Abstract
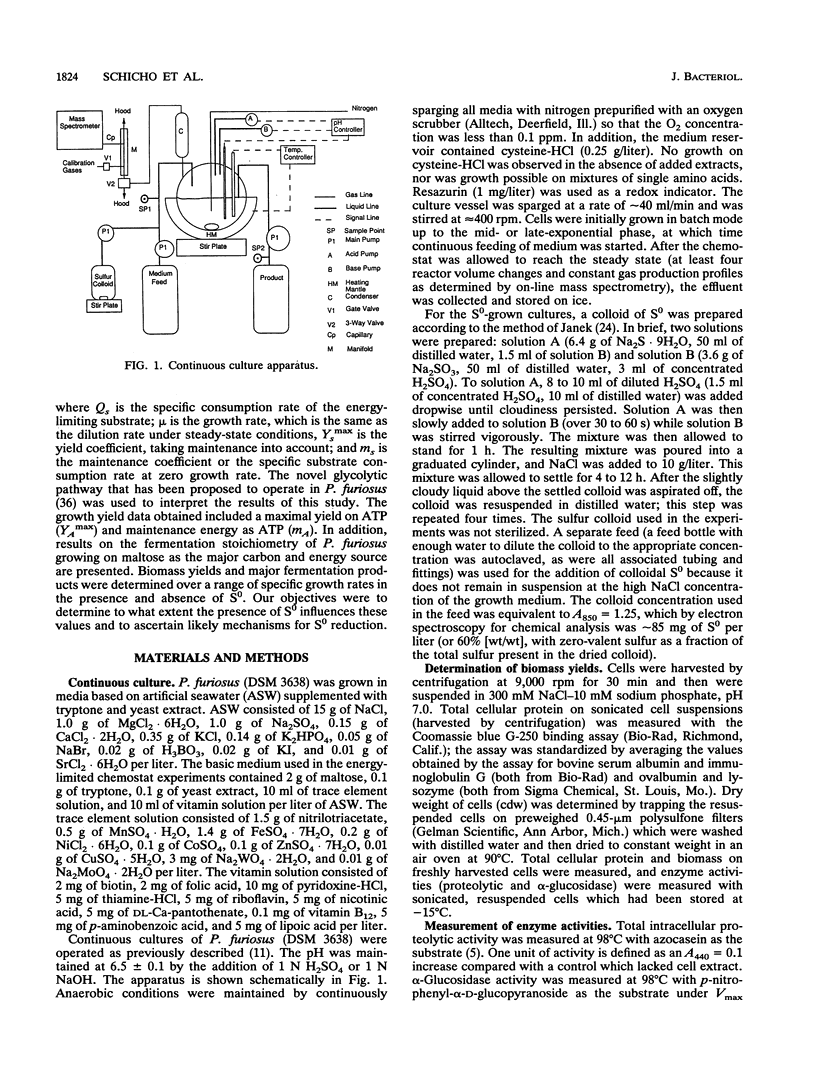
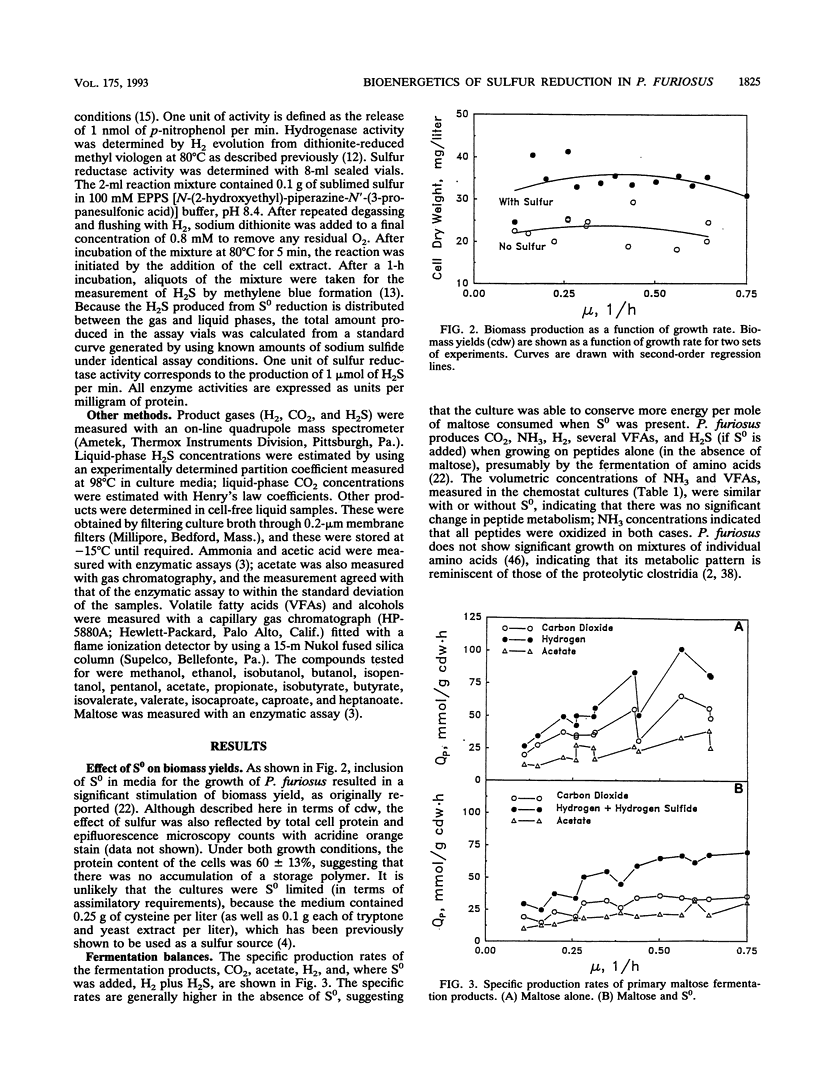
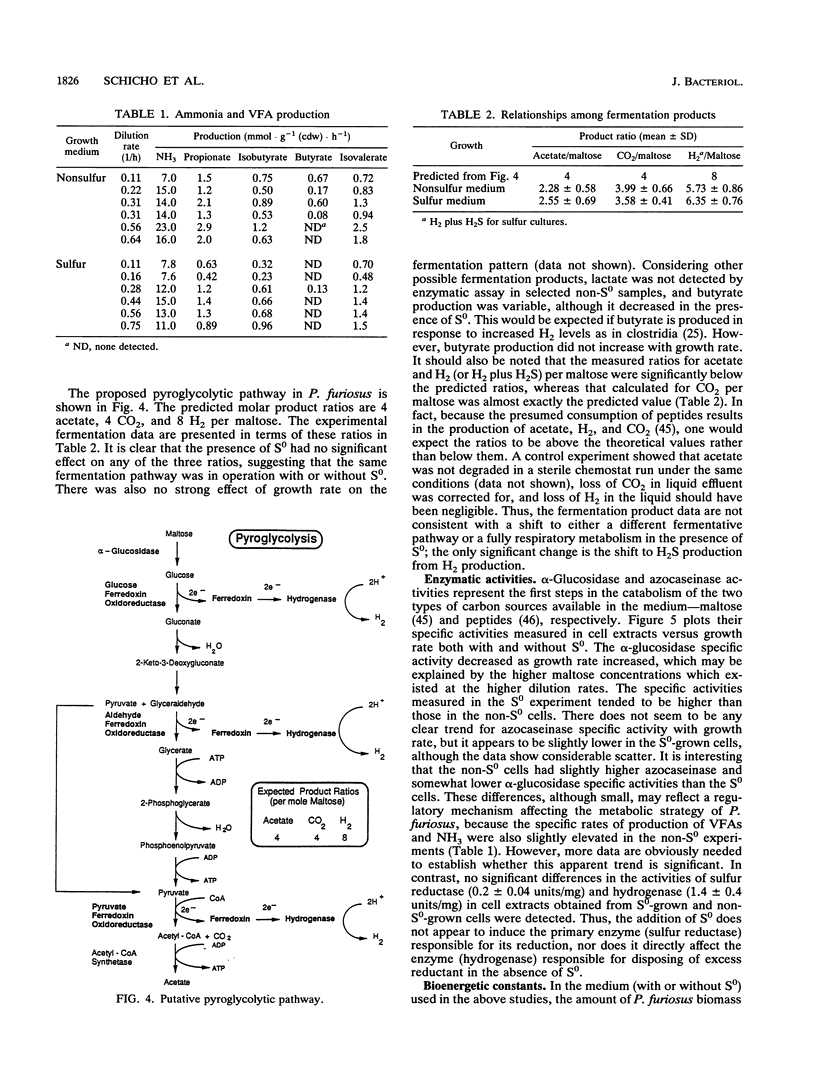
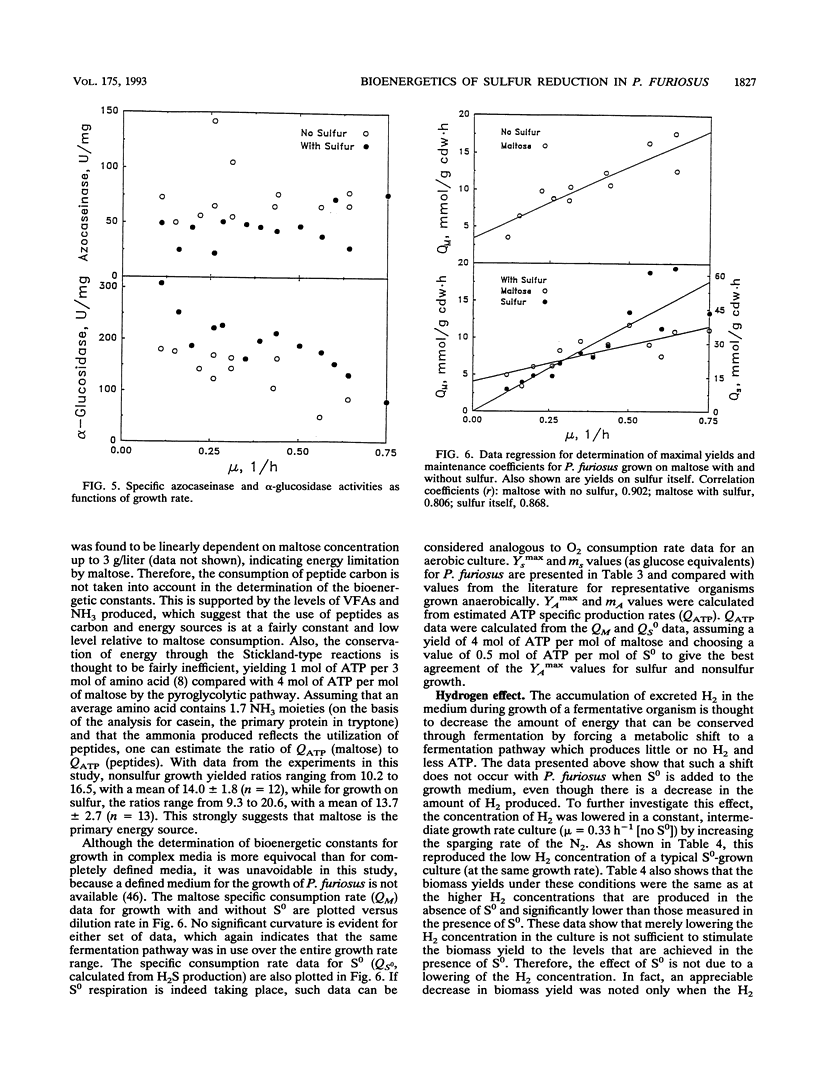
The bioenergetic role of the reduction of elemental sulfur (S0) in the hyperthermophilic archaeon (formerly archaebacterium) Pyrococcus furiosus was investigated with chemostat cultures with maltose as the limiting carbon source. The maximal yield coefficient was 99.8 g (dry weight) of cells (cdw) per mol of maltose in the presence of S0 but only 51.3 g (cdw) per mol of maltose if S0 was omitted. However, the corresponding maintenance coefficients were not found to be significantly different. The primary fermentation products detected were H2, CO2, and acetate, together with H2S, when S0 was also added to the growth medium. If H2S was summed with H2 to represent total reducing equivalents released during fermentation, the presence of S0 had no significant effect on the pattern of fermentation products. In addition, the presence of S0 did not significantly affect the specific activities in cell extracts of hydrogenase, sulfur reductase, alpha-glucosidase, or protease. These results suggest either that S0 reduction is an energy-conserving reaction, i.e., S0 respiration, or that S0 has a stimulatory effect on or helps overcome a process that is yield limiting. A modification of the Entner-Doudoroff glycolytic pathway has been proposed as the primary route of glucose catabolism in P. furiosus (S. Mukund and M. W. W. Adams, J. Biol. Chem. 266:14208-14216, 1991). Operation of this pathway should yield 4 mol of ATP per mol of maltose oxidized, from which one can calculate a value of 12.9 g (cdw) per mol of ATP for non-S0 growth. Comparison of this value to the yield data for growth in the presence of S0 reduction is equivalent to an ATP yield of 0.5 mol of ATP per mol of S0 reduced. Possible mechanism to account for this apparent energy conservation are discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barker H. A. Amino acid degradation by anaerobic bacteria. Annu Rev Biochem. 1981;50:23–40. doi: 10.1146/annurev.bi.50.070181.000323. [DOI] [PubMed] [Google Scholar]
- Blumentals I. I., Itoh M., Olson G. J., Kelly R. M. Role of Polysulfides in Reduction of Elemental Sulfur by the Hyperthermophilic Archaebacterium Pyrococcus furiosus. Appl Environ Microbiol. 1990 May;56(5):1255–1262. doi: 10.1128/aem.56.5.1255-1262.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumentals I. I., Robinson A. S., Kelly R. M. Characterization of sodium dodecyl sulfate-resistant proteolytic activity in the hyperthermophilic archaebacterium Pyrococcus furiosus. Appl Environ Microbiol. 1990 Jul;56(7):1992–1998. doi: 10.1128/aem.56.7.1992-1998.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannan D. K., Caldwell D. E. Growth Kinetics and Yield Coefficients of the Extreme Thermophile Thermothrix thiopara in Continuous Culture. Appl Environ Microbiol. 1983 Jan;45(1):169–173. doi: 10.1128/aem.45.1.169-173.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britz M. L., Wilkinson R. G. Leucine dissimilation to isovaleric and isocaproic acids by cell suspensions of amino acid fermenting anaerobes: the Stickland reaction revisited. Can J Microbiol. 1982 Mar;28(3):291–300. doi: 10.1139/m82-043. [DOI] [PubMed] [Google Scholar]
- Brown S. H., Costantino H. R., Kelly R. M. Characterization of Amylolytic Enzyme Activities Associated with the Hyperthermophilic Archaebacterium Pyrococcus furiosus. Appl Environ Microbiol. 1990 Jul;56(7):1985–1991. doi: 10.1128/aem.56.7.1985-1991.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown S. H., Kelly R. M. Cultivation Techniques for Hyperthermophilic Archaebacteria: Continuous Culture of Pyrococcus furiosus at Temperatures near 100 degrees C. Appl Environ Microbiol. 1989 Aug;55(8):2086–2088. doi: 10.1128/aem.55.8.2086-2088.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant F. O., Adams M. W. Characterization of hydrogenase from the hyperthermophilic archaebacterium, Pyrococcus furiosus. J Biol Chem. 1989 Mar 25;264(9):5070–5079. [PubMed] [Google Scholar]
- Chen J. S., Mortenson L. E. Inhibition of methylene blue formation during determination of the acid-labile sulfide of iron-sulfur protein samples containing dithionite. Anal Biochem. 1977 May 1;79(1-2):157–165. doi: 10.1016/0003-2697(77)90390-6. [DOI] [PubMed] [Google Scholar]
- Costantino H. R., Brown S. H., Kelly R. M. Purification and characterization of an alpha-glucosidase from a hyperthermophilic archaebacterium, Pyrococcus furiosus, exhibiting a temperature optimum of 105 to 115 degrees C. J Bacteriol. 1990 Jul;172(7):3654–3660. doi: 10.1128/jb.172.7.3654-3660.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbendam P. M., Neijssel O. M., Tempest D. W. Metabolic and energetic aspects of the growth of Clostridium butyricum on glucose in chemostat culture. Arch Microbiol. 1985 Sep;142(4):375–382. doi: 10.1007/BF00491907. [DOI] [PubMed] [Google Scholar]
- Farmer I. S., Jones C. W. The effect of temperature on the molar growth yield and maintenance requirement of Escherichia coli W during aerobic growth in continuous culture. FEBS Lett. 1976 Sep 1;67(3):359–363. doi: 10.1016/0014-5793(76)80564-9. [DOI] [PubMed] [Google Scholar]
- Jones D. T., Woods D. R. Acetone-butanol fermentation revisited. Microbiol Rev. 1986 Dec;50(4):484–524. doi: 10.1128/mr.50.4.484-524.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laanbroek H. J., Stal L. H., Veldkamp H. Utilization of hydrogen and formate by Campylobacter spec. under aerobic and anaerobic conditions. Arch Microbiol. 1978 Oct 4;119(1):99–102. doi: 10.1007/BF00407935. [DOI] [PubMed] [Google Scholar]
- Lacis L. S., Lawford H. G. Thermoanaerobacter ethanolicus Growth and Product Yield from Elevated Levels of Xylose or Glucose in Continuous Cultures. Appl Environ Microbiol. 1991 Feb;57(2):579–585. doi: 10.1128/aem.57.2.579-585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukund S., Adams M. W. The novel tungsten-iron-sulfur protein of the hyperthermophilic archaebacterium, Pyrococcus furiosus, is an aldehyde ferredoxin oxidoreductase. Evidence for its participation in a unique glycolytic pathway. J Biol Chem. 1991 Aug 5;266(22):14208–14216. [PubMed] [Google Scholar]
- NISMAN B. The Stickland reaction. Bacteriol Rev. 1954 Mar;18(1):16–42. doi: 10.1128/br.18.1.16-42.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neijssel O. M., Tempest D. W. The role of energy-spilling reactions in the growth of Klebsiella aerogenes NCTC 418 in aerobic chemostat culture. Arch Microbiol. 1976 Nov 2;110(23):305–311. doi: 10.1007/BF00690243. [DOI] [PubMed] [Google Scholar]
- Pfennig N., Biebl H. Desulfuromonas acetoxidans gen. nov. and sp. nov., a new anaerobic, sulfur-reducing, acetate-oxidizing bacterium. Arch Microbiol. 1976 Oct 11;110(1):3–12. doi: 10.1007/BF00416962. [DOI] [PubMed] [Google Scholar]
- Pihl T. D., Black L. K., Schulman B. A., Maier R. J. Hydrogen-oxidizing electron transport components in the hyperthermophilic archaebacterium Pyrodictium brockii. J Bacteriol. 1992 Jan;174(1):137–143. doi: 10.1128/jb.174.1.137-143.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihl T. D., Maier R. J. Purification and characterization of the hydrogen uptake hydrogenase from the hyperthermophilic archaebacterium Pyrodictium brockii. J Bacteriol. 1991 Mar;173(6):1839–1844. doi: 10.1128/jb.173.6.1839-1844.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihl T. D., Schicho R. N., Black L. K., Schulman B. A., Maier R. J., Kelly R. M. Hydrogen-sulfur autotrophy in the hyperthermophilic archaebacterium, Pyrodictium brockii. Biotechnol Genet Eng Rev. 1990;8:345–377. doi: 10.1080/02648725.1990.10647874. [DOI] [PubMed] [Google Scholar]
- Snowden L. J., Blumentals I. I., Kelly R. M. Regulation of Proteolytic Activity in the Hyperthermophile Pyrococcus furiosus. Appl Environ Microbiol. 1992 Apr;58(4):1134–1141. doi: 10.1128/aem.58.4.1134-1141.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stouthamer A. H., Bettenhaussen C. W. A continuous culture study of an ATPase-negative mutant of Escherichia coli. Arch Microbiol. 1977 Jun 20;113(3):185–189. doi: 10.1007/BF00492023. [DOI] [PubMed] [Google Scholar]
- Stouthamer A. H., Bettenhaussen C. W. Determination of the efficiency of oxidative phosphorylation in continuous cultures of Aerobacter aerogenes. Arch Microbiol. 1975 Mar 10;102(3):187–192. doi: 10.1007/BF00428367. [DOI] [PubMed] [Google Scholar]
- Thauer R. K. Citric-acid cycle, 50 years on. Modifications and an alternative pathway in anaerobic bacteria. Eur J Biochem. 1988 Oct 1;176(3):497–508. doi: 10.1111/j.1432-1033.1988.tb14307.x. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Uden N., Madeira-Lopes A. Yield and maintenance relations of yeast growth in the chemostat at superoptimal temperatures. Biotechnol Bioeng. 1976 Jun;18(6):791–804. doi: 10.1002/bit.260180603. [DOI] [PubMed] [Google Scholar]
- Woese C. R., Kandler O., Wheelis M. L. Towards a natural system of organisms: proposal for the domains Archaea, Bacteria, and Eucarya. Proc Natl Acad Sci U S A. 1990 Jun;87(12):4576–4579. doi: 10.1073/pnas.87.12.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe R. S., Penning N. Reduction of sulfur by spirillum 5175 and syntrophism with Chlorobium. Appl Environ Microbiol. 1977 Feb;33(2):427–433. doi: 10.1128/aem.33.2.427-433.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwickl P., Fabry S., Bogedain C., Haas A., Hensel R. Glyceraldehyde-3-phosphate dehydrogenase from the hyperthermophilic archaebacterium Pyrococcus woesei: characterization of the enzyme, cloning and sequencing of the gene, and expression in Escherichia coli. J Bacteriol. 1990 Aug;172(8):4329–4338. doi: 10.1128/jb.172.8.4329-4338.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries W., Kapteijn W. M., van der Beek E. G., Stouthamer A. H. Molar growth yields and fermentation balances of Lactobacillus casei L3 in batch cultures and in continuous cultures. J Gen Microbiol. 1970 Nov;63(3):333–345. doi: 10.1099/00221287-63-3-333. [DOI] [PubMed] [Google Scholar]



