Abstract
Time-trial performance deteriorates in the heat. This might potentially be the result of a temperature-induced decrease in gross-efficiency (GE). The effect of high ambient temperature on GE during cycling will be studied, with the intent of determining if a heat-induced change in GE could account for the performance decrements in time trial exercise found in literature. Ten well-trained male cyclists performed 20-min cycle ergometer exercise at 60%
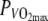 (power output at which VO2max was attained) in a thermo-neutral climate (N) of 15.6 ± 0.3°C, 20.0 ± 10.3% RH and a hot climate (H) of 35.5 ± 0.5°C, 15.5 ± 3.2% RH. GE was calculated based on VO2 and RER. Skin temperature (Tsk), rectal temperature (Tre) and muscle temperature (Tm) (only in H) were measured. GE was 0.9% lower in H compared to N (19.6 ± 1.1% vs. 20.5 ± 1.4%) (P < 0.05). Tsk (33.4 ± 0.6°C vs. 27.7 ± 0.7°C) and Tre (37.4 ± 0.6°C vs. 37.0 ± 0.6°C) were significantly higher in H. Tm was 38.7 ± 1.1°C in H. GE was lower in heat. Tm was not high enough to make mitochondrial leakage a likely explanation for the observed reduced GE. Neither was the increased Tre. Increased skin blood flow might have had a stealing effect on muscular blood flow, and thus impacted GE. Cycling model simulations showed, that the decrease in GE could account for half of the performance decrement. GE decreased in heat to a degree that could explain at least part of the well-established performance decrements in the heat.
(power output at which VO2max was attained) in a thermo-neutral climate (N) of 15.6 ± 0.3°C, 20.0 ± 10.3% RH and a hot climate (H) of 35.5 ± 0.5°C, 15.5 ± 3.2% RH. GE was calculated based on VO2 and RER. Skin temperature (Tsk), rectal temperature (Tre) and muscle temperature (Tm) (only in H) were measured. GE was 0.9% lower in H compared to N (19.6 ± 1.1% vs. 20.5 ± 1.4%) (P < 0.05). Tsk (33.4 ± 0.6°C vs. 27.7 ± 0.7°C) and Tre (37.4 ± 0.6°C vs. 37.0 ± 0.6°C) were significantly higher in H. Tm was 38.7 ± 1.1°C in H. GE was lower in heat. Tm was not high enough to make mitochondrial leakage a likely explanation for the observed reduced GE. Neither was the increased Tre. Increased skin blood flow might have had a stealing effect on muscular blood flow, and thus impacted GE. Cycling model simulations showed, that the decrease in GE could account for half of the performance decrement. GE decreased in heat to a degree that could explain at least part of the well-established performance decrements in the heat.
Keywords: Heat, Performance, Muscle temperature
Introduction
Performance decrements are widely observed during exercise in the heat compared to thermo-neutral circumstances (Febbraio et al. 1996; Gonzalez Alonso et al. 1999; Parkin et al. 1999; Romer et al. 2003; Tatterson et al. 2000; Tucker et al. 2004). It has been shown that fatigue at exhaustion is related to factors associated with thermoregulation and hyperthermia (Febbraio et al. 1996; Nielsen et al. 1990; Parkin et al. 1999). Time trial performance and fatigue (evidenced by decrements in power output over the race) and finish time have received much less attention in the literature. Accordingly, the present study will focus on the effect of a hot ambient temperature on thermal and cardio respiratory strain during the exercise that might contribute to the well-established decrease in power output.
Tatterson et al. (2000) measured time trial performance on a 30 min self-paced cycling time trial in 32°C, 60% RH versus 23°C, 60% RH. They observed that power output was reduced in the heat by 6.5% (345 ± 9 W vs. 323 ± 8 W). Tucker et al. (2004) compared 20 km time trials in 35 and 15°C and found a comparable reduction in average power output in the heat of about 6.3% (255 ± 47 W vs. 272 ± 45 W). This change in average power output led to a difference in final time on a 20 km time trial of about 48 s (29.6 ± 1.9 min vs. 28.8 ± 1.8 min), which equals about 2.8%. Possible causes for this deterioration in performance are associated with elevated body temperature (Tatterson et al. 2000) and anticipatory changes in pacing strategy to avoid hyperthermia (Tucker et al. 2004). Although most research has revolved around neuromuscular function, central drive and fatigue at the point of exhaustion, a plausible explanation for the deterioration in time trial performance in heat may also be found in a temperature-induced change in gross-efficiency (GE). GE is an important variable in cycling performance (Lucia et al. 2002; Moseley et al. 2004) and a linear relationship between body temperature and GE has been observed (Daanen et al. 2006).
A possible explanation for the decreased GE in the heat could be mitochondrial leakage. A temperature induced metabolic disruption caused by non-specific proton-leakage across the inner mitochondrial membrane has been shown to occur at high muscle temperatures (Brooks et al. 1971; Willis and Jackman 1994), resulting in a decrease in ADP:O ratio. Further, a heat induced skin vasodilatation could occur in heat. To prevent a resulting decrease in blood flow to exercising and respiratory muscles (Nielsen et al. 1990; Romer et al. 2003; Rowell et al. 1966) a higher cardiac output must exist to continue supplying the muscles with the same blood flow, but still sending extra blood to the skin for cooling.
In the present study we sought to determine the effect of heat on GE. GE can be assessed accurately during sub-maximal exercise at intensities as high as 60−80% VO2max by calculating the ratio between mechanical and metabolic (mainly dependent on aerobic energy metabolism) power output, as has been done in Daanen et al. (2006), Foster et al. (2003), Hettinga et al. (2006), Lucia et al. (2002), Moseley et al. (2001, 2004). The presence of a possible effect of ambient temperature on GE can serve as input for an energy flow model (De Koning et al. 1999) to quantify the impact of this effect on time trial performance.
Materials and methods
Subjects
Ten healthy, non-smoking, well-trained male subjects, familiar with cycling exercise at the club-level, participated in this study. They were informed of the nature of the experiment and provided written informed consent. Subject characteristics are presented in Table 1. The study was approved by the Medical Ethical Committee of the University Medical Centre Utrecht (The Netherlands).
Table 1.
Subject characteristics
| Mean value ± SD | |
|---|---|
| Age (years) | 23.5 ± 4.4 |
| Height (cm) | 179.7 ± 9.1 |
| Body mass (kg) | 72.5 ± 7.2 |
| VO2max (l min−1) | 4.78 ± 0.41 |
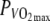 (W) (W) |
354 ± 29 |
Values are mean ± SD
Experimental design
Incremental test
The subjects first performed an incremental bicycle test to determine at which power output VO2max was attained 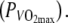 The incremental test was performed in the heat, under similar conditions as the constant intensity bouts, to make sure that
The incremental test was performed in the heat, under similar conditions as the constant intensity bouts, to make sure that 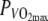 would not be overestimated in the hot condition. This incremental test was used solely to determine the relative intensities for the constant intensity cycling bouts. The test began at a power output (PO) of 150 W, after which PO was increased with 15 W every minute. Exercise was performed on an electronically braked cycle ergometer (Lode Excalibur, Lode NV, Groningen, The Netherlands) until exhaustion or until pedal frequency dropped below 80 rpm. Oxygen consumption (VO2) was measured breath by breath, using open circuit spirometry (Oxycon Pro-Delta, Jaeger, Hoechberg, Germany). The gas analyzer was calibrated using a Jaeger 2 l syringe, room air and a calibration gas mixture. Heart rate (HR) was monitored using radiotelemetry (Polar Electro, Kempele, Finland).
would not be overestimated in the hot condition. This incremental test was used solely to determine the relative intensities for the constant intensity cycling bouts. The test began at a power output (PO) of 150 W, after which PO was increased with 15 W every minute. Exercise was performed on an electronically braked cycle ergometer (Lode Excalibur, Lode NV, Groningen, The Netherlands) until exhaustion or until pedal frequency dropped below 80 rpm. Oxygen consumption (VO2) was measured breath by breath, using open circuit spirometry (Oxycon Pro-Delta, Jaeger, Hoechberg, Germany). The gas analyzer was calibrated using a Jaeger 2 l syringe, room air and a calibration gas mixture. Heart rate (HR) was monitored using radiotelemetry (Polar Electro, Kempele, Finland).
Constant intensity exercise bouts
On separate days, subjects performed a constant-intensity exercise bout at 60% of 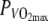 in a thermo-neutral climate (N) and in a hot, dry climate (H). Temperature in the thermo-neutral climate was 15.6 ± 0.3°C, relative humidity (RH) was 20.0 ± 10.3%. In the hot, dry climate, the temperature was 35.5 ± 0.5°C and RH was 15.5 ± 3.2%. These temperatures were equivalent to the temperatures in the studies of Tatterson et al. (2000) and Tucker et al. (2004). All tests were performed in a climate-controlled room with a continuous airflow of 0.2 m s−1. RH was set low to increase the evaporative capacity of the environment. Further, all trials in the H-climate were performed with a simulated wind velocity of 1.72 m s−1 (6.2 km/h) to further increase evaporative heat loss, which is the most important form of heat loss in cycling in hot conditions (Saunders et al. 2005). Prior to the experiments, the subjects were asked to refrain from vigorous exercise for at least 48 h. They were also asked not to consume coffee, alcohol or drugs after 10 p.m. the day before the exercise and not to eat for two hours prior to the experiments. Subjects drank water ad libitum before the experiment.
in a thermo-neutral climate (N) and in a hot, dry climate (H). Temperature in the thermo-neutral climate was 15.6 ± 0.3°C, relative humidity (RH) was 20.0 ± 10.3%. In the hot, dry climate, the temperature was 35.5 ± 0.5°C and RH was 15.5 ± 3.2%. These temperatures were equivalent to the temperatures in the studies of Tatterson et al. (2000) and Tucker et al. (2004). All tests were performed in a climate-controlled room with a continuous airflow of 0.2 m s−1. RH was set low to increase the evaporative capacity of the environment. Further, all trials in the H-climate were performed with a simulated wind velocity of 1.72 m s−1 (6.2 km/h) to further increase evaporative heat loss, which is the most important form of heat loss in cycling in hot conditions (Saunders et al. 2005). Prior to the experiments, the subjects were asked to refrain from vigorous exercise for at least 48 h. They were also asked not to consume coffee, alcohol or drugs after 10 p.m. the day before the exercise and not to eat for two hours prior to the experiments. Subjects drank water ad libitum before the experiment.
Before the constant intensity bout in the H-condition, the subject stabilized for 50 min in the 35°C, 20% RH room. For the comfort of the subjects and to prevent them from shivering, the stabilizing period was reduced to 35 min for the N-condition in the 15°C, 20% RH room. During the test, oxygen consumption (VO2), respiratory exchange ratio (RER) and ventilation (VE) were measured breath by breath. Power output (PO) and heart rate were registered continuously.
After stabilization, a 5-min warm-up was performed at 100 W with a pedal frequency of ∼100 rpm. After 1 min of rest, the constant intensity bout was started (∼100 rpm). The subjects cycled for 20 min at a constant power output of 60% of the power output at which maximal VO2 was attained at the incremental test 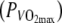 in the H condition. Directly after the end of exercise, blood lactate concentration (BLC) was measured (Lactate Pro, Arkray, Kyoto, Japan).
in the H condition. Directly after the end of exercise, blood lactate concentration (BLC) was measured (Lactate Pro, Arkray, Kyoto, Japan).
Rectal temperature (Tre) was monitored every 5 s during the entire test using a thermistor temperature probe (YSI 701, Yellow Springs Instrument, Dayton, USA) inserted about ten centimeters in the rectum. Skin temperature (Tsk) was measured every 5 s at 14 different skin loci, conform ISO 9886, using thermocouples (YSI 709B, Yellow Springs Instrument, Dayton, USA). Data were recorded with a Data Translation acquisition board (DT2821, Viewdac, Keithley Instruments, Cleveland, USA). Further, muscle temperature (Tm) was measured at a minimum depth of two centimeters and recorded every 5 s.
Since this measurement was invasive, measurements were restricted to the constant intensity bout in H (n = 6). Tm was measured with a sterile thermal thermocouple-probe (type MAC08170A275SM, Ellab A/S, Rodovre, Denmark) in the right vastus lateralis muscle, inserted by a physician. One hour before inserting the temperature probe, a lidocaine plaster was attached to the skin, as a local anaesthetic.
Calculating gross-efficiency (GE)
Metabolic power (Pmet) was calculated by multiplying oxygen consumption with the oxygen equivalent: Pmet (W) = VO2 × [(4,940 × RER + 16,040)/60] according to Garby and Astrup (1987), assuming that respiratory exchange ratio (RER) equaled respiratory quotient (RQ) at sub-maximal intensities. Further, we assumed that respiratory exchange ratios in excess of 1.00 were attributable to buffering of lactate by bicarbonate. Ratios in excess of 1.00 were thus treated as if they equaled 1.00. The measured mechanical power output (PO) divided by the calculated Pmet defined GE. GE was calculated from 90 s after the start of exercise until the end of exercise.
Energy flow model
The energy flow model as described by De Koning et al. (1999) was used to calculate the effect of changes in GE on performance, by simulating a 20 km time trial as is studied in literature (Tatterson et al. 2000; Tucker et al. 2004). This model is based on power equations and has been reasonably successful in predicting performance in cyclic events as cycling (De Koning et al. 1999) and speed skating (De Koning et al. 2005). The energy flow model, also referred to as power balance model, relates to power production and power dissipation: Pprod = Plost + dE/dt, where Pprod is total power that can be produced, Plost power that is used to overcome frictional losses, and dE/dt rate of change of kinetic energy of the mass centre of the body. These terms can be calculated as described in De Koning et al. (1999), and the influence of changing one single variable, in this case GE, can be predicted.
Statistics
Paired Student t test’s were performed to test if data were significantly different between the H-condition and N-condition, P values less than 0.05 were accepted as statistically significant.
Results
Power output at 60% 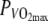 was 211.5 ± 18.6 W. All subjects were able to complete the trials in both H and N.
was 211.5 ± 18.6 W. All subjects were able to complete the trials in both H and N.
VO2 was significantly higher in H than in N, resulting in GE being significantly lower in H compared to N. Further, HR and VE were higher in H compared to N. For RER and BLC, no main effect of temperature was found. Mean values are shown in Table 2.
Table 2.
Gross-efficiency (GE), VO2, respiratory exchange ratio (RER), respiratory minute volume (VE), heart rate (HR) and blood lactate concentration (BLC) at 60% 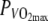 in the thermo-neutral (N) and in the hot dry (H) climate
in the thermo-neutral (N) and in the hot dry (H) climate
60% 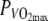
|
||
|---|---|---|
| N | H | |
| GE (%) | 20.5 ± 1.4 | 19.6 ± 1.1* |
| VO2 (ml min−1) | 3,002.8 ± 290.1 | 3,126.5 ± 268.3* |
| RER | 0.89 ± 0.03 | 0.90 ± 0.01 |
| VE (l min−1) | 76.9 ± 7.7 | 82.0 ± 9.4* |
| HR (bpm) | 145.1 ± 6.5 | 155.4 ± 12.0* |
| BLC (mmol l−1) | 2.6 ± 1.0 | 3.9 ± 2.1 |
Values are mean ± SD
* Significant differences with N (P < 0.05)
Mean GE over time is plotted for both conditions in Fig. 1. The difference in GE between conditions was 0.9% over the entire trial. From minute 5–8, the N − H difference was 0.6% ± 0.7%, from minute 15–18, the difference was 1.1 ± 1.3%, both significant. No significant change in GE within trials was observed comparing GE over the first half (min 5–8) with GE over the second half (min 15–18).
Fig. 1.
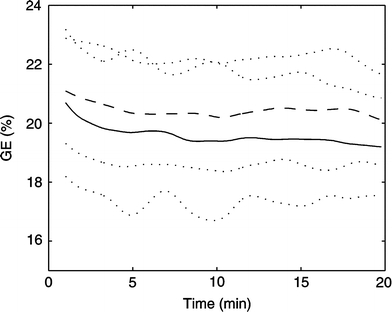
Gross-efficiency (GE) plotted over time at 60% 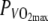 in the thermo-neutral (N) climate (dashed line) and in the hot dry (H) climate (solid line) ±SD (dotted lines)
in the thermo-neutral (N) climate (dashed line) and in the hot dry (H) climate (solid line) ±SD (dotted lines)
Tsk and Tre were significantly higher in H. Rectal and skin temperature changes combined, the difference in body heat content between N and H amounts to ∼138.5 ± 35.1 kJ, calculated assuming that the specific heat of the body tissue was 3.4 kJ kg−1°C−1. Maximal muscle temperature (Tmmax) in H was 38.7 ± 1.1°C. Mean temperature values are shown in Table 3. Figure 2 shows Tre, Tsk and Tm plotted over time for both conditions. Figure 3 shows the correlation between Tre and GE for both conditions. R2 in N was 0.04, R2 in H was 0.36.
Table 3.
Rectal temperature (Tre), skin temperature (Tsk) and maximal muscle temperature (Tmmax) at 60% 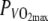 in the thermo-neutral (N) and in the hot dry (H) climate
in the thermo-neutral (N) and in the hot dry (H) climate
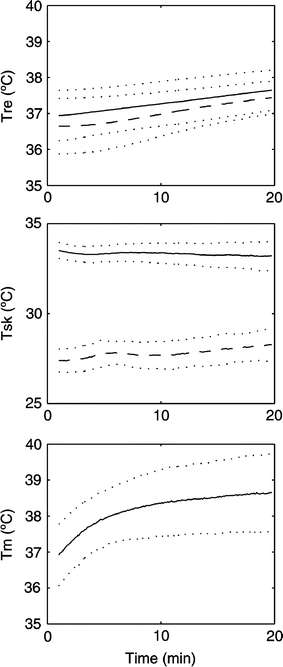
60% 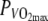
|
||
|---|---|---|
| N | H | |
| Tre (°C) | 37.03 ± 0.58 | 37.35 ± 0.63* |
| Tsk (°C) | 27.74 ± 0.71 | 33.39 ± 0.57* |
| Tmmax (°C) | 38.7 ± 1.1 (n = 6) | |
Values are mean ± SD
* Significant differences with N (P < 0.05)
Fig. 2.
Top panel Rectal temperature (Tre) plotted over time at 60% 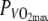 in the thermo-neutral (N) climate (dashed line) and in the hot dry (H) climate (solid line) ±SD (dotted lines). Center panel Skin temperature (Tsk) plotted over time at 60%
in the thermo-neutral (N) climate (dashed line) and in the hot dry (H) climate (solid line) ±SD (dotted lines). Center panel Skin temperature (Tsk) plotted over time at 60% 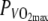 in the thermo-neutral (N) climate (dashed line) and in the hot dry (H) climate (solid line) ±SD (dotted lines). Bottom panel Muscle temperature (Tm) plotted over time at 60%
in the thermo-neutral (N) climate (dashed line) and in the hot dry (H) climate (solid line) ±SD (dotted lines). Bottom panel Muscle temperature (Tm) plotted over time at 60% 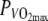 in the hot dry (H) climate (solid line) ±SD (dotted lines)
in the hot dry (H) climate (solid line) ±SD (dotted lines)
Fig. 3.
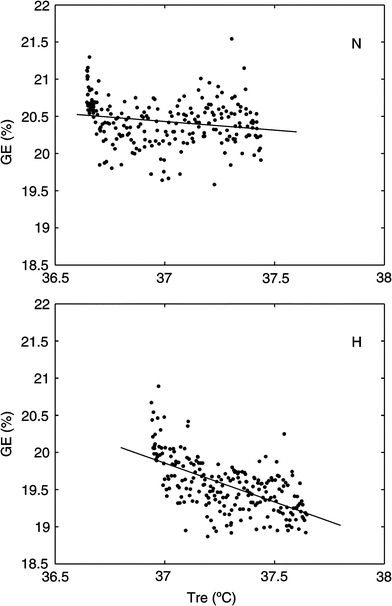
Top panel Correlation between gross-efficiency (GE) and rectal temperature (Tre) for the thermo-neutral (N) climate. Bottom panel Correlation between gross-efficiency (GE) and rectal temperature (Tre) for the hot, dry (H) climate
To quantify the effect of the decrease in GE of 0.9%, a simulation with an energy flow model (De Koning et al. 1999) was performed. This simulation showed that a change in GE of 0.9% would result in a difference in final time of 25.6 s over 20 km. Thus, about half of the decrement in performance reported in the literature (a difference in final time of 48 s over 20 km, Tucker et al. 2004) could be accounted for by the measured decrease in GE. To explain the entire deterioration in time trial performance in the heat, the mean decrease in GE had to be 2%.
Discussion
GE during sub-maximal cycling exercise in the heat was significantly reduced compared to exercise in N by 0.9%. Values for GE corresponded to values found in literature, between 17 and 22% (Foster et al. 2003; Moseley et al. 2001). It has to be kept in mind that in calculating GE at 60% 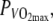 anaerobic contribution was assumed to be negligible. But it might have been influenced by the different climates. Gonzalez-Alonso et al. (1999) reported that exercise in a hot environment results in a higher anaerobic contribution and found an increase in carbohydrate utilization and lactate accumulation during exercise. Though not significant, BLC in the present study was higher in H. According to di Prampero et al. (1999), an increase of BLC of 1 mmol l−1 of blood is equivalent to an O2 consumption of 3 ml kg−1 body weight. Differences in BLC in the present study between climates (2.6 mmol l−1 in N vs. 3.9 mmol l−1 in H) thus correspond with a difference in O2 consumption equivalent of ∼14.1 ml min−1. Accordingly, differences in GE between climates might be even larger then reported in the present study. If one adds this ‘anaerobic O2 equivalent’ to the VO2, then GE decreases about ∼0.19% in N compared to ∼0.27% in H, which means that the difference in GE between N and H increases with ∼0.07%.
anaerobic contribution was assumed to be negligible. But it might have been influenced by the different climates. Gonzalez-Alonso et al. (1999) reported that exercise in a hot environment results in a higher anaerobic contribution and found an increase in carbohydrate utilization and lactate accumulation during exercise. Though not significant, BLC in the present study was higher in H. According to di Prampero et al. (1999), an increase of BLC of 1 mmol l−1 of blood is equivalent to an O2 consumption of 3 ml kg−1 body weight. Differences in BLC in the present study between climates (2.6 mmol l−1 in N vs. 3.9 mmol l−1 in H) thus correspond with a difference in O2 consumption equivalent of ∼14.1 ml min−1. Accordingly, differences in GE between climates might be even larger then reported in the present study. If one adds this ‘anaerobic O2 equivalent’ to the VO2, then GE decreases about ∼0.19% in N compared to ∼0.27% in H, which means that the difference in GE between N and H increases with ∼0.07%.
Mean Tre was about 0.3°C higher in H compared to N. Mean Tsk was about 5.7°C higher in H than in N. Comparable differences of 0.2–0.4°C in Tre (Tatterson et al. 2000; Tucker et al. 2004) and ∼6.0°C in Tsk (Tatterson et al. 2000) were reported during cycling bouts of ∼30 min in climates comparable to the present study. Although the difference in rectal temperature between H and N was significant, it was not a large difference compared to literature. The measured absolute values for Tre were somewhat low, presumably due to the relatively short period of exercise in our protocol and the use of a fan to optimize losing heat to the environment, as was done in the studies of Tatterson et al. (2000) and Tucker et al. (2004). The fan was used to simulate competitive circumstances, and although wind velocity in this study was not as high as the cycling speed, combined with the relatively low RH, the effect would have been considerable (Saunders et al. 2005).
The differences in ambient temperature between H and N represent a considerable difference in thermal stress. Thermal strain is mainly visible in the increased skin temperature, but although the difference of 0.3°C in core temperature is small, it is significant. Rectal and skin temperature changes combined, the difference in body heat content between N and H amounts to 138.5 ± 35.1 kJ, which is considerable. Further, the differences between climates have been shown to be large enough to evoke a difference in GE of 0.9%, the main purpose of the present study.
A possible explanation for the lower GE in the heat may be the higher core temperature. Daanen et al. (2006) found a strong linear relationship between body temperature, mainly determined by Tre, and GE at 60% 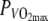 in 30°C. Their study showed that our difference in Tre of 0.3°C could account for a reduction in GE of ∼0.2%, and thus was not large enough to explain the entire reduction in GE of 0.9%. In the study of Daanen et al. (2006), the skin temperature however was almost unchanged, while in the present study skin temperature in the H condition was about 5.6°C higher, indicating an increased blood flow to the skin. The present study did not find a strong correlation in heat (r2 = of 0.36) between Tre and GE. Further, remarkably, in the N-condition, no correlation between Tre and GE was found at all, even though Tre reached values which are also observed in the H-condition, only later in time (see Fig. 3). Apparently, Tre does not seem to be the main cause of the reduced GE. This is supported by the observation that even though Tre rose significantly during the time trial with about 1°C, GE did not increase significantly comparing the first half with the second half within the trials.
in 30°C. Their study showed that our difference in Tre of 0.3°C could account for a reduction in GE of ∼0.2%, and thus was not large enough to explain the entire reduction in GE of 0.9%. In the study of Daanen et al. (2006), the skin temperature however was almost unchanged, while in the present study skin temperature in the H condition was about 5.6°C higher, indicating an increased blood flow to the skin. The present study did not find a strong correlation in heat (r2 = of 0.36) between Tre and GE. Further, remarkably, in the N-condition, no correlation between Tre and GE was found at all, even though Tre reached values which are also observed in the H-condition, only later in time (see Fig. 3). Apparently, Tre does not seem to be the main cause of the reduced GE. This is supported by the observation that even though Tre rose significantly during the time trial with about 1°C, GE did not increase significantly comparing the first half with the second half within the trials.
Two other explanations for the reduced GE in heat can be given. Firstly, an explanation for the temperature-induced reduction in GE might be a metabolic disruption that is known to occur at elevated muscle temperatures (Brooks et al. 1971; Willis and Jackman 1994). Though Ferguson et al. (2006) found no effect of increasing muscle temperature on energy turnover in dynamic exercise in a range of 34.2–38.3°C, ADP:O ratio has been shown to decrease at higher muscle temperatures (Brooks et al. 1971; Willis and Jackman 1994). During heavy exercise in the heat, non-specific proton leakage across the inner mitochondrial membrane is increased, resulting in a decrease in the efficiency of oxidative phosphorylation and increasing the resting metabolic rate (Willis and Jackman 1994). This will result in a reduction in GE. Willis and Jackman (1994) found a decrease of 10–20% in ADP:O ratio at muscle temperatures of 40°C and higher compared to 37°C. They found that this decrease resulted in a 400–800 ml min−1 increase in VO2. In the present study, a smaller but significant increase in VO2 of 124 ml min−1 was found. Mean Tmmax in heat was 38.7°C, lower than the 40°C as found in Willis and Jackman (1994), but higher than 34.2–38.3°C, the Tm range in which Ferguson et al. (2006) found no effect of increasing Tm on energy turnover in dynamic exercise. Since Tm was not measured in both climates, it cannot be confirmed if muscle temperature was significantly different. Tsk and Tre were significantly different but did not approach 40°C in either condition. It seems that, as suggested by Brooks (1971), the core of the body functions as a heat sink for the skeletal musculature helping to maintain Tm below the point where significant reductions in the ADP:O ratio occur.
Another potential cause for the decrease of GE in a hot ambient temperature was the larger vasodilatation of the skin to lose heat, as was shown by the significantly higher Tsk in H compared to N. The resulting decreased blood flow to exercising and respiratory muscles may be compensated by increasing cardiac output (Nielsen et al. 1990; Rowell et al. 1966), since a significant increase in heart rate was found in heat. The extra VO2 in the H condition is at least partially attributable to the extra myocardial VO2, since a higher cardiac output has to exist to continue supplying the muscles with the same blood flow, but have to send extra blood to the skin for cooling. Additionally, ventilation was increased, which may also lead to a reduction in GE. Assuming that the mechanical work per breath is 80–125 J (Milic-Amili et al. 2001), it can be estimated that the higher VE in this study can account for maximally 10% of the increase in VO2.
Lastly, it has to be noticed, that GE decreases if the proportion of energy expenditure that is used to maintain homeostasis is increased. Thus, the lower GE in H could have been solely due to an increase in resting metabolic rate, while net efficiency remained unchanged. This would be consistent with the hypothesis that muscle temperature in the hot conditions was not high enough to make mitochondrial leakage a likely explanation for the observed reduced GE. Unfortunately, resting metabolic rate has not been measured.
To determine the potential importance of the measured decrease in GE on time trial performance, a 20-km time trial, which was also studied by Tucker et al. (2004), was modeled by the use of the energy flow model (De Koning et al. 1999). A time trial of this distance can be seen as a mainly aerobic exercise bout. Tucker et al. (2004) found a difference in final time of 48 s between exercise in 35°C compared to 15°C. Using the energy flow model of De Koning et al. (1999), it was calculated that the measured difference in GE of 0.9% would lead to a difference in final time between H and N of 25.6 s. This explains about half of the 48 s found by Tucker et al. (2004). For the entire reduction in final time of 48 s, a decrease in GE of 2% would be necessary, which was not found. It can be concluded that about half of the decrease in time trial performance can be accounted for by the reduction in GE. It has to be noted that in this simulation, it is assumed that the difference in GE between H and N at higher intensities will not change. Although it has been shown that GE increases with exercise intensity, since the relative share of resting metabolism diminishes at higher sub-maximal intensities (Mosely and Jeukendrup 2001), this effect seems to be equal for both conditions and will at most have only a minor effect on the difference between conditions.
Conclusion
GE was lower in the heat. Tm was not high enough to make mitochondrial leakage a likely explanation for the observed reduced GE. Neither was the increased Tre. The extra VO2 in the H condition seems to be at least partially attributable to the extra myocardial VO2, since a higher cardiac output has to exist to continue supplying the muscles with the same blood flow, but have to send extra blood to the skin for cooling and thus impacted GE. Based on our findings under the current circumstances, it can be concluded that the temperature-induced change in GE could account for about half of the well-established performance decrements found during time trial exercise in the heat.
References
- Brooks GA, Hittelman KJ, Faulkner JA, Beyer RE (1971) Temperature, skeletal muscle mitochondrial functions and oxygen debt. Am J Physiol 220:1053–1059 [DOI] [PubMed]
- Daanen HA, van Es E, de Graaf JL (2006) Heat strain and gross efficiency during endurance exercise after lower, upper or whole body precooling in the heat. Int J Sport Med 27(5):379–388 [DOI] [PubMed]
- De Koning JJ, Bobbert MF, Foster C (1999) Determination of optimal pacing strategy in track cycling with an energy flow model. J Sci Med Sports 2(3):266–277 [DOI] [PubMed]
- De Koning JJ, Foster C, Lampen J, Hettinga F, Bobbert M (2005) Experimental evaluation of the power balance model of speed skating. J Appl Physiol 98(1):227–233 [DOI] [PubMed]
- Di Prampero PE, Feretti G (1999) The energetics of anaerobic muscle metabolism: a reappraisal of older and recent concepts. Resp Physiol 118:103–115 [DOI] [PubMed]
- Febbraio MA, Murton P, Selig SE, Clarke SA, Lambert DL, Angus DJ, Carey MF (1996) Effects of carbohydrate ingestion on exercise metabolism and performance in different ambient temperatures. Med Sci Sports Exerc 28:1380–1387 [DOI] [PubMed]
- Ferguson RA, Krustrup P, Kjaer M, Mohr M, Ball D, Bangsbo J (2006) Effect of temperature on skeletal muscle energy turnover during dynamic knee-extensor exercise in humans. J Appl Physiol 101(1):47–52 [DOI] [PubMed]
- Foster C, de Koning J, Hettinga F, Lampen J, La Clair KL, Dodge C, Bobbert M, Porcari JP (2003) Pattern of energy expenditure during simulated competition. Med Sci Sports Exerc 35(5):826–831 [DOI] [PubMed]
- Garby L, Astrup A (1987) The relationship between the respiratory quotient and the energy equivalent of oxygen during simultaneous glucose and lipid oxidation and lipogenesis. Acta Physiol Scand 129:443–444 [DOI] [PubMed]
- Gonzalez-Alonso J, Teller C, Andersen SL, Jensen FB, Hyldig T, Nielsen B (1999) Influence of body temperature on the development of fatigue during prolonged exercise in the heat. J Appl Physiol 86:1032–1039 [DOI] [PubMed]
- Hettinga FJ, DeKoning JJ, Broersen FT, van Geffen P, Foster C (2006) Pacing strategy and the occurrence of fatigue in 4000 m cycling time trials. Med Sci Sports Exerc 38(8):1484–1491 [DOI] [PubMed]
- Lucia A, Hoyos J, Perez M, Santalla A, Chicharro JL (2002) Inverse relationship between VO2max and economy/efficiency in world class cyclists. Med Sci Sports Exerc 34:2079–2084 [DOI] [PubMed]
- Milic-Emili J, Kayser B, Gautier H (2001) Mechanics of breathing. In: Hornbein TF, Schoene RB (eds) High altitude. An exploration of human adaptation. Marcel Dekker Inc., New York/Basel, p 191
- Moseley L, Jeukendrup AE (2001) The reliability of cycling efficiency. Med Sci Sports Exerc 33(4):621–627 [DOI] [PubMed]
- Moseley L, Achten J, Martin JC, Jeukendrup AE (2004) No differences in cycling efficiency between world-class and recreational cyclists. Int J Sports Med 25:374–379 [DOI] [PubMed]
- Nielsen B, Savard G, Richter EA, Hargreaves M, Saltin B (1990) Muscle blood flow and muscle metabolism during exercise and heat stress. J Appl Physiol 69(3):1040–1046 [DOI] [PubMed]
- Parkin JM, Carey MF, Zhao S, Febbraio MA (1999) Effect of ambient temperature on human skeletal muscle metabolism during fatiguing sub-maximal exercise. J Appl Physiol 86:902–908 [DOI] [PubMed]
- Romer LM, Bridge MW, McConnell AK, Jones DA (2003) Influence of environmental temperature on exercise-induced inspiratory muscle fatigue. Eur J Appl Physiol 91:656–663 [DOI] [PubMed]
- Rowell LB, Marx HJ, Bruce RA, Conn RD, Kusumi F (1966) Reductions in cardiac output, central blood volume and stroke volume with thermal stress in normal men during exercise. J Clin Invest 45:1801–1816 [DOI] [PMC free article] [PubMed]
- Saunders AG, Dugas JP, Tucker R, Lambert MI, Noakes TD (2005) The effects of different air velocities on heat storage and body temperature in humans cycling in a hot, humid environment. Acta Physiol Scand 183(3):241–255 [DOI] [PubMed]
- Tatterson AJ, Hahn AG, Martin DT, Febbraio MA (2000) Effects of heat stress on physiological responses and exercise performance in elite cyclists. J Sci Med Sport 3(2):186–193 [DOI] [PubMed]
- Tucker R, Rauch L, Harley YX, Noakes TD (2004) Impaired exercise performance in the heat is associated with an anticipatory reduction in skeletal muscle recruitment. Pflugers Arch 448(4):422–430 [DOI] [PubMed]
- Willis WT, Jackman MR (1994) Mitochondrial function during heavy exercise. Med Sci Sports Exerc 26(11):1347–1354 [PubMed]


