Abstract
Background
The authors compared the microtensile bond strength of teeth restored with four adhesives at the gingival and pulpal cavity walls of Class II resin-based composite restorations.
Methods
Five pairs of extracted third molars received two Class II preparations/restorations in each tooth. The authors randomly assigned each preparation to one of four adhesive groups: Adper Scotchbond Multipurpose Dental Adhesive (SBMP) (3M ESPE, St. Paul, Minn.), Clearfil SE Bond (CFSE) (Kuraray America, New York City), Prime & Bond NT (PBNT) (Dentsply Caulk, Milford, Del.) and PQ1 (Ultradent, South Jordan, Utah). They restored the teeth and obtained microtensile specimens from each cavity wall. Specimens were tested on a testing machine until they failed.
Results
The mean (± standard deviation) bond strengths (in megapascals) were as follows: SBMP (pulpal), 36.4 (17.2); SBMP (gingival), 29.7 (15.3); CFSE (pulpal), 50.8 (13.6); CFSE (gingival), 50.2 (14.0); PBNT (pulpal), 38.3 (19.2); PBNT (gingival), 38.9 (17.7); PQ1 (pulpal), 58.7 (8.7); and PQ1 (gingival), 54.5 (18.5). A two-way analysis of variance found an adhesive effect (P < .001) but no location effect (P > .05).
Conclusions
PQ1 and CFSE performed the best. The results showed no significant difference in microtensile bond strength at the gingival wall versus the pulpal wall.
Clinical Implications
Under in vitro conditions, a total-etch ethanol-based adhesive (PQ1) failed cohesively more often than did the other adhesives tested.
Keywords: Dentin bonding agents, restoration failure, microtensile test
Bonding to enamel is highly predictable but bonding to dentin is less predictable, especially when the bonding is to the gingival cavity wall of Class II posterior resin-based composite preparations. A previous study1 that used a water-based adhesive in Class II preparations found that the bond to the gingival wall was weaker than the bond to the axial wall. One possible reason for this weaker bond strength was an increased number of voids at the gingival wall, probably due to increased wetness at the gingival wall compared with the axial wall.
BONDSTRENGTH
Excessive surface moisture may result in voids at the resin-dentin interface.2 If the dentin surface is too wet, an “overwet” phenomenon occurs whereby the adhesive resin does not fully penetrate the dentinal tubules or the demineralized dentin.3-9 The wetness may come from either intrinsic sources10 (for example, dentinal tubules) or extrinsic sources (for example, water primer, sulcular fluid, humidity of the operatory or water left on the dentin after the acid is washed off). Water that is not removed completely within the resin-dentin interdiffusion zone can interfere with the polymerization of the resin.11 Any solvent remaining on a primed dentin surface will prevent complete adaptation of the bonding resin and may result in nonattachment.12
Dentin close to the pulp has a concentration of approximately 50,000 tubules/millimeter2, while dentin closer to the dentinoenamel junction has a density of approximately 20,000 tubules/mm2.13 Deeper dentin also has less intertubular dentin available to bond with the adhesive. Both factors result in weaker bonds to deeper dentin than to superficial dentin. As the amount of superficial intertubular dentin increases, the bond strength of the adhesive resin to the dentin increases.14 In addition, the dentinal tubules located 1.0 mm above the cementoenamel junction (CEJ) are 49 percent more dense on the gingival floor than on the axial wall.15 Dentin, by nature, is a heterogeneous substrate made of (by volume) 55 percent mineral, 30 percent collagen and 15 percent water; enamel consists of 97 percent inorganic matter and 1 to 2 percent water.16 More water in dentin could be another reason why adhesive bonding to dentin results in weaker bond strengths compared with those to enamel.
Other explanations for the variability of bond strengths to dentin include whether the dentin surface is wet or dry,5 the region of the tooth to which one is bonding,1,14,17 whether the surface being bonded is flat or a cavity preparation wall,14,17 the configuration factor (c-factor)(that is, the ratio of the number of bonded to unbonded surfaces) of the tooth preparation,18 the adhesive system used,14 the thickness of the composite increment placed in the cavity13 and the operator's clinical technique and skill level in placing the material.19
The gingival cavity wall of a Class II preparation is the location in which a resin-based composite restoration fails most frequently.20 If this failure is due to increased wetness from the primer, perhaps a different primer solvent or chemistry could yield a better bond of the adhesive to the gingival cavity wall.
We conducted a study to determine if adhesives with different chemistries bonded to pulpal versus gingival cavity walls resulted in increased microtensile bond strengths to dentin in Class II cavity preparations. We tested two null hypotheses:
■ There is no statistically significant difference in the microtensile bond strength of adhesive that is bonded to the pulpal wall versus the gingival wall of Class II resin-based composite restorations.
■ There is no statistically significant difference in the microtensile bond strength to dentin of four different adhesives with different chemical compositions and etching techniques.
MATERIALS AND METHODS
To test these hypotheses, we chose a controlled in vitro trial with two independent variables. The first independent variable was the location of bonding (that is, to the gingival wall and the pulpal wall of Class II resin-based composite preparations), and the second independent variable was the four adhesives. These consisted of a total-etch three-step water-based adhesive (Adper Scotchbond Multipurpose Dental Adhesive [SBMP], 3M ESPE, St. Paul, Minn.) (lot 20040607, expiration date March 2007), a self-etch two-step water-based adhesive (Clearfil SE Bond [CFSE], Kuraray America, New York City) (lot 61566, expiration date October 2005), a total-etch, two-step, acetone-based adhesive (Prime & Bond NT [PB], Dentsply Caulk, Milford, Del.) (lot 040220, expiration date February 2006) and a total-etch, two-step, ethanol-based adhesive (PQ1, Ultradent, South Jordan, Utah) (lot 62ZN, expiration date October 2005). The dependent variable was microtensile strength.
After we received approval from the University of Missouri-Kansas City Institutional Review Board, an oral surgeon extracted two third molars from five patients (a total of 10 teeth). Each pair of teeth yielded four interproximal preparations. We randomly assigned each interproximal preparation to one of four treatment groups. The extracted teeth had no previous restorations, caries or other disease.
One of us (J.H.P.) prepared the interproximal surface of each molar with an interproximal box and an occlusal extension. He used a two-surface preparation in each tooth, while leaving intact an isthmus of tooth structure between the two preparations. The gingival depth of the box was 4.0 mm, the facial-lingual width was approximately 5.0 mm and the axial depth was approximately 1.5 mm into the dentin. The dentist located the preparation 1.0 mm above the CEJ. He prepared the teeth using a no. 56 carbide bur (Brasseler USA, Savannah, Ga.). He did not bevel the enamel.
The dentist applied each adhesive according to the manufacturer's directions and light cured it with a halogen light-curing unit (XL 3000, 3M ESPE). The unit produced an intensity of at least 500 milliwatts/square centimeter within three seconds. The dentist measured the light output (Optilux Radiometer, Model 100, SDS Kerr, Danbury, Conn.) before and after placing the restorations. He placed the restorative material (Filtek Z250, 3M ESPE [shade A-3], lot 20040608, 4JH, expiration date May 2007) in an initial increment of approximately 1.0 to 2.0 mm at the gingival wall. The dentist placed a total of three increments (in layers no thicker than 2.0 mm) at oblique angles until the tooth was restored to its original contours. He did not use a matrix band and placed the curing light approximately 1.0 mm from the restorative material.
After placing the restorations, the dentist stored the teeth for 24 hours in tap water.
One of us (J.H.P.) cut the samples with a lowspeed saw blade (Isomet, Buehler, Lake Bluff, Ill.) under water to obtain a flat rectangular plane shape with a cross-sectional area of approximately 0.50 mm2, with no notch at the adhesive interface. He took three gingival sections from each of the proximal boxes and three pulpal sections from each occlusal surface. He determined the thickness of each section using a digital micrometer (1-micrometer resolution).
The dentist mounted the samples on the testing machine (Universal Istron testing machine, Model 1125, Istron, Norwood, Mass.) using a cyanoacrylate adhesive (Zapit, Ventures of America, Corona, Calif.), and he used an accelerator to attach the microtensile specimen to the opposing arms of a testing device (Bencor Multi-T, Danville Engineering, San Ramon, Calif.), which was attached to the Instron testing machine. Two of us (J.H.P., M.H.) measured the microtensile strength of the samples using a 500-kilogram load cell at a crosshead speed of 1.0 mm/minute.
We determined that 11 specimens were needed in each group to achieve alpha = 0.05 and beta = 0.2; a 25 percent difference in mean bond strength was statistically and clinically significant. We obtained five sets of paired third molars from patients and 119 microtensile specimens from these sets. We examined representative samples from each group under a scanning electron microscope (XL30, FEI, Hillsboro, Ore.) to determine the mode of failure.
RESULTS
Table 1 presents microtensile bond strength and debonding data. A two-way analysis of variance found an adhesive effect (P < .001) but no significant location or interaction effects (P > .05). A Tukey post hoc test (P < .05) (Table 2) revealed the following significant differences: SBMP was significantly different from CFSE and PQ1. PBNT was significantly different from PQ1. None of the other comparisons was significant. PQ1 and CFSE had the highest bond strengths to dentin. Of the 119 samples that we obtained, 18 debonded during specimen preparation. This left 101 samples that could be tested for microtensile strength. Therefore, we did not reject the first null hypothesis: there is no statistically significant difference in microtensile bond strength between gingival and pulpal walls. However, we rejected the second null hypothesis, because there was a statistically significant difference in microtensile bond strength between adhesive treatment groups.
Table 1.
Microtensile bond strength of adhesives tested.
| ADHESIVE | LOCATION | NO. OF SAMPLES |
MEAN (SD*) BOND STRENGTH, MEGAPASCALS |
NO. (%) OF DEBONDED SAMPLES |
NO. (%) OF COHESIVE FAILURES |
|---|---|---|---|---|---|
|
Adper Scotchbond Multipurpose Dental Adhesive† |
Pulpal wall | 13 | 36.4 (17.2) | 3 (18.7) | (7.7) |
| Gingival wall | 7 | 29.7 (16.3) | 9 (56.3) | 0 (0) | |
| Clearfil SE Bond‡ | Pulpal wall | 14 | 50.8 (13.6) | 0 (0) | 2 14.3) |
| Gingival wall | 12 | 60.2 (14.0) | 0 (0) | 1 (8.3) | |
|
Prime & Bond NT Adhesive§ |
Pulpal wall | 14 | 38.3 (19.2) | 2 (12.5) | 0 (0) |
| Gingival wall | 12 | 38.9 (17.7) | 4 (25) | 0 (0) | |
| PQ1¶ | Pulpal wall | 14 | 68.7 (8.7) | 0 (0) | 8 (57.1) |
| Gingival wall | 15 | 54.5 (18.5) | 0 (0) | 3 (20) |
SD: Standard deviation.
Adper Scotchbond Multipurpose Dental Adhesive is manufactured by 3M ESPE, S t Paul, Minn.
Clearfil SE Bond is manufactured by Kuraray America. Mew York City.
Prime & Bond NT Adhesive is manufactured by Dentsply Caulk, Milford, Del.
PQ1 is manufactured by Ultradent, South Jordan, Utah.
Table 2.
Tukey post hoc comparisons (alpha = .05).
| ADHESIVE | NO. OF SAMPLES |
MEAN BOND STRENGTH (MEGAPASCALS)* |
||
|---|---|---|---|---|
| 1 | 2 | 3 | ||
|
Adper Scotchbond Multipurpose Dental Adhesive† |
20 | 34.0 | — | — |
|
Prime & Bond NT Adhesive‡ |
26 | 38.6 | 38.6 | — |
| Clearfil SE Bond§ | 26 | — | 50.6 | 50.6 |
| PQ1¶ | 29 | — | — | 56.5 |
Means in the same column do not differ from one another in bond strength.
Adper Scotchbond Multipurpose Dental Adhesive is manufactured by 3M ESPE, St. Paul, Minn.
Prime & Bond NT Adhesive is manufactured by Dentsply Caulk, Milford, Del.
Clearfil SE Bond is manufactured by Kuraray America, New York City.
PQ1 is manufactured by Ultradent, South Jordan, Utah.
To determine the mode of failure in each treatment group, we examined the specimens under a scanning electron microscope. Eighty-six (85 percent) of 101 samples broke through the adhesive layer, as observed under the scanning electron microscope at magnifications of up to × 5,000. It is important to note that nine (56 percent) of 16 gingival SBMP samples debonded at the adhesive interface during specimen preparation and could not be tested. A χ2 analysis found more debonded samples than expected with SBMP (χ2 = 22.0, P < .001). Showing a remarkable strength were eight (57 percent) of 14 pulpal samples and three (20 percent) of 15 gingival samples in the PQ1 group that failed cohesively (through either the dentin or the resin-based composite). We observed more cohesive failures for samples in the PQ1 group than we expected (χ2 = 19.9, P < .001). A cohesive failure is a failure through the dentin or the resin-based composite, but it does not involve the adhesive interface.
The figure shows a representative sample from the PQ1 group that experienced an adhesive failure. This figure shows a mixed fracture pattern involving the dentin, adhesive and hybrid layer. Other samples in adhesive groups that experienced adhesive failures looked similar to the sample in the figure.
Figure.
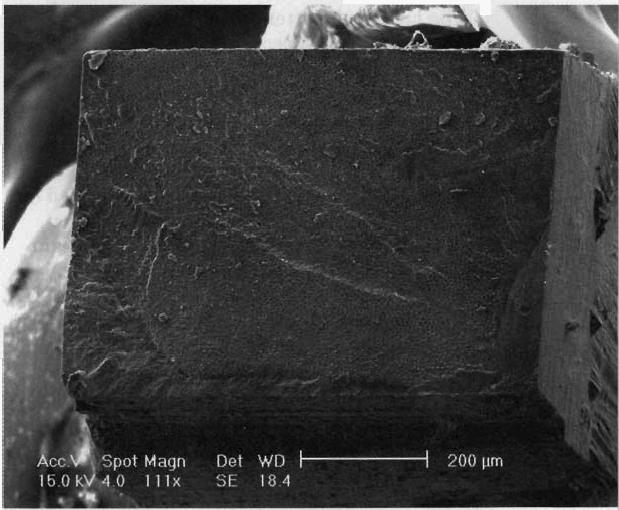
A sample from the PQ1 group (Ultradent, South Jordan, Utah) with an adhesive failure examined under a scanning electron microscope at ×111. The fracture is in the hybrid layer through the adhesive, dentin and resin-based composite restoration.
DISCUSSION
Increasing the adhesive bond strength to the dentinal gingival wall and reducing the number of debonded samples is a difficult task. Debonding may occur because of voids at the interface of the dentin and adhesive from either intrinsic wetness (for example, dentinal fluid) or extrinsic wetness (for example, too much moisture on the dentin surface from the water in the primer or water that remains after the acid is washed off). A debonding rate as high as 25 percent is clinically unacceptable and means that nearly one of four times, no bond occurs between the adhesive resin and the dentin at the gingival cavity wall. If the dentin has been acid-etched and the smear layer removed without hybridizing the demineralized dentin, the dentin could be forever “wounded.” This unhybridized dentin may be more susceptible to further degradation from hydrolytic breakdown and may be susceptible to penetration by bacterial enzymes or other toxic substances.21
The difference between the adhesive bond strength of water-based SBMP and that of PQ1 may be due to the ethanol-based chemistry in PQ1. The volatile nature of alcohol, as demonstrated by its quick evaporation, may have assisted in reducing wetness at the adhesive bonding site. The results of the microtensile bond strength test of PQ1 in this study are consistent with PQ1's ability to encapsulate collagen that has been acid-etched. The other adhesive that performed well in this study was CFSE. The self-etching primers in this adhesive may have the ability to reduce intrinsic moisture, because the smear layer is not removed entirely. Tubules still covered with a smear layer might not spill their fluid content onto the surface of the dentin. PQ1 and CFSE adhesives did not differ significantly from each other in bond strength.
The mean bond strength of SBMP probably was lower than that reported because of the significant number of debonded samples observed. The use of this adhesive system resulted in 56.3 percent of the samples' debonding at the gingival wall. We calculated bond strength only for the specimens that survived the preparation stage. As a result, the mean bond strengths reported for groups that had debonded samples are higher than they would have been if the debonded samples had been included in the data set.
On the other hand, the mean bond strength of PQ1 probably is higher than that reported, because of the significant number of cohesive failures observed. About 57 percent of the pulpal samples and 20 percent of the gingival samples had adhesive bond strengths greater than the cohesive strength of the dentin or resin-based composite. The number of cohesive failures for PQ1 indicates excellent bonding properties, which complement the high mean bond strengths noted above.
We cured the adhesive and the resin-based composite material as close to the halogen curing light as possible. We did not use a matrix band to simulate clinical use. In clinical applications, bond strengths may be lower because of positive pulpal pressure and limitations in the oral environment that do not allow for close placement of the light-curing unit, as well as limitations caused by the use of a matrix band.
CONCLUSION
The results of this study show that of the four adhesives tested, PQ1 and CFSE performed best. We found no significant difference in microtensile bond strength at the gingival wall versus the pulpal wall. However, resin-based composite may bond differently to dentin depending on the chemical composition of the adhesive used.
Acknowledgments
The authors thank 3M ESPE for donating adhesive materials, restorative materials and the curing light used in this study. They also thank Kuraray America, Dentsply Caulk and Ultradent for donating adhesive materials.
Footnotes
This study was supported in part by U.S. Public Health Service grants DE07294, DE09696 and 1-K23-DE016324-01A1.
Contributor Information
John H. Purk, An associate professor and section head in operative dentistry, Department of Restorative Dentistry, University of Missouri-Kansas City, School of Dentistry, 650 E. 25th St., Kansas City, Mo. 64108.
Matthew Healy, A fourth-year dental student, University of Missouri-Kansas City, School of Dentistry..
Vladimir Dusevich, A research assistant, Department of Oral Biology, University of Missouri-Kansas City, School of Dentistry..
Alan Glaros, Associate dean and a professor of basic medical sciences, Kansas City University of Medicine and Biosciences, Kansas City, Mo..
J. David Eick, Chair, Department of Oral Biology, and a curators' professor, University of Missouri-Kansas City, School of Dentistry..
References
- 1.Purk JH, Dusevich V, Glaros A, Spencer P, Eick JD. In vivo versus in vitro microtensile bond strength of axial versus gingival cavity preparation walls in Class II resin-based composite restorations. JADA. 2004;135(2):185–93. doi: 10.14219/jada.archive.2004.0150. quiz 228. [DOI] [PubMed] [Google Scholar]
- 2.Walshaw PR, McComb D. Clinical considerations for optimal dentinal bonding. Quintessence Int. 1996;27(9):619–25. [PubMed] [Google Scholar]
- 3.Pereira GD, Paulillo LA, De Goes MF, Dias CT. How wet should dentin be? Comparison of methods to remove excess water during moist bonding. J Adhes Dent. 2001;3(3):257–64. [PubMed] [Google Scholar]
- 4.Tay FR, Gwinnett JA, Wei SH. Micromorphological spectrum from overdrying to overwetting acid-conditioned dentin in acetone-baaed, single-bottle primer/adhesives. Dental Mater. 1996;12(4):236–44. doi: 10.1016/s0109-5641(96)80029-7. [DOI] [PubMed] [Google Scholar]
- 5.Tay FR, Gwinnett AJ, Wei SH. The overwet phenomenon: a transmission electron microscopic study of surface moisture in the acid-conditioned, resin-dentin interface. Am J Dent. 1996;9(4):161–6. [PubMed] [Google Scholar]
- 6.Tay FR, Gwinnett AJ, Wei SH. The overwet phenomenon: a scanning electron microscopic study of surface moisture in the add-conditioned, resin-dentin interface. Am J Dent. 1996;9(3):109–14. [PubMed] [Google Scholar]
- 7.Tay FR, Gwinnett AJ, Wei SH. The overwet phenomenon! an optical, micromorphological study of surface moisture in the acid-conditioned, resin-dentin interface. Am J Dent. 1996;9(1):43–8. [PubMed] [Google Scholar]
- 8.Van Meerbeek B, Yoshida Y, Lambrechts P, et al. A TEM study of two water-based adhesive systems bonded to dry and wet dentin. J Dent Res. 1998;77(1):50–9. doi: 10.1177/00220345980770010501. [DOI] [PubMed] [Google Scholar]
- 9.Spencer P, Wang Y, Walker MP, Wieliczka DM, Swafford JR. Interfacial chemistry of the dentin/adhesive bond. J Dent Res. 2000;79(7):1458–63. doi: 10.1177/00220345000790070501. [DOI] [PubMed] [Google Scholar]
- 10.Ogata M, Okuda M, Nakajima M, Pereira PN, Sano H, Tagami J. Influence of the direction of tubules on bond strength to dentin. Oper Dent. 2001;26(1):27–35. [PubMed] [Google Scholar]
- 11.Tanumiharja M, Burrow MF, Tyas MJ. Microtensile bond strengths of seven dentin adhesive systems. Dent Mater. 2000;16(3):180–7. doi: 10.1016/s0109-5641(00)00007-5. [DOI] [PubMed] [Google Scholar]
- 12.Walshaw PR, McComb D. Microscopic features of clinically successful dentine bonding. Dent Update. 1998;25(7):281–6. [PubMed] [Google Scholar]
- 13.Eick JD, Gwinnett AJ, Pashley DH, Robinson SJ. Current concepts on adhesion to dentin. Crit Rev Oral Biol Med. 1997;8(3):306–35. doi: 10.1177/10454411970080030501. [DOI] [PubMed] [Google Scholar]
- 14.Prati C, Pashley DH. Dentin wetness, permeability and thickness and bond strength of adhesive systems. Am J Dent. 1992;5(1):33–8. [PubMed] [Google Scholar]
- 15.Garberoglio P. The ratio of the densities of dentinal tubules on the cervical and axial walls in cavities. Quintessence Int. 1994;25(1):49–62. [PubMed] [Google Scholar]
- 16.Marshall GW., Jr. Dentin: microstructure and characterization. Quintessence Int. 1993;24(9):606–17. [PubMed] [Google Scholar]
- 17.Bouillaguet S, Ciucchi B, Jacoby T, Wataha JC, Pashley D. Bonding characteristics to dentin walls of Class II cavities, in vitro. Dent Mater. 2001;17(4):316–21. doi: 10.1016/s0109-5641(00)00089-0. [DOI] [PubMed] [Google Scholar]
- 18.Yoshikawa T, Sano H, Burrow MF, Tagami J, Pashley DH. Effects of dentin depth and cavity configuration on bond strength. J Dent Res. 1999;78(4):898–905. doi: 10.1177/00220345990780041001. [DOI] [PubMed] [Google Scholar]
- 19.Shono Y, Ogawa T, Terashita M, Carvalho RM, Pashley EL, Pashley DH. Regional measurement of resin-dentin bonding as an array. J Dent Res. 1999;78(2):699–705. doi: 10.1177/00220345990780021001. [DOI] [PubMed] [Google Scholar]
- 20.Mjor IA. The location of clinically diagnosed secondary caries. Quintessence Int. 1998;29(5):313–7. [PubMed] [Google Scholar]
- 21.Spencer P, Swafford JR. Unprotected protein at the dentin-adhesive interface. Quintessence Int. 1999;30(7):501–7. [PubMed] [Google Scholar]
- 22.Wang Y, Spencer P. Evaluation of the interface between one-bottle adhesive systems and dentin by Goldner's trichrome. Am J Dent. 2005;18(1):66–72. [PubMed] [Google Scholar]


