Abstract
GABA and glutamate, the main transmitters in the basal ganglia, exert their effects through ionotropic and metabotropic receptors. The dynamic activation of these receptors in response to released neurotransmitter depends, among other factors, on their precise localization in relation to corresponding synapses. The use of high resolution quantitative electron microscope immunocytochemical techniques has provided in-depth description of the subcellular and subsynaptic localization of these receptors in the CNS. In this article, we review recent findings on the ultrastructural localization of GABA and glutamate receptors and transporters in the basal ganglia, at synaptic, extrasynaptic and presynaptic sites. The anatomical evidence supports numerous potential locations for receptor-neurotransmitter interactions, and raises important questions regarding mechanisms of activation and function of synaptic versus extrasynaptic receptors in the basal ganglia.
INTRODUCTION
The basal ganglia are a group of subcortical structures involved in the integration and processing of sensorimotor, cognitive and limbic information. Dysfunction of these nuclei results in a wide variety of motor neurological disorders, including Parkinson's disease, Huntington's disease, dystonia, hemiballism and Tourette's syndrome. In primates, the basal ganglia include the dorsal striatum (caudate nucleus and putamen), the external and internal segments of the globus pallidus (GPe and GPi, respectively), the substantia nigra pars reticulata (SNr), the substantia nigra pars compacta (SNc) and the subthalamic nucleus (STN). In addition, the limbic or ventral division of the basal ganglia includes the ventral striatum or nucleus accumbens, ventral pallidum and ventral tegmental area.
Basal ganglia connectivity
The striatum (Fig. 1A) receives glutamatergic projections from virtually all areas of the cerebral cortex and several thalamic nuclei. Dopaminergic inputs from SNc interact with glutamatergic afferents to regulate striatal activity. In addition, striatal projection cells, the medium-sized spiny neurons, receive GABAergic inputs from interneurons, local axon collaterals of other projection neurons, and GPe; as well as input from cholinergic interneurons (Bolam and Smith, 1990; Tepper and Bolam, 2004). The medium-sized spiny neurons project directly to the basal ganglia output nuclei (GPi and SNr), or indirectly via the GPe and STN (DeLong, 1990; Smith et al., 1998). Although all striatal spiny neurons use GABA as their transmitter, those cells that give rise to the direct pathway express preferentially the neuropeptides substance P and dynorphin, and D1 dopamine receptors; whereas striatal cells projecting to GPe express primarily enkephalin and D2 dopamine receptors (Gerfen and Wilson, 1996).
Figure 1.
Schematic representation of the main afferents and efferents of basal ganglia neurons. Minor connections have been omitted for the sake of clarity. Abbreviations: GPe: globus pallidus, external segment; GPi: globus pallidus, internal segment; PPN: Pedunculopontine nucleus; RF: Reticular Formation; SC: superior colliculus; SNc: substantia nigra pars compacta; SNr: substantia nigra pars reticulata; STN: subthalamic nucleus.
GPe cells (Fig. 1B) receive GABAergic innervation from striatal afferents and local axon collaterals, as well as glutamatergic inputs from the STN and the thalamus. Subthalamic cells (Fig. 1C) are, in turn, contacted by GABAergic GPe afferents, and receive glutamatergic inputs from the cerebral cortex, thalamus and brainstem pedunculopontine nucleus. GPi and SNr neurons (Fig. 1D) receive their main glutamatergic afferents from the STN, and massive GABAergic inputs from striatum and GPe. GPi and SNr are considered the output nuclei of the basal ganglia, because their GABAergic projections target motor and intralaminar nuclei of the thalamus, the habenula, the superior colliculus, the pedunculopontine nucleus and the reticular formation (Gerfen and Wilson, 1996).
Dopaminergic cells in the SNc (Fig. 1E) receive a significant glutamatergic input from the pedunculopontine nucleus and more discrete inputs from the cerebral cortex and STN. The striatum, the GPe and ventral pallidum, as well as axon collaterals from SNr, contribute to their GABAergic innervation. SNc cells project back to the striatum, and also provide significant innervation to extrastriatal basal ganglia, cerebral cortex and thalamus. The raphe contributes serotoninergic innervation to all basal ganglia structures. (for more extensive reviews of basal ganglia connectivity, readers are referred to Alexander and Crutcher, 1990; DeLong, 1990; Parent, 1990; Joel and Weiner, 1994; Parent and Hazrati, 1995; Gerfen and Wilson, 1996; Smith et al., 1998; Bolam et al., 2000).
Synaptic localization of neurotransmitter receptors
The effects of neurotransmitters depend not only on the pharmacology or molecular structure of their targeted receptors, but also on the spatial arrangement of receptors and transporters in relation to the sites of transmitter release. For the past ten years, the use of highly sensitive electron microscopic (EM) immunocytochemical methods has provided exquisite information about the subcellular and subsynaptic localization of receptors in the basal ganglia.
The localization of receptor in relation to the anatomically defined synapse can be termed synaptic, if they are located within the synaptic specialization; perisynaptic, if located at the periphery of the synapse; or extrasynaptic, if attached to nonsynaptic sites along the plasma membrane. Synaptic, perisynaptic and extrasynaptic receptors are exposed to different neurotransmitter transients. Following release, synaptic receptors sense a rapid increase in neurotransmitter concentration. The neurotransmitter diffuses out of the synaptic cleft; its concentration is attenuated as the distance required to reach the most remote extrasynaptic receptors increases, and thus, extrasynaptic receptors will be exposed to different concentrations of transmitter, depending on their distance to the release site (Kullmann, 2000; Barbour, 2001). The spatial and temporal properties of transmitter diffusion is not only a function of the amount of released transmitter, but also depends on many other factors, including the geometry of the synapse, the tortuosity of the extracellular space (Sykova, 2004), as well as the activity and spatial arrangement of plasma membrane transporters (Amara and Fontana, 2002; Rusakov and Lehre, 2002; Semyanov et al., 2003; Huang and Bergles, 2004). Together with the functional properties of the receptors (e. g., affinity for the neurotransmitter, or number of bound transmitter molecules required for activation), the subsynaptic localization contributes to the physiological conditions for receptor activation following neurotransmitter release.
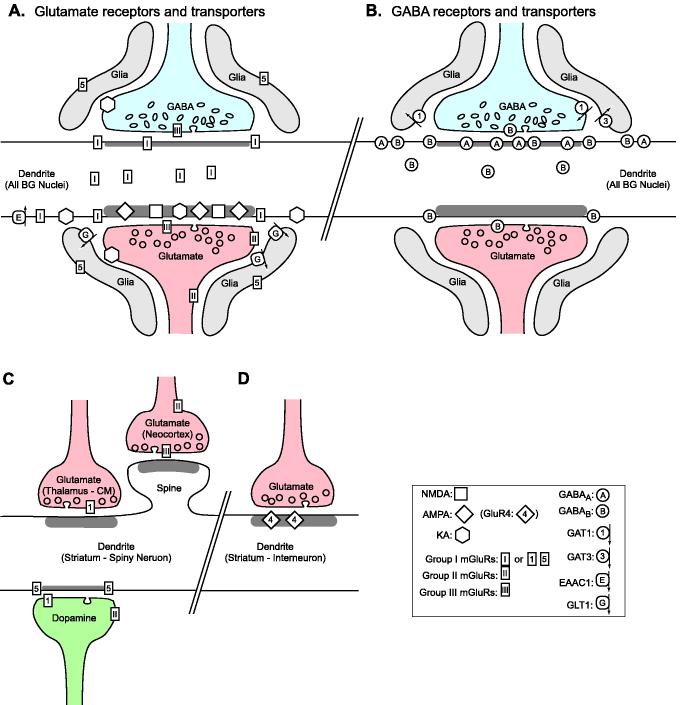
The aim of this review is to summarize recent findings on the subcellular and subsynaptic localization of receptors and transporters of the GABA and glutamate transmitter systems in the basal ganglia; and attempts to relate the ultrastructural localization with functions of these receptors (description of other neurotransmitter systems is beyond the scope of this review, for more information on these transmitter systems see Inglis and Winn, 1995; Nicola et al., 2000; Pickel et al., 2002; Quik and Kulak, 2002; Rosin et al., 2003). We propose that ionotropic and metabotropic GABA and glutamate receptors are largely segregated to different subsynaptic domains of basal ganglia neurons; so that ionotropic receptors are clustered at synapses close to the main release site of their activating transmitters, whereas metabotropic receptors are mostly peri- or extrasynaptic (Fig. 7).
Fig. 7.
Summary figure of the subsynaptic localization of glutamate (panel A) and GABA (panel B) receptors and transporters in the basal ganglia, as revealed by immunogold EM studies. The schematic representation shows exemplary glutamate and GABA terminals that form axo-dendritic synapses. Most of the features depicted in A and B are valid for a majority of basal ganglia neurons; except for the spines of projection neurons and interneurons in the striatum, which are depicted in panels C and D respectively.
The present review focuses on high resolution EM data obtained with immunogold techniques, since this is the only approach currently available that allows reliable localization of membrane proteins at the nanometer level. Additional information on the localization of receptors and transporters gathered through light microscopic immunocytochemical techniques, mRNA in situ hybridization methods and autoradiographic ligand binding techniques will not be discussed here, but recent reviews provide extensive coverage of these findings (Ravenscroft and Brotchie, 2000; Smith et al., 2000; Smith et al., 2001; Conn et al., 2005).
TECHNICAL CONSIDERATIONS
The interpretation of receptor localization data gathered through EM immunohistochemical analyses is greatly dependent on the sensitivity and spatial resolution of the methods used. In this section, we briefly describe different techniques to study receptor localization at the subcellular and subsynaptic levels (for more details on these techniques the reader is referred to Ingham, 1992; Totterdell and Ingham, 1992; Merighi and Polak, 1993; Ribeiro-Da-Silva et al., 1993). These techniques rely on the use of highly specific antibodies that bind to the molecules of interest. To localize the antibodies at the ultrastructural level, it is necessary to compromise between technical conditions that preserve the antigenic sites and conditions that preserve the tissue ultrastructure. The bound antibodies are labeled using electron dense markers that allow detection with an electron microscope; the most commonly used markers for EM analysis are peroxidase reaction products and colloidal gold particles.
It is important to emphasize that, in any immunocytochemical technique, the use of adequate controls is critical to reduce erroneous results. The optimal control is to test the antibodies specificity in tissue devoid of the target antigen (i. e., in gene knock-out mice). Additional controls include immunoblotting of brain tissue or transfected cells, pre-adsorption of primary antibodies with their corresponding antigenic peptides, and omission of the primary and/or secondary antibodies (Saper and Sawchenko, 2003; Rhodes and Trimmer, 2006).
Immunoperoxidase method
The immunoperoxidase method (Fig. 2 “Immunoperoxidase”), is a highly sensitive technique that permits identification of low level of antigens. The peroxidase reaction results in an insoluble, easy to visualize, electron dense reaction product. However, this method suffers of important disadvantages that limit considerably its use for protein localization at the subsynaptic level. The main drawback is the diffusion of the peroxidase reaction product from the actual site of enzymatic reaction, making it difficult to assess the exact localization of antigenic binding sites. In addition, the amorphous reaction product can obscure ultrastructural details of intracellular organelles and synaptic specializations. Furthermore, since the peroxidase reaction is not linear, this method is not suitable for quantitative analysis. Thus, although the immunoperoxidase method is a good starting tool to identify cellular elements that express a particular receptor or transporter protein, or whether the antigenic site is located on the internal or external face of the plasma membrane, it does not offer the level of spatial resolution necessary to assess the precise subcellular and subsynaptic distribution of these proteins.
Figure 2.
Schematic representation of the three main techniques used for EM immunocytochemistry. The three examples show immunostaining for α1 subunits of the GABAA receptor in the monkey STN. AT, axon terminal; den, dendrite
Pre-embedding immunogold method
For precise localization of proteins at the subsynaptic level, it is necessary to employ immunolabeling techniques in which the antigenic sites are labeled with non-diffusible markers. In this sense, colloidal gold provides a much higher level of spatial resolution than the amorphous peroxidase reaction product. Gold particles are conspicuous in the tissue, and do not obstruct observation of ultrastructural details. In addition, immunogold particles are easily quantified. Hence, the immunogold technique is the method of choice to study the spatial relationship between synapses and receptors or transporter proteins.
The immunocytochemical labeling with gold particles can be performed before or after the tissue has been fixed, dehydrated and embedded in a hard material (often acrylic resins), and thus the techniques are known as pre-embedding or postembedding immunogold, respectively. In the pre-embedding immunogold technique (Fig. 2 “Pre-embedding immunogold”), 1-1.4 nm gold particles coupled to secondary antibodies are used as markers, and the particles are intensified with silver after the immunoreaction, to increase their size to a level suitable with EM observation (around 20-30 nm).
In spite of the improved spatial resolution, the pre-embedding immunogold method is not as sensitive as the immunoperoxidase approach, because it lacks the amplification steps inherent to the immunoperoxidase technique. Furthermore, the penetration of gold-conjugated secondary antibodies into the tissue is more limited in the immunogold method, compared to immunoperoxidase. Another important limitation, most relevant to the present discussion, is that the pre-embedding immunogold method may lead to false negative synaptic labeling for ionotropic GABA and glutamate receptors (e. g. Baude et al., 1993; Nusser et al., 1994; Baude et al., 1995). This might be due to hindrance for the antibodies to access antigenic sites located in the dense material of the postsynaptic density.
Postembedding immunogold
The postembedding immunogold technique overcomes some of the limitations of pre-embedding methods. In the postembedding technique (Fig. 2, “Postembedding immunogold”), the ultra-thin sections are directly in contact with antibodies, providing an even exposure of the antigenic sites and minimizing problems of penetration. The postembedding immunogold method offers the highest level of spatial resolution to localize synaptic receptors. Other advantages of this approach are the use of 5-20 nm gold particles, which avoids the need for silver-enhancement, and the potential for the use of secondary antibodies conjugated to different sizes of gold particles to assess co-localization of 2 or 3 proteins at individual synaptic sites.
However, many receptor proteins cannot be detected using the postembedding immunogold technique, because their antigenicity is lost following fixation, dehydration and embedding procedures. To help preserve antigenic sites, the tissue can be rapidly frozen and kept at very low temperature during dehydration and embedding in resin (‘freeze-substitution’); but the ultrastructural features are less well defined in this material than in osmium-treated, epoxy resin-embedded tissue. The freeze-substitution technique, nevertheless, has been instrumental for the localization of ionotropic GABA and glutamate receptors in various brain regions, including the basal ganglia (e.g. Nusser et al., 1994; Baude et al., 1995; Nusser et al., 1995a; Matsubara et al., 1996; Bernard et al., 1997; Ottersen and Landsend, 1997; Fujiyama et al., 2002; Wu et al., 2005).
In conclusion, both the pre- and postembedding immunogold methods provide a high spatial resolution for the localization of antigens, at the expense of decreased sensitivity, compared to the immunoperoxidase method. Since all these methods offer complementary information on protein localization, a detailed comprehensive assessment of receptors and transporter distribution at the cellular, subcellular and subsynaptic levels is usually achieved through a combination of techniques. Other promising high-resolution approaches that combine immunogold and freeze-fracture techniques (Torrisi and Mancini, 1996), have recently been used to study localization of receptor proteins (e. g. Hagiwara et al., 2005; Tanaka et al., 2005; Kulik et al., 2006).
Immunocytochemical methods do not take into account that receptor and transporter proteins are extremely labile and constantly undergo dynamic regulatory changes in trafficking and pharmacological properties (Triller and Choquet, 2003; Triller and Choquet, 2005). Immunocytochemistry rather provides a snapshot of the distribution of proteins in the plasma membrane at a given moment. Recent advances in two-photon excitation microscopy have facilitated the study of the dynamics of glutamate receptors at the single synapse level in live cells (reviewed in Svoboda and Yasuda, 2006).
GLUTAMATERGIC TRANSMISSION
All basal ganglia nuclei receive glutamatergic inputs from external sources (cerebral cortex, thalamus, pedunculopontine nucleus) or from the STN (Fig. 1). Glutamate exerts its effects through various subtypes of ionotropic (iGluRs) or metabotropic (mGluRs) receptors.
Ionotropic receptors
Ionotropic glutamate receptors form cation-selective channels, with permeability for Na+ and K+, and with differing degrees of permeability to Ca2+ and Mg2+.. They are categorized into three classes, named according to their affinity for their selective agonists: α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA), kainic acid or kainate (KA), and N-methyl-D-aspartate (NMDA). These receptors assemble as homo- or hetero-oligomeric complexes of protein subunits. The AMPA receptor family includes subunits GluR1- GluR4; KA receptors are formed by GluR5-GluR7 and KA1/KA2 subunits; and NMDA receptors are composed of NR1, NR2A–NR2D and NR3A-NR3B subunits (Madden, 2002; Mayer and Armstrong, 2004; Kew and Kemp, 2005).
AMPA and NMDA receptors
At most CNS synapses, both AMPA and NMDA receptors are activated during synaptic transmission. AMPA receptors mediate fast neurotransmission, while NMDA receptors mediate slower and long-lasting responses (Ozawa et al., 1998). The subsynaptic localization of various AMPA and NMDA receptor subunits has been studied with preand postembedding immunogold methods in the rat basal ganglia (Bernard et al., 1997; Bernard and Bolam, 1998; Clarke and Bolam, 1998; Chatha et al., 2000; Fujiyama et al., 2004) (Table 1), but much less is known in primates (Paquet and Smith, 1996).
Table 1.
Summary of the main ultrastructural features of the subcellular and subsynaptic localization of NMDA and AMPA receptor subunits in the basal ganglia, as revealed by high resolution immunogold procedures.
| Structure | Technique | GluR1 | GluR2/3* | GluR4 | NR1 | NR2A/B* | Colocalization |
|---|---|---|---|---|---|---|---|
| Striatum (refs. 1, 2, 3) |
Pre- and postembedding (refs. 1,2 |
Synaptic Extrasynaptic (refs. 1,3) |
Synaptic Extrasynaptic (refs. 1,2,3) |
Restricted to interneurons Synaptic and extrasynaptic (refs. 1, 3) |
Synaptic Extrasynaptic (very few) (refs. 2,3) |
Synaptic (ref. 3) |
NR1 & GluR2/3 (ref. 2) |
| Postembedding (ref. 3) |
Presynaptic (ref. 3) |
Presynaptic (ref. 3) |
|||||
| Presynaptic (ref. 3) |
|||||||
| GP (ref. 2) |
Pre- and postembedding |
-- | Synaptic Extrasynaptic |
-- | Synaptic Extrasynaptic (very few) |
-- | NR1 & GluR2/3 |
| STN (ref. 4) |
Postembedding | Synaptic | Synaptic | Not detected | Synaptic | -- | GluR1 & GluR2/3, NR1 & GluR2/3 |
| Entopeduncular nucleus (EP, rodent analog of the GPi) (ref. 4) |
Postembedding | Synaptic | Synaptic | Synaptic | Synaptic | -- | GluR1 & GluR2/3 GluR4 & GluR2/3 NR1 & GluR2/3 |
| SNr (ref. 5) |
Pre- and postembedding |
-- | Synaptic Extrasynaptic |
-- | Synaptic Extrasynaptic |
Synaptic | NR1 & GluR2/3 NR1 & NR2 |
| SNc (ref. 5) |
Pre- and postembedding |
-- | Synaptic Extrasynaptic |
-- | Synaptic Extrasynaptic |
Synaptic | NR1 & GluR2/3 NR2 & GluR2/3 NR1 & NR2 |
Data not available
The immunolocalization of GluR2 and GluR3 subunits or NR2A/B was achieved using antibodies that recognize both subunits, providing no distinction in the localization of each respective subunits.
References:
There are common salient features that characterize the subsynaptic distribution of NMDA and AMPA receptors in the basal ganglia: (1) The AMPA and NMDA receptor subunits are primarily located at asymmetric (glutamatergic) synapses. (2) AMPA or NMDA receptor subunits are not expressed at GABAergic synapses. (3) A small proportion of AMPA or NMDA receptor subunits is found at extrasynaptic locations. (4) AMPA and NMDA receptors are evenly distributed along the length of glutamatergic synapses. (5) AMPA and NMDA receptor subunits often co-localize at individual glutamatergic synapses.
Synaptic AMPA and NMDA receptors
The highest density of AMPA and NMDA receptors is found at glutamatergic synapses; a location that allows for rapid exposure to glutamate released by the presynaptic terminal, and facilitates the role of these receptors in mediating fast glutamatergic transmission. Indeed, fast AMPA and NMDA-mediated excitatory responses have been described extensively in the striatum (e. g. Wilson, 1993; Calabresi et al., 1996; Kita, 1996; Gotz et al., 1997; Carter and Sabatini, 2004; Kerr and Plenz, 2004), both segments of GP (e.g. Soltis et al., 1994; Gotz et al., 1997; Kita et al., 2004; Kita et al., 2005), STN (e. g. Mouroux and Feger, 1993; Gotz et al., 1997; Nambu et al., 2000; Wilson et al., 2004; Zhu et al., 2004) and SNc (e.g. Mereu et al., 1991; Christoffersen and Meltzer, 1995). Further supporting the notion that glutamatergic transmission is mediated by a combination of AMPA and NMDA receptor, both types of receptors subunits co-localize at individual glutamatergic synapses (Fig. 3). The two types of receptors are intermingled, rather than segregated, and display an even distribution along the synaptic plasma membrane (Bernard et al., 1997; Bernard and Bolam, 1998; Clarke and Bolam, 1998; Chatha et al., 2000).
Figure 3.
Co-localization of the GluR2/3 AMPA and NR1 NMDA receptor subunits at an asymmetric, putatively glutamatergic synapse, in the rat SNr, as revealed by double-labeling postembedding immunogold. Arrowhead points to NR1 subunits (10 nm gold particles), arrow indicates GluR2/3 subunits (15 nm particles) d, dendrite, b, bouton. Scale bar: 0.5 μm. From Chatha et al., 2000, with permission.
Variability in the subunit composition of iGluRs plays an important role in determining the specific properties of the native receptors, including calcium permeability and receptor desensitization (Ozawa et al., 1998; Dingledine et al., 1999; Kew and Kemp, 2005). The subunit composition of individual channels cannot be determined with immunohistochemistry, but the presence of more than one type of subunit at individual synapses can be revealed with double labeling experiments. Using this technique, Clarke and Bolam (1998) described that GluR2/3 and GluR4 are expressed at individual synapses in the entopeduncular nucleus (EP, rodent analog of the GPi); while GluR1 and GluR2/3 subunits co-localize in a significant proportion of glutamatergic synapses in the STN. On the other hand, NR1 and NR2A/B subunits coexist in a large proportion of glutamatergic synapses in both segments of the SN (Chatha et al., 2000), consistent with the fact that functional NMDA receptors require at least one NR1 and one NR2 subunit (Mayer and Armstrong, 2004). An in-depth characterization of the subsynaptic localization of these subunits, including distinction of NR2A and NR2B with specific antibodies, could be useful to interpret behavioral pharmacology studies that showed antiparkinsonian properties of NR2B selective compounds in animal models of Parkinson's disease (for a review see Marino et al., 2003).
As neurons in the basal ganglia receive glutamatergic inputs from different sources (Fig. 1), the question arises whether neurons are capable of targeting selective AMPA and NMDA receptor subtypes to specific synaptic inputs, a mechanism that could result in fine-tuning and strengthening of particular postsynaptic responses (Nusser, 2000). Examples of such selective targeting have been described in the dorsal cochlear nucleus and hippocampus (Rubio and Wenthold, 1997; Nusser et al., 1998a; Watanabe et al., 1998). The gathering of such information depends on rigorous quantitative analysis of individual synaptic labeling through serial ultra thin sections, an approach that has not yet been applied in basal ganglia.
Extrasynaptic AMPA and NMDA receptors
AMPA and NMDA receptor subunits are localized at extrasynaptic locations in basal ganglia neurons, but there are discrepancies between pre- and postembedding immunogold data regarding the degree of extrasynaptic labeling for AMPA and NMDA receptor subunits. The pre-embedding method certainly reveals a much higher level of nonsynaptic immunoreactivity than the postembedding technique (Bernard et al., 1997; Bernard and Bolam, 1998; Fujiyama et al., 2004). The apparent scarcity of extrasynaptic AMPA and NMDA labeling could be an artifact due to the reduced sensitivity of the postembedding immunogold method to detect low levels of antigens (see Technical Considerations above); and thus, the proportion of extrasynaptic NMDA and AMPA receptor subunits reported in studies that rely only on the postembedding method (Clarke and Bolam, 1998; Fujiyama et al., 2004) could be an underestimate.
Extrasynaptic iGluRs could potentially serve a number of roles in glutamatergic transmission. Glutamate receptors can switch between extrasynaptic and synaptic localizations via lateral diffusion in the plasma membrane (Triller and Choquet, 2003; Triller and Choquet, 2005), a process that allows rapid regulation of synaptic strength (Rao and Craig, 1997). Extrasynaptic iGluRs can also contribute to glutamate-mediated signaling at nonsynaptic locations, when glutamate release is sufficient to diffuse out of the synaptic space and activate extrasynaptic receptors (Kullmann, 2000). They could also be critical for the glutamate-mediated responses induced by nonsynaptic glial release of neurotransmitter (Araque et al., 1999). It is noteworthy that the responses of extrasynaptic receptors can be different, or even opposite, to the responses evoked by synaptic glutamate receptors. For instance, in hippocampal cultures, activation of synaptic NMDA receptors promotes cell-survival events; whereas activation of extrasynaptic receptors triggers neuronal degeneration and induces cell death (Hardingham et al., 2002). It is not clear if such events occur in vivo, but detailed analysis of the abundance and subunit composition of synaptic vs. extrasynaptic receptors seems warranted to better understand the possible roles of these two different types of receptors in glutamatergic transmission.
Presynaptic AMPA and NMDA receptors
Even though they are usually associated with postsynaptic effects, NMDA and AMPA receptors are also located presynaptically and modulate release of glutamate and other transmitters (MacDermott et al., 1999; Engelman and MacDermott, 2004). In the basal ganglia, however, AMPA and NMDA receptors are not usually located presynaptically.
Only scarce presynaptic NR1 or NR2A immunoreactivity is observed in the striatum (Bernard and Bolam, 1998; Fujiyama et al., 2004) (but see Wang and Pickel, 2000). Nevertheless, physiological studies indicate that striatal NMDA receptors are involved in the regulation of dopamine and glutamate release (e. g. Krebs et al., 1991; Micheletti et al., 1993; Morari et al., 1993; Cheramy et al., 1996; Sesack et al., 2003). The lack of anatomical evidence for presynaptic NMDA receptors suggests that the effects observed in physiological studies are likely mediated by mechanisms that do not require presynaptic NMDA receptors. The presynaptic localization of AMPA receptors in the striatum remains controversial (Bernard et al., 1997; Fujiyama et al., 2004).
Kainate receptors
Although AMPA and NMDA receptors are the primary mediators of glutamatergic transmission in the CNS, kainate (KA) receptors also contribute to neuronal excitability (for reviews, see Frerking and Nicoll, 2000; Huettner, 2003; Lerma, 2003).
The localization of KA receptors at the EM level has been studied in the striatum and GP (Charara et al., 1999; Kieval et al., 2001; Kane-Jackson and Smith, 2003; Jin and Smith, 2005; Jin et al., 2006), but not in other basal ganglia. In the monkey striatum, pre- and postembedding immunogold methods have revealed that the largest proportion of GluR6/7 and KA2 plasma membrane-bound subunits is located at extrasynaptic sites, while only one third is located at glutamatergic synapses (Kieval et al., 2001), a strikingly different pattern from that of AMPA and NMDA receptors distributions (Bernard et al., 1997; Bernard and Bolam, 1998; Fujiyama et al., 2004).
Despite the strong postsynaptic expression of the KA receptor subunits GluR6/7 and KA2 in the striatum (Kieval et al., 2001), no evidence has been obtained for synaptic activation of these receptors after electrical stimulation of glutamatergic fibers, in dorsal striatum or nucleus accumbens (Chergui et al., 2000; Casassus and Mulle, 2002). Postsynaptic KA receptors may depress GABAergic transmission indirectly, by release of adenosine acting on A2A receptors (Chergui et al., 2000).
KA receptor subunits are expressed presynaptically in cortical and thalamic terminals in the striatum (Charara et al., 1999; Kieval et al., 2001). Activation of KA receptors by exogenous agonist inhibits glutamatergic transmission through presynaptic mechanisms, in the nucleus accumbens (Casassus and Mulle, 2002; Crowder and Weiner, 2002). Also, in rat SNc dopaminergic neurons, activation of KA receptors results in modulation of GABAergic transmission, probably through a presynaptic mechanism (Nakamura et al., 2003); yet the precise location of KA receptors in SNc remains to be determined.
In the rat and monkey GP, KA receptor subunits are expressed in pre- and postsynaptic elements, the former include unmyelinated axons and putative glutamatergic and GABAergic terminals (Kane-Jackson and Smith, 2003; Jin et al., 2006). Activation of KA receptors, indeed, has dual effects on glutamatergic transmission in slices of rat GP: it evokes postsynaptic currents with small amplitude and slower kinetics than AMPA receptor-mediated currents; and reduces glutamatergic synaptic transmission through presynaptic, G-protein-coupled, protein kinase C (PKC)-dependent, metabotropic mechanisms (Jin et al., 2006).
Metabotropic glutamate receptors
The metabotropic glutamate receptors (mGluRs) are members of the class C G-protein-coupled receptors. To date, eight mGluR subtypes have been cloned, and based on sequence homology, pharmacological profile, and second messenger coupling they are classified into three groups: group I (mGluRs 1 and 5), group II (mGluRs 2 and 3) and group III (mGluRs 4, 6, 7 and 8). Group I mGluRs are mainly coupled to the Gq/11 family of heterotrimeric G-proteins, which activates phospholipase C and subsequently increases intracellular Ca2+. Group I receptors can also induce slow direct depolarization by decreasing K+ currents. In contrast, group II and group III receptors are coupled to the Gi/o family, which negatively regulates adenylyl cyclase and cyclic AMP production. Group II and group III receptors can also increase K+ and decrease Ca2+ currents (Conn and Pin, 1997; Marino et al., 2003; Kew and Kemp, 2005).
The three subgroups of mGluRs are widely distributed in the basal ganglia circuitry, where they modulate synaptic transmission through both pre and postsynaptic mechanisms (Awad et al., 2000; Smith et al., 2001; Valenti et al., 2002; Conn et al., 2005). In general, group I mGluRs are localized in postsynaptic elements, and mediate excitatory effects, whereas group II and group III receptors are localized presynaptically, and modulate neurotransmitter release. Table 2 presents a summary of the subcellular and subsynaptic localization of mGluR subtypes in the basal ganglia.
Table 2.
Summary of the main ultrastructural features of the subcellular and subsynaptic localization of mGluR subtypes in the basal ganglia, as revealed by high resolution pre-embedding immunogold procedures.
| Structure | Presynaptic | Postsynaptic | References | |||
|---|---|---|---|---|---|---|
| Intracellular | Membrane-bound | |||||
| Extrasynaptic | Perisynaptic | Synaptic | ||||
| Striatum | 1a (Thal, Ctx) | 1a | 1a | 1a (Glu) | 1a (GABA) | 1a, 5: Paquet and Smith, 2003 |
| 3 (Ctx) | 5 | 5 | 5 (Glu, DA) | 5 (GABA) | 3: Tamaru et al., 2001 | |
| 4 (Glu, GABA) | 4: Corti et al., 2002 | |||||
| GP | 4 (GABA) | 5 | 1a | 1a (Glu) | 1a (GABA) | 1a, 5: Hanson and Smith, 1999 |
| 5 | 5 (Glu) | 5 (GABA) | 4: Corti et al., 2002 | |||
| STN | - | 1a | 1a | 1a (Glu, GABA) | - | 1a, 5: Kuwajima et al., 2004b |
| 5 | 5 | 5 (Glu, GABA) | ||||
| SNr | 4 (Glu, GABA) | 5 | 1a | 1a (Glu) | 1a (GABA) | 1a, 5: Hubert et al., 2001 |
| 5 | 5 (Glu) | 5 (GABA) | 4: Corti et al., 2002 | |||
Source(s) of afferents and chemical phenotype of synapses are indicated in parentheses. Ctx– cortex; DA – dopamine; GABA – γ-aminobutyric acid; Glu – glutamate; Thal – thalamus.
In all basal ganglia, most of the plasma membrane-bound group I mGluRs is extrasynaptic.
No published data is available on the immunogold localization of mGluRs 2, 7, and 8 in all basal ganglia
Group I mGluRs
The subcellular and subsynaptic distribution of group I mGluRs has been studied in detail in the monkey striatum (Paquet and Smith, 2003), GP (Hanson and Smith, 1999), and STN (Kuwajima et al., 2004b), as well as in the rat and monkey SN (Hubert et al., 2001; Marino et al., 2001).
The localization of mGluR1a and mGluR5 in the basal ganglia shares some common characteristics: (1) Subsets of neurons in all basal ganglia nuclei co-express both mGluR1a and mGluR5. This pattern is different from other brain regions such as the cerebellum or hippocampus, where either mGluR1 or mGluR5 predominates (Baude et al., 1993). (2) Both receptor subtypes are largely expressed in postsynaptic elements (i.e., dendrites, spines and perikarya). (3) A substantial, though variable, pool of receptors is associated with the intracellular compartments of dendritic processes and cell bodies. (4) The vast majority of plasma membrane-associated receptors are found at extrasynaptic locations. (5) When associated with synapses, both receptors are located at the edges of glutamatergic synapses. (6) Both receptor subtypes are also selectively expressed at GABAergic striatal synapses in GP and SNr, and at the edges of putative pallidal synapses in STN. Although the two group I mGluRs share common ultrastructural characteristics, it is important to highlight the fact that they also display specific ultrastructural features that may contribute to their different functions whenever they co-exist in individual basal ganglia neurons (Valenti et al., 2002; Conn et al., 2005).
Postsynaptic expression of group I mGluRs
In the monkey striatum, both mGluR1a and mGluR5 are primarily expressed in dendrites and spines (Paquet and Smith, 2003). Perikarya of many medium-sized spiny neurons and some large cholinergic interneurons are also immunolabeled for mGluR5, but much less frequently for mGluR1a. In postsynaptic elements, both group I mGluR subtypes exhibit a similar pattern of subsynaptic distribution: 30-60% of immunoreactivity is apposed to the plasma membrane, while the rest is found in the intracellular compartments. Of the plasma membrane-bound receptors, more than 80% are extrasynaptic. The remaining 20% is localized at the edges of putative glutamatergic synapses or within the active zone of symmetric synapses. In addition to putative GABAergic synapses, mGluR5, but not mGluR1a, is also perisynaptic to a majority of symmetric synapses established by dopaminergic terminals in the monkey striatum (Fig. 4C) (Paquet and Smith, 2003).
Figure 4.
Subcellular and subsynaptic localization of group I mGluRs in the basal ganglia. A: A dendrite (den) in the monkey STN displays perisynaptic immunogold labeling for mGluR5 (small arrows) at a symmetric synapse (arrowhead) formed by a GABA-containing terminal (GABA+), and at an asymmetric synapse (large arrow) formed by a GABA-negative terminal (GABA−). From Kuwajima et al., 2004b, with permission B: Immunoreactivity for mGluR5 (white arrowheads) in the monkey GP is expressed within the postsynaptic specialization of symmetric axo-dendritic synapses established by putative striatal terminals (AT1, AT2). From Hanson and Smith, 1999, with permission. C: Immunoreactivity for mGluR5 (small arrows) in the monkey striatum is localized perisynaptically at a symmetric synapse (black arrowhead) established by a tyrosine hydroxylase-immunopositive (dopaminergic) axon terminal (TH+). From Paquet and Smith, 2003 with permission. D-E: Subcellular distribution of immunogold labeling for mGluR1a (D) and mGluR5 (E) in the rat SNr. Note that most mGluR1a labeling is apposed to the dendritic plasma membrane (D), whereas most of mGluR5 labeling is intracellular (E). From Hubert et al., 2001 with permission. F: In the monkey putamen, a biotin-dextran-amine-labeled terminal bouton (asterisk) from the centromedian nucleus of the thalamus contains mGluR1a immunogold labeling. From Paquet and Smith, 2003, with permission. G: Presynaptic mGluR1a immunogold labeling is also found in a TH-positive terminal (asterisk) in the monkey putamen. From Paquet and Smith, 2003, with permission. Abbreviations: AT, axonal terminal; den, dendrite; Ut, unlabeled terminal. Scale bars: A, 200 nm; B, 500 nm; C, 250 nm; D, 500 nm (valid for E); F, 500 nm; G, 250 nm.
The subsynaptic localization of postsynaptic mGluR1a and mGluR5 is also similar in STN neurons (Wang et al., 2000; Kuwajima et al., 2004b), the majority (60-70%) of immunolabeling for both group I mGluR subtypes being found in the intracellular compartment, while 30-40% is expressed on the plasma membrane. Of the plasma membrane-bound immunolabeling, more than 90% is extrasynaptic, and less than 10% is associated with synapses. Most of such synapse-associated labeling is localized at the edges of postsynaptic specializations (Fig. 4A) (Kuwajima et al., 2004b).
Unlike the striatum and STN, the subsynaptic localization of mGluR1a and mGluR5 is different in postsynaptic elements of the monkey GP (both internal and external segments Hanson and Smith, 1999), as well as the monkey and rat SN (Hubert et al., 2001). In SN dendrites, a large proportion of mGluR1a immunolabeling is on the plasma membrane, whereas mGluR5 is mostly expressed in the intracellular compartment (Fig. 4D-E). Similar to the striatum, however, the vast majority of the plasma membrane-bound immunolabeling for both mGluR1a and mGluR5 is extrasynaptic, and the remaining immunoreactivity is localized at the edges of putative glutamatergic synapses or within the active zone of symmetric synapses established by putative striatal GABAergic terminals (Fig. 4B) (Hanson and Smith, 1999; Hubert et al., 2001; Kuwajima et al., 2004a).
Differential distribution of mGluR1a and mGluR5: plasma membrane vs. intracellular receptors
There are some noticeable differences in the subcellular and subsynaptic distribution of mGluR1a and mGluR5, and it has been proposed that these differences in subsynaptic localization may underlie the functional specificity of the two group I mGluRs subtypes whenever they coexist in individual basal ganglia neurons (Valenti et al., 2002). For instance, in GP and SN, mGluR1a immunoreactivity is predominantly associated with the plasma membrane, whereas the majority of mGluR5 immunoreactivity is localized in the cytoplasm (Fig. 4E-D) (Hanson and Smith, 1999; Hubert et al., 2001; Kuwajima et al., 2004a). This is an interesting finding, especially because group I mGluR-mediated direct depolarization in SNr and GP neurons depends solely on mGluR1 (Marino et al., 2001; Poisik et al., 2003). However, differential receptor distribution alone is probably not sufficient to explain such differences in physiologic functions because activation of predominantly intracellular mGluR5 does, indeed, evoke an increase in the intracellular level of Ca2+ in SNr neurons (Marino et al., 2002). In addition, mGluR5 mediates cross-desensitization of mGluR1a in a PKC-dependent manner in GP (Poisik et al., 2003).
Intracellular group I mGluR: functions and receptor trafficking
The intracellular pool of mGluR5 expressed on the nuclear envelope of striatal neurons is functional and activated by glutamate transported into the lumen of the envelope. Once activated, the nuclear mGluR5 modulates intranuclear level of Ca2+, and subsequently increases phosphorylation of the transcription factor CREB (Jong et al., 2005), which could then result in gene transcription. Intranuclear Ca2+ is also known to influence other important cellular functions such as protein import into the nucleus (Rogue and Malviya, 1999).
Since some immunolabeling is associated with microtubules and intracellular organelles such as the endoplasmic reticulum, the intracellular pool of group I mGluRs may also represent receptors in the process of trafficking to or from the plasma membrane. The intracellular trafficking of group I mGluRs can be regulated by scaffolding proteins such as Homer (Xiao et al., 2000; de Bartolomeis and Iasevoli, 2003) and tamalin (Kitano et al., 2002), as well as specific amino acid residues within the intracellular C-terminal tail of the receptors (Francesconi and Duvoisin, 2002; Das and Banker, 2006).
Studies using cell culture systems have demonstrated that Homer proteins can regulate translocation of the receptor to the plasma membrane (Ango et al., 2000; Ango et al., 2002); the clustering of group I mGluRs on the cell surface (Serge et al., 2002; Das and Banker, 2006), as well as translocation of receptors to different cellular compartments (e.g., soma vs. dendrites vs. axons) (Ango et al., 2000; Francesconi and Duvoisin, 2002; Das and Banker, 2006). Tamalin increases the cell-surface expression of mGluR1a in heterologous cells (Kitano et al., 2002). However, the precise role of Homer or tamalin proteins in the intracellular trafficking of group I mGluRs in vivo is still unknown. β-tubulin may also contribute to the differential plasma membrane distribution pattern of the two group I mGluR subtypes (Ciruela et al., 1999; Chan et al., 2001; Serge et al., 2003).
While mGluR1a possesses the long C-terminal intracellular domain that mediates direct interactions with a variety of proteins like Homer and tamalin, such is not the case for other splice variants of mGluR1 (i. e., mGluR1b, c, and d). Although little is known about the subsynaptic localization of the short mGluR1 splice variants, there is light microscopic evidence that the immunolabeling for mGluR1b in the rat forebrain is usually restricted to neuronal perikarya and the very proximal portion of dendrites, while mGluR1a is localized in both the perikarya and the entire dendritic tree (Ferraguti et al., 1998), suggesting the existence of differential trafficking mechanisms for mGluR1 splice variants.
Postsynaptic group I mGluRs at non-glutamatergic synapses
In the monkey striatum, mGluR5, but not mGluR1a, is expressed at the edges of symmetric synaptic specializations established by dopaminergic terminals (Paquet and Smith, 2003). These results provide anatomical support for possible receptor interactions that could underlie synaptic plasticity in the striatum (Calabresi et al., 1996). An interesting possibility described in the cerebral cortex, is that dopamine receptors and group I mGluRs cooperate to induce long-term depression (LTD) by convergence on common signaling pathways (Otani et al., 1999).
Both group I mGluR subtypes are found postsynaptically at the striatopallidal and striatonigral synapses. Activation of group I mGluRs at these synapses could potentially modulate GABAergic neurotransmission through the Gq signaling cascade. For instance, the activation of mGluR5 enhances GABAA-mediated currents in a Ca2+- and PKC-dependent manner in retinal amacrine cells (Hoffpauir and Gleason, 2002). Also, GABAB receptors contain consensus sequence for PKC phosphorylation sites in the C-terminal tail, which can lead to suppression of GABAB-mediated K+ conductance in hippocampus (Couve et al., 2000). Activation of GABAB receptors enhances mGluR1-mediated excitatory postsynaptic currents in cerebellar Purkinje neurons (Hirono et al., 2001; Tabata et al., 2004), from which these receptors co-immunoprecipitate (Tabata et al., 2004). Although such interactions between group I mGluRs and GABA receptors have not yet been demonstrated in the basal ganglia, the fact that both receptor families display common patterns of subsynaptic localization at GABAergic synapses offer potential sites where they could functionally interact (Hanson and Smith, 1999; Galvan et al., 2004; Kuwajima et al., 2004b; Charara et al., 2005).
Presynaptic group I mGluRs
In the monkey striatum, a subpopulation of thalamostriatal terminals displays immunoreactivity for mGluR1a (Fig. 4F). Less frequently, corticostriatal and dopaminergic terminals also show mGluR1a immunolabeling (Fig. 4G) (Paquet and Smith, 2003). These findings suggest that group I mGluRs have a presynaptic role modulating dopaminergic and glutamatergic transmission in the monkey striatum, which is consistent with microdialysis studies showing that local application of group I mGluR agonists facilitates dopamine release in the rat striatum (Verma and Moghaddam, 1998; Bruton et al., 1999). Also, the pharmacological blockade of glutamate transporters in the rat striatum inhibits evoked DA release, an effect likely mediated by the activation of presynaptic mGluR1a on dopaminergic terminals via glutamate spilled over from neighboring cortical and/or thalamic afferents (Zhang and Sulzer, 2003).
Presynaptic effects of group I mGluRs inhibit synaptic transmission in slices of young (P14-18) rat SNr, despite light presynaptic mGluR1 and mGluR5 immunolabeling in adult SNr (Marino et al., 2001). This discrepancy between the electrophysiological data and receptor localization can be explained by a developmental regulation of group I mGluR in rat SNr. In contrast to adults, both mGluR1 and mGluR5 are highly expressed in unmyelinated axons in P14-18 rats (Hubert and Smith, 2004) suggesting that presynaptic effects of group I mGluRs in SNr may be an age-dependent phenomenon. Alternatively, the activation of postsynaptic group I mGluRs may lead to the synthesis and release of endocannabinoids, which, in turn, may retrogradely activate presynaptic CB1 cannabinoid receptors to modulate excitatory neurotransmission, as demonstrated in the cerebellum and hippocampus (reviewed in Doherty and Dingledine, 2003).
Glial expression of group I mGluRs
Immunoreactivity for mGluR5 is found in glial processes in the monkey striatum (Paquet and Smith, 2003), the mouse GP (Kuwajima et al., 2004a), and the monkey and mouse STN (Kuwajima et al., 2004a; Kuwajima et al., 2004b). Glial processes in the adult rat SNr do not express mGluR5, although mGluR5-immunoreactive processes are very abundant in young rats (Hubert and Smith, 2004), suggesting a role for this receptor in CNS development (Romano et al., 1995; Catania et al., 2001). mGluR1a is also expressed in glial processes in the monkey STN (Kuwajima et al., 2004b), and the monkey and rat SNr (Hubert et al., 2001). A growing body of evidence suggests that group I mGluRs expressed in glial cells are involved in intracellular signaling and glial function (e. g., Ca2+ mobilization and regulation of K+ conductance; for a review see Winder and Conn, 1996).
Group II and Group III mGluRs
In contrast to the extensive information currently available for group I mGluRs in the basal ganglia, little is known about the subsynaptic localization of group II and group III mGluRs. The use of the immunogold technique has been restricted to a couple of reports on mGluR3 and mGluR4 (Tamaru et al., 2001; Corti et al., 2002). Aside from these, the available electron microscope studies have used the immunoperoxidase technique to describe the subcellular distribution of these receptors. These studies have been extensively reviewed elsewhere (Smith et al., 2001). Here, they will be discussed briefly, along with the limited information that has been obtained using immunogold methods.
Both group II and group III mGluRs are mainly expressed in presynaptic elements in basal ganglia nuclei, where they act as auto- or heteroreceptors. Overall, group II and III mGluRs display a significantly different pattern of expression in presynaptic elements; group II mGluRs being preferentially expressed in pre-terminal axons and nonsynaptic sites of the plasma membrane of axon terminals, whereas group III mGluRs are confined to the presynaptic active zone of labeled boutons, close to the transmitter release site (Petralia et al., 1996; Bradley et al., 2000; Corti et al., 2002; Poisik et al., 2005).
Both mGluR2 and mGluR3 are found in glutamatergic terminals making asymmetric synapses in the striatum (Tamaru et al., 2001) and SNr (Bradley et al., 2000). In the striatum, some of the mGluR2-containing elements arise from the cerebral cortex, while in SNr, a significant proportion likely originates in the STN (Bradley et al., 2000; Tamaru et al., 2001). Immunolabeling for mGluR3 is found mostly on the extrasynaptic plasma membrane, and only occasionally in the presynaptic active zone (Tamaru et al., 2001). Like group II receptors, mGluR7 is localized in putative cortical terminals making axo-dendritic and axo-spinous asymmetric synapses in the striatum (Kinoshita et al., 1998; Kosinski et al., 1999). Another group III receptor, mGluR4, is also present at asymmetric synapses albeit to a lesser extent, in the striatum and SNr (Corti et al., 2002). In line with these findings, both group II and group III mGluRs regulate glutamate release at excitatory corticostriatal (Calabresi et al., 1992; Lombardi et al., 1993; East et al., 1995; Lovinger and McCool, 1995; Pisani et al., 1997), and subthalamonigral synapses (Bradley et al., 2000; Wittmann et al., 2001). In GP, group II mGluRs are also expressed in small unmyelinated pre-terminal axons and glutamatergic terminal boutons (Poisik et al., 2005). In the midbrain, activation of group II and III mGluRs modulates glutamatergic transmission onto DA neurons (Wigmore and Lacey, 1998; Valenti et al., 2005). Functional mGluR4 and mGluR7a autoreceptors are both expressed in the STN (Kosinski et al., 1999; Awad-Granko and Conn, 2001; Corti et al., 2002), although the detailed subsynaptic localization is yet to be characterized.
Group III mGluRs also act as heteroreceptors that modulate the release of GABA in the basal ganglia. The presence of these receptors in the active zones of putative GABAergic terminals has been described in the striatum (Corti et al., 2002), GP (Bradley et al., 1999a; Bradley et al., 1999b; Corti et al., 2002) and SNr (Corti et al., 2002). Immunoreactivity for mGluR4 and mGluR7 is present at symmetric (probably striatal) synapses in GP and SNr (Kosinski et al., 1999). Accordingly, electrophysiological evidence indicates that the activation of group III mGluRs regulates GABA release in these brain regions (Wittmann et al., 2001; Valenti et al., 2003). Extrasynaptic glutamate can also modulate dopamine release via group II mGluRs in nigrostriatal terminals (Baker et al., 2002).
Glutamate transporters
After synaptic release, clearance of glutamate from the extracellular space is accomplished by high-affinity transporters. Through regulation of extracellular glutamate concentration, glutamate transporters can influence the activation of synaptic and extrasynaptic glutamate receptors (Rusakov and Lehre, 2002; Huang and Bergles, 2004).
To date, five different glutamate or excitatory amino acid transporters (EAATs) have been cloned: GLAST (GluT, EAAT1), GLT1 (EAAT2, GLAST2, GLTR), EAAC1 (EAAT3), EAAT4 and EAAT5. GLAST and GLT1 are primarily found in glial cells, while EAAC1, EAAT4 and EAAT5 are expressed in neurons. GLT1 is widely expressed throughout the brain, and is considered as the major glutamate transporter in the CNS. GLAST has a more restricted distribution, being expressed only at low levels in most brain regions. EAAC1 is found in most areas of the brain, the highest levels are detected in the hippocampus, cerebellum and basal ganglia. EAAT4 is expressed primarily in Purkinje cell dendrites, while EAAT5 expression is restricted to the retina. In the CNS, EAATs are rarely expressed on glutamatergic terminals (for an in-depth review of glutamate transporters, the reader is referred to Danbolt, 2001).
The available information regarding the distribution of EAATs in the basal ganglia derives mostly from in situ hybridization (Torp et al., 1994; Velaz-Faircloth et al., 1996; Lievens et al., 2001) and light microscopic immunohistochemical studies (Rothstein et al., 1994; Lehre et al., 1995); except for EM data from our group on the ultrastructural localization of GLT1, GLAST and EAAC1 in monkey (Charara et al., 2002).
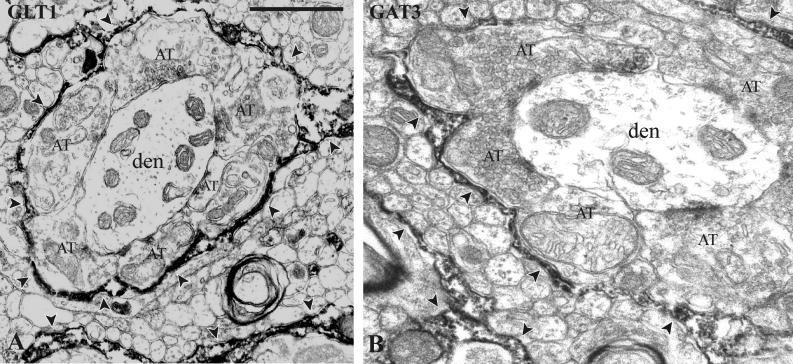
In the basal ganglia, GLT1 is localized for the most part in glial processes, except in the striatum, where is also expressed in a subset of dendritic spines (Charara et al., 2002). Although GLT1 is considered as a glial transporter (Danbolt, 2001), the GLT1b isoform is expressed in neurons (Chen et al., 2002b), and could represent a neuronal component for the rapid clearance of glutamate from the synaptic cleft. In GPe and GPi, GLT1-immunopositive glial processes form a tight sheath surrounding axo-dendritic synaptic complexes (Fig. 5) (Charara et al., 2002). Remarkably, these GLT1-containing glial processes do not extend between terminals to reach the synaptic cleft, as they do in the striatum (Charara et al., 2002) and other brain regions (Lehre and Danbolt, 1998). One could speculate that this arrangement allows glutamate released from axon terminals to persist in the extracellular space long enough to diffuse and activate extrasynaptic targets distant from neurotransmitter release sites.
Figure 5.
Localization of GLT1 (A) and GAT3 (B) in the monkey GPe, as revealed by immunoperoxidase. Both transporters display a similar pattern of distribution, being confined to glial processes (arrowheads) that surround groups of axonal terminals (AT) in contact with a dendrite (den). Scale bar 1 μm (valid for A and B). B from Galvan et al., 2005, with permission.
EAAC1 is found mostly in postsynaptic elements, expressed at extrasynaptic locations, or associated with the intracellular compartment of labeled elements. Presynaptically, EAAC1 is expressed only in small subpopulations of terminals in the GP and SN, suggesting limited extent of glutamate reuptake in nerve terminals. GLAST immunoreactivity is confined to thin glial processes (Charara et al., 2002).
Besides their importance in neurotransmitter clearance, glutamate transporters play a role in preventing glutamate-induced neurotoxicity (Danbolt, 2001). Interestingly, the density of GLT1 in striatal astrocytes is decreased in a rodent model of PD (Dervan et al., 2004). This deficiency suggests a compromised ability of glial cells to regulate glutamate and its associated synaptic functions in parkinsonism, which appears to be reversed after levodopa treatment (Lievens et al., 2001).
Glutamate transporters may also be involved in non-vesicular release of glutamate (Nedergaard et al., 2002), a process that has been documented in the rat striatum, where both glutamate and dopamine transmission are modulated by glutamate released nonsynaptically from the cysteine-glutamate antiporter (Baker et al., 2002; Moran et al., 2005).
Overview of Glutamate transmission in the basal ganglia
Figure 7A summarizes our current knowledge about the subsynaptic localization of iGluRs, mGluRs and EAATs in the basal ganglia. AMPA and NMDA receptors are enriched in the postsynaptic densities of glutamatergic synapses; while KA receptors and group I mGluRs are prominent at peri- and extrasynaptic locations (as described in other brain regions, e. g. Ottersen and Landsend, 1997; Takumi et al., 1999). Group II and III mGluRs and KA receptors contribute to the presynaptic regulation of glutamatergic and GABAergic activity throughout the basal ganglia. EAATs are located mostly in glial processes (GLT1), or at extrasynaptic locations on the plasma membrane of the postsynaptic elements (EAAC1).
Extrasynaptic glutamate receptors could be activated after spillover of synaptically released glutamate. Since mGluRs exhibit a higher affinity for glutamate than AMPA/kainate receptors (Conn and Pin, 1997; Dingledine et al., 1999), they can be activated by relatively small concentrations of glutamate. Consistent with this notion, evoked postsynaptic group I mGluR responses require high-frequency or tetanic stimulation of glutamatergic afferents (Batchelor et al., 1994; Tempia et al., 1998; Zhang and Sulzer, 2003), and strongly respond to glutamate transporters blockade (Brasnjo and Otis, 2001; Reichelt and Knopfel, 2002).
GABAERGIC TRANSMISSION
Except for the projections from the STN, SNc and striatal cholinergic interneurons, all intrinsic connections and output projections of the basal ganglia are GABAergic (Fig. 1). In recent years, several reports have come to light regarding the ultrastructural localization of GABAA and GABAB receptors in the basal ganglia, and information has also started to emerge regarding the localization of the GABA transporters. These studies have contributed to the understanding of GABAergic transmission in the basal ganglia circuitry.
GABAA receptors
The rapid inhibitory responses characteristic of GABAergic transmission are mediated by the activation of GABAA receptors, which are ligand-gated chloride channels formed by five subunits. The GABAA receptor subunits characterized to date are α1-α6, β1-β4, γ1-γ3, ρ1-ρ3, δ, ε, θ, π and their combinations result in specific physiological and pharmacological profiles. The most common GABAA receptor stoichiometry is two α, two β and one γ subunits (Barnard et al., 1998; Korpi et al., 2002; Sieghart and Sperk, 2002).
The ultrastructural localization of some of the most prevalent subunits (α1, β2/3, γ2) has been analyzed using immunogold in rat striatum (Fujiyama et al., 2000), monkey and rat GP (Somogyi et al., 1996; Charara et al., 2005), in rat SN (Fujiyama et al., 2002), as well as in the monkey STN (Galvan et al., 2004), as detailed in Table 3.
Table 3.
Summary of the main ultrastructural features of the subsynaptic localization of GABAA receptor subunits in the basal ganglia, as revealed by high resolution immunogold procedures.
| Structure | Technique | α1 | α2 | β2/3 | γ2 | Colocalization |
|---|---|---|---|---|---|---|
| Striatum (ref. 1) |
Postembedding | Synaptic | Synaptic | Synaptic | -- | α1, β2/3 and γ2 |
| GPe/GPi (refs. 2 and 3) |
Postembedding (ref. 2) |
Synaptic (ref. 2,3) |
-- | Synaptic (refs. 2,3) |
Synaptic (ref. 2) |
α1, β2/3 and γ2 (ref. 2) |
| Pre- and postembedding (ref. 3) |
Extrasynaptic (ref. 3) |
Extrasynaptic (ref. 3) |
α1 and β2/3 (ref. 3) |
|||
| STN (ref. 4) |
Pre- and postembedding |
Synaptic Extrasynaptic |
-- | -- | -- | -- |
| SN (ref. 5) |
Postembedding | Synaptic | -- | Synaptic | Synaptic | α1, β2/3 andγ2 |
Data not available
References:
Various common features characterize the subsynaptic localization of GABAA receptors in the basal ganglia: (1) A large proportion of GABAA α1, β2/3 and γ2 subunits are localized at symmetric synapses which, in many cases, have been clearly identified as GABAergic. (2) GABAA α1, β2/3 and γ2 subunits often co-localize at the level of individual synapses. (3) GABAA receptor subunits are also localized at extrasynaptic locations. (4) GABAA receptor subunits are not expressed at asymmetric (glutamatergic) synapses.
Synaptic GABAA receptors
The most prominent immunogold labeling for GABAA receptor subunits is clustered at symmetric (putatively GABAergic) synapses (Fig. 6B). GABAA receptor α1, β2/3 and γ2 subunits are often co-localized and evenly distributed along synaptic specializations (Somogyi et al., 1996; Fujiyama et al., 2000; Fujiyama et al., 2002; Galvan et al., 2004; Charara et al., 2005), suggesting that fast GABAA-mediated IPSPs, reported extensively in various basal ganglia structures (e. g. Kita et al., 1983; Nakanishi et al., 1985; Nakanishi et al., 1987; Kita and Kitai, 1991; Tepper et al., 1995; Paladini et al., 1999; Kita, 2001; Bevan et al., 2002; Hallworth and Bevan, 2005), are mostly mediated by synaptic GABAA receptors containing α1, β2/3 and γ2 subunits.
Figure 6.
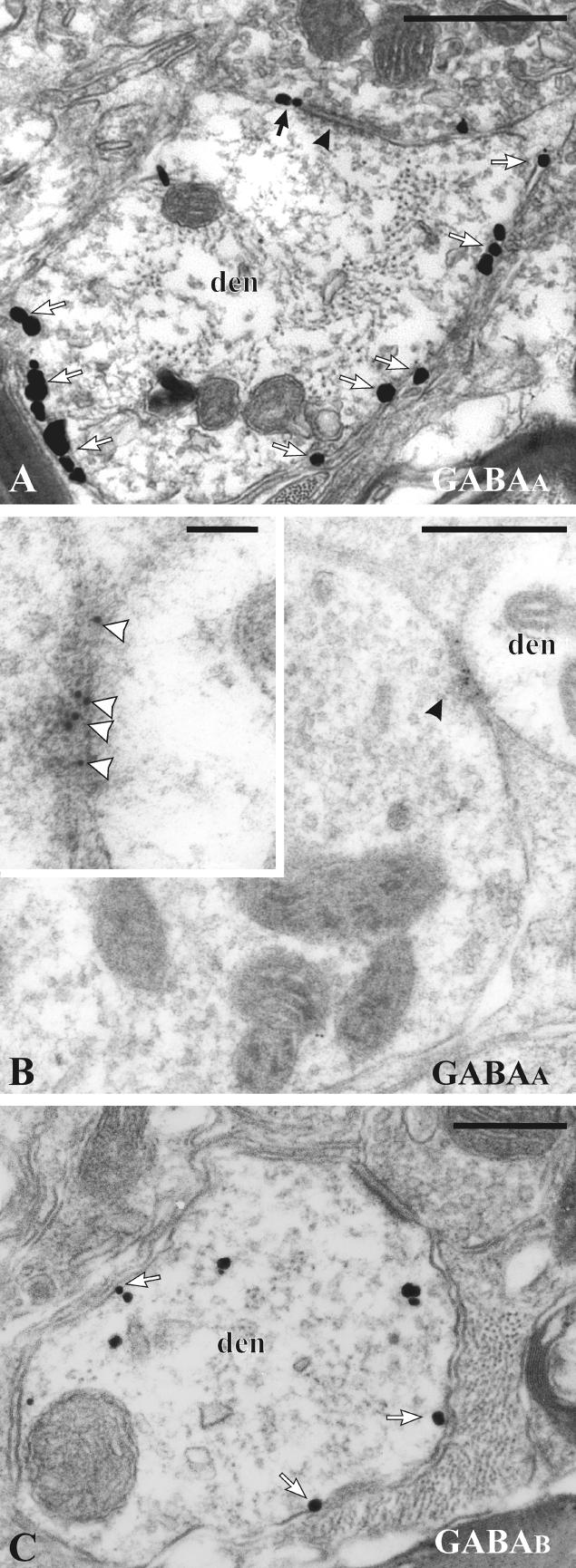
Subsynaptic localization of GABAA and GABAB receptors in the monkey STN. A. GABAA receptors revealed by pre-embedding immunogold. Most of the immunogold particles are located at extrasynaptic sites (white arrows). Some particles are at the edge (black arrow) of a symmetric synapse (arrowhead). B. GABAA receptors revealed by postembedding immunogold. The majority of gold particles (white arrowheads) are located at symmetric synapses (black arrowhead). C. Pre-embedding immunogold to reveal GABAB receptors. Gold particles at extrasynaptic locations on the plasma membrane are indicated by white arrows. Abbreviation: den, dendrite. Scale bars: A, B 0.5 μm; inset in B 0.1 μm; C, 0.25 μm. From Galvan et al. 2004, with permission.
For the most part, GABAA-positive synapses are formed by terminals enriched in GABA (Fujiyama et al., 2000, 2002), but in the rat striatum, about half of these terminals present no detectable GABA levels (Fujiyama et al., 2000). These could represent non-GABAergic terminals associated with GABAA receptors, including dopaminergic and cholinergic boutons (Izzo and Bolam, 1988; Pickel and Chan, 1990; Smith et al., 1994). Those terminals containing GABA and forming GABAA-positive synapses originate from local axon collaterals of striatal projection neurons or GABAergic interneurons. A small, albeit significant, GABAergic contribution from the GPe, SNr and VTA should also be considered (Smith and Parent, 1986; Rajakumar et al., 1994; Bevan et al., 1998; Gonzalez-Hernandez et al., 2004).
Projections from GABA/parvalbumin-containing interneurons exert a powerful inhibition on spiny projection neurons through synapses on proximal dendrites or perikarya (Bennett and Bolam, 1994; Koos and Tepper, 1999; Koos et al., 2004; Tepper et al., 2004); whereas local axon collaterals terminate more distally; mainly on spines (Wilson and Groves, 1980), and generate weak inhibitory responses (Jaeger et al., 1994; Koos et al., 2004). The differences between the inhibitory strength of the spiny cell axon collaterals and GABA interneurons arise mainly from the location and the number of synapses formed by each type of afferents (Tepper et al., 2004). Yet, another factor that could contribute to the strength of GABAA-mediated inhibition, which has not been explored in detail, is the density and the subunit composition of postsynaptic GABAA receptors at specific synapses. Compartmentalized distribution of GABAA receptors at different GABAergic afferents has been described in the cerebellum and hippocampus (Nusser et al., 1995a; Nusser et al., 1995b; Nusser et al., 1996; Nyiri et al., 2001).
GABAA receptor density at individual synapses varies in response to plastic changes induced by normal physiological activity (Christie and de Blas, 2002), or pathological conditions. Such plasticity is observed in the rat SNr, after striatal lesions (an animal model of Huntington's disease), which results in an increased density of GABAA receptor subunits at synapses established by degenerating striatonigral terminals (Fujiyama et al., 2002). This represents an elegant example of the reliability and sensitivity of ultrastructural analysis to characterize changes of receptor proteins at subsynaptic levels in pathological conditions.
Extrasynaptic GABAA receptors
Extrasynaptic GABAA receptors have been described in many brain regions (Nusser et al., 1995a; Nusser et al., 1995b; Nusser et al., 1998b). In the basal ganglia, a significant level of GABAA receptor subunits immunoreactivity is located at nonsynaptic sites, as revealed with the pre-embedding immunogold method (Fig. 6A) (Somogyi et al., 1996; Fujiyama et al., 2000; Fujiyama et al., 2002; Galvan et al., 2004; Charara et al., 2005). However, because of the relatively lower density of extrasynaptic receptors compared to synaptic receptors, the postembedding method usually fails to reveal significant extrasynaptic GABAA receptor labeling (as described above for AMPA and NMDA receptors). This emphasizes the importance of using various immunocytochemical approaches to achieve a clear and accurate picture of the subcellular and subsynaptic localization of receptor proteins.
While the exact subunit composition and the functional relevance of extrasynaptic GABAA receptors in the basal ganglia remain to be established, data gathered in the cerebellum and dentate gyrus indicates that extrasynaptic GABAA receptors likely contain α4, α5, α6 or δ subunits (Mody, 2001; Semyanov et al., 2004). Of these, α4 is strongly expressed in the striatum; while moderate to high levels of the δ subunit are present in all basal ganglia nuclei (Wisden et al., 1992; Fritschy and Mohler, 1995; Kultas-Ilinsky et al., 1998; Schwarzer et al., 2001). These subunits confer specific pharmacological properties. Receptors containing the δ subunit, in particular, have a higher affinity for GABA than other GABAA receptors, and little or no desensitization to prolonged presence of agonist, making them ideal candidates to sense the low concentrations of GABA that persist in the extracellular space (Mody, 2001; Semyanov et al., 2004). Extrasynaptic GABAA receptors have generated increasing interest in recent years because of their prominent role in mediating tonic GABA-mediated currents that can significantly affect the membrane conductance and impact neuronal excitability (Semyanov et al., 2004; Mody, 2005). To date, GABAA receptor-mediated tonic currents have not been studied in basal ganglia neurons, except in the STN, where such conductances have not been found (Hallworth and Bevan, 2005).
GABAB receptors
Metabotropic GABAB receptors are heterodimers formed by GABAB R1 and GABAB R2 subunits (Marshall et al., 1999). Postsynaptic GABAB receptors mediate slow hyperpolarization, while presynaptic receptors inhibit neurotransmitter release (Bowery et al., 2002; Bettler et al., 2004).
Detailed subsynaptic localization of GABAB R1 and R2 receptors has been described in the rat SNr, SNc, GP and striatum (Boyes and Bolam, 2003; Chen et al., 2004; Lacey et al., 2005), and that of GABAB R1 in the monkey STN and GP (Galvan et al., 2004; Charara et al., 2005) (see Table 4). These studies reveal that the subcellular localization of GABAB receptors shares several similarities among basal ganglia across species: (1) The majority of GABAB receptors are located intracellularly. (2) The majority of plasma membrane-bound GABAB receptors are associated with extrasynaptic sites. (3) A small proportion of GABAB receptors is located at symmetric (GABAergic) synapses. (4) A small proportion of GABAB receptors is found at the edges of asymmetric (glutamatergic) postsynaptic specializations. (5) Presynaptic GABAB receptors are encountered on subpopulations of glutamatergic and GABAergic terminals.
Table 4.
Summary of the main ultrastructural features of the subsynaptic localization of GABAB receptors in the basal ganglia, as revealed by high resolution immunogold procedures.
| Structure | GABABR1 | GABABR2 | ||
|---|---|---|---|---|
| Presynaptic* | Postsynaptic | Presynaptic* | Postsynaptic | |
| Striatum (ref. 1) |
Ctx, Thal and GABA | Intracellular Extrasynaptic |
CTX, Thal and GABAergic terminals |
Intracellular Extrasynaptic |
| GP (ref. 2, rat) |
Glu and Str | Intracellular Extrasynaptic |
Glu terminals Striatal terminals |
Extrasynaptic Intracellular |
| GPe/GPi (ref. 3, monkey) |
Glu | Intracellular Extrasynaptic |
-- | -- |
| STN (ref. 4) |
Intracellular Extrasynaptic |
-- | -- | |
| SN (ref. 5) |
Str, GP and Glu | Intracellular Extrasynaptic |
Str, GP and Glu | Intracellular Extrasynaptic |
Source(s) of afferents and chemical phenotype of synapses: Ctx – cortex; Glu – glutamate; GP – globus pallidus; Str – Striatum; Thal – thalamus.
Data not available
References:
Postsynaptic GABAB receptors
In contrast to the hippocampus, cerebellum and thalamus, where most GABAB R1 labeling is bound to the plasma membrane (Kulik et al., 2002; Kulik et al., 2003; Villalba et al., 2006), the largest proportion of GABAB R1 labeling is intracellular in basal ganglia neurons (Boyes and Bolam, 2003; Chen et al., 2004; Galvan et al., 2004; Charara et al., 2005; Lacey et al., 2005). Intracellular GABAB R1 immunoreactivity could represent GABAB R1/R2 heterodimers trafficking to the plasma membrane (Couve et al., 2000), or non-functional GABAB R1 subunits (Margeta-Mitrovic et al., 2000). The larger proportion of intracellular GABAB R1 subunits in the basal ganglia, compared to other brain regions, suggests that basal ganglia neurons are exposed to specific conditions that affect the trafficking and internalization of GABAB R1subunits.
In contrast to the abundance of synaptic GABAA receptors, the largest proportion of postsynaptic plasma membrane-bound GABAB R1 and GABAB R2 immunoreactivity is extrasynaptic (Fig. 6C) (Boyes and Bolam, 2003; Chen et al., 2004; Galvan et al., 2004; Charara et al., 2005; Lacey et al., 2005); a general feature of metabotropic GABAergic receptors throughout the brain (Ige et al., 2000; Gonchar et al., 2001; Kulik et al., 2002; Kulik et al., 2003; Lujan et al., 2004; Villalba et al., 2006). In all basal ganglia structures, smaller proportions of plasma membrane-bound GABAB receptors are found either at the edges of asymmetric synapses formed by glutamatergic afferents, or at symmetric GABAergic synapses (Boyes and Bolam, 2003; Chen et al., 2004; Galvan et al., 2004; Charara et al., 2005; Lacey et al., 2005). As discussed above (see Group I mGluRs section), there is a striking similarity between the distribution patterns of group I mGluRs and GABAB receptors in the STN, GP and SN.
GABAB-mediated postsynaptic effects have been reported in striatum (Seabrook et al., 1991); SNc (Engberg et al., 1993; Erhardt et al., 1999); and in a subpopulation of GP neurons (Stefani et al., 1999); while other studies described no significant GABAB-mediated electrophysiological responses in SNr (Shen and Johnson, 1997; Chan et al., 1998) and STN neurons (Shen and Johnson, 2001; Urbain et al., 2002). The functions and mechanisms of activation of postsynaptic GABAB receptors remained unclear, and the abundance of postsynaptic GABAB receptors seemed to be at odds with the weak postsynaptic responses observed, until recent data provided important insights into the physiological conditions under which postsynaptic GABAB receptors can be activated (Hallworth and Bevan, 2005; Kaneda and Kita, 2005; Kita et al., 2006). In both STN and GP, high frequency stimulation of GABAergic afferents is necessary to elicit postsynaptic GABAB-mediated effects, while single pulses evoke GABAA-mediated responses (Hallworth and Bevan, 2005; Kaneda and Kita, 2005; Kita et al., 2006). Furthermore, the activation of GABAB receptors elicit burst firing in STN neurons (Hallworth and Bevan, 2005). These findings suggest that GABAB receptors exert their effects only in response to synchronous firing of large populations of afferent projections.
Presynaptic GABAB receptors
Presynaptic GABAB R1 and R2 immunoreactivity is found in a subset of putative glutamatergic terminals forming asymmetric synapses in the striatum, GP, STN and SN (Charara et al., 2000; Boyes and Bolam, 2003; Charara et al., 2004; Chen et al., 2004; Charara et al., 2005; Lacey et al., 2005). In the striatum, presynaptic GABAB receptors are expressed in cortical and thalamic terminals, identified with vesicular glutamate transporters 1 and 2 (vGluT1 and vGluT2, respectively), indicating that the two main sources of excitatory inputs to striatal neurons can be regulated by GABAB receptors (Lacey et al., 2005). GABAB agonists, indeed, depress glutamate-mediated postsynaptic potentials in slices of rat striatum, GP and STN (Calabresi et al., 1990; Seabrook et al., 1990; Calabresi et al., 1991; Nisenbaum et al., 1992, 1993; Shen and Johnson, 2001; Chen et al., 2002a).
Besides their role as heteroreceptors, presynaptic GABAB receptors are also expressed in GABAergic terminals in the rat striatum, GP, SNr and SNc (Boyes and Bolam, 2003; Chen et al., 2004; Lacey et al., 2005). Thus, GABAB receptors are in position to modulate the release of GABA, as shown in the rat striatum and SN (Wilson and Wilson, 1985; Floran et al., 1988; Giralt et al., 1990; Seabrook et al., 1991; Nisenbaum et al., 1992, 1993; Hausser and Yung, 1994; Stanford and Lacey, 1996; Shen and Johnson, 1997; Chan et al., 1998; Chen et al., 1998; Giustizieri et al., 2005). Surprisingly, despite the strong presynaptic expression of GABAB receptors in GABAergic terminals in the GP (Chen et al., 2004), presynaptic modulation of GABA release does not appear to be mediated by GABAB receptors (Hashimoto and Kuriyama, 1997).
GABAB receptors are located in subsets of en passant, putatively dopaminergic, boutons in the striatum (Charara et al., 2000; Lacey et al., 2005), which provides direct support for presynaptic GABAB regulation of dopamine release in the striatum (Reimann, 1983; Arias Montano et al., 1992; Smolders et al., 1995) (but see Santiago et al., 1993).
GABA transporters
GABA transporters take up GABA from the synaptic cleft to terminate the synaptic events evoked by released GABA. In addition, these transporters can mediate non-vesicular release of GABA, by reversing the direction of transport (Isaacson et al., 1993; Richerson and Wu, 2003). To date, four GABA plasma membrane transporters have been characterized, namely GAT1, GAT2, GAT3 (GABA transporter 1-3), and BGT1 (Betaine-GABA transporter). Both GAT1 and GAT3 are widely expressed in the brain. Originally, GAT1 and GAT3 were considered as exclusive neuronal and glial transporters, respectively, but this distinction is far from absolute, since GAT1 is expressed in glial cells, and instances of neuronal GAT3 expression have been reported (Borden, 1996). In the brain, GAT2 expression is restricted to the meninges and the ependymal cells lining the ventricles; while low to moderate levels of BGT1 are expressed in most brain regions (Borden, 1996; Dalby, 2003). Scant information, derived mostly from in situ mRNA hybridization and light microscopy immunocytochemistry, is available on the distribution of GABA transporters in the basal ganglia (Durkin et al., 1995; Yasumi et al., 1997; Augood et al., 1999). The subcellular localization of GAT1 and GAT3 has been analyzed in the monkey GPe using immunoperoxidase (Galvan et al. 2005).
GAT1 immunoreactivity is primarily found in pre-terminal axons and glial elements, whereas GAT3 is restricted to glial processes (Galvan et al., 2005). Furthermore, most GAT1- or GAT3-labeled processes are distant from GABAergic synapses. Only scarce GAT1- and none GAT3-positive axon terminals are found in GPe, indicating that limited uptake of GABA occurs in presynaptic terminals. Interestingly, the GAT3-labeled glial processes in the monkey GPe display a pattern of distribution remarkably similar to that of GLT1 (Charara et al., 2002); i. e., both ensheath complexes or rosettes of dendrites and terminals (Fig. 5).
The functions of GABA transporters in the basal ganglia have been studied to a limited extent; however the information available indicates that their activity is essential to regulate GABA-mediated transmission. In rat GP slices, for instance, blockade of GAT1 inhibits GABA uptake (Gonzalez et al., 2006) and alters GABAA-mediated responses (Chen and Yung, 2003). In vivo, intrapallidal injections of GAT1 blockers result in altered motor behavior in the rat (Chen and Yung, 2003); while in the monkey, both GAT1 and GAT3 modulate the levels of extracellular GABA and the spontaneous activity of GPe and GPi cells (Galvan et al., 2005). SNr express functional GAT1 (Bahena-Trujillo and Arias-Montano, 1999), and blockade of GAT1 or GAT3 in this area reduces electroshock-induced convulsions (Dalby, 2000). In the striatum, GABA transporters contribute to action potential-independent release of GABA (Del Arco et al., 1998; Schoffelmeer et al., 2000).
It is currently unknown if GABA transporters exhibit plastic changes under pathological conditions. For instance, GABA transporters could be up- or downregulated in response to the altered levels of GABA observed in GPe (Robertson et al., 1991) and STN (Soares et al., 2004) in parkinsonism. Ultrastructural immunogold studies are needed to determine the localization of these transporters at the synaptic level in the basal ganglia, in normal and disease conditions.
Overview of GABAergic transmission in the basal ganglia
A schematic representation of the ultrastructural localization of GABA receptors and transporters is shown in Fig. 7B. In general, the subsynaptic distribution of GABAA and GABAB receptors in basal ganglia nuclei is consistent with the current view that GABAA receptors are localized to mediate fast phasic inhibition, while GABAB receptors are mostly extrasynaptic and mediate slow long term inhibition (Mody et al., 1994). GABAB receptors display a high affinity for GABA (Jones et al., 1998) which makes them well suited to detect extrasynaptic neurotransmitter spillover. On the other hand, synaptic GABAA receptors have a much lower affinity for GABA and desensitize much more rapidly than GABAB receptors (Macdonald and Olsen, 1994). However, the subunit composition of specific subtypes of extrasynaptic GABAA receptors makes them more sensitive to GABA and less prone to desensitization in response to prolonged exposure to the agonist (Semyanov et al., 2004).
More information is needed on the distribution of GABA transporters in the basal ganglia. The glial and nonsynaptic localization of GAT1 and GAT3 in the monkey GPe suggests potential for diffusion of GABA from synaptic release sites, to activate extrasynaptic targets.
CONCLUDING REMARKS
High resolution EM immunocytochemistry has provided the basic elements to construct a working model of the subsynaptic localization of glutamate and GABA receptors and transporters in the basal ganglia (Fig. 7). This scenario, as complex as it may appear, is by necessity a simplification, particularly because other neurotransmitter systems have yet to be incorporated. An important concept that stands out from these findings is the significant proportion of metabotropic and ionotropic receptors at extrasynaptic sites in basal ganglia neurons, which raises interesting issues about the activation and roles of extrasynaptic receptors in neuronal communication. In this review, we have highlighted the possibility that activation of extrasynaptic GABA and glutamate receptors throughout the basal ganglia relies on neurotransmitter diffusion outside the synaptic cleft, a phenomenon that has been demonstrated in vitro in cerebellar and hippocampal neurons (Isaacson et al., 1993; Mitchell and Silver, 2000; Scanziani, 2000; Hamann et al., 2002). The extent to which this occurs in vivo, however, remains unclear.
Several other possibilities for extrasynaptic receptor activation must be considered: (1) Non-vesicular release of transmitters, mediated by reversed activity of plasma membrane transporters (Attwell et al., 1993; Baker et al., 2002; Moran et al., 2005). (2) Glial release of transmitters (Araque et al., 1998). (3) Direct receptor-receptor interactions. Several examples of such interactions have now been described in transfected cells; including GABAA γ2 and dopamine D5 receptors (Liu et al., 2000), GABAA γ2 and GABAB R1 receptors (Balasubramanian et al., 2004), and NMDA NR1 and D1 receptors (Lee et al., 2002; Pei et al., 2004). (4) Common signal transduction mechanisms, as demonstrated for GABAB and group I mGluRs in the cerebellum (Hirono et al., 2001; Tabata et al., 2002; Tabata et al., 2004). (5) Agonist-independent modulation, as described for group I mGluRs (Ango et al., 2001). (6) Spare receptors. Extrasynaptic GABA and glutamate subunits can act as a reserve pool, which can potentially be inserted in the postsynaptic density (Triller and Choquet, 2003; Triller and Choquet, 2005). Alternatively, they could represent synaptic receptors translocated to nonsynaptic regions prior to internalization (Barnes, 2000; Ashby et al., 2004; Racz et al., 2004). It cannot be excluded that extrasynaptic receptors are non-functional under basal physiological conditions. However, they could be activated by exogenous receptor modulators, or the endogenous ligand in toxic or pathological conditions, which makes them highly relevant as potential targets for novel pharmacological therapies.
Although significant progress has been made in the characterization of glutamate and GABA receptor localization in the basal ganglia, a substantial amount of work remains to be done. Additional, quantitative measurements of receptor density at identified synapses from specific sources are needed; as are detailed comparative analyses of receptor localization in normal and pathological conditions. These future studies will provide critical information on the level of plasticity glutamate and GABA receptors are endowed with, and pave the way for the refinement of available therapies, and development of novel pharmacotherapeutic approaches for basal ganglia diseases.
Acknowledgements
The authors thank Jean-Francois Paré and Susan Maxson for technical assistance. This work was supported by grants to Y. Smith from the National Institutes of Health (R01 NS037423, R01 NS037948, R01 NS42937) and the US Army Medical Research and Material Command, as well as the Yerkes National Primate Research Center base grant (RR00165).
REFERENCES
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Amara SG, Fontana AC. Excitatory amino acid transporters: keeping up with glutamate. Neurochem Int. 2002;41:313–318. doi: 10.1016/s0197-0186(02)00018-9. [DOI] [PubMed] [Google Scholar]
- Ango F, Pin JP, Tu JC, Xiao B, Worley PF, Bockaert J, Fagni L. Dendritic and axonal targeting of type 5 metabotropic glutamate receptor is regulated by homer1 proteins and neuronal excitation. J Neurosci. 2000;20:8710–8716. doi: 10.1523/JNEUROSCI.20-23-08710.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ango F, Prezeau L, Muller T, Tu JC, Xiao B, Worley PF, Pin JP, Bockaert J, Fagni L. Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein Homer. Nature. 2001;411:962–965. doi: 10.1038/35082096. [DOI] [PubMed] [Google Scholar]
- Ango F, Robbe D, Tu JC, Xiao B, Worley PF, Pin J-P, Bockaert J, Fagni L. Homer-Dependent Cell Surface Expression of Metabotropic Glutamate Receptor Type 5 in Neurons. Mol Cell Neurosci. 2002;20:323–329. doi: 10.1006/mcne.2002.1100. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci. 1998;10:2129–2142. doi: 10.1046/j.1460-9568.1998.00221.x. [DOI] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends Neurosci. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Arias Montano JA, Martinez-Fong D, Aceves J. GABAB receptor activation partially inhibits Nmethyl-D-aspartate-mediated tyrosine hydroxylase stimulation in rat striatal slices. Eur J Pharmacol. 1992;218:335–338. doi: 10.1016/0014-2999(92)90187-9. [DOI] [PubMed] [Google Scholar]
- Ashby MC, De La Rue SA, Ralph GS, Uney J, Collingridge GL, Henley JM. Removal of AMPA Receptors (AMPARs) from Synapses Is Preceded by Transient Endocytosis of Extrasynaptic AMPARs. J Neurosci. 2004;24:5172–5176. doi: 10.1523/JNEUROSCI.1042-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwell D, Barbour B, Szatkowski M. Nonvesicular release of neurotransmitter. Neuron. 1993;11:401–407. doi: 10.1016/0896-6273(93)90145-h. [DOI] [PubMed] [Google Scholar]
- Augood SJ, Waldvogel HJ, Munkle MC, Faull RL, Emson PC. Localization of calcium-binding proteins and GABA transporter (GAT-1) messenger RNA in the human subthalamic nucleus. Neuroscience. 1999;88:521–534. doi: 10.1016/s0306-4522(98)00226-7. [DOI] [PubMed] [Google Scholar]
- Awad-Granko H, Conn PJ. Activation of groups I or III metabotropic glutamate receptors inhibits excitatory transmission in the rat subthalamic nucleus. Neuropharmacology. 2001;41:32–41. doi: 10.1016/s0028-3908(01)00047-8. [DOI] [PubMed] [Google Scholar]
- Awad H, Hubert GW, Smith Y, Levey AI, Conn PJ. Activation of metabotropic glutamate receptor 5 has direct excitatory effects and potentiates NMDA receptor currents in neurons of the subthalamic nucleus. J Neurosci. 2000;20:7871–7879. doi: 10.1523/JNEUROSCI.20-21-07871.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahena-Trujillo R, Arias-Montano JA. [3H] gamma-aminobutyric acid transport in rat substantia nigra pars reticulata synaptosomes: pharmacological characterization and phorbol ester-induced inhibition. Neurosci Lett. 1999;274:119–122. doi: 10.1016/s0304-3940(99)00692-8. [DOI] [PubMed] [Google Scholar]
- Baker DA, Xi Z-X, Shen H, Swanson CJ, Kalivas PW. The Origin and Neuronal Function of In Vivo Nonsynaptic Glutamate. J Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasubramanian S, Teissere JA, Raju DV, Hall RA. Hetero-oligomerization between GABA-A and GABA-B receptors regulates GABA-B receptor trafficking. J Biol Chem. 2004 doi: 10.1074/jbc.M313470200. [DOI] [PubMed] [Google Scholar]
- Barbour B. An Evaluation of Synapse Independence. J Neurosci. 2001;21:7969–7984. doi: 10.1523/JNEUROSCI.21-20-07969.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard EA, Skolnick P, Olsen RW, Mohler H, Sieghart W, Biggio G, Braestrup C, Bateson AN, Langer SZ. International Union of Pharmacology. XV. Subtypes of gamma-aminobutyric acidA receptors: classification on the basis of subunit structure and receptor function. Pharmacol Rev. 1998;50:291–313. [PubMed] [Google Scholar]
- Barnes EM., Jr. Intracellular trafficking of GABA(A) receptors. Life Sci. 2000;66:1063–1070. doi: 10.1016/s0024-3205(99)00469-5. [DOI] [PubMed] [Google Scholar]
- Batchelor AM, Madge DJ, Garthwaite J. Synaptic activation of metabotropic glutamate receptors in the parallel fibre-Purkinje cell pathway in rat cerebellar slices. Neuroscience. 1994;63:911–915. doi: 10.1016/0306-4522(94)90558-4. [DOI] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Roberts JD, Mulvihill E, McIlhinney RA, Somogyi P. The metabotropic glutamate receptor (mGluR1 alpha) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. doi: 10.1016/0896-6273(93)90086-7. [DOI] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Molnar E, McIlhinney RA, Somogyi P. High-resolution immunogold localization of AMPA type glutamate receptor subunits at synaptic and non-synaptic sites in rat hippocampus. Neuroscience. 1995;69:1031–1055. doi: 10.1016/0306-4522(95)00350-r. [DOI] [PubMed] [Google Scholar]
- Bennett BD, Bolam JP. Synaptic input and output of parvalbumin-immunoreactive neurons in the neostriatum of the rat. Neuroscience. 1994;62:707–719. doi: 10.1016/0306-4522(94)90471-5. [DOI] [PubMed] [Google Scholar]
- Bernard V, Somogyi P, Bolam JP. Cellular, subcellular, and subsynaptic distribution of AMPA-type glutamate receptor subunits in the neostriatum of the rat. J Neurosci. 1997;17:819–833. doi: 10.1523/JNEUROSCI.17-02-00819.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard V, Bolam JP. Subcellular and subsynaptic distribution of the NR1 subunit of the NMDA receptor in the neostriatum and globus pallidus of the rat: co- localization at synapses with the GluR2/3 subunit of the AMPA receptor. Eur J Neurosci. 1998;10:3721–3736. doi: 10.1046/j.1460-9568.1998.00380.x. [DOI] [PubMed] [Google Scholar]
- Bettler B, Kaupmann K, Mosbacher J, Gassmann M. Molecular structure and physiological functions of GABA(B) receptors. Physiol Rev. 2004;84:835–867. doi: 10.1152/physrev.00036.2003. [DOI] [PubMed] [Google Scholar]
- Bevan MD, Booth PA, Eaton SA, Bolam JP. Selective innervation of neostriatal interneurons by a subclass of neuron in the globus pallidus of the rat. J Neurosci. 1998;18:9438–9452. doi: 10.1523/JNEUROSCI.18-22-09438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MD, Magill PJ, Hallworth NE, Bolam JP, Wilson CJ. Regulation of the Timing and Pattern of Action Potential Generation in Rat Subthalamic Neurons In Vitro by GABA-A IPSPs. J Neurophysiol. 2002;87:1348–1205. doi: 10.1152/jn.00582.2001. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Smith Y. The GABA and substance P input to dopaminergic neurones in the substantia nigra of the rat. Brain Res. 1990;529:57–78. doi: 10.1016/0006-8993(90)90811-o. [DOI] [PubMed] [Google Scholar]
- Bolam JP, Hanley JJ, Booth PA, Bevan MD. Synaptic organisation of the basal ganglia. J Anat. 2000;196:527–542. doi: 10.1046/j.1469-7580.2000.19640527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery NG, Bettler B, Froestl W, Gallagher JP, Marshall F, Raiteri M, Bonner TI, Enna SJ. International Union of Pharmacology. XXXIII. Mammalian gamma -Aminobutyric AcidB Receptors: Structure and Function. Pharmacol Rev. 2002;54:247–264. doi: 10.1124/pr.54.2.247. [DOI] [PubMed] [Google Scholar]
- Boyes J, Bolam JP. The subcellular localization of GABA(B) receptor subunits in the rat substantia nigra. Eur J Neurosci. 2003;18:3279–3293. doi: 10.1111/j.1460-9568.2003.03076.x. [DOI] [PubMed] [Google Scholar]
- Bradley SR, Standaert DG, Levey AI, Conn PJ. Distribution of group III mGluRs in rat basal ganglia with subtype-specific antibodies. Ann N Y Acad Sci. 1999a;868:531–534. doi: 10.1111/j.1749-6632.1999.tb11322.x. [DOI] [PubMed] [Google Scholar]
- Bradley SR, Standaert DG, Rhodes KJ, Rees HD, Testa CM, Levey AI, Conn PJ. Immunohistochemical localization of subtype 4a metabotropic glutamate receptors in the rat and mouse basal ganglia. J Comp Neurol. 1999b;407:33–46. [PubMed] [Google Scholar]
- Bradley SR, Marino MJ, Wittmann M, Rouse ST, Awad H, Levey AI, Conn PJ. Activation of group II metabotropic glutamate receptors inhibits synaptic excitation of the substantia Nigra pars reticulata. J Neurosci. 2000;20:3085–3094. doi: 10.1523/JNEUROSCI.20-09-03085.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasnjo G, Otis TS. Neuronal glutamate transporters control activation of postsynaptic metabotropic glutamate receptors and influence cerebellar long-term depression. Neuron. 2001;31:607–616. doi: 10.1016/s0896-6273(01)00377-4. [DOI] [PubMed] [Google Scholar]
- Bruton RK, Ge J, Barnes NM. Group I mGlu receptor modulation of dopamine release in the rat striatum in vivo. Eur J Pharmacol. 1999;369:175–181. doi: 10.1016/s0014-2999(99)00072-2. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Mercuri NB, De Murtas M, Bernardi G. Endogenous GABA mediates presynaptic inhibition of spontaneous and evoked excitatory synaptic potentials in the rat neostriatum. Neurosci Lett. 1990;118:99–102. doi: 10.1016/0304-3940(90)90258-b. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Mercuri NB, De Murtas M, Bernardi G. Involvement of GABA systems in feedback regulation of glutamate-and GABA-mediated synaptic potentials in rat neostriatum. J Physiol. 1991;440:581–599. doi: 10.1113/jphysiol.1991.sp018726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabresi P, Mercuri NB, Bernardi G. Activation of quisqualate metabotropic receptors reduces glutamate and GABA-mediated synaptic potentials in the rat striatum. Neurosci Lett. 1992;139:41–44. doi: 10.1016/0304-3940(92)90852-x. [DOI] [PubMed] [Google Scholar]
- Calabresi P, Pisani A, Mercuri NB, Bernardi G. The corticostriatal projection: from synaptic plasticity to dysfunctions of the basal ganglia. Trends Neurosci. 1996;19:19–24. doi: 10.1016/0166-2236(96)81862-5. [DOI] [PubMed] [Google Scholar]
- Carter AG, Sabatini BL. State-dependent calcium signaling in dendritic spines of striatal medium spiny neurons. Neuron. 2004;44:483–493. doi: 10.1016/j.neuron.2004.10.013. [DOI] [PubMed] [Google Scholar]
- Casassus G, Mulle C. Functional characterization of kainate receptors in the mouse nucleus accumbens. Neuropharmacology. 2002;42:603–611. doi: 10.1016/s0028-3908(02)00011-4. [DOI] [PubMed] [Google Scholar]
- Catania MV, Bellomo M, Di Giorgi-Gerevini V, Seminara G, Giuffrida R, Romeo R, De Blasi A, Nicoletti F. Endogenous activation of group-I metabotropic glutamate receptors is required for differentiation and survival of cerebellar Purkinje cells. J Neurosci. 2001;21:7664–7673. doi: 10.1523/JNEUROSCI.21-19-07664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan PK, Leung CK, Yung WH. Differential expression of pre- and postsynaptic GABA(B) receptors in rat substantia nigra pars reticulata neurones. Eur J Pharmacol. 1998;349:187–197. doi: 10.1016/s0014-2999(98)00194-0. [DOI] [PubMed] [Google Scholar]
- Chan WY, Soloviev MM, Ciruela F, McIlhinney RA. Molecular determinants of metabotropic glutamate receptor 1B trafficking. Mol Cell Neurosci. 2001;17:577–588. doi: 10.1006/mcne.2001.0965. [DOI] [PubMed] [Google Scholar]
- Charara A, Blankstein E, Smith Y. Presynaptic kainate receptors in the monkey striatum. Neuroscience. 1999;91:1195–1200. doi: 10.1016/s0306-4522(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Charara A, Heilman TC, Levey AI, Smith Y. Pre- and postsynaptic localization of GABA(B) receptors in the basal ganglia in monkeys. Neuroscience. 2000;95:127–140. doi: 10.1016/s0306-4522(99)00409-1. [DOI] [PubMed] [Google Scholar]
- Charara A, Paquet M, Rothstein JD, Smith Y. Subcellular and Subsynaptic Localization of Glutamate Transporters in the Monkey Basal Ganglia. In: ea Graybiel., editor. The Basal Ganglia VI. Kluwer Academic/Plenum Publishers; New York: 2002. [Google Scholar]
- Charara A, Galvan A, Kuwajima M, Hall RA, Smith Y. An electron microscope immunocytochemical study of GABAB R2 receptors in the monkey basal ganglia: A comparative analysis with GABAB R1 receptor distribution. J Comp Neurol. 2004;476:65–79. doi: 10.1002/cne.20210. [DOI] [PubMed] [Google Scholar]
- Charara A, Pare JF, Levey AI, Smith Y. Synaptic and extrasynaptic GABA-A and GABA-B receptors in the globus pallidus: an electron microscopic immunogold analysis in monkeys. Neuroscience. 2005;131:917–933. doi: 10.1016/j.neuroscience.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Chatha BT, Bernard V, Streit P, Bolam JP. Synaptic localization of ionotropic glutamate receptors in the rat substantia nigra. Neuroscience. 2000;101:1037–1051. doi: 10.1016/s0306-4522(00)00432-2. [DOI] [PubMed] [Google Scholar]
- Chen L, Chan SCY, Yung WH. Rotational behavior and electrophysiological effects induced by GABAB receptor activation in rat globus pallidus. Neuroscience. 2002a;114:417–425. doi: 10.1016/s0306-4522(02)00299-3. [DOI] [PubMed] [Google Scholar]
- Chen L, Yung WH. Effects of the GABA-uptake inhibitor tiagabine in rat globus pallidus. Exp Brain Res. 2003;152:263–269. doi: 10.1007/s00221-003-1549-7. [DOI] [PubMed] [Google Scholar]
- Chen L, Boyes J, Yung WH, Bolam JP. Subcellular localization of GABAB receptor subunits in rat globus pallidus. J Comp Neurol. 2004;474:340–352. doi: 10.1002/cne.20143. [DOI] [PubMed] [Google Scholar]
- Chen Q, Veenman L, Knopp K, Yan Z, Medina L, Song WJ, Surmeier DJ, Reiner A. Evidence for the preferential localization of glutamate receptor-1 subunits of AMPA receptors to the dendritic spines of medium spiny neurons in rat striatum. Neuroscience. 1998;83:749–761. doi: 10.1016/s0306-4522(97)00452-1. [DOI] [PubMed] [Google Scholar]
- Chen W, Aoki C, Mahadomrongkul V, Gruber CE, Wang GJ, Blitzblau R, Irwin N, Rosenberg PA. Expression of a variant form of the glutamate transporter GLT1 in neuronal cultures and in neurons and astrocytes in the rat brain. J Neurosci. 2002b;22:2142–2152. doi: 10.1523/JNEUROSCI.22-06-02142.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheramy A, Godeheu G, L'Hirondel M, Glowinski J. Cooperative contributions of cholinergic and NMDA receptors in the presynaptic control of dopamine release from synaptosomes of the rat striatum. J Pharmacol Exp Ther. 1996;276:616–625. [PubMed] [Google Scholar]
- Chergui K, Bouron A, Normand E, Mulle C. Functional GluR6 kainate receptors in the striatum: indirect downregulation of synaptic transmission. J Neurosci. 2000;20:2175–2182. doi: 10.1523/JNEUROSCI.20-06-02175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie SB, de Blas AL. alpha5 Subunit-containing GABA(A) receptors form clusters at GABAergic synapses in hippocampal cultures. Neuroreport. 2002;13:2355–2358. doi: 10.1097/00001756-200212030-00037. [DOI] [PubMed] [Google Scholar]
- Christoffersen CL, Meltzer LT. Evidence for N-methyl-D-aspartate and AMPA subtypes of the glutamate receptor on substantia nigra dopamine neurons: possible preferential role for N-methyl-D-aspartate receptors. Neuroscience. 1995;67:373–381. doi: 10.1016/0306-4522(95)00047-m. [DOI] [PubMed] [Google Scholar]
- Ciruela F, Robbins MJ, Willis AC, McIlhinney RA. Interactions of the C terminus of metabotropic glutamate receptor type 1alpha with rat brain proteins: evidence for a direct interaction with tubulin. J Neurochem. 1999;72:346–354. [PubMed] [Google Scholar]
- Clarke NP, Bolam JP. Distribution of glutamate receptor subunits at neurochemically characterized synapses in the entopeduncular nucleus and subthalamic nucleus of the rat. J Comp Neurol. 1998;397:403–420. [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Battaglia G, Marino MJ, Nicoletti F. Metabotropic glutamate receptors in the basal ganglia motor circuit. Nat Rev Neurosci. 2005;6:787–798. doi: 10.1038/nrn1763. [DOI] [PubMed] [Google Scholar]
- Corti C, Aldegheri L, Somogyi P, Ferraguti F. Distribution and synaptic localisation of the metabotropic glutamate receptor 4 (mGluR4) in the rodent CNS. Neuroscience. 2002;110:403–420. doi: 10.1016/s0306-4522(01)00591-7. [DOI] [PubMed] [Google Scholar]
- Couve A, Moss SJ, Pangalos MN. GABAB receptors: a new paradigm in G protein signaling. Mol Cell Neurosci. 2000;16:296–312. doi: 10.1006/mcne.2000.0908. [DOI] [PubMed] [Google Scholar]
- Crowder TL, Weiner JL. Functional characterization of kainate receptors in the rat nucleus accumbens core region. J Neurophysiol. 2002;88:41–48. doi: 10.1152/jn.2002.88.1.41. [DOI] [PubMed] [Google Scholar]
- Dalby NO. GABA-level increasing and anticonvulsant effects of three different GABA uptake inhibitors. Neuropharmacology. 2000;39:2399–2407. doi: 10.1016/s0028-3908(00)00075-7. [DOI] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Das SS, Banker GA. The role of protein interaction motifs in regulating the polarity and clustering of the metabotropic glutamate receptor mGluR1a. J Neurosci. 2006;26:8115–8125. doi: 10.1523/JNEUROSCI.1015-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bartolomeis A, Iasevoli F. The Homer family and the signal transduction system at glutamatergic postsynaptic density: potential role in behavior and pharmacotherapy. Psychopharmacol Bull. 2003;37:51–83. [PubMed] [Google Scholar]
- Del Arco A, Castaneda TR, Mora F. Amphetamine releases GABA in striatum of the freely moving rat: involvement of calcium and high affinity transporter mechanisms. Neuropharmacology. 1998;37:199–205. doi: 10.1016/s0028-3908(98)00013-6. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- Dervan AG, Meshul CK, Beales M, McBean GJ, Moore C, Totterdell S, Snyder AK, Meredith GE. Astroglial plasticity and glutamate function in a chronic mouse model of Parkinson's disease. Exp Neurol. 2004;190:145–156. doi: 10.1016/j.expneurol.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Doherty J, Dingledine R. Functional interactions between cannabinoid and metabotropic glutamate receptors in the central nervous system. Curr Opin Pharmacol. 2003;3:46–53. doi: 10.1016/s1471-4892(02)00014-0. [DOI] [PubMed] [Google Scholar]
- Durkin MM, Smith KE, Borden LA, Weinshank RL, Branchek TA, Gustafson EL. Localization of messenger RNAs encoding three GABA transporters in rat brain: an in situ hybridization study. Brain Res Mol Brain Res. 1995;33:7–21. doi: 10.1016/0169-328x(95)00101-w. [DOI] [PubMed] [Google Scholar]
- East SJ, Hill MP, Brotchie JM. Metabotropic glutamate receptor agonists inhibit endogenous glutamate release from rat striatal synaptosomes. Eur J Pharmacol. 1995;277:117–121. doi: 10.1016/0014-2999(95)00119-6. [DOI] [PubMed] [Google Scholar]
- Engberg G, Kling-Petersen T, Nissbrandt H. GABAB-receptor activation alters the firing pattern of dopamine neurons in the rat substantia nigra. Synapse. 1993;15:229–238. doi: 10.1002/syn.890150308. [DOI] [PubMed] [Google Scholar]
- Engelman HS, MacDermott AB. Presynaptic ionotropic receptors and control of transmitter release. Nat Rev Neurosci. 2004;5:135–145. doi: 10.1038/nrn1297. [DOI] [PubMed] [Google Scholar]
- Erhardt S, Nissbrandt H, Engberg G. Activation of nigral dopamine neurons by the selective GABA(B)-receptor antagonist SCH 50911. J Neural Transm. 1999;106:383–394. doi: 10.1007/s007020050166. [DOI] [PubMed] [Google Scholar]
- Ferraguti F, Conquet F, Corti C, Grandes P, Kuhn R, Knopfel T. Immunohistochemical localization of the mGluR1beta metabotropic glutamate receptor in the adult rodent forebrain: evidence for a differential distribution of mGluR1 splice variants. J Comp Neurol. 1998;400:391–407. [PubMed] [Google Scholar]
- Floran B, Silva I, Nava C, Aceves J. Presynaptic modulation of the release of GABA by GABAA receptors in pars compacta and by GABAB receptors in pars reticulata of the rat substantia nigra. Eur J Pharmacol. 1988;150:277–286. doi: 10.1016/0014-2999(88)90008-8. [DOI] [PubMed] [Google Scholar]
- Francesconi A, Duvoisin RM. Alternative splicing unmasks dendritic and axonal targeting signals in metabotropic glutamate receptor 1. J Neurosci. 2002;22:2196–2205. doi: 10.1523/JNEUROSCI.22-06-02196.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frerking M, Nicoll RA. Synaptic kainate receptors. Curr Opin Neurobiol. 2000;10:342–351. doi: 10.1016/s0959-4388(00)00094-5. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Mohler H. GABAA-receptor heterogeneity in the adult rat brain: differential regional and cellular distribution of seven major subunits. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- Fujiyama F, Fritschy JM, Stephenson FA, Bolam JP. Synaptic localization of GABA(A) receptor subunits in the striatum of the rat. J Comp Neurol. 2000;416:158–172. [PubMed] [Google Scholar]
- Fujiyama F, Stephenson FA, Bolam JP. Synaptic localization of GABA(A) receptor subunits in the substantia nigra of the rat: effects of quinolinic acid lesions of the striatum. Eur J Neurosci. 2002;15:1961–1975. doi: 10.1046/j.1460-9568.2002.02017.x. [DOI] [PubMed] [Google Scholar]
- Fujiyama F, Kuramoto E, Okamoto K, Hioki H, Furuta T, Zhou L, Nomura S, Kaneko T. Presynaptic localization of an AMPA-type glutamate receptor in corticostriatal and thalamostriatal axon terminals. Eur J Neurosci. 2004;20:3322–3330. doi: 10.1111/j.1460-9568.2004.03807.x. [DOI] [PubMed] [Google Scholar]
- Galvan A, Charara A, Pare JF, Levey AI, Smith Y. Differential subcellular and subsynaptic distribution of GABA(A) and GABA(B) receptors in the monkey subthalamic nucleus. Neuroscience. 2004;127:709–721. doi: 10.1016/j.neuroscience.2004.05.014. [DOI] [PubMed] [Google Scholar]
- Galvan A, Villalba RM, West SM, Maidment NT, Ackerson LC, Smith Y, Wichmann T. GABAergic Modulation of the Activity of Globus Pallidus Neurons in Primates: In Vivo Analysis of the Functions of GABA Receptors and GABA Transporters. J Neurophysiol. 2005;94:990–1000. doi: 10.1152/jn.00068.2005. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Wilson CJ. The Basal Ganglia. In: Björklund A, Hökfeld T, Swanson L, editors. Handbook of Chemical Neuroanatomy, Integrated Systems of the CNS. Part III. Elsevier; Amsterdam: 1996. pp. 369–466. [Google Scholar]
- Giralt MT, Bonanno G, Raiteri M. GABA terminal autoreceptors in the pars compacta and in the pars reticulata of the rat substantia nigra are GABAB. Eur J Pharmacol. 1990;175:137–144. doi: 10.1016/0014-2999(90)90224-t. [DOI] [PubMed] [Google Scholar]
- Giustizieri M, Bernardi G, Mercuri NB, Berretta N. Distinct Mechanisms of Presynaptic Inhibition at GABAergic Synapses of the Rat Substantia Nigra Pars Compacta. J Neurophysiol. 2005;94:1992–2003. doi: 10.1152/jn.00171.2005. [DOI] [PubMed] [Google Scholar]
- Gonchar Y, Pang L, Malitschek B, Bettler B, Burkhalter A. Subcellular localization of GABA(B) receptor subunits in rat visual cortex. J Comp Neurol. 2001;431:182–197. doi: 10.1002/1096-9861(20010305)431:2<182::aid-cne1064>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Hernandez T, Barroso-Chinea P, Rodriguez M. Response of the GABAergic and dopaminergic mesostriatal projections to the lesion of the contralateral dopaminergic mesostriatal pathway in the rat. Mov Disord. 2004;19:1029–1042. doi: 10.1002/mds.20206. [DOI] [PubMed] [Google Scholar]
- Gonzalez B, Paz F, Floran L, Aceves J, Erlij D, Floran B. Adenosine A2A receptor stimulation decreases GAT-1-mediated GABA uptake in the globus pallidus of the rat. Neuropharmacology. 2006;51:154–159. doi: 10.1016/j.neuropharm.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Gotz T, Kraushaar U, Geiger J, Lubke J, Berger T, Jonas P. Functional properties of AMPA and NMDA receptors expressed in identified types of basal ganglia neurons. J Neurosci. 1997;17:204–215. doi: 10.1523/JNEUROSCI.17-01-00204.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara A, Fukazawa Y, Deguchi-Tawarada M, Ohtsuka T, Shigemoto R. Differential distribution of release-related proteins in the hippocampal CA3 area as revealed by freeze-fracture replicalabeling. J Comp Neurol. 2005;489:195–216. doi: 10.1002/cne.20633. [DOI] [PubMed] [Google Scholar]
- Hallworth NE, Bevan MD. Globus Pallidus Neurons Dynamically Regulate the Activity Pattern of Subthalamic Nucleus Neurons through the Frequency-Dependent Activation of Postsynaptic GABAA and GABAB Receptors. J Neurosci. 2005;25:6304–6315. doi: 10.1523/JNEUROSCI.0450-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann M, Rossi DJ, Attwell D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron. 2002;33:625–633. doi: 10.1016/s0896-6273(02)00593-7. [DOI] [PubMed] [Google Scholar]
- Hanson JE, Smith Y. Group I metabotropic glutamate receptors at GABAergic synapses in monkeys. J Neurosci. 1999;19:6488–6496. doi: 10.1523/JNEUROSCI.19-15-06488.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham GE, Fukunaga Y, Bading H. Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat Neurosci. 2002;5:405–414. doi: 10.1038/nn835. [DOI] [PubMed] [Google Scholar]
- Hashimoto T, Kuriyama K. GABAA receptor-mediated K(+)-evoked GABA release from globus pallidus-- analysis using microdialysis. Neurochem Int. 1997;30:247–252. doi: 10.1016/s0197-0186(96)00100-3. [DOI] [PubMed] [Google Scholar]
- Hausser MA, Yung WH. Inhibitory synaptic potentials in guinea-pig substantia nigra dopamine neurones in vitro. J Physiol (Lond) 1994;479:401–422. doi: 10.1113/jphysiol.1994.sp020305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirono M, Yoshioka T, Konishi S. GABA(B) receptor activation enhances mGluR-mediated responses at cerebellar excitatory synapses. Nat Neurosci. 2001;4:1207–1216. doi: 10.1038/nn764. [DOI] [PubMed] [Google Scholar]
- Hoffpauir BK, Gleason EL. Activation of mGluR5 modulates GABA(A) receptor function in retinal amacrine cells. J Neurophys. 2002;88:1766–1776. doi: 10.1152/jn.2002.88.4.1766. [DOI] [PubMed] [Google Scholar]
- Huang YH, Bergles DE. Glutamate transporters bring competition to the synapse. Curr Opin Neurobiol. 2004;14:346–352. doi: 10.1016/j.conb.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Hubert GW, Paquet M, Smith Y. Differential Subcellular Localization of mGluR1a and mGluR5 in the Rat and Monkey Substantia Nigra. J Neurosci. 2001;21:1838–1847. doi: 10.1523/JNEUROSCI.21-06-01838.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert GW, Smith Y. Age-related changes in the expression of axonal and glial group I metabotropic glutamate receptor in the rat substantia nigra pars reticulata. J Comp Neurol. 2004;475:95–106. doi: 10.1002/cne.20163. [DOI] [PubMed] [Google Scholar]
- Huettner JE. Kainate receptors and synaptic transmission. Prog Neurobiol. 2003;70:387–407. doi: 10.1016/s0301-0082(03)00122-9. [DOI] [PubMed] [Google Scholar]
- Ige AO, Bolam JP, Billinton A, White JH, Marshall FH, Emson PC. Cellular and sub-cellular localisation of GABA(B1) and GABA(B2) receptor proteins in the rat cerebellum. Brain Res Mol Brain Res. 2000;83:72–80. doi: 10.1016/s0169-328x(00)00199-6. [DOI] [PubMed] [Google Scholar]
- Ingham C. Immunocytochemistry II: post-embeding staining. In: Bolam JP, editor. Experimental Neuroanatomy A Practical Approach. Oxford University Press; New York: 1992. pp. 129–151. [Google Scholar]
- Inglis WL, Winn P. The pedunculopontine tegmental nucleus: where the striatum meets the reticular formation. Prog Neurobiol. 1995;47:1–29. doi: 10.1016/0301-0082(95)00013-l. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Solis JM, Nicoll RA. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron. 1993;10:165–175. doi: 10.1016/0896-6273(93)90308-e. [DOI] [PubMed] [Google Scholar]
- Izzo PN, Bolam JP. Cholinergic synaptic input to different parts of spiny striatonigral neurons in the rat. J Comp Neurol. 1988;269:219–234. doi: 10.1002/cne.902690207. [DOI] [PubMed] [Google Scholar]
- Jaeger D, Kita H, Wilson CJ. Surround inhibition among projection neurons is weak or nonexistent in the rat neostriatum. J Neurophysiol. 1994;72:2555–2558. doi: 10.1152/jn.1994.72.5.2555. [DOI] [PubMed] [Google Scholar]
- Jin XT, Smith Y. Bolam JP, Magill PJ, Ingham C, editors. Localization and functions of kainate receptors in the rat globus pallidus. The Basal Ganglia VIII. 2005 [Google Scholar]
- Jin XT, Pare JF, Raju DV, Smith Y. Localization and function of pre- and postsynaptic kainate receptors in the rat globus pallidus. Eur J Neurosci. 2006;23:374–386. doi: 10.1111/j.1460-9568.2005.04574.x. [DOI] [PubMed] [Google Scholar]
- Joel D, Weiner I. The organization of the basal ganglia-thalamocortical circuits: open interconnected rather than closed segregated. Neuroscience. 1994;63:363–379. doi: 10.1016/0306-4522(94)90536-3. [DOI] [PubMed] [Google Scholar]
- Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, Yao WJ, Johnson M, Gunwaldsen C, Huang LY, Tang C, Shen Q, Salon JA, Morse K, Laz T, Smith KE, Nagarathnam D, Noble SA, Branchek TA, Gerald C. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- Jong YJ, Kumar V, Kingston AE, Romano C, O'Malley KL. Functional metabotropic glutamate receptors on nuclei from brain and primary cultured striatal neurons. Role of transporters in delivering ligand. J Biol Chem. 2005;280:30469–30480. doi: 10.1074/jbc.M501775200. [DOI] [PubMed] [Google Scholar]
- Kane-Jackson R, Smith Y. Pre-synaptic kainate receptors in GABAergic and glutamatergic axon terminals in the monkey globus pallidus. Neuroscience. 2003;122:285–289. doi: 10.1016/s0306-4522(03)00596-7. [DOI] [PubMed] [Google Scholar]
- Kaneda K, Kita H. Synaptically Released GABA Activates Both Pre- and Postsynaptic GABAB Receptors in the Rat Globus Pallidus. J Neurophysiol. 2005;94:1104–1114. doi: 10.1152/jn.00255.2005. [DOI] [PubMed] [Google Scholar]
- Kerr JND, Plenz D. Action Potential Timing Determines Dendritic Calcium during Striatal Up-States. J Neurosci. 2004;24:877–885. doi: 10.1523/JNEUROSCI.4475-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kew JN, Kemp JA. Ionotropic and metabotropic glutamate receptor structure and pharmacology. Psychopharmacology (Berl) 2005;179:4–29. doi: 10.1007/s00213-005-2200-z. [DOI] [PubMed] [Google Scholar]
- Kieval JZ, Hubert GW, Charara A, Pare JF, Smith Y. Subcellular and subsynaptic localization of presynaptic and postsynaptic kainate receptor subunits in the monkey striatum. J Neurosci. 2001;21:8746–8757. doi: 10.1523/JNEUROSCI.21-22-08746.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita A, Shigemoto R, Ohishi H, van der Putten H, Mizuno N. Immunohistochemical localization of metabotropic glutamate receptors, mGluR7a and mGluR7b, in the central nervous system of the adult rat and mouse: a light and electron microscopic study. J Comp Neurol. 1998;393:332–352. [PubMed] [Google Scholar]
- Kita H, Chang HT, Kitai ST. Pallidal inputs to subthalamus: intracellular analysis. Brain Res. 1983;264:255–265. doi: 10.1016/0006-8993(83)90823-5. [DOI] [PubMed] [Google Scholar]
- Kita H, Kitai ST. Intracellular study of rat globus pallidus neurons: membrane properties and responses to neostriatal, subthalamic and nigral stimulation. Brain Res. 1991;564:296–305. doi: 10.1016/0006-8993(91)91466-e. [DOI] [PubMed] [Google Scholar]
- Kita H. Glutamatergic and GABAergic postsynaptic responses of striatal spiny neurons to intrastriatal and cortical stimulation recorded in slice preparations. Neuroscience. 1996;70:925–940. doi: 10.1016/0306-4522(95)00410-6. [DOI] [PubMed] [Google Scholar]
- Kita H. Neostriatal and globus pallidus stimulation induced inhibitory postsynaptic potentials in entopeduncular neurons in rat brain slice preparations. Neuroscience. 2001;105:871–879. doi: 10.1016/s0306-4522(01)00231-7. [DOI] [PubMed] [Google Scholar]
- Kita H, Nambu A, Kaneda K, Tachibana Y, Takada M. Role of ionotropic glutamatergic and GABAergic inputs on the firing activity of neurons in the external pallidum in awake monkeys. J Neurophysiol. 2004;92:3069–3084. doi: 10.1152/jn.00346.2004. [DOI] [PubMed] [Google Scholar]
- Kita H, Tachibana Y, Nambu A, Chiken S. Balance of monosynaptic excitatory and disynaptic inhibitory responses of the globus pallidus induced after stimulation of the subthalamic nucleus in the monkey. J Neurosci. 2005;25:8611–8619. doi: 10.1523/JNEUROSCI.1719-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kita H, Chiken S, Tachibana Y, Nambu A. Origins of GABA(A) and GABA(B) receptor-mediated responses of globus pallidus induced after stimulation of the putamen in the monkey. J Neurosci. 2006;26:6554–6562. doi: 10.1523/JNEUROSCI.1543-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano J, Kimura K, Yamazaki Y, Soda T, Shigemoto R, Nakajima Y, Nakanishi S. Tamalin, a PDZ domain-containing protein, links a protein complex formation of group 1 metabotropic glutamate receptors and the guanine nucleotide exchange factor cytohesins. J Neurosci. 2002;22:1280–1289. doi: 10.1523/JNEUROSCI.22-04-01280.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koos T, Tepper JM. Inhibitory control of neostriatal projection neurons by GABAergic interneurons. Nat Neurosci. 1999;2:467–472. doi: 10.1038/8138. [DOI] [PubMed] [Google Scholar]
- Koos T, Tepper JM, Wilson CJ. Comparison of IPSCs evoked by spiny and fast-spiking neurons in the neostriatum. J Neurosci. 2004;24:7916–7922. doi: 10.1523/JNEUROSCI.2163-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korpi ER, Grunder G, Luddens H. Drug interactions at GABA(A) receptors. Prog Neurobiol. 2002;67:113–159. doi: 10.1016/s0301-0082(02)00013-8. [DOI] [PubMed] [Google Scholar]
- Kosinski CM, Risso Bradley S, Conn PJ, Levey AI, Landwehrmeyer GB, Penney JB, Jr., Young AB, Standaert DG. Localization of metabotropic glutamate receptor 7 mRNA and mGluR7a protein in the rat basal ganglia. J Comp Neurol. 1999;415:266–284. [PubMed] [Google Scholar]
- Krebs MO, Desce JM, Kemel ML, Gauchy C, Godeheu G, Cheramy A, Glowinski J. Glutamatergic control of dopamine release in the rat striatum: evidence for presynaptic N-methyl-D-aspartate receptors on dopaminergic nerve terminals. J Neurochem. 1991;56:81–85. doi: 10.1111/j.1471-4159.1991.tb02565.x. [DOI] [PubMed] [Google Scholar]
- Kulik A, Nakadate K, Nyiri G, Notomi T, Malitschek B, Bettler B, Shigemoto R. Distinct localization of GABA(B) receptors relative to synaptic sites in the rat cerebellum and ventrobasal thalamus. Eur J Neurosci. 2002;15:291–307. doi: 10.1046/j.0953-816x.2001.01855.x. [DOI] [PubMed] [Google Scholar]
- Kulik A, Vida I, Lujan R, Haas CA, Lopez-Bendito G, Shigemoto R, Frotscher M. Subcellular localization of metabotropic GABA(B) receptor subunits GABA(B1a/b) and GABA(B2) in the rat hippocampus. J Neurosci. 2003;23:11026–11035. doi: 10.1523/JNEUROSCI.23-35-11026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik A, Vida I, Fukazawa Y, Guetg N, Kasugai Y, Marker CL, Rigato F, Bettler B, Wickman K, Frotscher M, Shigemoto R. Compartment-dependent colocalization of Kir3.2-containing K+ channels and GABAB receptors in hippocampal pyramidal cells. J Neurosci. 2006;26:4289–4297. doi: 10.1523/JNEUROSCI.4178-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM. Spillover and synaptic cross talk mediated by glutamate and GABA in the mammalian brain. In: Agnati LF, Fuxe K, Nicholson C, Sykova E, editors. Volume Transmission Revisited. Elsevier; Amsterdam: 2000. pp. 339–351. [DOI] [PubMed] [Google Scholar]
- Kultas-Ilinsky K, Leontiev V, Whiting PJ. Expression of 10 GABA(A) receptor subunit messenger RNAs in the motor- related thalamic nuclei and basal ganglia of Macaca mulatta studied with in situ hybridization histochemistry. Neuroscience. 1998;85:179–204. doi: 10.1016/s0306-4522(97)00634-9. [DOI] [PubMed] [Google Scholar]
- Kuwajima M, Dehoff MH, Collins SC, Hall RA, Worley PF, Smith Y. Homer proteins regulate the subcellular and subsynaptic localization of group I metabotropic glutamate receptors in the mouse pallido-subthalamic complex. Soc Neurosci Abstr. 2004a;162:4. [Google Scholar]
- Kuwajima M, Hall RA, Aiba A, Smith Y. Subcellular and subsynaptic localization of group I metabotropic glutamate receptors in the monkey subthalamic nucleus. J Comp Neurol. 2004b;474:589–602. doi: 10.1002/cne.20158. [DOI] [PubMed] [Google Scholar]
- Lacey CJ, Boyes J, Gerlach O, Chen L, Magill PJ, Bolam JP. GABA(B) receptors at glutamatergic synapses in the rat striatum. Neuroscience. 2005;136:1083–1095. doi: 10.1016/j.neuroscience.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Lee FJ, Xue S, Pei L, Vukusic B, Chery N, Wang Y, Wang YT, Niznik HB, Yu XM, Liu F. Dual regulation of NMDA receptor functions by direct protein-protein interactions with the dopamine D1 receptor. Cell. 2002;111:219–230. doi: 10.1016/s0092-8674(02)00962-5. [DOI] [PubMed] [Google Scholar]
- Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci. 1995;15:1835–1853. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehre KP, Danbolt NC. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J Neurosci. 1998;18:8751–8757. doi: 10.1523/JNEUROSCI.18-21-08751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J. Roles and rules of kainate receptors in synaptic transmission. Nat Rev Neurosci. 2003;4:481–495. doi: 10.1038/nrn1118. [DOI] [PubMed] [Google Scholar]
- Lievens JC, Salin P, Nieoullon A, Kerkerian-Le Goff L. Nigrostriatal denervation does not affect glutamate transporter mRNA expression but subsequent levodopa treatment selectively increases GLT1 mRNA and protein expression in the rat striatum. J Neurochem. 2001;79:893–902. doi: 10.1046/j.1471-4159.2001.00644.x. [DOI] [PubMed] [Google Scholar]
- Liu F, Wan Q, Pristupa ZB, Yu XM, Wang YT, Niznik HB. Direct protein-protein coupling enables cross-talk between dopamine D5 and gamma-aminobutyric acid A receptors. Nature. 2000;403:274–280. doi: 10.1038/35002014. [DOI] [PubMed] [Google Scholar]
- Lombardi G, Alesiani M, Leonardi P, Cherici G, Pellicciari R, Moroni F. Pharmacological characterization of the metabotropic glutamate receptor inhibiting D-[3H]-aspartate output in rat striatum. Br J Pharmacol. 1993;110:1407–1412. doi: 10.1111/j.1476-5381.1993.tb13977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, McCool BA. Metabotropic glutamate receptor-mediated presynaptic depression at corticostriatal synapses involves mGLuR2 or 3. J Neurophysiol. 1995;73:1076–1083. doi: 10.1152/jn.1995.73.3.1076. [DOI] [PubMed] [Google Scholar]
- Lujan R, Shigemoto R, Kulik A, Juiz JM. Localization of the GABAB receptor 1a/b subunit relative to glutamatergic synapses in the dorsal cochlear nucleus of the ra. J Comp Neurol. 2004;475:36–46. doi: 10.1002/cne.20160. [DOI] [PubMed] [Google Scholar]
- MacDermott AB, Role LW, Siegelbaum SA. Presynaptic ionotropic receptors and the control of transmitter release. Annu Rev Neurosci. 1999;22:443–485. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Olsen RW. GABAA receptor channels. Annu Rev Neurosci. 1994;17:569–602. doi: 10.1146/annurev.ne.17.030194.003033. [DOI] [PubMed] [Google Scholar]
- Madden DR. The structure and function of glutamate receptor ion channels. Nat Rev Neurosci. 2002;3:91–101. doi: 10.1038/nrn725. [DOI] [PubMed] [Google Scholar]
- Margeta-Mitrovic M, Jan YN, Jan LY. A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron. 2000;27:97–106. doi: 10.1016/s0896-6273(00)00012-x. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Wittmann M, Bradley SR, Hubert GW, Smith Y, Conn PJ. Activation of group I metabotropic glutamate receptors produces a direct excitation and disinhibition of GABAergic projection neurons in the substantia nigra pars reticulata. J Neurosci. 2001;21:7001–7012. doi: 10.1523/JNEUROSCI.21-18-07001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino MJ, Awad H, Poisik O, Wittmann M, Conn PJ. Localization and physiological roles of metabotropic glutamate receptors in the direct and indirect pathways of the basal ganglia. Amino Acids. 2002;23:185–191. doi: 10.1007/s00726-001-0127-1. [DOI] [PubMed] [Google Scholar]
- Marino MJ, Valenti O, Conn PJ. Glutamate receptors and Parkinson's disease: opportunities for intervention. Drugs Aging. 2003;20:377–397. doi: 10.2165/00002512-200320050-00006. [DOI] [PubMed] [Google Scholar]
- Marshall FH, Jones KA, Kaupmann K, Bettler B. GABAB receptors - the first 7TM heterodimers. Trends Pharmacol Sci. 1999;20:396–399. doi: 10.1016/s0165-6147(99)01383-8. [DOI] [PubMed] [Google Scholar]
- Matsubara A, Laake JH, Davanger S, Usami S, Ottersen OP. Organization of AMPA receptor subunits at a glutamate synapse: a quantitative immunogold analysis of hair cell synapses in the rat organ of Corti. J Neurosci. 1996;16:4457–4467. doi: 10.1523/JNEUROSCI.16-14-04457.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer ML, Armstrong N. Structure and function of glutamate receptor ion channels. Annu Rev Physiol. 2004;66:161–181. doi: 10.1146/annurev.physiol.66.050802.084104. [DOI] [PubMed] [Google Scholar]
- Mereu G, Costa E, Armstrong DM, Vicini S. Glutamate receptor subtypes mediate excitatory synaptic currents of dopamine neurons in midbrain slices. J Neurosci. 1991;11:1359–1366. doi: 10.1523/JNEUROSCI.11-05-01359.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merighi A, Polak JM. Post-embedding immunogold staining. In: Cuello AC, editor. Immunohistochemistry II. John Wiley & Sons; Chichester: 1993. pp. 229–264. [Google Scholar]
- Micheletti G, Lannes B, Warter JM, Zwiller J. Evidence for NMDA/D2 receptor-receptor interactions in the rat striatum. Adv Neurol. 1993;60:128–132. [PubMed] [Google Scholar]
- Mitchell SJ, Silver RA. GABA Spillover from Single Inhibitory Axons Suppresses Low-Frequency Excitatory Transmission at the Cerebellar Glomerulus. J Neurosci. 2000;20:8651–8658. doi: 10.1523/JNEUROSCI.20-23-08651.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody I, De Koninck Y, Otis TS, Soltesz I. Bridging the cleft at GABA synapses in the brain. Trends Neurosci. 1994;17:517–525. doi: 10.1016/0166-2236(94)90155-4. comment. [DOI] [PubMed] [Google Scholar]
- Mody I. Distinguishing between GABA(A) receptors responsible for tonic and phasic conductances. Neurochem Res. 2001;26:907–913. doi: 10.1023/a:1012376215967. [DOI] [PubMed] [Google Scholar]
- Mody I. Aspects of the homeostaic plasticity of GABAA receptor-mediated inhibition. J Physiol. 2005;562:37–46. doi: 10.1113/jphysiol.2004.077362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran MM, McFarland K, Melendez RI, Kalivas PW, Seamans JK. Cystine/Glutamate Exchange Regulates Metabotropic Glutamate Receptor Presynaptic Inhibition of Excitatory Transmission and Vulnerability to Cocaine Seeking. J Neurosci. 2005;25:6389–6393. doi: 10.1523/JNEUROSCI.1007-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morari M, O'Connor WT, Ungerstedt U, Fuxe K. N-methyl-D-aspartic acid differentially regulates extracellular dopamine, GABA, and glutamate levels in the dorsolateral neostriatum of the halothane-anesthetized rat: an in vivo microdialysis study. J Neurochem. 1993;60:1884–1893. doi: 10.1111/j.1471-4159.1993.tb13416.x. [DOI] [PubMed] [Google Scholar]
- Mouroux M, Feger J. Evidence that the parafascicular projection to the subthalamic nucleus is glutamatergic. Neuroreport. 1993;4:613–615. doi: 10.1097/00001756-199306000-00002. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Jang IS, Ishibashi H, Watanabe S, Akaike N. Possible Roles of Kainate Receptors on GABAergic Nerve Terminals Projecting to Rat Substantia Nigra Dopaminergic Neurons. J Neurophysiol. 2003;90:1662–1670. doi: 10.1152/jn.01165.2002. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Hori N, Kastuda N. Neostriatal evoked inhibition and effects of dopamine on globus pallidal neurons in rat slice preparations. Brain Res. 1985;358:282–286. doi: 10.1016/0006-8993(85)90972-2. [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Kita H, Kitai ST. Electrical membrane properties of rat subthalamic neurons in an in vitro slice preparation. Brain Res. 1987;437:35–44. doi: 10.1016/0006-8993(87)91524-1. [DOI] [PubMed] [Google Scholar]
- Nambu A, Tokuno H, Hamada I, Kita H, Imanishi M, Akazawa T, Ikeuchi Y, Hasegawa N. Excitatory cortical inputs to pallidal neurons via the subthalamic nucleus in the monkey [In Process Citation] J Neurophysiol. 2000;84:289–300. doi: 10.1152/jn.2000.84.1.289. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Takano T, Hansen AJ. Beyond the role of glutamate as a neurotransmitter. Nat Rev Neurosci. 2002;3:748–755. doi: 10.1038/nrn916. [DOI] [PubMed] [Google Scholar]
- Nicola SM, Surmeier J, Malenka RC. Dopaminergic modulation of neuronal excitability in the striatum and nucleus accumbens. Annu Rev Neurosci. 2000;23:185–215. doi: 10.1146/annurev.neuro.23.1.185. [DOI] [PubMed] [Google Scholar]
- Nisenbaum ES, Berger TW, Grace AA. Presynaptic modulation by GABAB receptors of glutamatergic excitation and GABAergic inhibition of neostriatal neurons. J Neurophys. 1992;67:477–481. doi: 10.1152/jn.1992.67.2.477. [DOI] [PubMed] [Google Scholar]
- Nisenbaum ES, Berger TW, Grace AA. Depression of glutamatergic and GABAergic synaptic responses in striatal spiny neurons by stimulation of presynaptic GABAB receptors. Synapse. 1993;14:221–242. doi: 10.1002/syn.890140306. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mulvihill E, Streit P, Somogyi P. Subsynaptic segregation of metabotropic and ionotropic glutamate receptors as revealed by immunogold localization. Neuroscience. 1994;61:421–427. doi: 10.1016/0306-4522(94)90421-9. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Roberts JD, Baude A, Richards JG, Sieghart W, Somogyi P. Immunocytochemical localization of the alpha 1 and beta 2/3 subunits of the GABAA receptor in relation to specific GABAergic synapses in the dentate gyrus. Eur J Neurosci. 1995a;7:630–646. doi: 10.1111/j.1460-9568.1995.tb00667.x. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Roberts JD, Baude A, Richards JG, Somogyi P. Relative densities of synaptic and extrasynaptic GABAA receptors on cerebellar granule cells as determined by a quantitative immunogold method. J Neurosci. 1995b;15:2948–2960. doi: 10.1523/JNEUROSCI.15-04-02948.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Benke D, Fritschy JM, Somogyi P. Differential synaptic localization of two major gamma-aminobutyric acid type A receptor alpha subunits on hippocampal pyramidal cells. Proc Natl Acad Sci U S A. 1996;93:11939–11944. doi: 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Lujan R, Laube G, Roberts JD, Molnar E, Somogyi P. Cell type and pathway dependence of synaptic AMPA receptor number and variability in the hippocampus. Neuron. 1998a;21:545–559. doi: 10.1016/s0896-6273(00)80565-6. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Somogyi P. Segregation of different GABAA receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998b;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z. AMPA and NMDA receptors: similarities and differences in their synaptic distribution. Curr Opin Neurobiol. 2000;10:337–341. doi: 10.1016/s0959-4388(00)00086-6. [DOI] [PubMed] [Google Scholar]
- Nyiri G, Freund TF, Somogyi P. Input-dependent synaptic targeting of alpha(2)-subunit-containing GABA(A) receptors in synapses of hippocampal pyramidal cells of the rat. Eur J Neurosci. 2001;13:428–442. doi: 10.1046/j.1460-9568.2001.01407.x. [DOI] [PubMed] [Google Scholar]
- Otani S, Auclair N, Desce JM, Roisin MP, Crepel F. Dopamine receptors and groups I and II mGluRs cooperate for long-term depression induction in rat prefrontal cortex through converging postsynaptic activation of MAP kinases. J Neurosci. 1999;19:9788–9802. doi: 10.1523/JNEUROSCI.19-22-09788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottersen OP, Landsend AS. Organization of glutamate receptors at the synapse. Eur J Neurosci. 1997;9:2219–2224. doi: 10.1111/j.1460-9568.1997.tb01640.x. [DOI] [PubMed] [Google Scholar]
- Ozawa S, Kamiya H, Tsuzuki K. Glutamate receptors in the mammalian central nervous system. Prog Neurobiol. 1998;54:581–618. doi: 10.1016/s0301-0082(97)00085-3. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Celada P, Tepper JM. Striatal, pallidal, and pars reticulata evoked inhibition of nigrostriatal dopaminergic neurons is mediated by GABA(A) receptors in vivo. Neuroscience. 1999;89:799–812. doi: 10.1016/s0306-4522(98)00355-8. [DOI] [PubMed] [Google Scholar]
- Paquet M, Smith Y. Differential localization of AMPA glutamate receptor subunits in the two segments of the globus pallidus and the substantia nigra pars reticulata in the squirrel monkey. Eur J Neurosci. 1996;8:229–233. doi: 10.1111/j.1460-9568.1996.tb01184.x. [DOI] [PubMed] [Google Scholar]
- Paquet M, Smith Y. Group I Metabotropic Glutamate Receptors in the Monkey Striatum: Subsynaptic Association with Glutamatergic and Dopaminergic Afferents. J Neurosci. 2003;23:7659–7669. doi: 10.1523/JNEUROSCI.23-20-07659.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent A. Extrinsic connections of the basal ganglia. Trends Neurosci. 1990;13:254–258. doi: 10.1016/0166-2236(90)90105-j. [DOI] [PubMed] [Google Scholar]
- Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- Pei L, Lee FJS, Moszczynska A, Vukusic B, Liu F. Regulation of Dopamine D1 Receptor Function by Physical Interaction with the NMDA Receptors. J Neurosci. 2004;24:1149–1158. doi: 10.1523/JNEUROSCI.3922-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wang YX, Niedzielski AS, Wenthold RJ. The metabotropic glutamate receptors, mGluR2 and mGluR3, show unique postsynaptic, presynaptic and glial localizations. Neuroscience. 1996;71:949–976. doi: 10.1016/0306-4522(95)00533-1. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J. Spiny neurons lacking choline acetyltransferase immunoreactivity are major targets of cholinergic and catecholaminergic terminals in rat striatum. J Neurosci Res. 1990;25:263–280. doi: 10.1002/jnr.490250302. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Garzon M, Mengual E. Electron microscopic immunolabeling of transporters and receptors identifies transmitter-specific functional sites envisioned in Cajal's neuron. Prog Brain Res. 2002;136:145–155. doi: 10.1016/s0079-6123(02)36014-x. [DOI] [PubMed] [Google Scholar]
- Pisani A, Calabresi P, Centonze D, Bernardi G. Activation of group III metabotropic glutamate receptors depresses glutamatergic transmission at corticostriatal synapse. Neuropharmacology. 1997;36:845–851. doi: 10.1016/s0028-3908(96)00177-3. [DOI] [PubMed] [Google Scholar]
- Poisik O, Raju DV, Verreault M, Rodriguez A, Abeniyi OA, Conn PJ, Smith Y. Metabotropic glutamate receptor 2 modulates excitatory synaptic transmission in the rat globus pallidus. Neuropharmacology. 2005;49(Suppl 1):57–69. doi: 10.1016/j.neuropharm.2005.03.006. [DOI] [PubMed] [Google Scholar]
- Poisik OV, Mannaioni G, Traynelis S, Smith Y, Conn PJ. Distinct functional roles of the metabotropic glutamate receptors 1 and 5 in the rat globus pallidus. J Neurosci. 2003;23:122–130. doi: 10.1523/JNEUROSCI.23-01-00122.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Kulak JM. Nicotine and nicotinic receptors; relevance to Parkinson's disease. Neurotoxicology. 2002;23:581–594. doi: 10.1016/s0161-813x(02)00036-0. [DOI] [PubMed] [Google Scholar]
- Racz B, Blanpied TA, Ehlers MD, Weinberg RJ. Lateral organization of endocytic machinery in dendritic spines. Nat Neurosci. 2004;7:917–918. doi: 10.1038/nn1303. [DOI] [PubMed] [Google Scholar]
- Rajakumar N, Elisevich K, Flumerfelt BA. The pallidostriatal projection in the rat: a recurrent inhibitory loop? Brain Res. 1994;651:332–336. doi: 10.1016/0006-8993(94)90714-5. [DOI] [PubMed] [Google Scholar]
- Rao A, Craig AM. Activity regulates the synaptic localization of the NMDA receptor in hippocampal neurons. Neuron. 1997;19:801–812. doi: 10.1016/s0896-6273(00)80962-9. [DOI] [PubMed] [Google Scholar]
- Ravenscroft P, Brotchie J. NMDA receptors in the basal ganglia. J Anat 196. 2000;(Pt 4):577–585. doi: 10.1046/j.1469-7580.2000.19640577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichelt W, Knopfel T. Glutamate uptake controls expression of a slow postsynaptic current mediated by mGluRs in cerebellar Purkinje cells. J Neurophys. 2002;87:1974–1980. doi: 10.1152/jn.00704.2001. [DOI] [PubMed] [Google Scholar]
- Reimann W. Inhibition by GABA, baclofen and gabapentin of dopamine release from rabbit caudate nucleus: are there common or different sites of action? Eur J Pharmacol. 1983;94:341–344. doi: 10.1016/0014-2999(83)90425-9. [DOI] [PubMed] [Google Scholar]
- Rhodes KJ, Trimmer JS. Antibodies as valuable neuroscience research tools versus reagents of mass distraction. J Neurosci. 2006;26:8017–8020. doi: 10.1523/JNEUROSCI.2728-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro-Da-Silva A, Priestly JV, Cuello AC. Pre-embedding Ultrastructural Immunocytochemistry. In: Cuello AC, editor. Immunohistochemistry II. John Wiley & Sons; Chichester: 1993. pp. 190–227. [Google Scholar]
- Richerson GB, Wu Y. Dynamic equilibrium of neurotransmitter transporters: not just for reuptake anymore. J Neurophysiol. 2003;90:1363–1374. doi: 10.1152/jn.00317.2003. [DOI] [PubMed] [Google Scholar]
- Robertson RG, Graham WC, Sambrook MA, Crossman AR. Further investigations into the pathophysiology of MPTP-induced parkinsonism in the primate: an intracerebral microdialysis study of gamma-aminobutyric acid in the lateral segment of the globus pallidus. Brain Res. 1991;563:278–280. doi: 10.1016/0006-8993(91)91545-c. [DOI] [PubMed] [Google Scholar]
- Rogue PJ, Malviya AN. Calcium signals in the cell nucleus. Strasbourg, France, August 20-23, 1998. EMBO Journal. 1999;18:5147–5152. doi: 10.1093/emboj/18.19.5147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano C, Sesma MA, McDonald CT, O'Malley K, Van den Pol AN, Olney JW. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J Comp Neurol. 1995;355:455–469. doi: 10.1002/cne.903550310. [DOI] [PubMed] [Google Scholar]
- Rosin DL, Hettinger BD, Lee A, Linden J. Anatomy of adenosine A2A receptors in brain: morphological substrates for integration of striatal function. Neurology. 2003;61:S12–18. doi: 10.1212/01.wnl.0000095205.33940.99. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- Rubio ME, Wenthold RJ. Glutamate receptors are selectively targeted to postsynaptic sites in neurons. Neuron. 1997;18:939–950. doi: 10.1016/s0896-6273(00)80333-5. [DOI] [PubMed] [Google Scholar]
- Rusakov DA, Lehre KP. Perisynaptic asymmetry of glia: new insights into glutamate signalling. Trends Neurosci. 2002;25:492–494. doi: 10.1016/s0166-2236(02)02230-0. [DOI] [PubMed] [Google Scholar]
- Santiago M, Machado A, Cano J. In vivo release of dopamine from rat striatum, substantia nigra and prefrontal cortex: differential modulation by baclofen. Br J Pharmacol. 1993;109:814–818. doi: 10.1111/j.1476-5381.1993.tb13647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Sawchenko PE. Magic peptides, magic antibodies: guidelines for appropriate controls for immunohistochemistry. J Comp Neurol. 2003;465:161–163. doi: 10.1002/cne.10858. [DOI] [PubMed] [Google Scholar]
- Scanziani M. GABA Spillover Activates Postsynaptic GABAB Receptors to Control Rhythmic Hippocampal Activity. Neuron. 2000;25:673–681. doi: 10.1016/s0896-6273(00)81069-7. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer AN, Vanderschuren LJ, De Vries TJ, Hogenboom F, Wardeh G, Mulder AH. Synergistically interacting dopamine D1 and NMDA receptors mediate nonvesicular transporter-dependent GABA release from rat striatal medium spiny neurons. J Neurosci. 2000;20:3496–3503. doi: 10.1523/JNEUROSCI.20-09-03496.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzer C, Berresheim U, Pirker S, Wieselthaler A, Fuchs K, Sieghart W, Sperk G. Distribution of the major gamma-aminobutyric acid(A) receptor subunits in the basal ganglia and associated limbic brain areas of the adult rat. J Comp Neurol. 2001;433:526–549. doi: 10.1002/cne.1158. [DOI] [PubMed] [Google Scholar]
- Seabrook GR, Howson W, Lacey MG. Electrophysiological characterization of potent agonists and antagonists at pre- and postsynaptic GABAB receptors on neurones in rat brain slices. Br J Pharmacol. 1990;101:949–957. doi: 10.1111/j.1476-5381.1990.tb14186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabrook GR, Howson W, Lacey MG. Subpopulations of GABA-mediated synaptic potentials in slices of rat dorsal striatum are differentially modulated by presynaptic GABAB receptors. Brain Res. 1991;562:332–334. doi: 10.1016/0006-8993(91)90642-9. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci. 2003;6:484–490. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM, Silver RA. Tonically active GABAA receptors: modulating gain and maintaining the tone. Trends Neurosci. 2004;27:262–269. doi: 10.1016/j.tins.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Serge A, Fourgeaud L, Hemar A, Choquet D. Receptor activation and homer differentially control the lateral mobility of metabotropic glutamate receptor 5 in the neuronal membrane. J Neurosci. 2002;22:3910–3920. doi: 10.1523/JNEUROSCI.22-10-03910.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serge A, Fourgeaud L, Hemar A, Choquet D. Active surface transport of metabotropic glutamate receptors through binding to microtubules and actin flow. J Cell Sci. 2003;116:5015–5022. doi: 10.1242/jcs.00822. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate-dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann N Y Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- Shen KZ, Johnson SW. Presynaptic GABAB and adenosine A1 receptors regulate synaptic transmission to rat substantia nigra reticulata neurones. J Physiol (Lond) 1997;505:153–163. doi: 10.1111/j.1469-7793.1997.153bc.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen KZ, Johnson SW. Presynaptic GABA(B) receptors inhibit synaptic inputs to rat subthalamic neurons. Neuroscience. 2001;108:431–436. doi: 10.1016/s0306-4522(01)00424-9. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Smith Y, Parent A. Differential connections of caudate nucleus and putamen in the squirrel monkey (Saimiri sciureus) Neuroscience. 1986;18:347–371. doi: 10.1016/0306-4522(86)90159-4. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bennett BD, Bolam JP, Parent A, Sadikot AF. Synaptic relationships between dopaminergic afferents and cortical or thalamic input in the sensorimotor territory of the striatum in monkey. J Comp Neurol. 1994;344:1–19. doi: 10.1002/cne.903440102. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86:353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- Smith Y, Charara A, Hanson JE, Paquet M, Levey AI. GABA(B) and group I metabotropic glutamate receptors in the striatopallidal complex in primates. J Anat. 2000;196(Pt 4):555–576. doi: 10.1046/j.1469-7580.2000.19640555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith Y, Charara A, Paquet M, Kieval JZ, Pare JF, Hanson JE, Hubert GW, Kuwajima M, Levey AI. Ionotropic and metabotropic GABA and glutamate receptors in primate basal ganglia. J Chem Neuroanat. 2001;22:13–42. doi: 10.1016/s0891-0618(01)00098-9. [DOI] [PubMed] [Google Scholar]
- Smolders I, De Klippel N, Sarre S, Ebinger G, Michotte Y. Tonic GABA-ergic modulation of striatal dopamine release studied by in vivo microdialysis in the freely moving rat. Eur J Pharmacol. 1995;284:83–91. doi: 10.1016/0014-2999(95)00369-v. [DOI] [PubMed] [Google Scholar]
- Soares J, Kliem MA, Betarbet R, Greenamyre JT, Yamamoto B, Wichmann T. Role of external pallidal segment in primate parkinsonism: comparison of the effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism and lesions of the external pallidal segment. J Neurosci. 2004;24:6417–6426. doi: 10.1523/JNEUROSCI.0836-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis RP, Anderson LA, Walters JR, Kelland MD. A role for non-NMDA excitatory amino acid receptors in regulating the basal activity of rat globus pallidus neurons and their activation by the subthalamic nucleus. Brain Res. 1994;666:21–30. doi: 10.1016/0006-8993(94)90278-x. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Fritschy JM, Benke D, Roberts JD, Sieghart W. The gamma 2 subunit of the GABAA receptor is concentrated in synaptic junctions containing the alpha 1 and beta 2/3 subunits in hippocampus, cerebellum and globus pallidus. Neuropharmacology. 1996;35:1425–1444. doi: 10.1016/s0028-3908(96)00086-x. [DOI] [PubMed] [Google Scholar]
- Stanford IM, Lacey MG. Differential actions of serotonin, mediated by 5-HT1B and 5-HT2C receptors, on GABA-mediated synaptic input to rat substantia nigra pars reticulata neurons in vitro. J Neurosci. 1996;16:7566–7573. doi: 10.1523/JNEUROSCI.16-23-07566.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani A, Spadoni F, Giacomini P, Lavaroni F, Bernardi G. The modulation of calcium current by GABA metabotropic receptors in a sub-population of pallidal neurons. Eur J Neurosci. 1999;11:3995–4005. doi: 10.1046/j.1460-9568.1999.00836.x. [DOI] [PubMed] [Google Scholar]
- Svoboda K, Yasuda R. Principles of two-photon excitation microscopy and its applications to neuroscience. Neuron. 2006;50:823–839. doi: 10.1016/j.neuron.2006.05.019. [DOI] [PubMed] [Google Scholar]
- Sykova E. Extrasynaptic volume transmission and diffusion parameters of the extracellular space. Neuroscience. 2004;129:861–876. doi: 10.1016/j.neuroscience.2004.06.077. [DOI] [PubMed] [Google Scholar]
- Tabata T, Aiba A, Kano M. Extracellular calcium controls the dynamic range of neuronal metabotropic glutamate receptor responses. Mol Cell Neurosci. 2002;20:56–68. doi: 10.1006/mcne.2002.1118. [DOI] [PubMed] [Google Scholar]
- Tabata T, Araishi K, Hashimoto K, Hashimotodani Y, van der Putten H, Bettler B, Kano M. Ca2+ activity at GABAB receptors constitutively promotes metabotropic glutamate signaling in the absence of GABA. Proc Natl Acad Sci U S A. 2004;101:16952–16957. doi: 10.1073/pnas.0405387101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumi Y, Matsubara A, Rinvik E, Ottersen OP. The arrangement of glutamate receptors in excitatory synapses. Ann N Y Acad Sci. 1999;868:474–482. doi: 10.1111/j.1749-6632.1999.tb11316.x. [DOI] [PubMed] [Google Scholar]
- Tamaru Y, Nomura S, Mizuno N, Shigemoto R. Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience. 2001;106:481–503. doi: 10.1016/s0306-4522(01)00305-0. [DOI] [PubMed] [Google Scholar]
- Tanaka J, Matsuzaki M, Tarusawa E, Momiyama A, Molnar E, Kasai H, Shigemoto R. Number and density of AMPA receptors in single synapses in immature cerebellum. J Neurosci. 2005;25:799–807. doi: 10.1523/JNEUROSCI.4256-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempia F, Miniaci MC, Anchisi D, Strata P. Postsynaptic current mediated by metabotropic glutamate receptors in cerebellar Purkinje cells. J Neurophys. 1998;80:520–528. doi: 10.1152/jn.1998.80.2.520. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Martin LP, Anderson DR. GABAA receptor-mediated inhibition of rat substantia nigra dopaminergic neurons by pars reticulata projection neurons. J Neurosci. 1995;15:3092–3103. doi: 10.1523/JNEUROSCI.15-04-03092.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Bolam JP. Functional diversity and specificity of neostriatal interneurons. Curr Opin Neurobiol. 2004;14:685–692. doi: 10.1016/j.conb.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Tepper JM, Koos T, Wilson CJ. GABAergic microcircuits in the neostriatum. Trends Neurosci. 2004;27:662–669. doi: 10.1016/j.tins.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Torp R, Danbolt NC, Babaie E, Bjoras M, Seeberg E, Storm-Mathisen J, Ottersen OP. Differential expression of two glial glutamate transporters in the rat brain: an in situ hybridization study. Eur J Neurosci. 1994;6:936–942. doi: 10.1111/j.1460-9568.1994.tb00587.x. [DOI] [PubMed] [Google Scholar]
- Torrisi MR, Mancini P. Freeze-fracture immunogold labeling. Histochem Cell Biol. 1996;106:19–30. doi: 10.1007/BF02473199. [DOI] [PubMed] [Google Scholar]
- Totterdell S, Ingham CA. Immunocytochemistry I: pre-embedding staining. In: Bolam JP, editor. Experimental Neuroanatomy A Practical Approach. Oxford University Press; New York: 1992. pp. 104–127. [Google Scholar]
- Triller A, Choquet D. Synaptic structure and diffusion dynamics of synaptic receptors. Biol Cell. 2003;95:465–476. doi: 10.1016/j.biolcel.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Triller A, Choquet D. Surface trafficking of receptors between synaptic and extrasynaptic membranes: and yet they do move! Trends Neurosci. 2005;28:133–139. doi: 10.1016/j.tins.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Urbain N, Rentero N, Gervasoni D, Renaud B, Chouvet G. The Switch of Subthalamic Neurons From an Irregular to a Bursting Pattern Does Not Solely Depend on Their GABAergic Inputs in the Anesthetic-Free Rat. J Neurosci. 2002;22:8665–8675. doi: 10.1523/JNEUROSCI.22-19-08665.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti O, Conn PJ, Marino MJ. Distinct physiological roles of the Gq-coupled metabotropic glutamate receptors Co-expressed in the same neuronal populations. J Cell Physiol. 2002;191:125–137. doi: 10.1002/jcp.10081. [DOI] [PubMed] [Google Scholar]
- Valenti O, Marino MJ, Wittmann M, Lis E, DiLella AG, Kinney GG, Conn PJ. Group III Metabotropic Glutamate Receptor-Mediated Modulation of the Striatopallidal Synapse. J Neurosci. 2003;23:7218–7226. doi: 10.1523/JNEUROSCI.23-18-07218.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenti O, Mannaioni G, Seabrook GR, Conn PJ, Marino MJ. Group III metabotropic glutamate-receptor-mediated modulation of excitatory transmission in rodent substantia nigra pars compacta dopamine neurons. J Pharmacol Exp Ther. 2005;313:1296–1304. doi: 10.1124/jpet.104.080481. [DOI] [PubMed] [Google Scholar]
- Velaz-Faircloth M, McGraw TS, alandro MS, Fremeau RT, Jr., Kilberg MS, Anderson KJ. Characterization and distribution of the neuronal glutamate transporter EAAC1 in rat brain. Am J Physiol. 1996;270:C67–75. doi: 10.1152/ajpcell.1996.270.1.C67. [DOI] [PubMed] [Google Scholar]
- Verma A, Moghaddam B. Regulation of striatal dopamine release by metabotropic glutamate receptors. Synapse. 1998;28:220–226. doi: 10.1002/(SICI)1098-2396(199803)28:3<220::AID-SYN5>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Villalba RM, Dinesh RV, Hall RA, Smith Y. GABA B Receptors in the Centromedian/Parafascicular Thalamic Nuclear Complex: An Ultrastructural Analysis of GABA BR1 and GABA BR2 in the Monkey Thalamus. J Comp Neurol. 2006 doi: 10.1002/cne.20950. In press. [DOI] [PubMed] [Google Scholar]
- Wang H, Pickel VM. Presence of NMDA-type glutamate receptors in cingulate corticostriatal terminals and their postsynaptic targets. Synapse. 2000;35:300–310. doi: 10.1002/(SICI)1098-2396(20000315)35:4<300::AID-SYN8>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Wang XS, Ong WY, Lee HK, Huganir RL. A light and electron microscopic study of glutamate receptors in the monkey subthalamic nucleus. J Neurocytol. 2000;29:743–754. doi: 10.1023/a:1010990404833. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Fukaya M, Sakimura K, Manabe T, Mishina M, Inoue Y. Selective scarcity of NMDA receptor channel subunits in the stratum lucidum (mossy fibre-recipient layer) of the mouse hippocampal CA3 subfield. Eur J Neurosci. 1998;10:478–487. doi: 10.1046/j.1460-9568.1998.00063.x. [DOI] [PubMed] [Google Scholar]
- Wigmore MA, Lacey MG. Metabotropic glutamate receptors depress glutamate-mediated synaptic input to rat midbrain dopamine neurones in vitro. Br J Pharmacol. 1998;123:667–674. doi: 10.1038/sj.bjp.0701662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson CJ, Groves PM. Fine structure and synaptic connections of the common spiny neuron of the rat neostriatum: a study employing intracellular inject of horseradish peroxidase. J Comp Neurol. 1980;194:599–615. doi: 10.1002/cne.901940308. [DOI] [PubMed] [Google Scholar]
- Wilson CJ. The generation of natural firing patterns in neostriatal neurons. Prog Brain Res. 1993;99:277–297. doi: 10.1016/s0079-6123(08)61352-7. [DOI] [PubMed] [Google Scholar]
- Wilson CL, Puntis M, Lacey MG. Overwhelmingly asynchronous firing of rat subthalamic nucleus neurones in brain slices provides little evidence for intrinsic interconnectivity. Neuroscience. 2004;123:187–200. doi: 10.1016/j.neuroscience.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Wilson JS, Wilson JA. Baclofen attenuates hyperpolarizing not depolarizing responses of caudate neurons in cat. Brain Res. 1985;342:396–400. doi: 10.1016/0006-8993(85)91145-x. [DOI] [PubMed] [Google Scholar]
- Winder DG, Conn PJ. Roles of metabotropic glutamate receptors in glial function and glial-neuronal communication. J Neurosci Res. 1996;46:131–137. doi: 10.1002/(SICI)1097-4547(19961015)46:2<131::AID-JNR1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann M, Marino MJ, Bradley SR, Conn PJ. Activation of group III mGluRs inhibits GABAergic and glutamatergic transmission in the substantia nigra pars reticulata. J Neurophysiol. 2001;85:1960–1968. doi: 10.1152/jn.2001.85.5.1960. [DOI] [PubMed] [Google Scholar]
- Wu Y, Kawakami R, Shinohara Y, Fukaya M, Sakimura K, Mishina M, Watanabe M, Ito I, Shigemoto R. Target-cell-specific left-right asymmetry of NMDA receptor content in schaffer collateral synapses in epsilon1/NR2A knock-out mice. J Neurosci. 2005;25:9213–9226. doi: 10.1523/JNEUROSCI.2134-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Tu JC, Worley PF. Homer: a link between neural activity and glutamate receptor function. Curr Opin Neurobiol. 2000;10:370–374. doi: 10.1016/s0959-4388(00)00087-8. [DOI] [PubMed] [Google Scholar]
- Yasumi M, Sato K, Shimada S, Nishimura M, Tohyama M. Regional distribution of GABA transporter 1 (GAT1) mRNA in the rat brain: comparison with glutamic acid decarboxylase67 (GAD67) mRNA localization. Brain Res Mol Brain Res. 1997;44:205–218. doi: 10.1016/s0169-328x(96)00200-8. [DOI] [PubMed] [Google Scholar]
- Zhang H, Sulzer D. Glutamate spillover in the striatum depresses dopaminergic transmission by activating group I metabotropic glutamate receptors. J Neurosci. 2003;23:10585–10592. doi: 10.1523/JNEUROSCI.23-33-10585.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu ZT, Munhall A, Shen KZ, Johnson SW. Calcium-dependent subthreshold oscillations determine bursting activity induced by N-methyl-D-aspartate in rat subthalamic neurons in vitro. Eur J Neurosci. 2004;19:1296–1304. doi: 10.1111/j.1460-9568.2004.03240.x. [DOI] [PubMed] [Google Scholar]