ABSTRACT
Objective: Carotid artery (CA) invasion by head and neck tumors is a challenging problem for the cranial base surgeon. Proposed methods for management have the disadvantage of constant or temporary interruption of the arterial blood flow and, as a consequence, cerebral ischemic complications. The objective of the study was to investigate the long-term effects of a novel technique, “extarterectomy,” on the vascular wall and the arterial blood flow in an ovine model. Methods: Wallstents were implanted bilaterally in the common CA of 5 sheep by the Seldinger technique. Six weeks after stent implantation, a segment of the arterial wall of about 2 cm in length was peeled off the external surface of the stent. Six months later, control angiography was performed. The animals were sacrificed after 18 months and the “extarterectomized” arteries were removed for the microscopic and macroscopic evaluation. Results: There were no acquired neurological deficits in the study group. Extarterectomy was performed without any complication in every case. Control angiography confirmed patent CAs. Evaluation by light microscopy showed the “neointimal” layer within the interstices of the stent. Conclusion: Extarterectomy allows aggressive tumor removal together with the maintenance of blood flow through the CA and offers hope for those with tumors invading the CA.
Keywords: Carotid artery, head and neck cancer, carotid stenting
Carotid artery (CA) involvement in patients with head and neck cancer is one of the most challenging issues for the head and neck surgeon. Resection of the CA with or without reconstruction and “stripping” the tumor off the CA are the current management options.1,2,3,4,5,6,7 However, constant or temporary interruption of the arterial blood flow through the CA causes potential cerebral ischemic complications with high mortality and morbidity rates. “Extarterectomy,” first described by Lopes and Wakhloo,8,9 is a novel technique that allows radical tumor removal while avoiding the need to interrupt arterial blood flow through the internal CA. The purpose of this study was to investigate the effects on the vascular wall in an ovine model.
MATERIALS AND METHODS
Five 1-1/2-year-old male sheep (39 to 48 kg weight; mean, 42 kg) were used in the study. The animals were fed a standard laboratory ovine diet with water provided ad libitum. Experimental surgery was performed in accordance with the guidelines for animal research established by the Animal Ethics Committee of Ege University.
The animal was premedicated with intramuscular injection of 0.22 mg/kg of atropine. Anesthesia was induced with intramuscular application of ketamine and xylazine (5 mg/kg and 0.1 mg/kg, respectively).
After induction of anesthesia, the sheep was positioned on an angiography table in a right lateral decubitus position and the left hind limb with a moderate abduction exposing the right inguinal area. After cleaning and draping, a standard 7-French (F) introducer sheath was placed in the right femoral artery percutaneously by the Seldinger technique. A 5F multipurpose catheter was positioned in the common CA and digital subtraction angiography was performed. After identification of the internal and external CAs, the carotid bifurcation was marked externally with a metal marker. Using digital road-mapping, a commercially available 0.0035-inch, 300-cm exchange guidewire with floppy tip was inserted and positioned in the external CA, and the catheter was withdrawn. The stent was inserted over the guidewire and deployed in the common CA just below the carotid bifurcation. A final angiogram was obtained to show the position of the stent in the common CA. Ten Wallstents (Boston Scientific, Natick, MA) 7 mm in diameter with various lengths (3 to 7 cm) were implanted bilaterally in five animals. At the end of the procedure, the femoral introducer sheath was removed and puncture bleeding was controlled by manual compression and secured by tight bandage. No anticoagulation or antiplatelet therapy was used in the follow-up period to facilitate “neointima” formation on the internal surface of the stent.
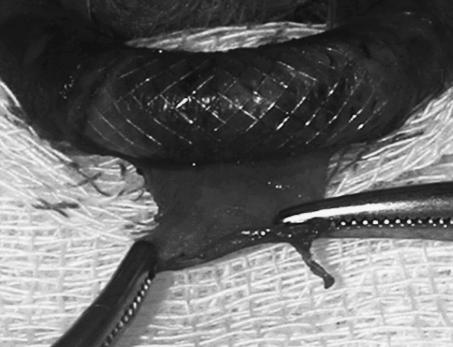
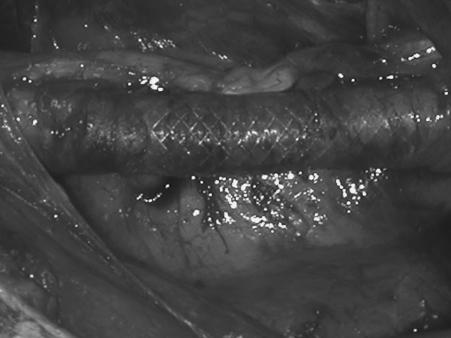
Six weeks after the CA stenting, the animal was anesthetized using the same protocol. After performing an oblique cervicotomy incision parallel to the anterior border of the right sternocleidomastoid muscle, the neurovascular bundle of the carotid sheath was exposed medial of the muscle. The CA, internal jugular vein, and vagus nerve were exposed by blunt dissection. The stented segment of the CA was identified with gentle palpation. A longitudinal arteriotomy was performed. Approximately 2 cm of arterial wall was peeled off the external surface of the mesh interstices of the stent (Figs. 1 and 2). The cervicotomy incision was then closed and the procedure repeated on the other side. Parenteral prophylactic antibiotherapy by daily intramuscular ceftriaxone (50 mg/kg) injection and the daily wound care were performed for 7 days.
Figure 1.
A segment of full-thickness arterial wall being peeled off the external surface of the implanted stent.
Figure 2.
Neointima and the stent struts seen in the extarterectomized segment of the carotid artery.
Six months after the extarterectomy, control angiography using the Seldinger technique was performed to evaluate the patency of the arterial blood flow through the CAs and the surface characteristics of the neointima in the stented segment.
After 18 months the animals were sacrificed. The stented segment of the CA was resected and cut in half longitudinally to examine the status of the neointima and the arterial wall. Hematoxylin and eosin-stained cross-sectional specimens of the extarterectomized segments were obtained to demonstrate the neointima formation.
RESULTS
Stent implantation was technically successful for all cases. All animals were symptom-free after the implantation.
The extarterectomy step of the study was uneventful. The neointima within the stent and the connective tissue between the interstices of the stent formed a tight barrier preventing arterial bleeding after the extarterectomy in all cases. In the follow-up period there were no neurologic deficits in the study group.
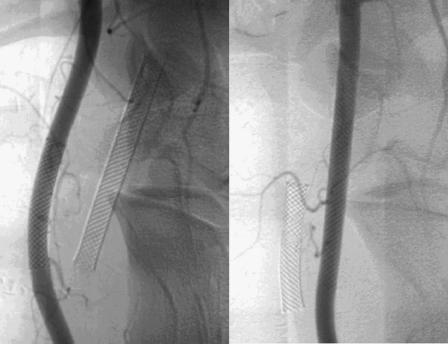
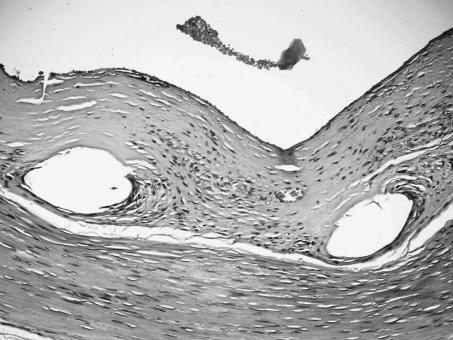
Control angiographies at 6 months revealed patent CAs without angiographically visible intimal thickening or aneurysm formation (Fig. 3). Light microscopic evaluation of the hematoxylin and eosin-stained cross-sectional specimens demonstrated a distinct layer of neointima within the extarterectomized segment (Fig. 4). Gross evaluation of the arteries after sacrifice of the animals at 18 months showed a smooth vascular surface on the internal surface of the stent without wall-adherent thrombi. Devoid of the arterial wall, the stented extarterectomized segment of the CA was minimally dilated with respect to the nonextarterectomized segment (Fig. 5). Pseudoaneurysm was not present in any case.
Figure 3.
Control angiograms reveal patent carotid arteries without angiographically visible intimal thickening and aneurysm formation.
Figure 4.
Photomicrograph shows distinct neointima formation (hematoxylin & eosin, × 20).
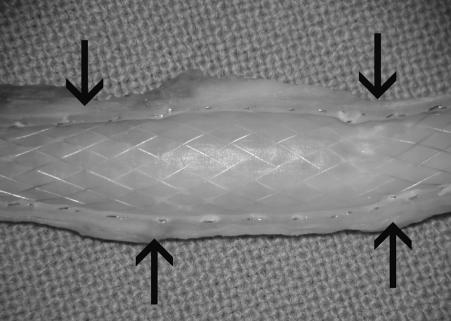
Figure 5.
Half-cut view of the extarterectomized segment with the neointimal layer covering the stent. Arrows show the borders of extarterectomy.
DISCUSSION
Complete resection of benign and malignant tumors of the head and neck is the most reliable method to achieve a cure. Curability of skull base tumors remains elusive due to difficulties in achieving complete tumor excision. The team approach using the skills of a neurosurgeon, otolaryngologist, and neuroradiologist is revolutionizing the concept of resectability, and therefore, curability.1
Nevertheless, controversy still exists about the advisability of resecting a CA invaded by tumor. The prognosis of patients with such lesions is generally poor. In addition, resection of the CA is associated with morbidity and mortality. Unselected carotid ligation causes significant cerebral ischemia in a large number of patients with associated mortality rates as high as 58%.7,10 Thus, some surgeons think that involvement of the CA prohibits surgical resection.11
On the other hand, some others advocate the resection of the CA when involved with tumor to improve cure rates.12,13 Methods of assessing the adequacy of cerebral circulation, modern anesthesia reducing the risk of intraoperative hypotension, and improved techniques of vascular reconstruction have made it possible to resect the CA.
There exist several methods to predict the safety of CA resection. Measurement of the back pressure in the distal CA stump has been used.14,15 Balloon test occlusion (BTO) has also been described.16 However, 5 to 20% of patients who are asymptomatic during BTO develop cerebral infarction following permanent CA occlusion.17 Other promising methods are xenon computed tomography cerebral blood flow imaging, technetium 99m hexamethylpropylenamine oxine single-proton emission computed tomography imaging, positron emission tomographic scanning, induced hypotension at the time of BTO, and intraoperative EEG.5 However, no test or combination of tests has been able to exclude definitively the possibility of cerebral ischemia after CA resection.18
“Stripping” the tumor off the CA has been proposed to avoid these ischemic complications,11,19 but this represents a subtotal resection. In 42% of cases, microscopic invasion of the adventitia and elastic media has been demonstrated.14 In addition, the question also exists as to whether radiation affects vasospasm and stripping of the adventitia might increase the susceptibility of the CA to malignant invasion and rupture.12,17
CA resection and vascular reconstruction using autogenous or synthetic material has been recommended to minimize the risk of ischemic complications.17,20 In a meta-analysis of 22 studies on vascular resection techniques by Snyderman and D'Amico,10 permanent neurologic sequelae were recorded in 17%. It was concluded that vascular reconstruction was not shown to significantly alter the risk of neurologic events following CA resection. Graft infection and anastomotic disruption with serious hemorrhage are the other risks associated with this technique.14,18 In addition, the idea that vascular reconstruction is not necessary in all patients has fueled the argument about the indication of this technique.1
The new technique studied in this experimental model has been named as extarterectomy by Nussbaum and colleagues,18 since the procedure entails removal of the complete external arterial wall. The technique was first described in a swine model by Lopes and Wakhloo.8,9 The concept relies on the endothelization that takes place rapidly after placement of the stent within the CA. This neointima can support the blood flow through the artery inside the stent, despite complete removal of the CA wall.18 Thus, cerebral blood flow is maintained without interruption during the entire procedure. This, in turn, minimizes the risk of ischemic complications.
Endothelization of the inner surface after intra-arterial stent implantation is a well-known and fully understood phenomenon.21 There are many animal studies and some applications on humans derived mainly from histopathologic examination of the resected coronary arteries that were stented previously. While the stent struts are engulfed by the intima in conjunction with neointima formation, endothelialization continues on the inner aspect of the vessel and is completed within 1 to 2 months. Histopathologic findings after human CA stenting, however, have recently been reported in an autopsy case.22 The authors demonstrated that after 8 months, the stented portion of the internal CA shows neointima formation ranging in thickness from 300 to 500 μm over the stent struts. This case gives further support to our hypothesis that endothelization and neointima formation after stent implantation in CA forms a stable and permanent inner layer and can bear arterial blood pressure even with the elimination of the vessel wall exterior to the stent struts.
The study of Lopes and Wakhloo8,9 and the first clinical application by Nussbaum and associates18 have demonstrated that neurologic sequelae were not experienced in any of the cases as in our experimental study. Complications reported in these previous studies were pseudoaneurysm in one animal8,9 and hemorrhage between the interstices of the stent.18 The latter was controlled with external pressure. Neither of these complications nor any other has been experienced in the present experimental study.
CONCLUSION
Although high morbidity and mortality rates have been reduced by improved patient selection criteria using tests of cerebral blood flow, modern anesthesia techniques with avoidance of intraoperative hypotension, and the benefits of CA reconstruction, the risk of cerebral ischemic complications still exists. The new technique, extarterectomy, which allows aggressive tumor removal together with the maintenance of blood flow through the CA, offers a promising aspect for this issue. However, further clinical application and experience are required.
REFERENCES
- de Vries E J, Sekhar L N, Janecka I P, Schramm V L, Jr, Horton J A, Eibling D E. Elective resection of the internal carotid artery without reconstruction. Laryngoscope. 1988;98:960–966. doi: 10.1288/00005537-198809000-00010. [DOI] [PubMed] [Google Scholar]
- Karam F, Schaefer S, Cherryholmes D, Dagher F J. Carotid artery resection and replacement in patients with head and neck malignant tumors. J Cardiovasc Surg (Torino) 1990;31:697–701. [PubMed] [Google Scholar]
- Lore J M, Boulos E J. Resection and reconstruction of the carotid artery in metastatic squamous cell carcinoma. Am J Surg. 1981;142:437–442. doi: 10.1016/0002-9610(81)90370-6. [DOI] [PubMed] [Google Scholar]
- Meleca R J, Marks S C. Carotid artery resection for cancer of the head and neck. Arch Otolaryngol. 1994;120:974–978. doi: 10.1001/archotol.1994.01880330056010. [DOI] [PubMed] [Google Scholar]
- Nayak U K, Donald P J, Stevens D. Internal carotid artery resection for invasion of malignant tumors. Arch Otolaryngol. 1995;121:1029–1033. doi: 10.1001/archotol.1995.01890090067013. [DOI] [PubMed] [Google Scholar]
- Reilly M K, Perry M O, Netterville J L, Meacham P W. Carotid artery replacement in conjunction with resection of squamous cell carcinoma of the neck. J Vasc Surg. 1992;15:324–330. doi: 10.1067/mva.1992.33808. [DOI] [PubMed] [Google Scholar]
- Wright J G, Nicholson R, Schuller D E, Smead W L. Resection of the internal carotid artery and replacement with greater saphenous vein: a safe procedure for en bloc cancer resections with carotid involvement. J Vasc Surg. 1996;23:775–780. doi: 10.1016/s0741-5214(96)70239-2. [DOI] [PubMed] [Google Scholar]
- Lopes D K, Wakhloo A K. Intravascular stents for endoluminal cerebrovascular bypass. San Diego, CA: Paper presented at: Annual Meeting of the American Society of Neuroradiology; May 23–29, 1999.
- Lopes D K, Wakhloo A K. Intravascular stents for endoluminal cerebrovascular bypass. Boston, MA: Paper presented at: Annual Meeting of the Congress of Neurological Surgeons; October 30–November 4, 1999.
- Snyderman C H, D'Amico F. Outcome of carotid artery resection for neoplastic disease: a meta-analysis. Am J Otolaryngol. 1992;13:373–380. doi: 10.1016/0196-0709(92)90079-9. [DOI] [PubMed] [Google Scholar]
- Kennedy J T, Krause C J, Loevy S. The importance of tumor attachment to the carotid artery. Arch Otolaryngol. 1977;103:70–73. doi: 10.1001/archotol.1977.00780190050002. [DOI] [PubMed] [Google Scholar]
- Biller H F, Urken M, Lawson W, Haimov M. Carotid artery resection and bypass for neck carcinoma. Laryngoscope. 1988;98:181–183. doi: 10.1288/00005537-198802000-00012. [DOI] [PubMed] [Google Scholar]
- Urken M, Biller H F, Lawson W, Haimov M. Salvage surgery for recurrent neck carcinoma after multimodality therapy. Head Neck Surg. 1986;8:332–342. doi: 10.1002/hed.2890080504. [DOI] [PubMed] [Google Scholar]
- Atkinson D P, Jacobs L A, Weaver A W. Elective carotid resection for squamous cell carcinoma of the head and neck. Am J Surg. 1984;148:483–488. doi: 10.1016/0002-9610(84)90374-x. [DOI] [PubMed] [Google Scholar]
- Moore W S, Hall A D. Carotid artery back pressure. Arch Surg. 1969;99:702–710. doi: 10.1001/archsurg.1969.01340180026005. [DOI] [PubMed] [Google Scholar]
- Meinig G, Gunther P, Ulrich P, et al. Reduced risk of carotid artery ligation after balloon occlusion test. Neurosurg Rev. 1982;5:95–98. doi: 10.1007/BF01743481. [DOI] [PubMed] [Google Scholar]
- Gonzalez C F, Moret J. Balloon occlusion of the carotid artery prior to surgery for neck tumors. AJNR Am J Neuroradiol. 1990;11:649–652. [PMC free article] [PubMed] [Google Scholar]
- Nussbaum E S, Levine S C, Hamlar D, Madison M T. Carotid stenting and “extarterectomy” in the management of head and neck cancer involving the internal carotid artery: technical case report. Neurosurgery. 2000;47:981–984. doi: 10.1097/00006123-200010000-00041. [DOI] [PubMed] [Google Scholar]
- Stell P M, Dalby J E, Singh S D, Taylor W. The fixed cervical lymph node. Cancer. 1984;53:336–341. doi: 10.1002/1097-0142(19840115)53:2<336::aid-cncr2820530227>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Huvos A G, Learning R H, Moore O S. Clinicopathologic study of the resected carotid artery: analysis of 64 cases. Am J Surg. 1973;126:570–574. doi: 10.1016/s0002-9610(73)80051-0. [DOI] [PubMed] [Google Scholar]
- Cejna M, Virmani R, Jones R, et al. Biocompatibility and performance of the Wallstent and several covered stents in a sheep iliac artery model. J Vasc Interv Radiol. 2001;12:351–358. doi: 10.1016/s1051-0443(07)61916-2. [DOI] [PubMed] [Google Scholar]
- Toma N, Matsushima S, Murao K, et al. Histopathological findings in a human carotid artery after stent implantation. J Neurosurg. 2003;98:199–204. doi: 10.3171/jns.2003.98.1.0199. [DOI] [PubMed] [Google Scholar]