Abstract
Objectives
Streptococcus pneumoniae is the leading cause of bacterial pneumonia and associated bacteremia during HIV infection. Rapid diagnostic assays may limit inappropriate therapy.
Methods
Clinical signs and symptoms and sera and urine were collected prospectively from 70 adults with pneumococcal pneumonia, including 47 with HIV co-infection. Pneumococcal C-polysaccharide antigen was detected in urine using the Binax® immunochromatographic test (ICT). A systematic review of 24 published studies was conducted.
Results
Clinical symptoms, signs, and laboratory parameters except leukocytosis, were similar in HIV-infected and HIV-seronegative pneumonia. The performance of the urine antigen ICT was independent of HIV-status (sensitivity 81%, specificity 98%, positive (PPV) and negative predictive values (NPV) 98%, and 82%, respectively). The sensitivity of sputum Gram’s stain was 58% [34/59] with sputum unable to be provided by 16%. The CRP response was identical in HIV-infected (mean ± SD) 133 ± 88 vs. seronegative 135 ± 104 mg/L (p=0.9). In the systematic review, the ICT performance revealed 74% sensitivity (95% CI: 72% to 77%) and 94% specificity (95% CI: 93% to 95%). Urine antigen testing increases etiologic diagnosis by 23% (Range: 10% –59%) when testing adults with community acquired pneumonia of unknown etiology.
Conclusions
Urinary antigen detection provides a credible rapid diagnostic test for pneumococcal pneumonia regardless of HIV-status. CRP response to acute infection is similar in HIV co-infection and increases diagnostic certainty.
Keywords: HIV, pneumonia, community acquired pneumonia, streptococcus pnuemoniae, pneumococcal, diagnosis, c-reactive protein, sensitivity
INTRODUCTION
Streptococcus pneumoniae is the leading cause of community acquired pneumonia (CAP), the 6th leading cause of death overall and the leading cause of infectious disease deaths.1,2,3,4,5 In England and Wales, the rates of invasive pneumococcal disease are at near 10 per 100,000 general population.6 Among HIV-infected adults, rates of CAP are 5–10 fold higher than in age-matched populations, and bacteremic pneumonia occurs 35–50 times more commonly.7,8,9,10 Rates in an HIV population approach 0.4–8 per 100 patient years in the U.S. and 1–4 per 100 person years in Sub-Saharan Africa.8,10,11,12 Despite the beneficial effects of HAART and the paediatric conjugate pneumococcal vaccination on the reducing the rates of CAP in the past decade, HIV-infected adults still comprise 24% of U.S. invasive pneumococcal disease and 28% of pneumonia deaths in British young-adults13,14
The persistently high incidence and morbidity of HIV-associated pneumococcal infections highlight the need for tools for rapid and accurate diagnosis. In part, this limitation is related to the suboptimal availability and poor quality of sputum specimens provided to laboratories.15 Rates of pathogen detection in CAP are only 30–50%.16,17,18 Musher et al. emphasized the importance of collecting adequate sputum specimens promptly, prior to antibiotic administration for diagnosis of pneumococcal pneumonia,18 but such specimens are often neither sought nor provided.15 Moreover, the decline in accessible microbiologic facilities in developed countries and the dearth of microbiological laboratories in much of the developing world, where the burden of HIV disease predominates, highlights the need for rapid diagnostic tests for S. pneumoniae infection at the point of care. The inability to establish specific aetiologic diagnoses leads expert panels to recommend broad-spectrum empiric antimicrobials for CAP. These well-intentioned efforts likely increase cost and facilitate the emergence of resistant organisms.
The Binax NOW® immunochromatographic test (ICT) is a rapid urine antigen test with reasonable performance characteristics among adults with clinical pneumonia.19,20 However, immunocompromised persons, including those with HIV-infection, have been largely excluded from prior urine antigen studies. In this report we characterize the performance characteristics of this ICT among HIV-infected patients with documented S. pneumoniae pneumonia and bacteremia.
PATIENTS AND METHODS
Study population and setting
We prospectively enrolled 230 adult patients from a study of the natural history of pulmonary complications of HIV infection at San Francisco General Hospital during 1990–92, as previously described.10,21,22 As a sub-study, urine was collected from 70 o these patients for urine antigen testing. After written informed consent, patients were selected for inclusion if they presented with pneumococcal pneumonia, defined by a compatible clinical scenario, a chest radiograph demonstrating parenchymal opacities, and microbiologic criteria. The microbiologic criteria included either: 1) S. pneumoniae cultured from a sterile site, i.e. blood, CSF, or tracheal aspirate; 2) acceptable sputum with Gram’s stain revealing a predominance of Gram-positive cocci in pairs and chains at 1,000x magnification with or without a positive S. pneumoniae sputum culture. Sputum samples were considered acceptable with >25 WBC and <10 epithelial cells per 400x field. Individuals were identified prospectively via microbiologic reporting.
Cases were defined as either 1) pneumococcal pneumonia or 2) pneumococcal bacteremic pneumonia. Exclusion criteria for this study included: lack of urine collection (n= 160) at hospital admission, nosocomial pneumonia or history of prior pneumococcal pneumonia, bacteremia, or hospitalization for S. pneumoniae. Control samples were obtained from 17 HIV+ and 46 HIV− healthy ambulatory patients without clinical evidence of pneumococcal infection. An exclusion criterion for controls was the presence of an active, undiagnosed pulmonary infection at the study visit. Clinical characteristics as well as serum, urine, sputum and blood for culture were collected at time of diagnosis. C-reactive protein (CRP) was measured from collected serum by antigen-antibody agglutination (Abbott). Urine samples were immediately frozen and stored at −70°C for a median of 13.5 years. Convalescent urine was collected from 64% [45/70] one month after illness. The urine was collected with the express a priori intent for urine antigen testing; however, this was not possible until after August 31, 1999 when the current ICT became available.
Urine antigen detection
The Binax® NOW ICT (Binax Inc. Portland, Maine) was performed per the manufacturer’s instructions.23 Unconcentrated aliquots (1 mL) of urine were thawed to room temperature. Briefly, the manufacturer-provided swab was dipped into a gently swirled urine sample and inserted into the test device. The Binax ICT utilizes a colloidal gold-labeled antibody immobilized on a nitrocellulose membrane. When the device is closed, the sample is brought in contact with the test strip. Any pneumococcal antigen present binds with conjugated rabbit anti-C-polysaccharide S. pneumoniae antibody creating an antigen-antibody complex. A colorimetric reaction occurs when those complexes are captured by anti-S. pneumoniae antibodies immobilized on the sample line (i.e. positive) on the test device. Photography documented results at 15 minutes, and three masked readers read the results independently. This step was undertaken to determine inter-observer variability, previously suggested as a cause of variation in ICT performance.24 Readers quantified test results based on their visual intensity as: negative (no detectable test band), weak positive (any visible test band <control band intensity), or strong positive (test band ≥control band intensity) [Appendix 1].
To exclude false negative reactions, all presumed false negative results (ICT negative but with bacteremic pneumococcal infection) were confirmed first by repeat urine ICT. In addition, the urine was acidified with buffered NH4Cl to dissociate potential antigen-antibody complexes as previously described,25 then pH neutralized, and the samples were re-tested.
Systematic Review
We identified and reviewed study design, performance characteristics and the microbiologic “gold-standard” used in 20 studies in adults from the PubMed database (1950–June, 2007) identified using the keywords Binax, urine antigen, and Streptococcus pneumoniae and 5 additional studies from cross-references. Selected studies showed heterogeneity in the populations and gold-standards used to assess the test, but most used standard sputum Gram’s stain, sputum culture, and blood culture for diagnosis. In the published studies, when the ICT was tested in patients with pneumonia of unknown aetiology, this is reported separately. Following the STARD initiative recommendations, we present positive and negative likelihood ratios (LR+, LR−). Data were analyzed using Meta-DiSc software (v1.4).26 Summary values of the test performance were calculated from pooled Mantel-Haenszel weighted means.
Data Analysis
The sensitivity and specificity of the Binax® urinary ICT for diagnosing pneumococcal infection was determined, stratified by HIV-status. A double blind was maintained with test performance, readers, and data analyses. Statistical analysis was performed with SPSS 14.0 (Chicago, IL). Continuous, normally distributed variables are expressed as mean ±standard deviation (SD) with comparisons via Student’s t-test. For categorical variables, Fisher’s exact test was used. For comparisons between inter-observer or multiple measurements, the Kappa statistic is presented. Statistical significance was defined as P<.05.
RESULTS
Diagnosis
Seventy pneumococcal infections occurred during the study (47 HIV+, 23 HIV−). Sixty infections were bacteremic pneumonia. Diagnostic methods of included sputum Gram’s stain (n=34), sputum culture (n=32), and/or both bacteremia and sputum positive by either Gram’s stain and/or culture (n=32) [Figure 1].
Figure 1.
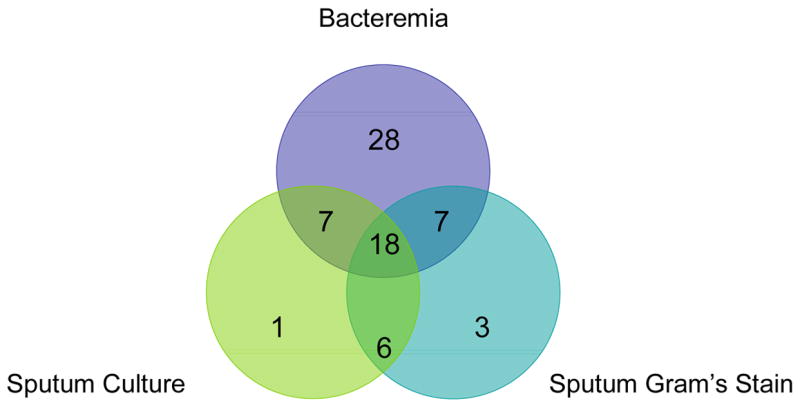
Distribution of the microbiologic determination of pneumococcal pneumonia
Clinical features of pneumococcal infection
Presenting symptoms included: cough, tactile fever, pleuritic chest pain and chills were present in ≥75% of each group (Table 1). The majority of patients with and without HIV infection showed symptoms at comparable frequencies. The median duration of symptoms prior to admission was 4 days (IQR: 2–5 days). Cough was present longer than other symptoms by 1.1 days (95% CI: 0.4–1.8 days; P=.0004). No patient reported receiving antibiotics in the 30 days prior to admission, including trimethoprim-sulfamethoxazole prophylaxis.
Table 1.
Clinical characteristics of patients with pneumococcal pneumonia and control subjects.
| Demographic | HIV-infected S. pneumoniae Pneumonia | HIV-negative S. pneumoniae Pneumonia | HIV-infected Control Subjects | HIV-negative Controls |
|---|---|---|---|---|
| No. of patients | 47 | 23 | 17 | 46 |
| Age (mean ± SD) | 35 ± 6 | 38 ± 7 | 34 ± 6 | 42 ± 15 |
| Sex (M:F) | 39 : 8 | 18 : 5 | 12 : 5 | 29 : 17 |
| Intravenous drug use | 21 (45%) | 11 (48%) | 11 (64%) | 0% |
| Smoking, current‡ | 72% | 86% | 79% | |
| MSM | 26 (55%)* | 1 (4%) | 6 (35%) | 0% |
| CD4+ T cells/μL (mean ±SD) | 218 ± 207 | 593 ± 316 | 360 ± 230 | |
| CD4+ T cells <200/μL (%)§ | 27 (56%) | 5 (22%) | 6 (33%) | |
| Clinical Symptoms & Signs | ||||
|
| ||||
| Cough | 93% | 95% | 28% | N/A† |
| Fever (subjective) | 90% | 89% | 11% | N/A |
| Pleural pain | 91% | 80% | 0% | N/A |
| Chills | 74% | 83% | 0% | N/A |
| Haemoptysis | 51% | 36% | 0% | N/A |
| Temperature >38°C | 63% | 71% | 0% | N/A |
p<05
Numbers (%) unless otherwise specified. MSM = men who have sex with men
Clinical symptoms and signs were not collected from HIV-negative controls.
Tobacco status known for 43 HIV-positive with pneumonia, 21 HIV-negative with pneumonia, 14 HIV-controls, and 1 HIV-negative control.
7 HIV pneumonia patients and 3 HIV controls patients were being treated with zidovudine (AZT) monotherapy.
HIV-infected patients with pneumonia were equally likely to generate an inflammatory response to the infection (fever, elevated CRP), although the proportion with elevated neutrophil counts was lower (Table 2). Chest radiographs revealed parenchymal opacities in all patients. These opacities were multi-lobar in 44% [31/70] with bilateral involvement in 30% [21/70] of persons with pneumococcal pneumonia. The most commonly infected lobes included the left lower (n=37) and right lower (n=36) lung lobes. The frequency and distribution of infiltrates was similar regardless of HIV-status. The interval between collection of blood for culture and final identification of positive cultures averaged 2.1 ±1.2 days with 33% [24/60] formally identified on hospital day two or three. A majority of HIV-infected patients with pneumonia had advanced HIV disease (56% < 200 CD4+ T cells/μL).
Table 2.
Presenting Laboratory Findings:
| Laboratory Parameters | Pneumococcal Pneumonia: HIV-infected (n = 47) | Pneumococcal Pneumonia: HIV-negative (n = 23) | Healthy: HIV-infected Controls (n = 17) ‡ |
|---|---|---|---|
| Haematologic parameters | |||
|
| |||
| WBC cells × 106/μL (mean ± SD) | 11.0 ± 6.9* | 15.6 ± 6.6 | 4.7 ± 1.7 |
| WBC >15 cells × 106/μL | 31%* | 54% | 0% |
| Absolute neutrophil count ×106/μL(mean ± SD) | 8.9 ± 5.9* | 14.4 ± 7.2 | 2.9 ± 1.3 |
| CRP mg/L (mean ± SD) § | 133 ± 88 | 135 ± 104 | 5 ± 6 |
| CRP > 40 mg/L § | 90% | 96% | 1% |
|
| |||
| Microbiology | |||
|
| |||
| Bacteremia | 41 (87%) | 19 (83%) | - |
| Diagnostic Sputum Gram’s Stain† | 57% [24/41] | 56% [10/18] | - |
| Unable to provide acceptable sputum† | 7 (15%) | 6 (26%) | - |
| Sputum culture % [positive / acceptable† collected] | 55% [22 / 40] | 59% [10/17] | - |
| Binax Urine Antigen positive | 39 (83%) | 18 (78%) | 1 (6%)** |
p<.05 compared with HIV-seronegative patients with pneumococcal pneumonia
The frequency of Binax positive urine antigen was 0% [0/46] in HIV-seronegative control subjects for whom other laboratory values were not available.
Diagnostic sputum Gram’s stain had a predominance of Gram positive cocci in pairs/chains in acceptable sputum. An acceptable sputum specimen for culture had >25 WBC and <10 epithelial cells per 400x field.
Data obtained for CRP from 90 HIV+ controls, WBC values from 63 HIV+ controls.
CRP normal reference value <8 mg/L
Sputum Specimens
Among pneumonia patients, sputum Gram’s stains were obtained in 59 (84%) subjects, and acceptable sputum cultured in 57 (81%) subjects (Table 2). The sensitivity of the sputum Gram’s stain was 58% (34/59) among all pneumonia patients and 51% (25/49) in bacteremic pneumonia. Only 37% (18/49) had sputum positive both by Gram’s stain and positive by sputum culture among bacteremic pneumonia cases and 41% (22/54) among all pneumonia patients (Kappa=0.42). Sputum collected >24 hours after antibiotic therapy rarely yielded either a positive Gram’s stain [4/12] or culture [4/11] compared with those collected within 24 hours of admission, 68% (30/44) for Gram’s stain (P=.03). Timing of sputum collection was unavailable for three subjects. Over one-third of patients with bacteremic pneumonia submitting sputum had non-diagnostic sputum specimens (18/50; 36%) either by Gram’s stain or culture and an additional 16% (10/60) of bacteremic pneumonias submitted no sputum sample, suggesting the need for an adjunctive diagnostic test at the point of care when making therapeutic decisions.
Urine antigen detection (ICT)
Among 70 cases of pneumococcal pneumonia, the urine ICT was positive in 81% (57/70), including 85% (51/60) of bacteremic pneumonias. The urine antigen performance was similar regardless of HIV status: 83% HIV+ vs. 78% HIV− (Table 2). Among control subjects without clinical evidence of current pneumococcal disease, the ICT showed positive results in none of 46 HIV-negative control subjects and 6% (1/17) HIV-infected control subjects. Overall, the urine antigen ICT sensitivity was 81% (95% CI: 71% to 89%) for pneumococcal infection, and specificity was 98% [62/63] (95% CI: 92% to 100%), with similar sensitivity among HIV-infected (83%) as seronegative (78%) persons. The urine antigen ICT LR+ was 51.3 (95% CI: 7.3 to 360), and the LR− was 0.19 (95% CI: 0.12 to 0.31). S. pneumoniae serotypes 1, 3, 4 (n=3), 9, 12 (n=3), 14 (n=4), 18, 19 (n=2), 20 (n=2), and 23 were detected by the Binax ICT. One bacteremic episode with serotype 16 yielded a false negative. There were no differences in urine antigen sensitivity based on the duration of symptoms nor on the time to positive blood culture (83% on hospital day ≤1 versus 87% on days ≥2). Proteinuria did not affect the ICT performance. The inter-observer validity was excellent with 99% agreement on the Binax ICT positivity among three readers.
False Negatives
Among patients with pneumococcal pneumonia, false negative results did not differ by clinical characteristics (symptom duration, fever), bacteremia, WBC count, or CRP level. Urine immune complexes were not responsible for false negative results as no new positive results were detected after urine acid dissociation of potential antibody-antigen complexes.
Antigen Clearance
Persistently positive results in convalescent samples collected at one month were detected in 40% (18/45) of pneumonia patients tested. Persistent antigen was more likely detected among those with initial strong positive results (58% [14/24]; RR 3.1, 95% CI: 1.4 to 6.9, P =.01) compared with those with initial weak positives (19% [4/21]) The frequency of persistent antigenuria at one month was comparable in pneumonia patients with and without HIV infection (40% [12/30] vs. 42% [6/14], respectively).
C-reactive protein
Results of CRP and ICT paralleled one another. Among patients with pneumonia, CRP was elevated (mean 132 ±93 mg/L, range 35–479 mg/L; 90% >40 mg/L). The frequency and magnitude of CRP responses were independent of HIV status (P=.6) (Figure 2). Using both tests together (Binax ICT and CRP >40 mg/L) increased the test performance modestly (Table 3) by raising the NPV (83% to 96%) and only slightly lowering the sensitivity (81% to 75%) compared with ICT alone. A negative Binax ICT and CRP <40 mg/L effectively excluded pneumococcal pneumonia (NPV 96%).
Figure 2.
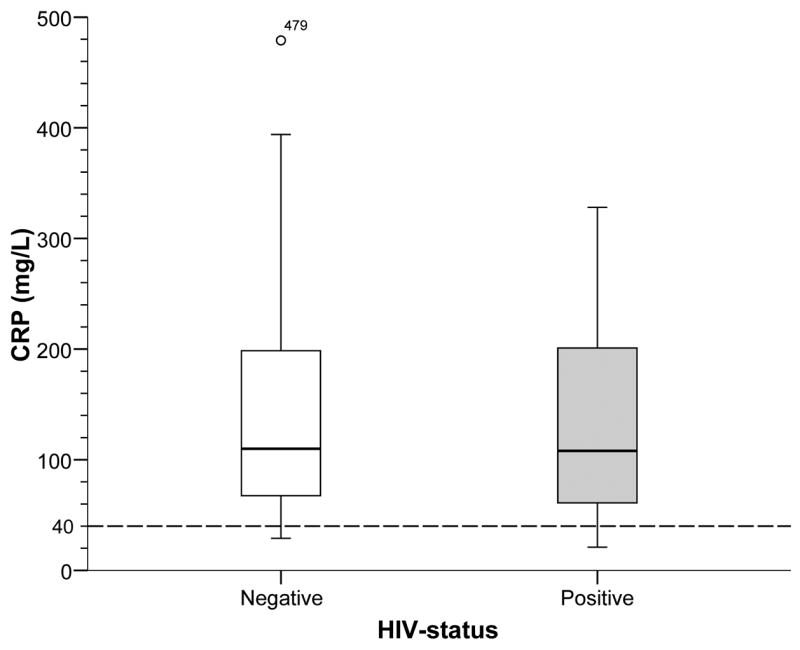
CRP Response among patients with pneumococcal pneumonia by HIV-status
Table 3.
Performance of Diagnostic Methodologies
| Test | Number of Samples | Number Positive | Sensitivity % | Specificity % | PPV % | NPV % |
|---|---|---|---|---|---|---|
| Sputum Gram’s Stain | 59 | 34 | 58 | 100* | 100* | -- |
| Sputum Culture | 57 | 32 | 56 | 100* | 100* | -- |
| Binax Urine Antigen | 70 | 57 | 81 | 98 | 98 | 83 |
By inclusion definition; in a clinical setting, the specificity is not 100% due to potential S. pneumoniae colonization.
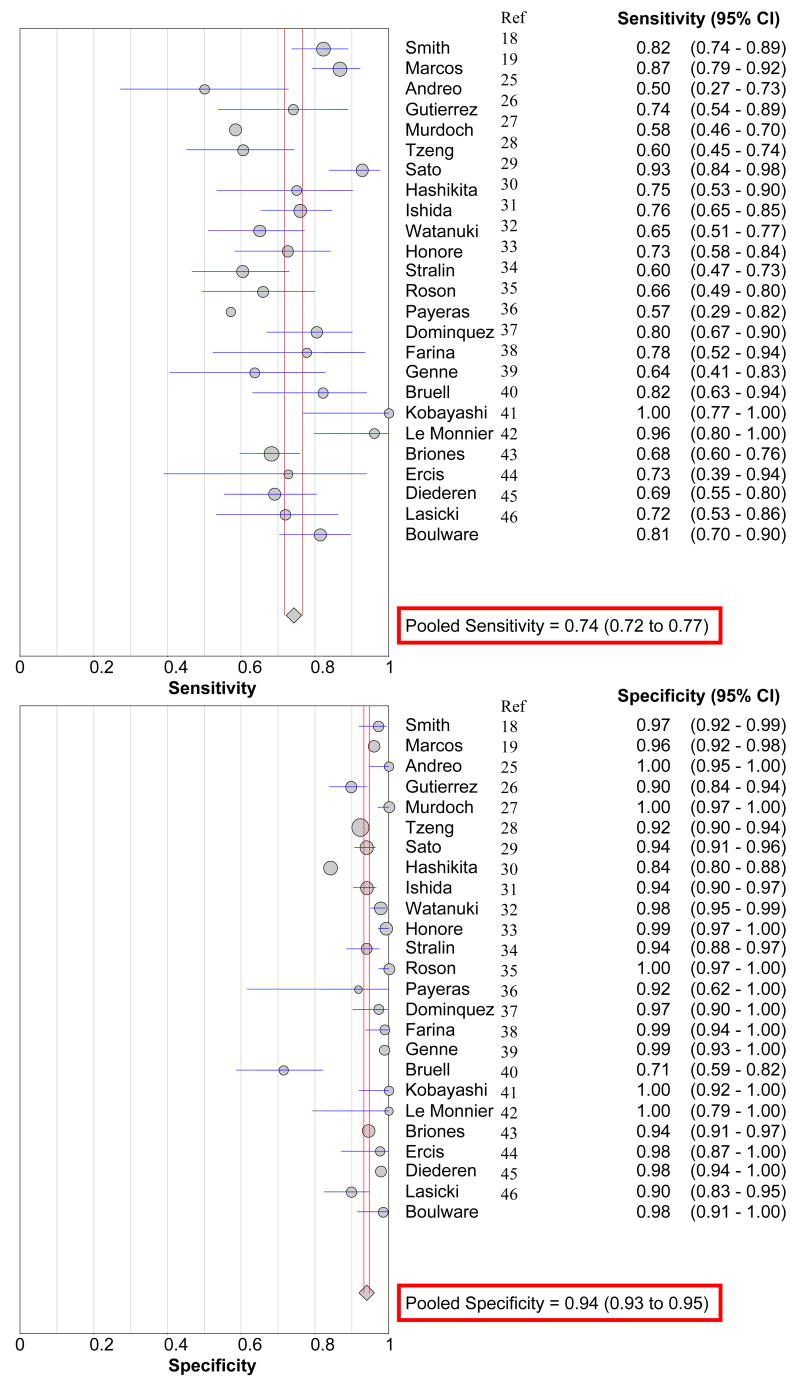
Systematic Review
Among the 25 published studies in HIV-negative adults, the pooled sensitivity for the Binax urine ICT was 74% (95% CI: 72% to 77%) and specificity 94% (95% CI: 93% to 95%) compared with traditional microbiology in adults with pneumococcal infection (Figure 3). The summary PPV is 79% (95% CI: 70% to 88%) and NPV is 92% (95% CI: 89% to 96%) using a gold standard of a positive sputum and/or blood culture with a consistent clinical syndrome. The LR+ for the ICT is 17 (95% CI: 11 to 26), and the LR− is 0.29 (95% CI: 0.24 to 0.39). Among the 17 studies testing the urine ICT test among pneumonias of unknown aetiology, an additional 23 ±14% were putatively diagnosed as S. pneumoniae pneumonia by urinary antigen testing (Table 4).
Figure 3.
Forest plot of the sensitivity and specificity of Binax urine antigen ICT performance.
Table 4.
Review of Binax Immunochromatographic urinary antigen test performance in adults
| Study | N | Methodology | Clinical Scenario | Gold Standard Microbiology technique | Sensitivity % | Specificity % | PPV % | NPV % | Additional Yield %† |
|---|---|---|---|---|---|---|---|---|---|
| Smith 19 | 213 | P | Bacteremia | Blood culture | 82 | 97 | 97 | 84 | N/A |
| Marcos 20 | 295 | P | CAP, 68 HIV Controls | Blood or sputum culture | 87 | 96 | 94 | 91 | 25 |
| Andreo 27 | 34 | P | CAP | Blood, sputum culture | 50 | 100 | 100 | 87 | 9 |
| Gutierrez 28 | 183 | P | CAP | Blood or sputum* | 70 | 90 | 85 | 98 | 26 |
| Murdoch 29 | 192 | P | CAP | Blood or sputum culture | 58 | 100 | 100 | 80 | 23 |
| Tzeng 30 | 859 | P | CAP | Blood or sputum culture | 64 | 92 | 27 | 98 | 10 |
| Sato 31 | 384 | P | CAP | Blood culture | 93 | 94 | 77 | 98 | 42 |
| Hasikita 32 | 372 | P | CAP | Blood or sputum* | 75 | 84 | 25 | 98 | 16 |
| Ishida 33 | 349 | P | CAP | Blood or sputum* | 76 | 94 | 91 | 83 | 39 |
| Watanuki 33 | 313 | P | CAP | Sputum Gram’s stain | 65 | 98 | 86 | 93 | N/A |
| Honore 34 | 304 | R/P | Pneumonia | Blood or sputum* | 77 | 98 | 95 | 95 | 59 |
| Strålin 35 | 191 | P | CAP | Blood or sputum culture | 60 | 94 | 86 | 74 | N/A |
| Rosón 36 | 173 | P | CAP | Sputum Gram’s stain | 66 | 100 | 100 | 99 | 26 |
| Payeras 37 | 163 | P | CAP | Sputum culture | 57 | 92 | 42 | 95 | 15 |
| Dominquez 38 | 122 | R | Pneumonia | Blood or sputum* | 80 | 97 | 95 | 87 | 25 |
| Farina 39 | 104 | P | CAP | Blood or sputum* | 78 | 99 | 93 | 96 | N/A |
| Genne 40 | 103 | P | CAP | Blood or sputum* | 64 | 99 | 93 | 91 | 24 |
| Bruell 41 | 91 | P | CAP | Blood, sputum, pleural fluid | 82 | 71 | 62 | 90 | 22 |
| Kobayashi 42 | 58 | P | CAP | Blood or sputum* | 100 | 100 | 100 | 100 | 57 |
| Le Monnier 43 | 41 | P | Empyema | Pleural fluid culture, PCR | 96 | 100 | 100 | 94 | N/A |
| Briones 44 | 566 | P | CAP | Blood or sputum* | 68 | 88 | 64 | 90 | 26 |
| Erics45 | 52 | R | CAP | Blood or sputum* | 73 | 98 | 89 | 93 | N/A |
| Diederen46 | 194 | R | CAP | Blood or sputum* | 69 | 98 | 93 | 88 | N/A |
| Lasocki47 | 140 | R | ICU | Blood, Sputum, BAL | 72 | 90 | 68 | 92 | 12 |
| Boulware | 133 | P | HIV CAP | Blood or sputum* | 81 | 98 | 98 | 83 | N/A |
|
| |||||||||
| Total | 5629 | 74 | 94 | 79 | 92 | 23 | |||
Totals reflected pooled data;
Methodology: P = Prospective, R = Retrospective
CAP=community acquired pneumonia; Pneumonia= CAP + nosocomial pneumonia; sputum* = Gram’s stain and culture
Represents the additional percent yield in aetiologic diagnosis made when testing the Binax urine antigen among pneumonias without an aetiologic diagnosis.
DISCUSSION
Among HIV-infected and seronegative patients with acute pneumococcal pneumonia, we demonstrate that S. pneumoniae infections can be diagnosed with reasonable sensitivity and excellent specificity with a simple test available at the point of care. Just as symptoms and signs of pneumococcal pneumonia are comparable regardless of HIV-status,49 the Binax S. pneumoniae urine antigen test has comparable performance characteristics among both groups. This test may provide reliable, rapid diagnosis for pneumococcal infection in the absence of an acceptable sputum specimen or readily-accessible microbiologic facilities. The absence of microbiologic laboratories may be encountered not only in resource-limited regions, where HIV infection is prevalent, but also in primary care clinics, urgent visit centers, or after-hours in developed countries when trained microbiology technicians are unavailable.
An advantage of urine antigen testing is that prior antibiotics do not rapidly decrease the sensitivity of ICT.18 Thus, patients having received antibiotics may still receive a specific diagnosis despite the rapidly diminishing yield of sputum examination. In the U.S., more rapid delivery of antibiotics is being encouraged. One U.S. Medicare quality improvement measure is the “number of pneumonia patients who received their first antibiotic dose within four hours from hospital arrival.”50 Antigenuria can persist for days to weeks after therapy, thereby enhancing the potential role of urine antigen detection for research purposes, such as vaccination trials.28 Additionally, C-polysaccharide, the antigen detected in urine, appears to be cold stable at −70°C for extended periods (≥13 years in this study), a characteristic that will enhance epidemiologic studies.
Of greater consequence, false negative results occur in ≈20% of patients with pneumococcal pneumonia. Causes for false negative results include aetiologic misclassification, low levels of C-polysaccharide antigen present, or sequestration of the antigen by antibodies in serum or urine as immune-complexes. Misclassification, by inclusion of non-pneumococcal pneumonia cases, does not account for all the false negatives as 15% of bacteremic pneumonias yielded false negative urine antigen results. In our study, false negatives did not differ by duration of symptoms, temperature, WBC count, or CRP, thus “early” infection is an unlikely explanation. We hypothesize that sequestration of antigen-antibody immune complexes in serum may decrease antigen shedding into the urine and yield false negative results. In prior studies, urine concentration improves ICT sensitivity by 5–20% as low levels antigenuria may occur in some individuals.20,28,29 Immune complexes were not present in the urine as acid dissociation did not improve detection. A broad array of different serotypes were detected in this study, thus the antigenic variability of the C-polysaccharide appears low. This is important as the previous latex agglutination urine antigen tests, which were based on the capsular polysaccharide, were limited in being serotype specific.51
In contrast to the high frequency of false positives in children,52 false positive tests occurred in one HIV-infected adult control with a prior pulmonary infection of unknown aetiology. Sources of false positives are two-fold. First, S. viridans species of S. mitis and S. oralis contain the same C-polysaccharide as S. pneumoniae.53 Second, antigen shedding continues for weeks as 40% in this study and 70% in a Spanish cohort had detectable urine antigen one month following illness.20 This urine antigen test should not be used in persons with a recent pneumococcal infection. Marcos et al demonstrated that nasopharyngeal pneumococcal carriage in eight clinically stable HIV-infected adults, CD4 count unspecified, did not yield any false positive results.20
One difficulty with definitive CAP diagnosis is that there is no perfect gold standard for the aetiologic diagnosis. Even employing a range of diagnostic tests in an academic centre, a specific aetiology cannot be determined in 50–70% of cases.16,17,18 Because expectorated sputum samples were diagnostic in ≤60% of patients in this study, a new approach is necessary.
With traditional microbiological techniques, a 24–48 hours delay typically precedes confirmation of the aetiologic diagnosis. In this study, the average for microbiologic results was 2.1 days. This diagnostic delay may prompt physicians to initiate and continue broader spectrum antimicrobial agents or order more diagnostic tests in the interim. With shorter hospitalizations now commonplace, physicians may be reluctant to narrow the antibiotic spectrum 2–3 days into therapy near time of discharge. As a result, broader, more expensive antimicrobial therapy may well be continued to complete a therapeutic course.
β-lactam antibiotics remain the mainstay therapy for pneumococcal infection and non-severe CAP.54 In a meta-analysis of 18 trials with 6749 adults with non-severe CAP, treatment failure with β-lactams was identical to broader spectrum agents having atypical coverage (RR 0.97; 95% CI: 0.87 to 1.07).54 Prompt aetiologic diagnosis within 15 minutes should promote narrow spectrum antibiotics, e.g. amoxicillin 1000mg three times daily, sparing broader spectrum, more expensive antibiotics, e.g. “respiratory” fluoroquinolones or azithromycin/clarithromycin, in non-severe CAP.55 The utility of rapid diagnosis-driven therapy at the point of care has been prospectively verified among military recruits.56 The cost of Binax Now® ICT (~£15, US$30) outweighs the cost differential for 8 days of fluoroquinolones vs. amoxicillin (~£35, US$70).57 The European and U.S. cost of the Binax ICT is still exponentially more expensive than what is practical for use in resource-limited areas.
For calculating the assay’s specificity, controls without acute pulmonary infection were chosen. Among studies utilizing other non-pneumococcal CAP patients as controls, “false positives” are reported in up to 29%.20,32,33,35 Because the frequency of the pneumococcal pneumonia is high in HIV selecting non-pneumococcal hospitalized CAP controls is difficult.7,8 With such a high pre-test probability, a culture-negative CAP still has a high PPV for pneumococcal pneumonia. In this study, 47% [28/60] of bacteremic pneumonia did not have a positive sputum Gram’s stain or culture. Even with viral pneumonias, mixed infections with pneumococcus may occur in up to one-third of children.58
Burel et al. have suggested that more work is required to establish the true specificity of the Binax® NOW test by studying its performance using urine from healthy subjects.42 Other studies have used various controls with CAP of unknown aetiology or with positive serologic tests for Chlamydia, Mycoplasma, or Legionella to define the control group. Among HIV-infected patients without consistent history, clinical and laboratory features of pneumococcal pneumonia, “false positive” urine antigen detection may represent prior, recent CAP infection. In our study, we eliminated this confounder by utilizing controls without pulmonary infection. Although this choice of controls may be viewed as a limitation, confounding of results by a high rate of cases without a specific aetiologic diagnosis was avoided.
Conversely, whether these “false positives” represent actual false positives or mixed infections with unconfirmed S. pneumoniae is unclear. Emerging data suggest that even in viral childhood pneumonias, S. pneumoniae may contribute to symptoms as a co-pathogen.58,59 These putative “false positives” lower the specificity and PPV in some studies reviewed.33,45
In summary, urine ICT is a useful rapid diagnostic test for S. pneumoniae pneumonia and bacteremia in adults with HIV-infection. The urine antigen ICT may be quite valuable for establishing an aetiologic diagnosis in the absence of traditional microbiological testing, such as outpatient or rural clinical settings, for research in resource-limited nations to determine appropriate point-of-care algorithms, or for epidemiologic studies. Use of the urine antigen in non-severe pneumonia is also promising to promote the use of narrower spectrum, less expensive antibiotics.54,56
Supplementary Material
Acknowledgments
We thank Claudine Fasching for technical and clinical support of the project. This work supported in part by National Institutes of Health (AI48796; P30-AI054907; AI39445; T32-AI055433; L30-AI066779), the Colorado Center for AIDS Research, and the Veterans Affairs Research Service.
Footnotes
There are no conflicts of interest.
Binax was not involved in the design and conduct of this study. We thank Binax® NOW for providing the test kits for the study.”
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lim WS, Macfarlane JT, Boswell TC, et al. Study of community acquired pneumonia aetiology (SCAPA) in adults admitted to hospital: implications for management guidelines. Thorax. 2001;56:296–301. doi: 10.1136/thorax.56.4.296. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodhead MA, Macfarlane JT, McCracken JS, Rose DH, Finch RG. Prospective study of the aetiology and outcome of pneumonia in the community. Lancet. 1987;1:671–4. doi: 10.1016/s0140-6736(87)90430-2. PubMed. [DOI] [PubMed] [Google Scholar]
- 3.Kozak LJ, Owings MF, Hall MJ. National Hospital Discharge Survey: 2002 annual summary with detailed diagnosis and procedure data. National Center for Health Statistics. [Accessed Apr 10, 2007];Vital Health Stat. 2005 13(158) Available at: http://www.cdc.gov/nchs/data/series/sr_13/sr13_158.pdf. [PubMed]
- 4.Bartlett JG, Mundy LM. Community-acquired pneumonia. N Engl J Med. 1995;333:1618–1624. doi: 10.1056/NEJM199512143332408. PubMed. [DOI] [PubMed] [Google Scholar]
- 5.Brown PD, Lerner SA. Community-acquired pneumonia. Lancet. 1998;352:1295–1302. doi: 10.1016/S0140-6736(98)02239-9. [DOI] [PubMed] [Google Scholar]
- 6.Sleeman K, Knox K, George R, et al. Invasive pneumococcal disease in England and Wales: vaccination implications. J Infect Dis. 2001;183:239–246. doi: 10.1086/317924. PubMed. [DOI] [PubMed] [Google Scholar]
- 7.Nuorti JP, Butler JC, Gelling L, Kool JL, Reingold AL, Vugia DJ. Epidemiologic relation between HIV and invasive pneumococcal disease in San Francisco County, California. Ann Intern Med. 2000;132:182–90. doi: 10.7326/0003-4819-132-3-200002010-00003. PubMed. [DOI] [PubMed] [Google Scholar]
- 8.Feikin DR, Feldman C, Schuchat A, Janoff EN. Global strategies to prevent bacterial pneumonia in adults with HIV disease. Lancet Infect Dis. 2004;4:445–455. doi: 10.1016/S1473-3099(04)01060-6. PubMed. [DOI] [PubMed] [Google Scholar]
- 9.Heffernan RT, Barrett NL, Gallagher KM, et al. Declining incidence of invasive Streptococcus pneumoniae infections among persons with AIDS in an era of highly active antiretroviral therapy. J Infect Dis. 2005;191:2038–2045. doi: 10.1086/430356. PubMed. [DOI] [PubMed] [Google Scholar]
- 10.Hirschtick RE, Glassroth J, Jordan MC, et al. Bacterial pneumonia in persons infected with the human immunodeficiency virus. Pulmonary Complications of HIV Infection Study Group. N Engl J Med. 1995;333:845–51. doi: 10.1056/NEJM199509283331305. PubMed. [DOI] [PubMed] [Google Scholar]
- 11.French N, Nakiyingi J, Carpenter LM, et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet. 2000;355:2106–11. doi: 10.1016/s0140-6736(00)02377-1. PubMed. [DOI] [PubMed] [Google Scholar]
- 12.Kohli R, Lo Y, Homel P, et al. Bacterial pneumonia, HIV therapy, and disease progression among HIV-infected women in the HIV epidemiologic research (HER) study. Clin Infect Dis. 2006;43:90–8. doi: 10.1086/504871. [DOI] [PubMed] [Google Scholar]
- 13.Flanery B, Heffernan RT, Harrison LH, et al. Changes in invasive pneumococcal disease among HIV-infected adults living in the era of childhood pneumococcal immunization. Ann Intern Med. 2006;1444:1–9. doi: 10.7326/0003-4819-144-1-200601030-00004. PubMed. [DOI] [PubMed] [Google Scholar]
- 14.Simpson JC, Macfarlane JT, Watson J, Woodhead MA. A national confidential enquiry into community acquired pneumonia deaths in young adults in England and Wales. British Thoracic Society Research Committee and Public Health Laboratory Service. Thorax. 2000;55:1040–5. doi: 10.1136/thorax.55.12.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartlett JG. Decline in microbial studies for patients with pulmonary infections. Clin Infect Dis. 2004;39:170–2. doi: 10.1086/421498. [DOI] [PubMed] [Google Scholar]
- 16.Garcia Vazquez E, Mensa J, Martinez JA, et al. Lower mortality among patients with community-acquired pneumonia treated with a macrolide plus a beta-lactam agent versus a beta-lactam agent alone. Eur J Clin Microbiol Infect Dis. 2005;24:190–5. doi: 10.1007/s10096-005-1295-9. PubMed. [DOI] [PubMed] [Google Scholar]
- 17.Apisarnthanarak A, Mundy LM. Etiology of community-acquired pneumonia. Clin Chest Med. 2005;26:47–55. doi: 10.1016/j.ccm.2004.10.016. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musher DM, Montoya R, Wanahita A. Diagnostic value of microscopic examination of Gram-stained sputum and sputum cultures in patients with bacteremic pneumococcal pneumonia. Clin Infect Dis. 2004;39:165–9. doi: 10.1086/421497. PubMed. [DOI] [PubMed] [Google Scholar]
- 19.Smith MD, Derrington P, Evans R, et al. Rapid diagnosis of bacteremic pneumococcal infections in adults by using the Binax NOW Streptococcus pneumoniae urinary antigen test: a prospective, controlled clinical evaluation. J Clin Microbiol. 2003;41:2810–3. doi: 10.1128/JCM.41.7.2810-2813.2003. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marcos MA, Jimenez de Anta MT, de la Bellacasa JP, et al. Rapid urinary antigen test for diagnosis of pneumococcal community-acquired pneumonia in adults. Eur Respir J. 2003;21:209–14. doi: 10.1183/09031936.03.00058802. PubMed. [DOI] [PubMed] [Google Scholar]
- 21.Osmond DH, Chin DP, Glassroth J, et al. Impact of bacterial pneumonia and Pneumocystis carinii pneumonia on human immunodeficiency virus disease progression. Pulmonary Complications of HIV Study Group. Clin Infect Dis. 1999;29:536–43. doi: 10.1086/598629. PubMed. [DOI] [PubMed] [Google Scholar]
- 22.The Pulmonary Complications of HIV Infection Study Group. Design of a prospective study of the pulmonary complications of human immunodeficiency virus infection. J Clin Epidemiol. 1993;46:497–507. doi: 10.1016/0895-4356(93)90122-h. [DOI] [PubMed] [Google Scholar]
- 23.Binax; Portland, ME: [Accessed Apr 10, 2007]. Binax NOW® Streptococcus pneumoniae Urinary Antigen Test Product Instructions. Available at: http://binax.com/uploads/strep_pneumo_pi_3_16_06_001.pdf. [Google Scholar]
- 24.Hamer DH, Egas J, Estrella B, MacLeod WB, Griffiths JK, Sempertegui F. Assessment of the Binax NOW Streptococcus pneumoniae urinary antigen test in children with nasopharyngeal pneumococcal carriage. Clin Infect Dis. 2002;34:1025–8. doi: 10.1086/339446. PubMed. [DOI] [PubMed] [Google Scholar]
- 25.Amdahl BM, Rubins JB, Daley CL, Gilks CF, Hopewell PC, Janoff EN. Impaired natural immunity to pneumolysin during human immunodeficiency virus infection in the United States and Africa. Am J Respir Crit Care Med. 1995;152:2000–4. doi: 10.1164/ajrccm.152.6.8520768. PubMed. [DOI] [PubMed] [Google Scholar]
- 26.Zamora J, Abraira V, Muriel A, Khan KS, Coomarasamy A. Meta-DiSc: a software for meta-analysis of test accuracy data. BMC Medical Research Methodology. 2006;6:31. doi: 10.1186/1471-2288-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Andreo F, Dominguez J, Ruiz J, Blanco S, Arellano E, Prat C, Morera J, Ausina V. Impact of rapid urine antigen tests to determine the etiology of community-acquired pneumonia in adults. Respir Med. 2006;100:884–91. doi: 10.1016/j.rmed.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 28.Gutierrez F, Masia M, Rodriguez JC, et al. Evaluation of the immunochromatographic Binax NOW assay for detection of Streptococcus pneumoniae urinary antigen in a prospective study of community-acquired pneumonia in Spain. Clin Infect Dis. 2003;36:286–92. doi: 10.1086/345852. PubMed. [DOI] [PubMed] [Google Scholar]
- 29.Murdoch DR, Laing RT, Mills GD, et al. Evaluation of a rapid immunochromatographic test for detection of Streptococcus pneumoniae antigen in urine samples from adults with community-acquired pneumonia. J Clin Microbiol. 2001;39:3495–8. doi: 10.1128/JCM.39.10.3495-3498.2001. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tzeng DH, Lee YL, Lin YH, Tsai CA, Shi ZY. Diagnostic value of the Binax NOW assay for identifying a pneumococcal etiology in patients with respiratory tract infection. J Microbiol Immunol Infect. 2006;39:39–44. PubMed. [PubMed] [Google Scholar]
- 31.Sato N, Takayanagi N, Kurashima K, et al. [Usefulness of Streptococcus pneumoniae urinary antigen detection kit and the duration and intensity of reactivity with urinary antigen in patients with pneumonia] Japanese. Nihon Kokyuki Gakkai Zasshi. 2004;42:247–52. PubMed. [PubMed] [Google Scholar]
- 32.Hashikita G, Yamaguti T, Tachi Y, et al. Examination about utility of a Streptococcus pneumoniae capsular antigen swiftness search kit urine in a pneumonia patient. Rinsho Biseibutshu Jinsoku Shindan Kenkyukai Shi. 2005;16:153–61. Japanese. PubMed. [PubMed] [Google Scholar]
- 33.Ishida T, Hashimoto T, Arita M, Tojo Y, Tachibana H, Jinnai M. A 3-year prospective study of a urinary antigen-detection test for Streptococcus pneumoniae in community-acquired pneumonia: utility and clinical impact on the reported etiology. J Infect Chemother. 2004;10:359–63. doi: 10.1007/s10156-004-0351-1. PubMed. [DOI] [PubMed] [Google Scholar]
- 34.Watanuki Y, Takahashi H, Ogura T, Miyazawa N, Tomioka T, Odagiri S. Usefulness of urinary antigen and sputum Gram stain for rapid diagnosis of pneumococcal respiratory infections. Kansenshogaku Zasshi. 2005;79:13–9. doi: 10.11150/kansenshogakuzasshi1970.79.13. Japanese. PubMed. [DOI] [PubMed] [Google Scholar]
- 35.Honore S, Trillard M, Ould-Hocine Z, Lesprit P, Deforges L, Legrand P. [Contribution of urinary pneumococcal antigen detection combined with the research of legionella antigen for diagnosis of pneumonia in hospitalized patients] French. Pathol Biol. 2004;52:429–33. doi: 10.1016/j.patbio.2004.07.026. PubMed. [DOI] [PubMed] [Google Scholar]
- 36.Stralin K, Kaltoft MS, Konradsen HB, Olcen P, Holmberg H. Comparison of two urinary antigen tests for establishment of pneumococcal etiology of adult community-acquired pneumonia. J Clin Microbiol. 2004;42:3620–25. doi: 10.1128/JCM.42.8.3620-3625.2004. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roson B, Fernandez-Sabe N, Carratala J, et al. Contribution of a urinary antigen assay (Binax NOW) to the early diagnosis of pneumococcal pneumonia. Clin Infect Dis. 2004;38:222–6. doi: 10.1086/380639. PubMed. [DOI] [PubMed] [Google Scholar]
- 38.Payeras Cifre A, Llado Ferrer B, Ramis Morell F, et al. [Usefulness of a new fast technique for detection of pneumococcal antigen in the diagnosis of community pneumonia] Spanish. Rev Clin Esp. 2003;203:521–25. doi: 10.1157/13052583. PubMed. [DOI] [PubMed] [Google Scholar]
- 39.Farina C, Arosio M, Vailati F, Moioli F, Goglio A. Urinary detection of Streptococcus pneumoniae antigen for diagnosis of pneumonia. New Microbiol. 2002;25:259–63. PubMed. [PubMed] [Google Scholar]
- 40.Domínguez J, Galí N, Blanco S, et al. Detection of Streptococcus pneumoniae antigen by a rapid immunochromatographic assay in urine samples. Chest. 2001;119:243–249. doi: 10.1378/chest.119.1.243. PubMed. [DOI] [PubMed] [Google Scholar]
- 41.Genne D, Siegrist HH, Lienhard R. Enhancing the etiologic diagnosis of community-acquired pneumonia in adults using the urinary antigen assay (Binax NOW) Int J Infect Dis. 2006;10:124–8. doi: 10.1016/j.ijid.2005.03.006. PubMed. [DOI] [PubMed] [Google Scholar]
- 42.Burel E, Dufour P, Gauduchon V, Jarraud S, Etienne J. Evaluation of a rapid immunochromatographic assay for detection of Streptococcus pneumoniae antigen in urine samples. Eur J Clin Microbiol Infect Dis. 2001;20:840–1. doi: 10.1007/s100960100614. [DOI] [PubMed] [Google Scholar]
- 43.Kobayashi T, Matsumoto T, Tateda K, et al. [Evaluation of Streptococcus pneumoniae-urinary antigen detection kit in patients with community acquired pneumonia] Japanese. Kansenshogaku Zasshi. 2002;76:995–1002. doi: 10.11150/kansenshogakuzasshi1970.76.995. PubMed. [DOI] [PubMed] [Google Scholar]
- 44.Le Monnier A, Carbonnelle E, Zahar JR, et al. Microbiological diagnosis of empyema in children: comparative evaluations by culture, polymerase chain reaction, and pneumococcal antigen detection in pleural fluids. Clin Infect Dis. 2006;42:1135–40. doi: 10.1086/502680. PubMed. [DOI] [PubMed] [Google Scholar]
- 45.Briones ML, Blanquer J, Ferrando D, Blasco ML, Gimeno C, Marin J. Assessment of analysis of urinary pneumococcal antigen by immunochromatography for etiologic diagnosis of community-acquired pneumonia in adults. Clin Vaccine Immunol. 2006;13:1092–7. doi: 10.1128/CVI.00090-06. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ercis S, Ergin A, Sahin GO, Hascelik G, Uzun O. Validation of urinary antigen test for Streptococcus pneumoniae in patients with pneumococcal pneumonia. Jpn J Infect Dis. 2006;59:388–90. PubMed. [PubMed] [Google Scholar]
- 47.Lasocki S, Scanvic A, Le Turdu F, Restoux A, Mentec H, Bleichner G, Sollet JP. Evaluation of the Binax NOW Streptococcus pneumoniae urinary antigen assay in intensive care patients hospitalized for pneumonia. Intensive Care Med. 2006;32:1766–72. doi: 10.1007/s00134-006-0329-9. PubMed. [DOI] [PubMed] [Google Scholar]
- 48.Diederen BM, Peeters MF. Rapid diagnosis of pneumococcal pneumonia in adults using the Binax NOW Streptococcus pneumoniae urinary antigen test. Int J Infect Dis. 2007;11:284–5. doi: 10.1016/j.ijid.2006.07.006. PubMed. [DOI] [PubMed] [Google Scholar]
- 49.Janoff EN, Breiman RF, Daley CL, Hopewell PC. Pneumococcal disease during HIV infection. Epidemiologic, clinical, and immunologic perspectives. Ann Intern Med. 1992;117:314–24. doi: 10.7326/0003-4819-117-4-314. PubMed. [DOI] [PubMed] [Google Scholar]
- 50.Centers for Medicare & Medicaid Services. Quality Measures Management Information System. [Accessed Jan 20, 2007]; Available at: https://www.qualitynet.org/qmis/measureDetailView.htm?measureId=10280.
- 51.Scott JA, Hannington A, Marsh K, Hall AJ. Diagnosis of pneumococcal pneumonia in epidemiological studies: evaluation in Kenyan adults of a serotype-specific urine latex agglutination assay. Clin Infect Dis. 1999;28:764–9. doi: 10.1086/515198. PubMed. [DOI] [PubMed] [Google Scholar]
- 52.Dowell SF, Garman RL, Liu G, Levine OS, Yang YH. Evaluation of Binax NOW, an assay for the detection of pneumococcal antigen in urine samples, performed among pediatric patients. Clin Infect Dis. 2001;32:824–5. doi: 10.1086/319205. PubMed. [DOI] [PubMed] [Google Scholar]
- 53.Gillespie SH, McWhinney PH, Patel S, et al. Species of alpha-hemolytic streptococci possessing a C-polysaccharide phosphorylcholine-containing antigen. Infect Immun. 1993;61:3076–7. doi: 10.1128/iai.61.7.3076-3077.1993. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mills GD, Oehley MR, Arrol B. Effectiveness of beta lactam antibiotics compared with antibiotics active against atypical pathogens in non-severe community acquired pneumonia: meta-analysis. BMJ. 2005;330:456. doi: 10.1136/bmj.38334.591586.82. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.BTS guidelines for the management of community acquired pneumonia in adults - 2004 update. [Accessed May 5, 2007]; doi: 10.1136/thorax.56.suppl_4.iv1. Available at: http://www.brit-thoracic.org.uk/ [DOI] [PMC free article] [PubMed]
- 56.Guchev IA, Yu VL, Sinopalnikov A, et al. Management of nonsevere pneumonia in military trainees with the urinary antigen test for Streptococcus pneumoniae: an innovative approach to targeted therapy. Clin Infect Dis. 2005;40:1608–16. doi: 10.1086/429919. PubMed. [DOI] [PubMed] [Google Scholar]
- 57.Drug topics red book: pharmacy’s fundamental resource. Montvale (NJ): Medical Economics; 2004. [Google Scholar]
- 58.Madhi SA, Klugman KP Pneumococcal Vaccine Trialist Group. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat Med. 2004;10:811–813. doi: 10.1038/nm1077. PubMed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Klugman KP, Madhi SA, Huebner RE, Kohberger R, Mbelle N, Pierce N Vaccine Trialists Group. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N Engl J Med. 2003;349:1341–8. doi: 10.1056/NEJMoa035060. PubMed. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.