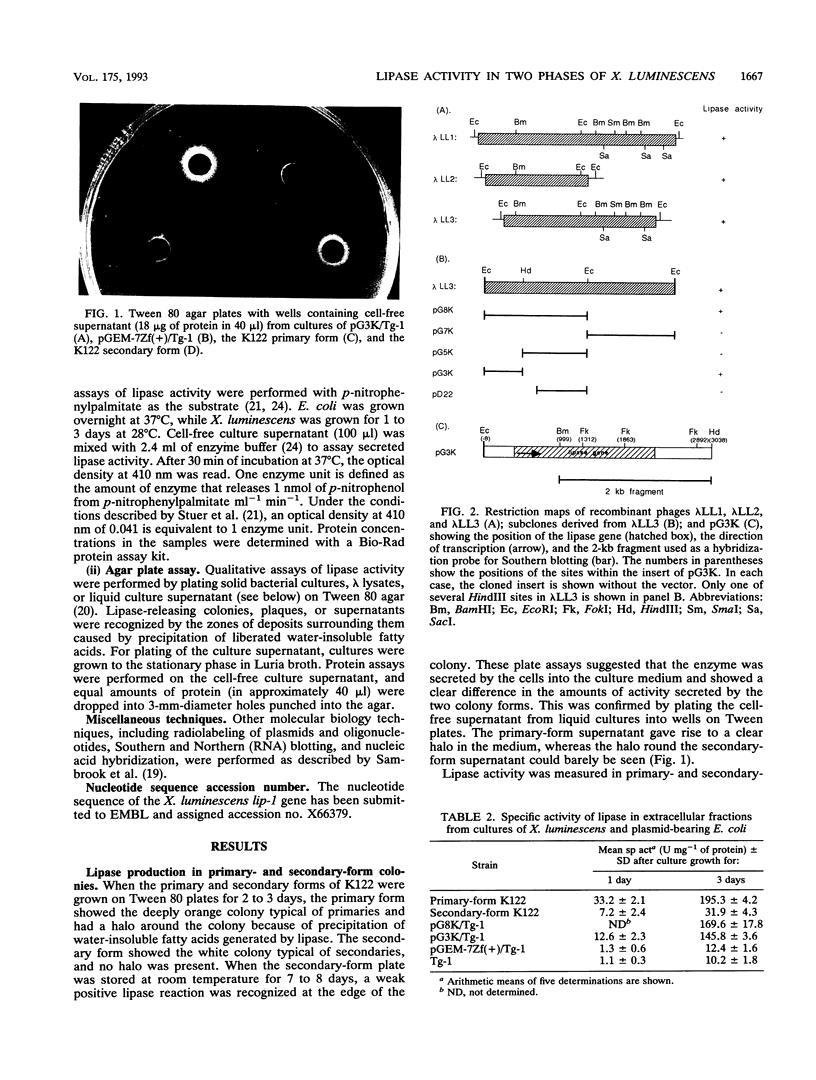
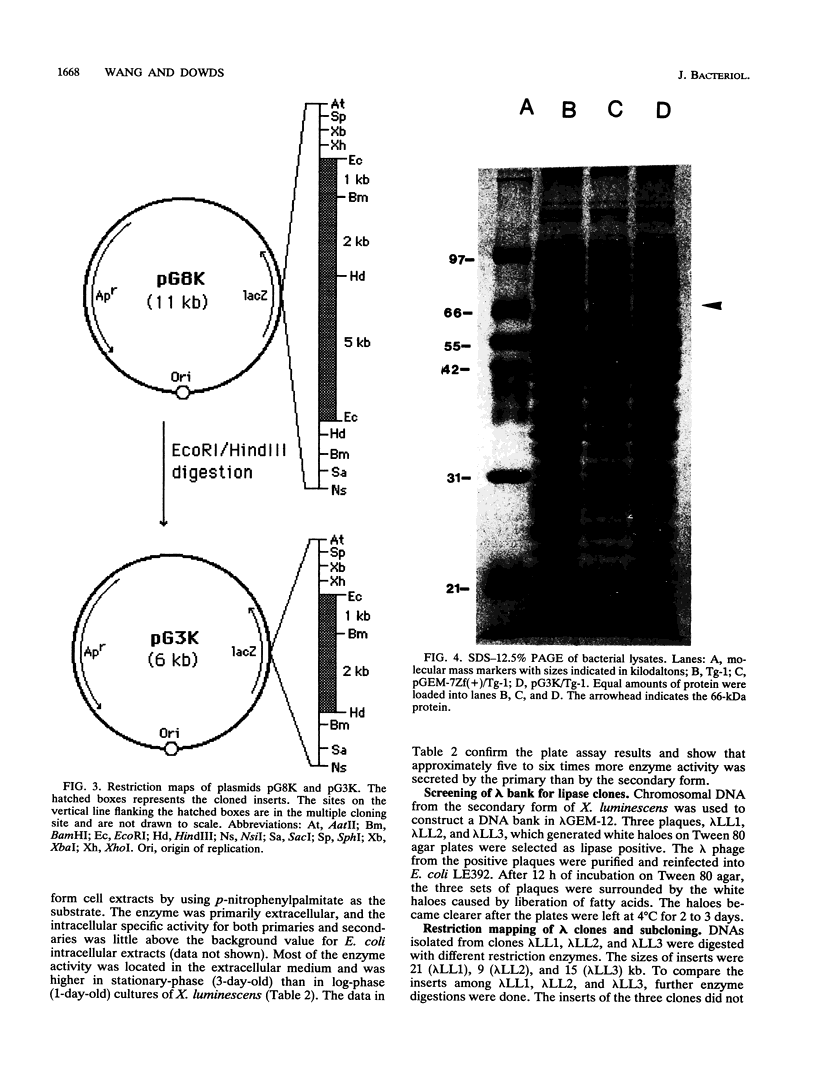

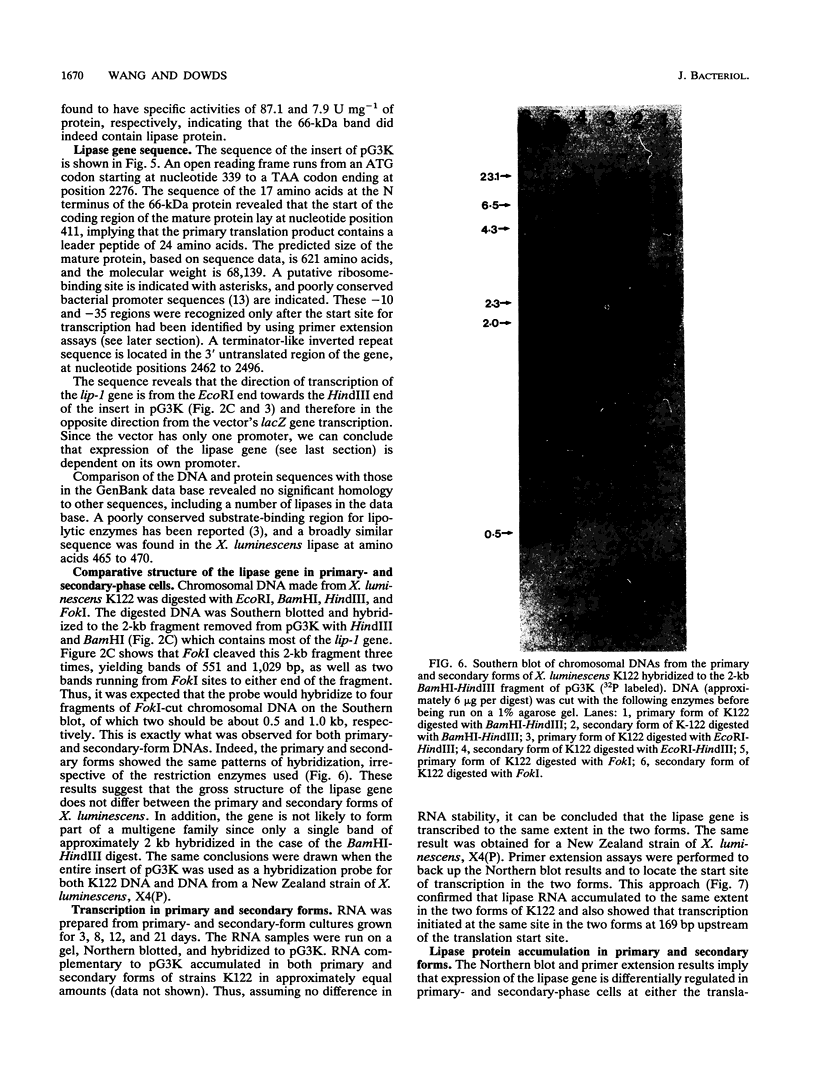


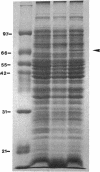
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonian E. Recent advances in the purification, characterization and structure determination of lipases. Lipids. 1988 Dec;23(12):1101–1106. doi: 10.1007/BF02535273. [DOI] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Foster S. J. Analysis of the autolysins of Bacillus subtilis 168 during vegetative growth and differentiation by using renaturing polyacrylamide gel electrophoresis. J Bacteriol. 1992 Jan;174(2):464–470. doi: 10.1128/jb.174.2.464-470.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frackman S., Anhalt M., Nealson K. H. Cloning, organization, and expression of the bioluminescence genes of Xenorhabdus luminescens. J Bacteriol. 1990 Oct;172(10):5767–5773. doi: 10.1128/jb.172.10.5767-5773.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager D. A., Burgess R. R. Elution of proteins from sodium dodecyl sulfate-polyacrylamide gels, removal of sodium dodecyl sulfate, and renaturation of enzymatic activity: results with sigma subunit of Escherichia coli RNA polymerase, wheat germ DNA topoisomerase, and other enzymes. Anal Biochem. 1980 Nov 15;109(1):76–86. doi: 10.1016/0003-2697(80)90013-5. [DOI] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlbert R. E., Xu J., Small C. L. Colonial and Cellular Polymorphism in Xenorhabdus luminescens. Appl Environ Microbiol. 1989 May;55(5):1136–1143. doi: 10.1128/aem.55.5.1136-1143.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lam H. M., Tancula E., Dempsey W. B., Winkler M. E. Suppression of insertions in the complex pdxJ operon of Escherichia coli K-12 by lon and other mutations. J Bacteriol. 1992 Mar;174(5):1554–1567. doi: 10.1128/jb.174.5.1554-1567.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc M. C., Boemare N. E. Plasmids and phase variation in Xenorhabdus spp. Appl Environ Microbiol. 1991 Sep;57(9):2597–2601. doi: 10.1128/aem.57.9.2597-2601.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIERRA G. A simple method for the detection of lipolytic activity of micro-organisms and some observations on the influence of the contact between cells and fatty substrates. Antonie Van Leeuwenhoek. 1957;23(1):15–22. doi: 10.1007/BF02545855. [DOI] [PubMed] [Google Scholar]
- Stuer W., Jaeger K. E., Winkler U. K. Purification of extracellular lipase from Pseudomonas aeruginosa. J Bacteriol. 1986 Dec;168(3):1070–1074. doi: 10.1128/jb.168.3.1070-1074.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Dowds B. C. Molecular cloning and characterization of the lux genes from the secondary form of Xenorhabdus luminescens, K122. Biochem Soc Trans. 1992 Feb;20(1):68S–68S. doi: 10.1042/bst020068s. [DOI] [PubMed] [Google Scholar]
- Winkler U. K., Stuckmann M. Glycogen, hyaluronate, and some other polysaccharides greatly enhance the formation of exolipase by Serratia marcescens. J Bacteriol. 1979 Jun;138(3):663–670. doi: 10.1128/jb.138.3.663-670.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Gabain A., Belasco J. G., Schottel J. L., Chang A. C., Cohen S. N. Decay of mRNA in Escherichia coli: investigation of the fate of specific segments of transcripts. Proc Natl Acad Sci U S A. 1983 Feb;80(3):653–657. doi: 10.1073/pnas.80.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]