Abstract
Although aspiration of contaminated amniotic fluid and gastric contents is common at birth, anecdotal evidence indicates that coughing occurs rarely if at all. Studies in which cough and other airway protective responses have been stimulated by introducing a small bolus of water or saline into the pharynx of sleeping infants have found that the predominant responses are swallowing, apnoea and laryngeal closure. Coughing is rare. Collectively these responses are known as the laryngeal chemoreflexes (LCR). These are mediated by receptors in the inter-arytenoid space. The LCR has been studied extensively in animal models. Upper airway infection increases the responses and in this case coughing becomes a common component. Studies in animal models indicate that with maturation, apnoea and swallowing components of the LCR decrease while cough becomes increasing prominent.
Keywords: Cough, Maturation, Infant, Laryngeal Chemoreflex
1. Introduction
The fetus is susceptible to aspiration of amniotic fluid. Whether or not the fetus coughs is open to speculation. Clearly cough could not be functional in protecting the airway in a lung filled with viscous fluid. The sudden cough-like movements frequently felt by mothers are hiccups, a laryngeal motor act that never-the-less is unrelated to cough. Intubated newborn infants appear to make coughing efforts during tracheal suctioning but whether this is true coughing is open to question; it may only represent gagging or some of the defensive reflex. Aspiration of amniotic fluid is common in infants during the brief period prior to and immediately after birth. A wide variety of neonatal aspiration syndromes with significant morbidity and mortality have been described [1,2]. These include aspiration of sterile fluid with exfoliated squamous cells, maternal blood, merconium or infectious bacteria. Although pharyngeal and laryngeal protective reflex responses are highly developed in newborn infants and aspiration of contaminated amniotic fluid or gastric contents is relatively common, this is only rarely if at all associated with cough (B.T. Thach personal observations).
One of the earliest occurrences of cough in the newborn is just at the beginning of a cry. This “cough cry sequence” appears at about 1 to 2 months (B.T. Thach, personal observations). Since crying is an emotional response, the cough at the beginning of a cry represents cortical or midbrain influences on the brainstem centres regulating coughing. The “cough cry sequence” is possibly protective since the cough could clear the airway of secretions just prior to the large inspirations that occur with crying.
By far the most research on the neonatal development of cough involves studies of the laryngeal chemoreflex (LCR), which is stimulated by fluids contacting the mucosa of the larynx. In studies of sleeping human infants we have found that a very small bolus (0.1 ml) of saline or water instilled into the pharynx via a nasal catheter stimulates this reflex (3, 4). The LCR reflex consists of several responses. The most common is one or more swallows. Somewhat less common is apnoea. This may or may not be accompanied by laryngeal closure as indicated by obstructed inspiratory efforts (Fig. 1). The least common response is cough, although, this increases in frequently with maturation [3] (Fig. 2).
Fig. 1.
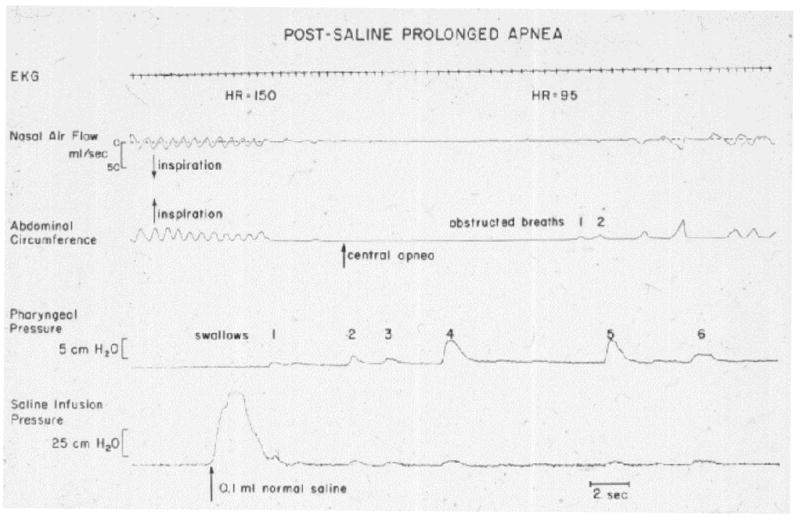
Prolonged apnoea in response to 0.1 ml saline (arrow), delivered to the pharynx of an infant. The response includes cessation of respiratory efforts, obstructed breaths and swallows [From 3].
Fig. 2.
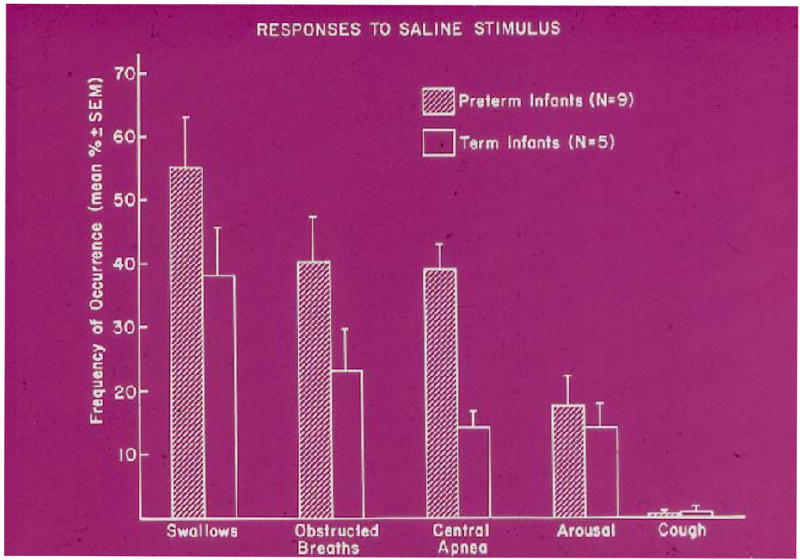
Frequency of responses when a small bolus of saline was introduced into the pharynx of sleeping term and preterm infants. [From 3]
Infants lose the protection of maternally acquired antibodies at 2 to 4 months of age. At this time they become susceptible to upper airway infections. Even otherwise benign upper respiratory infections can augment the LCR reflex and produce prolonged apnoea in both human infant and in neonatal animal models [5, 6, 7] Coughing is characteristic of certain of these airway infections. Respiratory syncytial virus (RCV) infection is extremely common during this period and coughing is a characteristic symptom. The pattern of responses in RSV infected infants is typical of the laryngeal chemoreflex apnoea [5] (Fig. 3). In keeping with the developmental pattern of the laryngeal reflex responses preterm infants who become infected with RSV often present first with apnoea [5]. Cough then comes later in the course of the infection. The same is true of pertussis infection where the classical “whoop” and cough can present as apnoea in young preterm infants.
Fig. 3.
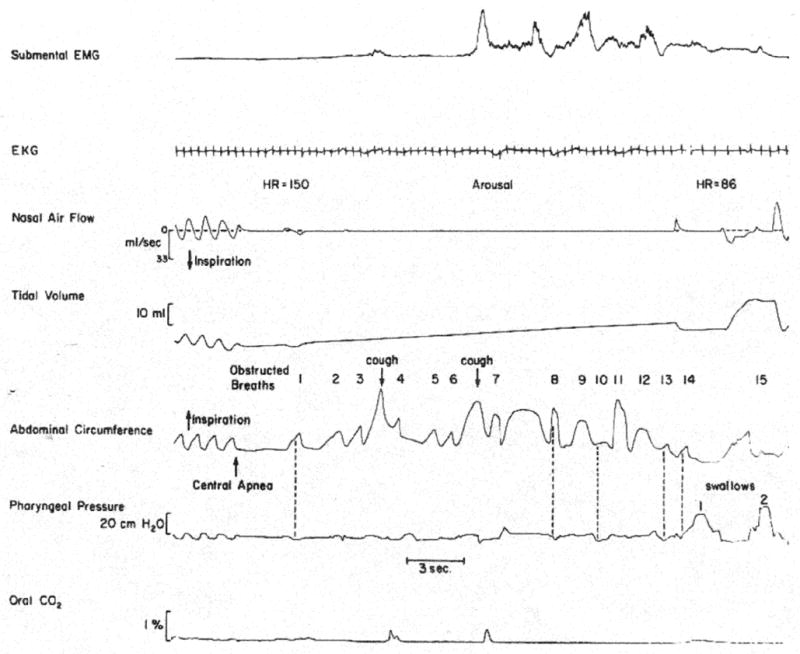
Polygraphic tracings showing a spontaneous prolonged apnoeic spell associated with respiratory syncytial viral infection in an infant. Note spell onset with a brief central apnoea followed by obstructed breaths, coughs and swallows. Cough was heard during the study and is associated with brief oral expiratory flow in the tracing [From 5].
In experimental studies of RSV infection it has been shown that infected laryngeal musocal cells secrete interleukins. These are retrogradly transported through sensory axons to brainstem respiratory controlling centres [7, 8, 9]. This is likely the mechanism whereby the local infection increases the sensitivity of the laryngeal chemoreflex. That is to say rather than local inflammation increasing receptor sensitivity, the increased activity of the LCR is centrally mediated. It has also been shown that intravenous or intrathecal injection of cytokines (I1 1B) in animal studies further augment the reflex and prolong the associated apnea [7], again indicating a central neural mechanism increasing LCR sensitivity. All in all it now seems clear that the LCR associated with laryngeal inflammation due to a respiratory syncytial virus infection, for example, strengthens the activity of the chemoreflex and also matures if from the standpoint that cough becomes a more frequent component.
2. Laryngeal chemoreflexes
In the mid 1970’s Johnson and colleagues discovered that when water is introduced into the larynx of the newborn lamb the response includes startle, arousal from sleep, rapid swallowing, prolonged apnoea and constriction of the airways [10]. Saline introduced into the larynx in the same manner had little or no affect. Others went on to confirm these findings in the neonatal lamb, dog, pig, cat and monkey [11–16]. Cough was not described due to the fact that the animals were tracheostomized. More recent studies in infants indicate that cough can be a component of the response [3, 5]. Others have found that this combination of reflexes arises from stimulation of sensory afferents in the superior laryngeal nerve [17]. Evidence was obtained by several other groups that nonmyelinated nerve endings terminating in the mucosal epithelium of the epiglottis, aryepiglotic folds and interarytenoid space were the primary, and probably sole receptors mediating these reflexes [13, 18, 19]. It has been shown that the concentration of chloride ion in solutions bathing the mucosal surface of the larynx is a critical factor determining receptor discharge [14]. Water and other solutions with low chloride content such as gastric fluid and saliva have a stimulating effect proportionate to their chloride whereas isotonic saline has little or no effect. It is also clear that acids stimulate these receptors even when chloride concentration equals that of plasma [14, 20, 21]. Of interest, the laryngeal water receptors are slowly adapting, meaning that they continue to discharge so long as the stimulant solution is present on the mucosal surface and cease firing only after it is washed away by saline [13, 14, 18, 19].
The laryngeal chemoreflexes (LCRs) perform a protective function. Clearly swallowing removes fluids from the pharyngeal airway, adjacent to the larynx, while vocal chord constriction combined with apnoea can prevent aspiration. Cough, when it occurs also has an obvious protective function. The rapidity of receptor response when water contacts the mucosa (50–250 ms) as well as the location of the receptors on the airway surface of the epiglottis, aryepiglottic folds and interarytenoid space also indicate an advantageous adaptation, one that is well suited to an airway protective role [19]. That is to say, appropriate protective responses can rapidly occur prior to any penetration of fluid into the laryngeal vestibule. Also the closure of the interarytenoid space during swallow, causing an apposition of the mucosal surfaces, can “blot” away fluids from the epithelium overlying the water receptors thereby reducing stimuli that might perpetuate reflex swallowing [3] (Fig. 4).
Fig. 4.
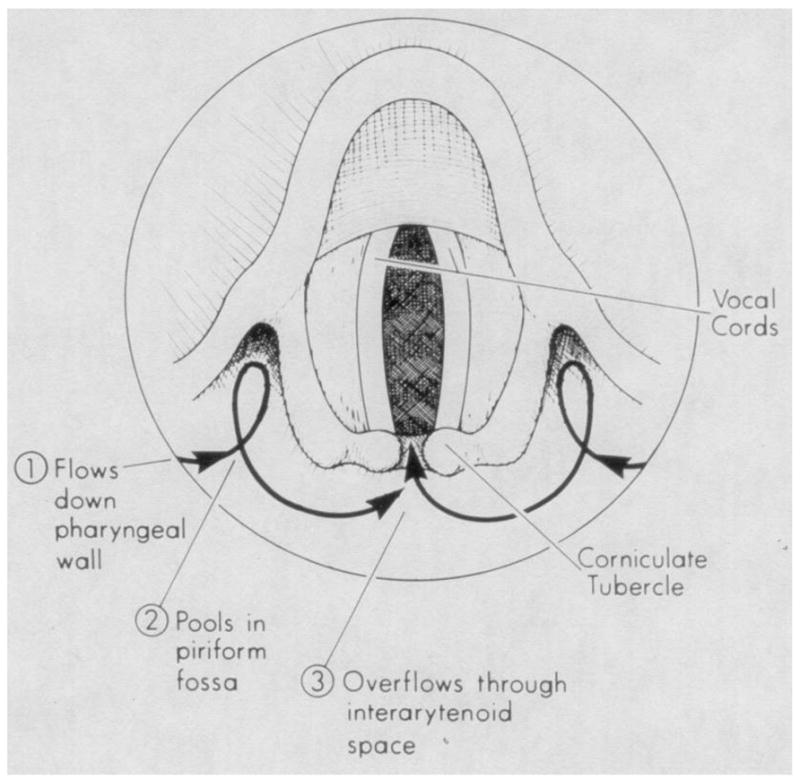
A stylized view of hypopharynx illustrating path of flowing saline using the stimulus technique for living subjects. After saline pools in piriform fossae, the first contact with laryngeal structures is by way of narrow channel in interarytenoid space or notch. The observations were made using endoscopy in infant cadavers [From 22].
In sleeping infants the LCR has been elicited by reflex instilling liquids into the pharynx until fluid levels in the piriform fossae gradually increase until they contact the LCR receptors at the entrance to the laryngeal airway [3, 22] (Fig. 4). Notably, in these studies a weak but often significant effect of saline in producing LCR responses was noted. This apparent effect of isotonic saline might be the result of the introduced fluids mixing with hypochloraemic pharyngeal secretions prior to reaching the laryngeal receptors. However, a weak stimulant effect of saline has been found in studies on the isolated superior laryngeal nerve. Additionally it cannot be ruled out that saline elicits mechanoreceptor responses as well. When laryngeal nerves are electrically stimulated prolonged apnoea is the predominant response in the tracheostomized piglet [23].
3. Maturation of the LCR responses
A reasonably clear picture of normal maturation of the various components of the LCR has emerged. This has come from studies of unanaesthetized dogs of various ages as well as studies of mature and immature human infants. Boggs and Bartlett found that prolonged apnoea and arousal were predominant responses to LCR stimulation in newborn puppies [14]. Apnoea duration diminished greatly after the first week of life. Swallowing was likely also prominent, as in other neonatal species, but this was not studied. In contrast, Sullivan and colleagues characterized LCR responses in sleeping adult dogs and found significant apnoea occurring only in REM sleep [24]. In contrast to findings in puppies in adult dogs cough, arousal and occasional swallows were the predominant responses in most situations with coughing being dependent on prior arousal [24]. Characterization of laryngeal airway closure with LCR stimulation has been made in the rabbit and dog [14, 21, 25]. Maturation increases this response in the canine model [25]. In studies of sleeping human infants stimulated with water infused into the pharynx, repeated swallowing, apnoea and airway closure resulting in obstructed inspiratory efforts are prominent in young premature infants [3, 22, 26, 27]. In infants born at term these responses are less frequent and less prolonged [22]. In these mature infants, a brief respiratory pause with one or two swallows is typical when a bolus of water was introduced into the pharynx, a situation unlike the more exaggerated response of the less mature infant born prematurely. Like the newborn puppy, coughing was infrequent in both preterm and term newborn infants. Arousal was also relatively infrequent. Apnoea of longer duration combined with bradycardia and airway obstruction was an infrequent response in term infants but was almost exclusively found in infants born prematurely [3, 22].
It is relevant that several studies in preterm infants have found that apnoea with bradycardia associated with swallowing and intermittent airway obstruction occur spontaneously during sleep in the absence of any detectable stimulus as well as during regurgitation of gastric contents [28]. This suggests that endogenous stimuli from accumulated pharyngeal secretions as well as regurgitated gastric fluid can elicit LCR responses in preterm infants and are a significant cause of apnoea of prematurity [3] (Fig. 4).
4. Conclusions
In summary, studies in animal models it indicate that maturation of the laryngeal chemoreflex is characterized by an increase in coughing, a decrease in swallowing and apnoea. Laryngeal nerve stimulation studies conducted by Lawson indicate that these changes likely are the result of central processing of afferent stimuli rather than reduction in sensitivity or change in receptor distribution within the larynx [14, 19]. Apnoea, swallowing, laryngeal closure and cough to some degree can be elicited postnatally by stimulating laryngeal water receptors, in the unanaesthetized, human newborn infant. Whereas, in the unanaesthetized adult dog introduction of a bolus of water into the larynx is a far more potent stimulus for coughing than it is for swallowing or apnoea. Both observations suggest that laryngeal receptors responsive both to water and acidity continue to be the primary defense against laryngeal aspiration of fluids long into adult life. In addition there is no indication that laryngeal water/acid receptors change in number, anatomical location or sensitivity during maturation in adults. Some authors have suggested that the postnatal disappearance of LCR-prolonged apnoea indicates that chemoreflexes have a diminished importance in defending the airway postnatally. The above considerations suggest another view, one in which the LCR continues into adult life with maturationally appropriate modifications and transitions in the several responses.
Acknowledgments
This work was funded by the National Institute of Child Health and Diseases
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Avery ME, Fletcher BD. The lung and its disorders in the newborn infant. 5. Philadelphia: W.B. Saunders; 1992. [Google Scholar]
- 2.Whitsett JA, Pryhuber GS, Rice WR, Warner B, West S. Acute respiratory disorders. In: Avery GBA, McDonald MG, editors. Neonatology, Pathophysiology and Management of the Newborn. 4. Philadelphia: J.B. Lippencott Company; 1994. pp. 429–52. [Google Scholar]
- 3.Pickens DL, Schefft GL, Thach BT. Prolonged apnea associated with upper airway protective reflexes in apnea of prematurity. Am Rev Respir Dis. 1988;137:113–8. doi: 10.1164/ajrccm/137.1.113. [DOI] [PubMed] [Google Scholar]
- 4.Perkett E, Vaughan R. Evidence for a laryngeal chemoreflex in some human preterm infants. Acta Paediatr Scand. 1982;71:969–72. doi: 10.1111/j.1651-2227.1982.tb09558.x. [DOI] [PubMed] [Google Scholar]
- 5.Pickens DL, Schefft GL, Storch GA, Thach BT. Characterization of prolonged apneic episodes associated with respiratory syncytial virus infection. Pediatr Pulmonol. 1989;6:195–201. doi: 10.1002/ppul.1950060314. [DOI] [PubMed] [Google Scholar]
- 6.Lindgren C, Grogaad J. Reflex apnoea response and inflammatory mediators in infants with respiratory tract infection. Acta Paediatr. 1996;85:798–803. doi: 10.1111/j.1651-2227.1996.tb14154.x. [DOI] [PubMed] [Google Scholar]
- 7.Lindgren C, Jing L, Graham B, Grogaard J, Sundell H. Respiratory syncytial virus infection reinforces reflex apnea in young lambs. Pediatr Res. 1992;31:381–5. doi: 10.1203/00006450-199204000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Maehlen J, Olsson T, Zachau A, Klareskog L, Kristensson K. Local enhancement of major histocompatibility complex (MHC) Class I and II expression and cell infiltration in experimental allergic encephalomyelitis around axotomized motor neutrons. J Neuroimmunology. 1989;24:105–12. doi: 10.1016/0165-5728(89)90031-3. [DOI] [PubMed] [Google Scholar]
- 9.Banks WA, Ortiz L, Plotkin SR, Kastin AJ. Human interleukin (IL) 1 alpha, murine IL-1alpha and murine I1-beta are transported from blood to brain in the mouse by a shared saturable mechanism. J Pharm Exper Therap. 1991;259:988–96. [PubMed] [Google Scholar]
- 10.Johnson P, Salisbury D, Storey A. Apnea induced by stimulation of sensory receptors in the larynx. In: Bosma JF, Showacre J, editors. Symposium on Development of Upper Respiratory Anatomy and Function. Implications for Sudden Infant Death Syndrome. Washington, DC: US Department of Health, Education and Welfare; 1975. pp. 160–85. [Google Scholar]
- 11.Harding R, Johnson P, McCelland ME. Liquid-sensitive laryngeal receptors in the developing sheep, cat and monkey. J Physiol (Lond) 1978;277:409–22. doi: 10.1113/jphysiol.1978.sp012281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Downing SE, Lee JC. Laryngeal chemosensitivity: A possible mechanism for Sudden Infant Death. Pediatrics. 1975;55:640–9. [PubMed] [Google Scholar]
- 13.Lucier GE, Storey AT, Sessle BJ. Effects of upper respiratory tract stimuli on neonatal respiration: reflex and single neuron analyses in the kitten. Biol Neonate. 1979;35:82–9. doi: 10.1159/000241157. [DOI] [PubMed] [Google Scholar]
- 14.Boggs DF, Bartlett D., Jr Chemical specificity of a laryngeal apneic reflex in puppies. J Appl Physiol Respirat Environ Exercise Physiol. 1882;53:455–462. doi: 10.1152/jappl.1982.53.2.455. [DOI] [PubMed] [Google Scholar]
- 15.Harned HS, Myracle J, Jr, Ferreiro J. Respiratory suppression and swallowing from introduction of fluids into the laryngeal region of the lamb. Pediat Res. 1978;12:1003–9. doi: 10.1203/00006450-197810000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Grogaard J, Lindstrom DP, Stahlman MT, Marchal F, Sundell H. The cardiovascular response to laryngeal water administration in young lambs. J Dev Physiol. 1982;4:353–70. [PubMed] [Google Scholar]
- 17.Sutton D, Taylor EM, Linderman RC. Prolonged apnea in infant monkeys resulting from stimulation of superior laryngeal nerve. Pediatrics. 1978;61:519–27. [PubMed] [Google Scholar]
- 18.Storey A, Johnson P. Laryngeal water receptors initiating apnea in the lamb. Exp Neurol. 1975;47:42–55. doi: 10.1016/0014-4886(75)90235-6. [DOI] [PubMed] [Google Scholar]
- 19.Storey A. A functional analysis of sensory units innervating epiglottis and larynx. Exp Neurol. 1968;20:366–83. doi: 10.1016/0014-4886(68)90080-0. [DOI] [PubMed] [Google Scholar]
- 20.Kovar I, Selstam U, Catterton WZ, Stahlman M, Sundell H. Laryngeal chemoreflex in newborn lambs: respiratory and swallowing response to salts, acids, and sugar. Pediat Res. 1979;13:1144–9. doi: 10.1203/00006450-197910000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Wetmore R. Effects of acid on the larynx of the maturing rabbit and their possible significance to the Sudden Infant Death Syndrome. Laryngoscope. 1993;103:1242–54. doi: 10.1288/00005537-199311000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Pickens DL, Schefft GL, Thach BT. Pharyngeal fluid clearance and aspiration preventive mechanisms in sleeping infants. J Appl Physiol. 1989;66:1164–71. doi: 10.1152/jappl.1989.66.3.1164. [DOI] [PubMed] [Google Scholar]
- 23.Lawson E. Prolonged central respiratory inhibition following reflex-induced apnea. J Appl Physiol Respirat Environ Exer Physiol. 1981;50:874–9. doi: 10.1152/jappl.1981.50.4.874. [DOI] [PubMed] [Google Scholar]
- 24.Sullivan CE, Murphy E, Kozar LF, Phillipson EA. Waking and ventilatory responses to laryngeal stimulation in sleeping dogs. J Appl Physiol Respirat Environ Exercise Physiol. 1978;45:682–9. doi: 10.1152/jappl.1978.45.5.681. [DOI] [PubMed] [Google Scholar]
- 25.Sasaki CT, Susucki M, Horiuchi M. Postnatal development of laryngeal reflexes in the dog. Arch Otolaryngol. 1977;103:138–43. doi: 10.1001/archotol.1977.00780200064005. [DOI] [PubMed] [Google Scholar]
- 26.Davies AM, Koenig JS, Thach BT. Characteristics of upper airway chemoreflex prolonged apnea in human infants. Am Rev Respir Dis. 1989;139:668–73. doi: 10.1164/ajrccm/139.3.668. [DOI] [PubMed] [Google Scholar]
- 27.Davies AM, Koenig JS, Thach BT. Upper airway chemoreflex responses to saline and water in preterm infants. J Appl Physiol. 1988;64:1412–20. doi: 10.1152/jappl.1988.64.4.1412. [DOI] [PubMed] [Google Scholar]
- 28.Menon AP, Schefft GL, Thach BT. Apnea associated with regurgitation in infants. J Pediatr. 1995;106:625–62. doi: 10.1016/s0022-3476(85)80091-3. [DOI] [PubMed] [Google Scholar]
- 29.Donnelly D, Haddad G. Respiratory changes induced by prolonged laryngeal stimulation in awake piglets. J Appl Physiol. 1986;61:1018–24. doi: 10.1152/jappl.1986.61.3.1018. [DOI] [PubMed] [Google Scholar]


