Abstract
Morphometric cerebral characteristics were studied in children with prenatal poly-substance exposure (n =14) compared to controls (n = 14) without such exposure. Ten of the substance exposed children were born to mothers who used opiates (heroin) throughout the pregnancy. Groups were compared across 16 brain measures: cortical gray matter, cerebral white matter, hippocampus, amygdala, thalamus, accumbens area, caudate, putamen, pallidum, brainstem, cerebellar cortex, cerebellar white matter, lateral ventricles, inferior lateral ventricles, and the 3rd and 4th ventricles. In addition, continuous measurement of thickness across the entire cortical mantle was performed. Volumetric characteristics were correlated with ability and questionnaire assessments 2 years prior to scan. Compared to controls, the substance-exposed children had smaller intracranial and brain volumes, including smaller cerebral cortex, amygdala, accumbens area, putamen, pallidum, brainstem, cerebellar cortex, cerebellar white matter, and inferior lateral ventricles, and thinner cortex of the right anterior cingulate and lateral orbitofrontal cortex. Pallidum and putamen appeared especially reduced in the subgroup exposed to opiates. Only volumes of the right anterior cingulate, the right lateral orbitofrontal cortex and the accumbens area, showed some association with ability and questionnaire measures. The sample studied is rare, and hence small, so conclusions cannot be drawn with certainty. Morphometric group differences were observed, but associations with previous behavioral assessment were generally weak. Some of the volumetric differences, particularly thinner cortex in part of the right lateral orbitofrontal cortex, may be moderately involved in cognitive and behavioral difficulties more frequently experienced by opiate and poly-substance exposed children.
Introduction
Prenatal exposure to various drugs and alcohol is associated with an increased risk of regulatory dysfunction and neuropsychological difficulties (e.g. Suess et al., 1997; Moe and Slinning, 2002; Moe, 2002; Slinning, 2004). This relationship can partly be explained by environmental correlates of maternal substance-abuse, including higher level of social risk (Hans and Jeremy, 2001), such as effects of poverty, stress, maternal psychopathology, disruptions in maternal care, and poorer interaction with the primary caregiver. However, developmental difficulties of children born to substance-abusing mothers likely also have potent biological foundations. To date, this is indicated by at least three lines of research: 1) Prenatally exposed children growing up under optimized social conditions, i.e. in carefully chosen foster or adoptive homes, also exhibit on average lower perceptual performance and increased rate of distractibility and hyperactivity (Moe and Slinning, 2002; Moe, 2002, Slinning, 2004, Ornoy et al., 2001). Both Ornoy et al. (2001) and Moe (2002) found that adopted substance-exposed children largely scored within normal limits on intellectual tests, but the performance IQ (and in Moe’s study, general IQ) was significantly lower than that of the comparison group. 2) In vitro studies of cell cultures and animal models have pointed to distinct cellular mechanisms by which exposure to alcohol, cocaine, amphetamine, and opiates cause brain alterations in the developing fetus (Bhat et al., 2006; Harlan and Song, 1994,Harvey, 2004; Hu et al., 2002; Malanga and Kosofsky, 2004; Vathy, 2002). 3) Imaging and autopsy studies of children prenatally exposed to alcohol, cocaine, and methamphetamine generally point to structural brain effects. Fetal Alcohol Syndrome (FAS) has been the most studied, and has been associated with microcephaly and smaller overall brain volumes, with a special volume reduction in the basal ganglia, corpus callosum and cerebellum (for an overview see Riley and McGee, 2005). In an MRI study of children exposed to methamphetamine (n = 13 exposed and 15 controls), Chang et al. (2004) found no significant reduction in total brain volume, but significantly smaller putamen and pallidum, a trend towards smaller caudate, and significantly smaller hippocampal volumes bilaterally. Smith et al. (2001) found cerebral metabolism differences, but no significant volumetric differences in either whole brain volume or any of the above-mentioned subcortical structures as measured in MR images of children exposed to cocaine in utero (n = 14) compared to controls (n = 12).
As seen above, structural brain effects of prenatal exposure to alcohol have been rather well documented in human beings and some human data also exist on structural brain characteristics in children prenatally exposed to cocaine and methamphetamine. Unfortunately, and despite heroin use being an increasing problem in young women relative to men (Greenfield et al., 2003), there is a complete lack of human studies on cerebral effects of prenatal exposure to opiates. The cell culture studies that have been conducted indicate potentially severe negative effects on the developing central nervous system. For instance, Hu et al. (2002) found that morphine triggers approximately a fourfold increase in programmed cell death (apoptosis) in human fetal microglia and neurons. Vathy (2002) points out that the highest concentrations of opioid receptors are present in several brain areas including the limbic system, thalamus, striatum, hypothalamus, midbrain and spinal cord, so a range of physiological mechanisms could be affected by opiate exposure. Along with such physiological effects, animal models have pointed to abnormalities in both subcortical and cortical areas following fetal opiate exposure, and as pointed out by Yanai et al. (2003), opioids, like many other neuroteratogens, affect numerous brain regions and processes. Studying embryos of rats injected with morphine, Harlan and Song (1994) found that prenatal exposure to opiates adversely affects migration and/or survival of neurons within a few days after neurogenesis. Their study suggested that the effect may be more evident in regions which contain neurons with mu opiate receptors, such as parts of the caudate-putamen area of the striatum and the nucleus accumbens.
The present study is targeted at morphometric brain characteristics in a sample of children with prenatal poly-substance exposure. The majority of these children were born to mothers whose main drug of choice was heroin, and who used this throughout the pregnancy. Morphometric characteristics are compared to a control sample without prenatal drug exposure. The effects of substance-exposure in utero is investigated in, and compared across, the thickness of the entire cortical mantle at a point by point basis, as well as in volumes of total cortical gray matter, cerebral white matter, hippocampus, amygdala, thalamus, the accumbens area, caudate, putamen, pallidum, the brainstem, cerebellar cortex, cerebellar white matter, the lateral ventricles, the inferior lateral ventricles, and the 3rd and 4th ventricles. Finally, we explore whether neuroanatomical volumes are related to selected behavioral variables as measured by the Wechsler Intelligence Scale for Children -Revised (WISC-R; Wechsler, 1974) and the Child Behavior Checklist (CBCL; Achenbach, 1991) at a previous assessment. To our knowledge, this is the first study of volumetric brain characteristics including persons exposed to heroin in utero. Heroin addiction constitutes a major problem in a number of societies and may have distinct consequences for the developing brain of the fetus. Therefore, analyses of brain characteristics are performed both for the total sample of poly-substance exposed children and for the subsample of children with known opiate exposure.
Materials and methods
Sample
The present sample is drawn from a longitudinal project on the development of children born to mothers who used illicit drugs during pregnancy (for details see Moe and Slinning, 2002; Moe 2002; Slinning, 2004). This project initially comprised 78 substance exposed children, and 58 control children born without known biological risk to mothers with no history or evidence of alcohol or illicit drug use. All were enrolled in the project in infancy. The majority of the biological mothers of the substance-exposed children (76.9%) were enrolled in a perinatal risk project at the Ullevål Municipal Hospital by the second or third trimester of pregnancy; 23.1% gave birth at other hospitals outside of Oslo. The risk project and an infant and family center [see 3] established contact with these families after the birth of their children. The biological mothers were referred to the perinatal risk project because of concerns about substance abuse by medical or social staff at the municipal health service. Information about substance use was based on the women’s medical and social record and on the information gathered by the perinatal risk project team. A limitation of many studies on prenatal substance exposure, including this one, is that regular toxicological test results for the mothers throughout the pregnancies are not available. Because of many of these women’s heavy substance abuse and the fact that they were polydrug and alcohol users, they often had trouble accounting for the amount, frequency, and timing of drug use during the pregnancy period, so we have no reliable measure of amount of drug exposure. For the reasons listed above, we have only included what may be the most reliable information: the women’s main drug of choice and information about additional drug use.
Among the children with prenatal substance exposure, the majority (84,6%) were placed in foster care during infancy. At the time of the present study, 10 (7 exposed and 3 controls) of the totally 136 families had withdrawn from the project. Further, the following in the exposed group were excluded from the present study: children living in foster homes (n = 29), children with FAS (n = 3), and children with unknown living conditions/address (n = 4). In addition, children in the control group were excluded if they were less than 9 years of age at the time of the present study (n = 6). The children living in foster homes were excluded because we found it too difficult to track their biological legal parents to ask for consent. The children with FAS were excluded because they may have distinct brain characteristics, and this group was so small. Thus, only children prenatally exposed to illicit drugs without a diagnosis of FAS were included in the present study. The control children less than 9 years of age were excluded to limit the age difference between the exposed and control group (see below). Thus, 35 exposed children (31 adopted, 4 living with biological mothers) and 49 control children who were 9 years of age or more at the time of study were invited to participate. 17 adopted exposed children and 17 control children accepted the invitation. In the exposed group, 1 child had a change of mind and 2 were excluded due to artifacts from dental braces in the scans. For 3 of the controls, we were not able to offer suitable scan appointments. Useable scan sets were thus obtained for 14 substance exposed and 14 control children. The sample characteristics of these are presented in Table 1.
Table 1.
Sample characteristics of the two groups.A
| Exposed group (7 F/ 7M) | Control group (5 F/ 9 M) | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Range | Mean | SD | Range | |
| Age at scan | 11.3 | 1.7 | 8.6-13.9 | 9.8 | 0.3 | 9.0-10.2 |
| Birth weight (g) | 2999 | 680 | 2015-4300 | 3864 | 426 | 3100-4615 |
| Birth length (cm)B | 47.6 | 3.0 | 44-52 | 51.5 | 1.4 | 49-54 |
| Birth head circumf. | 34.1 | 2.0 | 31.0-37.0 | 36.1 | 1.1 | 34.0-38.0 |
| Gestational age (w) | 38.1 | 2.7 | 31-40 | 40.7 | 1.1 | 39-42 |
| Abstinences at birthC | 2.1 | 1.1 | 0-3 | ----- | ----- | ------ |
| SES | 3.9 | 0.7 | 2.5-5.0 | 4.1 | 0.9 | 2.5-5.0 |
| McCarthy GCI 4 yrs | 108.4 | 15.3 | 87-136 | 116.1 | 9.4 | 100-128 |
| McCarthy PPSD 4yrs | 50.9 | 9.3 | 39-64 | 62.9 | 8.4 | 49-78 |
| WISC-R IQE 9yrs | 98.9 | 9.8 | 84-117 | 114.4 | 14.9 | 93-145 |
| Free. from distractibility | 27.6 | 4.8 | 17-34 | 34.9 | 4.9 | 24-40 |
| CBCL Total probl. | 29.0 | 17.9 | 5-72 | 15.0 | 12.1 | 0-41 |
| CBCL Externalizing | 8.6 | 5.2 | 1-15 | 5.4 | 5.4 | 0-16 |
| CBCL Attention probl. | 4.9 | 3.5 | 0-13 | 1.5 | 1.9 | 0-6 |
| CBCL Social probl. | 2-6 | 2-1 | 0-7 | 0.4 | 0.6 | 0-2 |
As seen, the means for the WISC-R and McCarthy scores are quite high. This likely partly reflects known elevation of scores since norms for these tests were developed some time ago.
Obtained for 12 children in the exposed group and 13 in the control group
At a rating scale where 0 = none, 1= mild, 2 = moderate, and 3 = severe
McCarthy Perceptual Performance Scale
WISC_R scores were obtained for 13 children in the exposed group, all in the control group
One-way ANOVAs showed significant differences between the groups in age at scan (F(1,27) = 10.973, p = .003), birth weight (F(1,27) = 16.247, p = .000), birth length (F(1,24) = 19.851, p = .000), head circumference at birth (F(1,27) = 11.962, p = .002), gestational age (F(1,27) = 11.579, p = .002), McCarthy Perceptual Performance Scale at appr. 4 yrs (F(1,27) = 12.927, p = .001), total WISC-R IQ at appr. 9 yrs (F(1,26) = 10.125, p = .004), the Freedom from Distractibility Factor from the WISC-R (F(1,26) = 14.959, p = .001), CBCL Total Problems (F (1,27) = 5.821, p = .023), CBCL Attention Problems (F(1,27) = 10.364, p = .003), and CBCL Social Problems (F (1,27) = 13.804, p = .001). There were, however, no significant differences across groups with respect to gender (F(1,27) = .553, p = .464), parental socioeconomic status (SES; F(1,27) = .720, p = .404), McCarthy General Cognitive Index (GCI) at appr. 4 yrs (F(1,27) = 2.577, p = .121), and CBCL Externalizing (F(1,27) = 2.567, p = .121).
In studies such as this, there is a risk of selective participation, i.e. the parents most concerned with their child’s health and behavior may be more willing to participate to get an MRI evaluation than those having no concerns. One-way ANOVAs were performed to see if there were significant differences on basic parameters between the children whose families chose to participate and those who refused the invitation. In the adoptive group, there were no significant differences or trends towards differences between refusers and participants, respectively, either in terms of birth weight (p = .429, F(1,29) = .642, M = 3199g (SD = 698g) vs M = 2999g (SD= 680g), respectively), parental socioeconomic status (SES; for a description of how this was calculated, see Moe, 2002; p = .261, F(1,29) = 1.315, M = 3.6 (SD = 0.8) vs M = 3.9 (SD = 0.7), respectively), McCarthy General Cognitive Index (McCarthy, 1972; see below for a description) at 4 years (p = .763, F(1,28) = .093, M = 107 (SD = 12.6) vs. M = 108, SD = 15.3), respectively), or McCarthy perceptual performance score at 4 years (p = .716, F(1,28) = .135, M = 52 (SD = 9) vs M = 51 (SD = 9), respectively). Hence, there is no reason to believe that selective recruitment of the more burdened substance-exposed children has unduly influenced the results. The same was true for the control group, where there were also no significant differences or trends towards such between refusers and participants, respectively, either in terms of birth weight (p = .192, F(1,47) = 1.754, M = 3673g (SD = 466g) vs M = 3864g (SD = 426g), respectively), parental socioeconomic status (p = .193, F(1,47) = 1.745, M = 3.8 (SD = 0.8) vs M = 4.1 (SD = 0.9), respectively), McCarthy General Cognitive Index at 4 years (p = .832, F(1,44) = .045, M = 115 (SD = 13) vs. M = 116 (SD = 9), respectively), or McCarthy perceptual performance score at 4 years (p = .544, F(1,45), M = 61 (SD = 10) vs M = 63 (SD = 8), respectively). A
As can be seen from Table 1, the controls that participated in the current study are on average 1.5 years younger than the exposed children. There are developmental effects, including pruning, i.e. natural developmental volume reductions, and to a lesser extent, some volume increase, occurring in a number of brain areas around the age of the present sample (Gogtay et al., 2004). Therefore, it would have been preferential if the groups were perfectly matched with regard to age. Unfortunately, if we were to achieve this, we would have lost the advantage of having controls that have been followed from birth, and regularly assessed with the same methods as have the substance-exposed children. Therefore, the study was performed on the original controls and age effects were controlled for statistically (see below).
The biological mothers of 10 of the substance exposed children reported opiates as their main drug of choice. However, 9 of the mothers using opiates reported use of a range of 1-5 other substances during pregnancy, including benzodiazepines, neuroleptics, cannabis, alcohol, cocaine and amphetamine. Of the remaining 4 mothers not reporting heroin use, 1 reported cocaine as main substance of choice, 2 reported psychopharmacological substances, and 1 reported alcohol. However, these 4 reported use of multiple substances, including benzodiazepines, neuroleptics, amphetamine and alcohol. 12 of the 14 children in the exposed group were described with symptoms associated with neonatal abstinence syndrome (NAS; Finnegan et al., 1975), and 10 of these were medicated during the first 8 weeks after birth. All the biological mothers of the exposed group reported smoking tobacco during the pregnancy. One control mother reported infrequent cigarette smoking during the pregnancy. HIV status was obtained from the medical records of 11 of the mothers using substances, and all were negative. None of the exposed children were affected with HIV. All substance exposed children moved from their biological mothers within 6 weeks after birth. 7 of the children had 1 placement prior to moving to permanent foster parents at or before 32 weeks. 6 children had 2 placements prior to moving to permanent foster parents at or before 26 weeks. One child had 3 placements prior to moving in with permanent foster parents at 48 weeks. That is, all substance exposed children were placed in permanent foster care within their first year of life, and all were later adopted by these same foster parents. The SES of the biological mothers was predominantly low, and can safely be assumed to be lower than that of the control and adoptive parents. SES of the birth mothers and prenatal substance exposure will therefore be closely related, and a statistical separation of these variables would unfortunately not be possible. However, as seen above, the SES of the adoptive and control parents was comparable. Variance in racial origin is another variable that may be of importance for possible group differences. However, race is not normally noted in medical journals in Norway, and this was not done in the present study either, so we can unfortunately not know this with certainty. However, all children appeared to be born by Norwegian caucasian mothers. There is not reason to believe that there were big differences in the racial origin of the control children and the exposed children.
Among the opiate exposed children, 1 was born with a myelomeningocele (MMC). All the MRI brain scans in the present study were inspected by a neuroradiologist. They were examined for anatomical abnormalities, including congenital changes, and the neuroradiologist looked for signal intensity differences/pathology in white and gray matter and examined CSF spaces. No signs of cerebral pathology were found. This was also the case with the scans of the child with MMC. They were carefully inspected, and there was no sign of intracerebral pathology, neither hydrocephalus nor any Chiari malformation. Another of the opiate exposed children had been diagnosed with Asperger’s syndrome (AS). After some consideration, we decided to include both of these children in the final sample. Maternal opiate addiction may be associated with significant prenatal risk both because of direct teratogenic factors and poor health and dietary conditions. Even though this has not been directly linked to either of the conditions in question, a higher rate of pathological conditions in prenatally opiate exposed children seems likely. For instance, MMC may be associated with inadequate maternal folic acid intake, low maternal vitamin B12 status, and exposure to some teratogens, and about 25 % of the incidences of AS is associated with pre- or perinatal risk (Gillberg and Cederlund, 2005; Mitchell et al., 2004). Two of the opiate exposed children were also born prematurely (gestational age (GA) < 37 weeks, namely 31 and 34 weeks), which is a known risk associated with prenatal substance exposure (Hans, 1992). Instead of excluding these children, whose conditions may be linked to the phenomenon being investigated, we chose to include them in the study, but perform analyses both including and excluding them. Prenatal substance exposure is associated with heightened risk of attention deficits (Slinning, 2004), and 4 of the children in the present sample had an Attention Deficit Hyperactivity Disorder (ADHD) diagnosis and were medicated for this.
The study was approved by the regional committee for medical research ethics. All parents signed informed consent. The children were given adapted information and all gave spoken consent. During scanning, the children listened to audio books or music through earphones calibrated to the scanner.
Behavioral data
As part of previous follow ups, the present sample (and the broader groups from which it is drawn) has been assessed with a number of cognitive and rating scale instruments (see Moe and Slinning 2002; Moe 2002; Slinning, 2004). Data from some of these are presented here. At approximately 4.5 years of age, the children were assessed with the McCarthy Scales of Children’s Abilities (McCarthy, 1972). This test yields a General Cognitive Index (GCI) and scores on five subscales labeled the Verbal scale, the Perceptual-Performance scale, the Quantitative scale, the Memory scale, and the Motor scale. The Verbal, Perceptual-Performance, and Quantitative scales are nonoverlapping and together constitute the GCI. The raw scores were converted to index scores (the GCI: M = 100, SD = 16; and the five Scale Indexes: M = 50, SD = 10). Data are presented here for the McCarthy GCI as well as the McCarthy Perceptual Performance Scale. The latter was the scale that correlated (Pearson’s correlation coefficient) the most with later IQ as measured, for all but 1 child, by the Wechsler Intelligence Scale for Children revised (WISC-R; Wechsler, 1974) at a mean age of 8.63 (SD 0.71) years (r(27) = .50, p = .008 for GCI-IQ, r(27) = .66, p = .000 for perceptual performance scale-IQ). WISC-R is a general ability measure that consists of a verbal and performance part, each composed of 6 subtests. The scores on these 12 subtests are converted to scaled scores and all together are used in calculating IQ (M = 100, SD = 15). As seen from table 1, the mean scores at the McCarthy and the WISC-R are quite high. It is true that none of the exposed children show profound cognitive deficits, but the scores for these ability tests are likely somewhat elevated since they are based on tests which norms were developed some time ago. While total IQ is calculated from all subtests, the Freedom from distractibility factor is comprised of the Arithmetic, Digit Span and Coding subtests. It has been suggested that in addition to resistance to the effects of distracting stimuli, this factor reflects executive and short term memory processes involved in planning, monitoring and evaluating task performance (Wielkiewicz, 1990). The Child Behavior Check List (CBCL; Achenbach, 1991) was also administered to all children’s families at the time of administration of WISC-R. The CBCL questionnaire is a well-validated, standardized assessment protocol for child behavior problems designed for completion by primary caregivers. CBCL produces nine clinical subscales which sums up to a total problem score. Here we report raw scores only, since Norwegian norms have not yet been developed, and it has been found that Nordic parents seem to assess their children similarly on the CBCL, but consistently lower than parents in other countries (Nøvik, 1999). The Attention Problems subscale of the CBCL is effective in identifying children with clinically diagnosed ADHD (Kadesjø et al., 2001).
MRI scanning
A Siemens (Erlangen, Germany) Sonata 1.5 T MR scanner with an 8 channel head coil was used. The pulse sequences used for morphometric analysis were: a range of 4 to7 3D magnetization prepared gradient echo (MPRAGE), T1-weighted sequences in succession (TR/TE/TI/FA = 2730ms/3.5ms/1000ms/7deg, matrix=256×256, FOV=256mm), with iPAT and a GRAPPA factor of 2, and a scan time of 4.3 min per volume. Each volume consisted of 128 sagittal slices with slice thickness = 1.33 mm, and in-plane pixel size of 1.3 mm × 1 mm. The image files in DICOM format were transferred to a Linux workstation for morphometric analysis. All scans were inspected for movement artifacts, and poor scans were excluded prior to averaging the MPRAGEs. The mean number of MPRAGEs obtained for the exposed group was 4.4 (SD 0.9), versus 4.3 (SD 0.5) for the controls. The mean number of MPRAGEs averaged was 3.1 (SD 0.7) for the exposed group and 3.4 (SD 0.6) for the controls, a non-significant difference (p = .18). The range of scans averaged was 2 to 4 in both groups. While numbers for total brain volume were calculated based on the averaged MPRAGE volume and all the structures segmented in this in the whole brain segmentation procedure (see below), intracranial volume (ICV) was calculated based on 3D double flip scans obtained during the same scanning session as the MPRAGEs (TR/TE/FA = 2.4ms/1.0ms/ 1 & 8 deg, matrix=128×128, FOV=320mm). Two such series were acquired with the body coil, and two such series were acquired with the head coil. A deformable template procedure, similar to the “Shrink Wrapping” procedure described by Dale and colleagues (Dale et al., 1999; Dale and Sereno, 1993), was used to obtain an estimate of the smooth surface surrounding the intracranial space (containing cerebrum and cerebellum, CSF, meninges, and brainstem to a level immediately below the pons).
Whole brain segmentation
The automated procedures for volumetric measures of the different brain structures are described by Fischl et al. (2002). The results of manual labeling using the validated techniques of the Center for Morphometric Analysis (e.g. Caviness et al., 1989; Kennedy et al., 1989; Seidman et al., 1999) are used to automatically extract the information required for automating the segmentation procedure. This procedure automatically assigns a neuroanatomical label to each voxel in an MRI volume based on probabilistic information automatically estimated from a manually labeled training set. The training set included adults only. A group-specific template or atlas would be considered ideal (Wilke et al., 2002), but was unfortunately not available for the present study. However, the present atlas was deemed adequate because even though intracranial volume and brain size undergoes dramatic changes in the first few years of life, and slight changes are also seen throughout the teens, the overall intracranial and brain volume at the age in question (8-13 yrs) is very similar to that of adults (see e.g. Sgouros et al., 1999). The classification technique used employs a registration procedure that is especially well suited to account for varying anatomies (Fischl et al., 2004) and was deemed to yield accurate results in children of the present age. Briefly, the segmentation is carried out as follows: First, an optimal linear transform is computed that maximizes the likelihood of the input image, given an atlas constructed from manually labeled images. Next, a nonlinear transform is initialized with the linear one, and the image is allowed to further deform to better match the atlas. Finally, a Bayesian segmentation procedure is carried out, and the maximum a posteriori (MAP) estimate of the labeling is computed. The segmentation uses three pieces of information to disambiguate labels: (1) the prior probability of a given tissue class occurring at a specific atlas location, (2) the likelihood of the image given that tissue class, and (3) the probability of the local spatial configuration of labels given the tissue class. This latter term represents a large number of constraints on the space of allowable segmentations, and prohibits label configurations that never occur in the training set (e.g. hippocampus is never anterior to amygdala). A correction for partial volume effects is performed by estimating the percentage of each voxel occupied by each tissue class that borders it based on the image intensity and the class means.
The technique has previously been shown to be comparable in accuracy to manual labeling (Fischl et al., 2002). The segmentations were visually inspected for accuracy. None were discarded, but a minor segmentation error where medial white matter (WM) of the temporal lobe (MTL) had been included in the hippocampal formation area was found for two subjects (one control and one substance exposed). These two errors were corrected manually. That is, the automated segmentation had in these two cases missed a few voxels of white matter that were misclassified as gray, but were actually part of the continuous white matter strings underlining the hippocampal area. The white matter voxels that constitute the caudal boundaries of the predominantly gray matter hippocampal area were then reattached manually to form a continuous string of WM by filling in a few voxels in some coronal slices. Accuracy of classification was also checked in saggital and transversal views. The mentioned segmentation errors may be due to a bias field in the scans in this area. A conventional Siemens B1 normalization was used, and the remaining inhomogeneties were in most cases benign, but may in these two cases have led to a few incorrectly labeled voxels that were then corrected by hand.
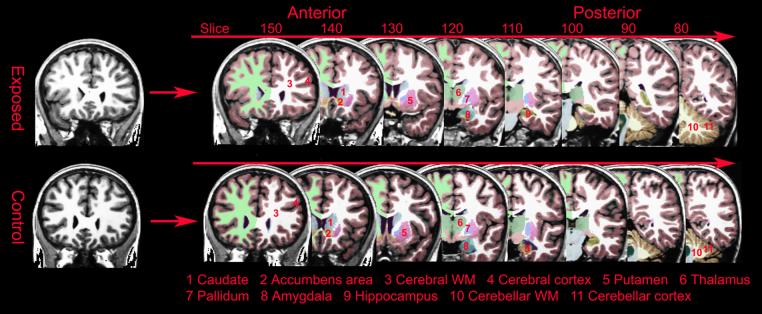
A sample of the segmentation in different coronal views is shown in Figure 1. Further images of the segmentation in coronal, horizontal, and saggital views can be seen in Supplementary Figure 1. General linear model analyses showed no interactions between group and hemisphere for any volume, so for each neuroanatomical volume, the values for the left and right hemispheres were summed.
Figure 1.
A sample of the automated segmentation of the brain volumes of two healthy 10 year old boys. Bottom: normal control. Top: opiate exposed. Voxel size is 1 mm3.
Cortical thickness analyses
The automated procedures for volumetric measurement of the entire cortical mantle are described by Salat et al. (2004). Cortical thickness measurements were obtained by reconstructing representations of the gray/white matter boundary (Dale et al., 1999; Dale and Sereno, 1993) and the cortical surface and then calculating the distance between these surfaces at each point across the cortical mantle. This method uses both intensity and continuity information from the entire 3D MR volume in segmentation and deformation procedures to construct representations of cortical thickness. The maps are created using spatial intensity gradients across tissue classes and are therefore not simply reliant on absolute signal intensity. The maps produced are not restricted to the voxel resolution of the original data and thus are capable of detecting submillimeter differences between groups (Fischl and Dale, 2000). This has been validated using histology and MR (Rosas et al., 2002; Kuperberg et al., 2003). Rosas et al. (2002), have showed that measurements on MR images of autopsy brains analyzed similarly were within 0.25 mm of those obtained using neuropathologic methods and were statistically indistinguishable. Thickness measures may be mapped on the ‘inflated’ surface of each participant’s reconstructed brain (Dale et al., 1999; Fischl et al., 1999), allowing visualization of data across the entire cortical surface without interference from cortical folding. Maps were smoothed using a circularly symmetric Gaussian kernel across the surface with a standard deviation of 12.6 mm and averaged across participants using a non-rigid high-dimensional spherical averaging method to align cortical folding patterns (Fischl et al., 1999). This procedure provides accurate matching of morphologically homologous cortical locations among participants on the basis of each individual’s anatomy while minimizing metric distortion, resulting in a mean measure of cortical thickness for each group at each point on the reconstructed surface. Statistical comparisons of global data and surface maps were generated by computing a general linear model of the effects of each variable on thickness at each vertex. Instead of using a corrected p-value threshold, a scale with the actual p-values is displayed in the figures. Since the number of participants in each group is limited, a standard correction for multiple comparisons will be too conservative and is not generally applied, and the results must be evaluated with this in mind, but see below for a description of permutation testing.
Statistical analyses
The groups differed with respect to a number of factors that we wanted to control for in the analyses. This included age at scan and gestational age. There were no significant gender differences between groups, but there was a slightly larger proportion of boys in the control group than in the substance-exposed group. Since gender influences brain size systematically also in childhood by means of larger brains for boys (Sgouros et al., 1999), we also wanted to regress out the effects of gender in some analyses. Further, since no former study has looked at cerebral morphometric characteristics in prenatal opiate exposure, we also wanted to perform analyses for this group alone, with cases of prenatal substance exposure not involving heroin excluded. In addition, two children in this group had relatively rare medical conditions, so analyses were also performed without them to see if they unduly influenced the results. Since the sample is relatively small and some of the potential confounds were strongly or exclusively associated with the substance exposed group, statistic separation of these variables and exclusion of cases of known pathology may mask true effects of prenatal substance exposure. In order to balance the pros of statistic control and the cons of systematically regressing out variables that may be of interest, and effectively further reducing the sample for analysis, we additionally chose to perform several separate analyses varying the number of variables to be regressed out (please note that this is not the same as classic stepwise regression): Analyses were performed comparing the groups with no other variables regressed out, then with the effect of sex regressed out, then with the effect of sex and age at scan regressed out, and then with the effect of sex, age at scan and gestational age regressed out. The reason for this analysis strategy is that the groups are so small that it is possible that statistical isolation of the variables cause undue influence from a very small number of cases and may create results which are not representative. We want the reader to be able to evaluate the effects in different circumstances. As a means of correcting the cortical analysis data for multiple comparisons, permutation testing was performed on the cortical GLM group analysis of exposed compared to controls.
Whole brain segmentation analyses
First, to study the effect group, exposed (E) vs. controls (C), on the neuroanatomical volumes, ANCOVA was performed for the whole sample (n = 14 E + 14 C) controlling for the effect of gestational age, age at scan, and gender. Second, this analysis was repeated with only the children exposed to heroin compared to controls (n = 10 E + 14 C), and third, the analysis was repeated with only cases of heroin exposure excluding cases of AS and MMC (n = 8 E + 14 C). Further, these analyses were repeated also controlling for the effect of total intracranial volume (ICV). Cortical thickness analyses: First, a general linear model was computed comparing the effect of group only on thickness at each vertex. Second, this analysis was repeated regressing out gestational age, age at scan, and gender, and third, the analysis was repeated with only those children exposed to heroin compared to controls (n = 10 E + 14 C), and last, the analysis was repeated with only cases of heroin exposure and excluding cases of AS and MMC (n = 8 E + 14 C). Correlations between neuroanatomical and behavioral variables: To explore possible functional significance of neuroanatomical variation, the studied neuroanatomical volumes, including selected cortical labels, were correlated with selected data collected at the last behavioral assessment (M = 8.62, SD 0.73 yrs of age). We chose broad ability variables, including total IQ and the Freedom from distractibility factor from WISC-R. In addition, correlations were performed for the CBCL Total problems score and selected CBCL variables that may be associated with regulatory function, and that showed group differences (see table 1), including the Attention problems and Social problems scores.
Results
Whole brain segmentation
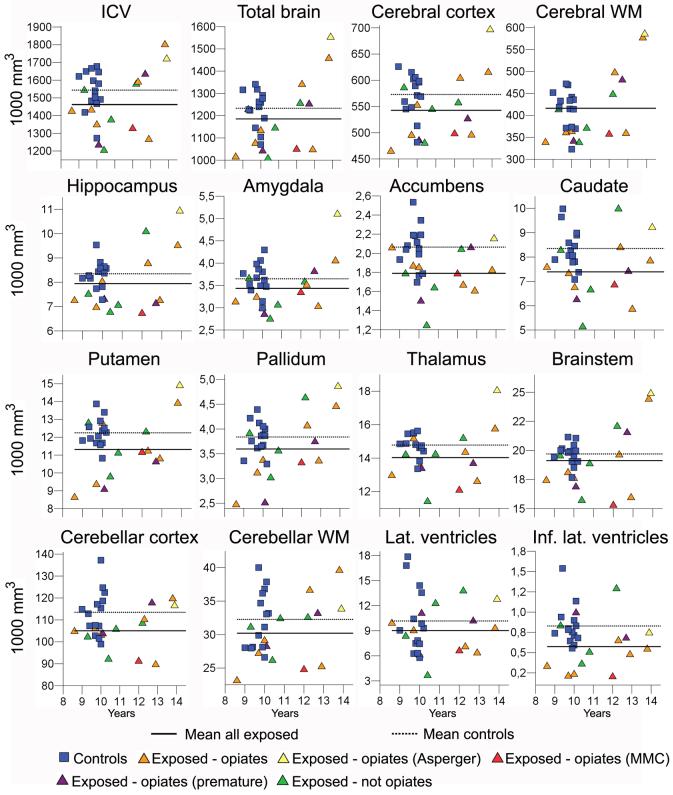
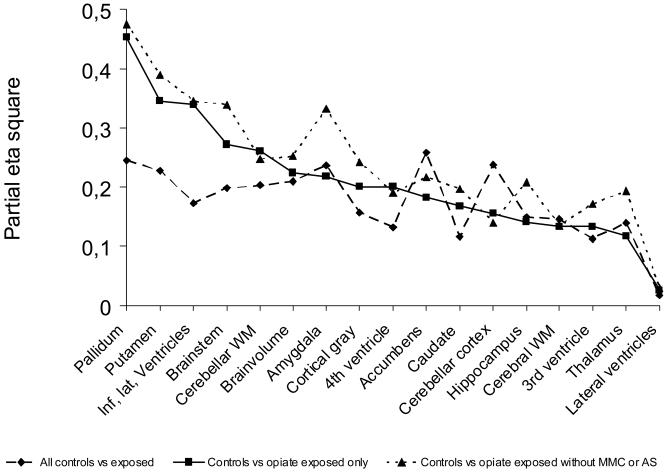
Table 2 shows mean volumes for all brain structures for controls and substance exposed children with and without exposure to opiates. Table 3 depicts ANCOVA results for the effect of group when gender, age at scan and gestational age were controlled for. The analyses were performed comparing controls and exposed as well as subgroups excluding non-opiate exposure and cases suffering from AS and MMC. There was a significant (p ≤ .05) effect of exposure group on ICV, total brain volume, cerebral cortical volume, and volumes of amygdala, accumbens, putamen, pallidum, the brainstem, cerebellar cortex and cerebellar white matter, as well as the inferior lateral ventricles. As seen from table 2, all effects were in the form of smaller volumes in the exposed group. The volume distributions for the different groups with exposure type and risk factors indicated are shown in Figure 2. With the exception of volumes of the lateral ventricles and the 3rd ventricle, trends (p ≤ .10) towards smaller volumes were also observed for the other structures investigated, namely for cerebral white matter, hippocampus, caudate, thalamus, and the 4th ventricle. The significant effects, with the exception of those for ICV, accumbens and cerebellar cortical volume, which were reduced to trends (p ≤ .10), remained significant when restricting the analyses to the smaller sample of children exposed to opiates. Further, these effects largely remained, or even increased in strength, also when excluding cases of AS and MMC, reducing the sample to n = 8. Effect sizes on brain volumes of different subsamples are plotted in Figure 3. While most effect sizes remained relatively stable across different subsamples of substance exposed children, it seems that effect sizes on the volume of pallidum, putamen, inferior lateral ventricles, and, to some extent, the brainstem, increased when restricting the analyses to the opiate exposed only compared to controls. The strongest effects for the opiate exposed group were observed in these structures. When controlling for the effect of intracranial volume in addition to gender, age at scan, and GA, the only significant group difference was for cerebellar cortical volume (F(1,22) = 5.090, p = .034, partial eta squared = .188), with a trend for accumbens area volume (F(1,22) = 4.005, p = .058, partial eta squared = .154) in the comparison of all controls and all exposed children. However, when comparing controls to opiate exposed children (n =10) only, there was a significant effect of group independent of intracranial volume on the pallidum (F(1,18) = 9.686, p = .006, partial eta squared = .350), the inferior lateral ventricles (F(1,18) = 5.885, p = .026, partial eta squared = .231), and the putamen (F(1,18) = 5.402, p = .032, partial eta squared = .231). The latter results were upheld also when excluding the cases with MMC and AS: Pallidum (F(1,16) = 9.950, p = .006, partial eta squared = .383), inferior lateral ventricles (F(1,16) = 5.810, p = .028, partial eta squared = .266, and putamen (F(1,16) = 6.410, p = .022, partial eta squared = .286) showed significant effects, and there were specific effects in this subgroup on amygdala (F(1,16) = 4.573, p = .048, partial eta squared = .222) and brainstem volume (F(1,16) = 4.916, p = .041, partial eta squared = .235).
Table 2.
Mean brain volumes in mm3 (SD) shown for all controls (n = 14) and all poly-substance exposed children without (n = 4) and with (n = 10) opiate exposure.
| Group Means and SDs in mm3 | ||||||
|---|---|---|---|---|---|---|
| Volume | Controls | Exposed non-opiate | Exposed opiate | |||
| M | SD | M | SD | M | SD | |
| ICV | 1543736 | 113627 | 1424438 | 171866 | 1477098 | 196827 |
| Total brain | 1233193 | 81300 | 1158444 | 111356 | 1196149 | 193924 |
| Cerebral cortex | 572950 | 40615 | 541721 | 44620 | 543194 | 73676 |
| Cerebral wm | 415927 | 40615 | 392284 | 48049 | 425926 | 99229 |
| Hippocampus | 8350 | 535 | 7852 | 1519 | 7982 | 1357 |
| Amygdala | 3649 | 352 | 3259 | 433 | 3503 | 675 |
| Accumbens | 2064 | 228 | 1676 | 333 | 1836 | 210 |
| Caudate | 8349 | 817 | 7506 | 2088 | 7344 | 996 |
| Putamen | 12248 | 787 | 11495 | 1345 | 11242 | 2070 |
| Pallidum | 3839 | 311 | 3777 | 676 | 3525 | 774 |
| Thalamus | 14780 | 642 | 13750 | 1631 | 14142 | 1769 |
| Brainstem | 19698 | 934 | 19036 | 2620 | 19165 | 3390 |
| Cerebellar cortex | 113464 | 10373 | 102095 | 7151 | 106204 | 10312 |
| Cerebellar wm | 32270 | 4338 | 30542 | 3006 | 30074 | 5479 |
| Lat. ventricles | 10166 | 3962 | 9494 | 4527 | 8824 | 2246 |
| Inf. lat. ventricles | 829 | 253 | 746 | 412 | 505 | 264 |
| 3rd ventricle | 1069 | 215 | 873 | 288 | 854 | 160 |
| 4th ventricle | 2488 | 654 | 2210 | 211 | 1651 | 382 |
Table 3.
Group differences in brain volumes when the effect of age at scan, gestational age at birth, and gender is controlled for. ANOVAs were performed with all controls (n = 14) compared to a) all substance exposed (n = 14, df = 1, 23), b) opiate exposed only (n =10, df = 1,19), c) opiate exposed without MMC or AS (n = 8, df = 1, 17).
| Volume | Controls/ all exposed | Controls/ opiate exposed | Controls/ healthy opiate exposed | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| ICV | 4.723 | .040 | 3.663 | .071 | 3.024 | .100 |
| Total brain | 6.094 | .021 | 5.478 | .030 | 5.740 | .028 |
| Cerebral cortex | 4.276 | .050 | 4.771 | .042 | 5.456 | .032 |
| Cerebral wm | 3.932 | .059 | 2.947 | .102 | 2.659 | .121 |
| Hippocampus | 4.012 | .057 | 3.129 | .093 | 4.452 | .050 |
| Amygdala | 7.096 | .014 | 5.292 | .033 | 8.443 | .010 |
| Accumbens | 8.047 | .009 | 4.268 | .053 | 4.723 | .044 |
| Caudate | 3.056 | .094 | 3.837 | .065 | 4.158 | .057 |
| Putamen | 6.797 | .016 | 9.991 | .005 | 10.851 | .004 |
| Pallidum | 7.453 | .012 | 15.790 | .001 | 15.440 | .001 |
| Thalamus | 3.744 | .065 | 2.539 | .128 | 4.091 | .059 |
| Brainstem | 5.684 | .026 | 7.111 | .015 | 8.716 | .009 |
| Cerebellar cortex | 7.184 | .013 | 3.520 | .076 | 2.779 | .114 |
| Cerebellar wm | 5.851 | .024 | 6.721 | .018 | 5.580 | .030 |
| Lat. ventricles | .403 | .532 | .505 | .486 | .507 | .486 |
| Inf. lat. ventricles | 4.806 | .039 | 9.785 | .006 | 8.948 | .008 |
| 3rd ventricle | 2.922 | .101 | 2.922 | .104 | 3.508 | .078 |
| 4th ventricle | 3.509 | .074 | 4.794 | .041 | 3.997 | .062 |
Figure 2.
Volume distributions measured in number of voxels (mm3, y-axis) and plotted according to age (x-axis) for the different groups with exposure type and risk factors indicated. The lines indicate group means for all exposed children together and controls, respectively.
Figure 3.
Partial eta squares plotted for comparison of effect sizes of group when the effect of age at scan, gestational age at birth, and gender is controlled for. ANOVAS were performed for all controls (n = 14) compared to A) all substance exposed (n = 14, df = 1, 23), B) opiate exposed only (n =10, df = 1,19), C) opiate exposed without MMC or AS (n = 8, df = 1, 17).
Cortical thickness
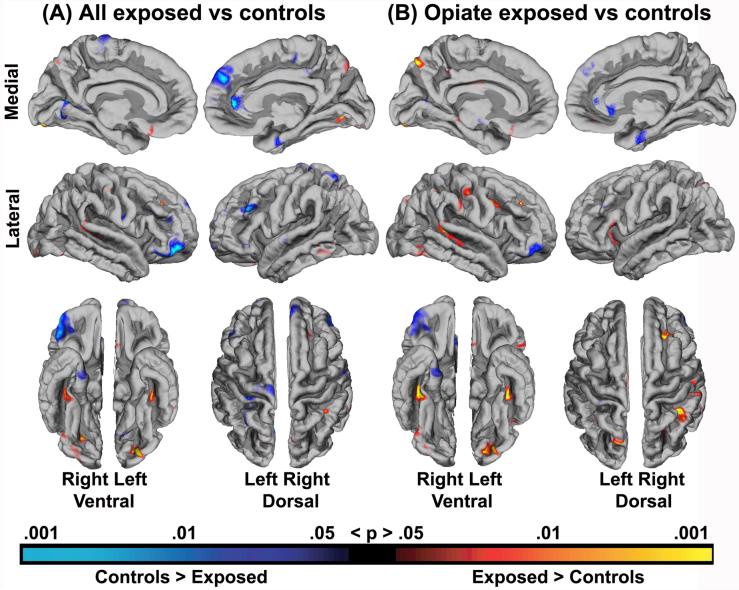
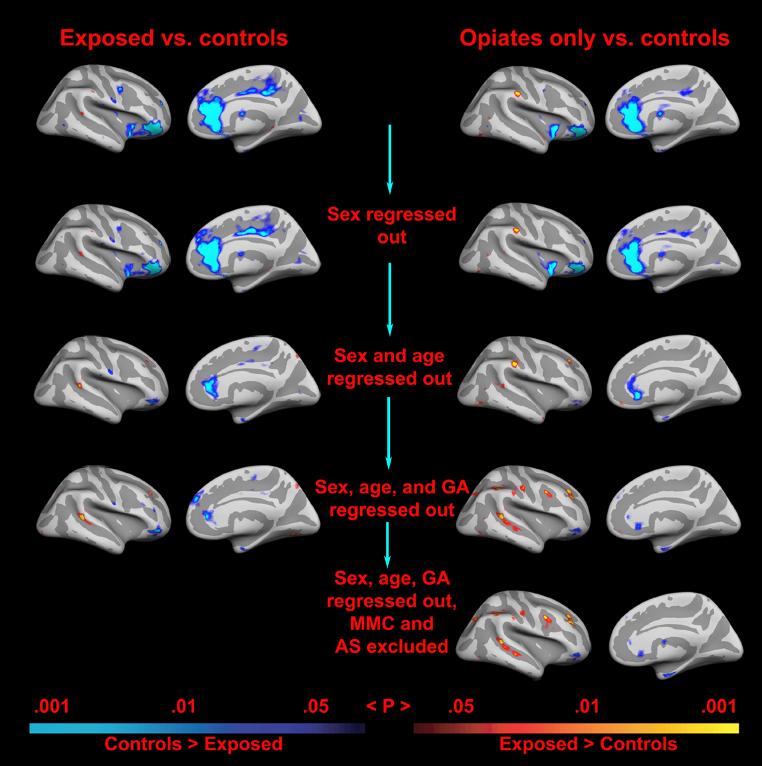
Results of the general linear model of the effect of exposure group on the cortical mantle with the effects of gender, age at scan and gestational age controlled for, are depicted in Figure 4a. Several areas were identified as being of significantly different cortical thickness across groups. Most noteworthy, areas in the anterior cingulate (ACC) and lateral orbitofrontal cortex (LOFC) of the right hemisphere were identified as thinner in the exposed group. As seen in figure 4b, this result remained significant when restricting the analysis to opiate exposed children only compared to controls. Most of the effects were seen in the form of relatively small areas of thinner cortex in the exposed group relative to controls, but as seen, a few scattered areas in the lateral cortex were identified as thicker in the exposed group. An analysis of effect of exposure group not regressing out other variables, depicted in Figure 5, showed almost exclusively effects in the form of thinner cortex in the exposed group, indicating that the spots of thicker cortex may to some degree be an artifact associated with the statistical separation of these variables. The broadest initial thinning effect, prior to regressing out other variables was seen in the right ACC, an area which has also been implicated in both substance exposure and ADHD. As seen from Figure 5, the right ACC and LOFC effects were limited by, but withstand, statistical control for potential confounds. The effects covered smaller areas, but remained significant also when excluding cases of AS and MMC, that is, when reducing the opiate exposed sample to n = 8.
Figure 4.
Results of a general linear model depicting the effect of exposure group on the cortical mantle when the effects of gender, age at scan and gestational age at birth were regressed out. Results are shown for group differences between controls and A) all exposed children, and B) opiate exposed children only.
Figure 5.
The scatterplots illustrate the effects of different combination of regressors on cortical thickness in the right hemisphere. The effects are projected onto an inflated brain (left: lateral view, right: medial view). Since there are several possible confounds that correlate with the primary variable of interest (prenatal exposure to opiates), the general linear model approach was repeated systematically with different variables regressed out. As can be seen, the effects are as strong when only the opiate exposed children are included as when all the exposed children are included, even though the sample size is smaller (10 vs. 14 exposed). Further, it can be seen that the effects in parts of the right anterior cingulate and lateral orbitofrontal cortex survive control for a number of possible confounds.
To see if any area of the right ACC and LOFC effects would survive correction for multiple spatial comparisons, a permutation approach was used to test the cluster-wise significance threshold. Since this test cannot be performed with non-orthogonal designs or continuous variables, only the effects of the initial GLM contrasting the exposed to the non-exposed participants were tested with this method. The smoothing level at which effects will and will not survive in such testing depends on the extent of the effects. Since the effects were deemed to be of unequal size, permutation testing was performed at two different smoothing levels: 10 mm and 20 mm Full-Width/Half-Maximum (FWHM) respectively. Clusters were identified using a vertex-wise p-value threshold of .01. Cluster-wise p-values were computed separately for each smoothing level according to Nichols and Holmes (2001). This analysis indicated a cluster corresponding to an area of the effect observed in the right ACC with a smoothing level of 10 mm FWHM. This cluster, which can be seen in Supplementary Figure 2, (Talairach x, y, z coordinates 7.9, 39.1, 9.2) was 523 mm2 / 847 vertices and had a clusterwise p-value of .032. The analysis also indicated a cluster corresponding to an area of the effect observed in the right LOFC with a smoothing level of 20 mm fwhm. This cluster, which can be seen in Supplementary Figure 3, (Talairach x, y, z coordinates 48.4, 33.7, -10.0) was 1744 mm2 / 2321 vertices and had a clusterwise p-value of .021. Thus, the permutation approach indicates that effects in the right ACC and LOFC survive a correction for multiple comparisons. In the strictest manner, one would further correct the significance levels here for having applied two smoothing levels, i.e. multiply the p-values by 2. This would render the clusterwise p-value of the ACC effect marginally insignificant in this small sample (p = .064), whereas the LOFC effect remains significant (p = .043) also when correcting for this.
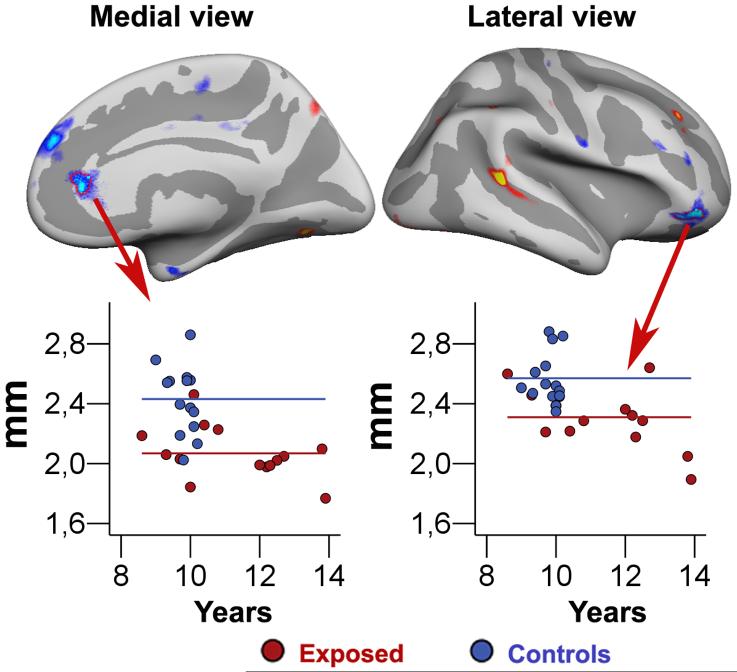
In order to further inspect the distribution of the groups within the right anterior cingulate and lateral orbitofrontal cortex, labels were drawn around the most continuous areas of these effect sites as observed for the sample as a whole when controlling for gestational age, age at scan, and gender. The results are shown in figure 6, and indicate that while there is some degree of overlap in cortical thickness across groups, both areas also show quite a few non-overlapping thickness values.
Figure 6.
Scatterplots showing the mean thickness of the cerebral cortex in two specific frontal brain areas: a part of anterior cingulate cortex and a part of the lateral orbitofrontal cortex in the right hemisphere. The areas are manually drawn on an inflated brain, which makes it possible to see inside the sulci of the brain surface. The lines represent the mean thickness in the two areas for the each of the two groups.
Correlations between neuroanatomical and behavioral variables
Correlations between neuroanatomical and behavioral variable are shown in table 4. In general, the observed relationships were rather weak, but in the expected directions. Smaller neuroanatomical volumes were related to lower ability scores on the WISC-R and a higher amount of problems as reported on the CBCL, but in most cases these relationships did not reach statistical significance. However, the cortical labels chosen and drawn on the basis of group differences, including parts of the right anterior cingulate (rACC) and right lateral orbitofrontal cortex (rLOFC) did show significant relationships with a number of behavioral variables. Thickness of the rACC correlated significantly (p < .05) negatively with Social problems, and showed a trend (p < .10) towards a negative relationship with Attention problems. These relationships were attenuated by other variables, and were not significant independently of gender, age at scan, and gestational age. However, thickness of the right lateral orbitofrontal cortex correlated signifcantly (p < .05) with all behavioral variables, except Total IQ, for which a positive trend (p < .10) was observed. When partialing out effects associated with gender, age at scan, and gestational age, thickness of this area of the lateral orbitofrontal cortex was still significantly related to Freedom from distractibility and showed trends towards relationships with Total problems and Social problems. The only other neuroanatomical variable showing relationships across behavioral variables, was accumbens area volume, which correlated significantly and positively with IQ, and significantly and negatively with Total problems and Social problems, and showed a trend towards a relationship with Freedom from distractibility.
Table 4.
Correlations for neuroanatomical and behavioral variables. rACC and rLOFC = parts of the right anterior cingulate and lateral orbitofrontal cortex as chosen and drawn on the basis of group effects as depicted in Figure 6. Numbers in parentheses: age at scan, gender, and gestational age partialed out, bold: p <.05, italics: p < .10.
| Volume | Total IQ | Free from distract. | Total problems | Attention problems | Social problems |
|---|---|---|---|---|---|
| rACC | .21 (-.04) | .27 (-.05) | -.08 (.22) | -.33 (.07) | -.39 (-.06) |
| rLOFC | .36 (.26) | .50 (.47) | -.41 (-.35) | -.50 (-.27) | -.59 (-.38) |
| ICV | .16 (.13) | .08 (.11) | -.13 (-.05) | .01 (.00) | -.12 (-.19) |
| Total brain | .12 (.18) | .00 (.11) | -.09 (-.08) | .10 (.04) | -.06 (-.17) |
| Cerebral cortex | .25 (.24) | .11 (.10) | -.15 (-.06) | .03 (.11) | -.11 (-.09) |
| Cerebral wm | .02 (.18) | -.10 (.14) | .06 (.00) | .24 (.06) | .08 (-.10) |
| Hippocampus | .15 (.20) | .05 (.08) | .21 (-.23) | -.02 (-.07) | -.18 (-.34) |
| Amygdala | .21 (.28) | .04 (.16) | -.05 (.02) | .20 (.23) | -.03 (-.12) |
| Accumbens | .40 (.29) | .36 (.32) | -.40 (-.27) | -.22 (-.07) | -.43 (-.42) |
| Caudate | .35 (.23) | .27 (.12) | -.28 (-.13) | -.18 (.00) | -.33 (-.33) |
| Putamen | .30 (.33) | .14 (.13) | -.23 (-.17) | -.05 (.03) | -.22 (-.23) |
| Pallidum | .20 (.30) | .14 (.27) | -.21 (-.25) | .01 (-.08) | -.12 (-.31) |
| Thalamus | .32 (.31) | .18 (.20) | -.26 (-.22) | -.01 (-.04) | -.15 (-.37) |
| Brainstem | .03 (.06) | -.08 (.04) | -.22 (-.30) | -.01 (-.23) | -.18 (-.47) |
| Cerebellar cortex | -.08 (-.30) | -.02 (-.13) | -.35 (-.26) | -.26 (-.28) | -.34 (-.39) |
| Cerebellar wm | -.05 (-.07) | .02 (.11) | -.29 (-.33) | -.16 (-.33) | -.15 (-.22) |
| Lat. Ventricles | -.03 (-.14) | .00 (-.08) | -.06 (.05) | -.08 (.00) | -.27 (-.29) |
| Inf. lat. ventricles | .08 (-.03) | .03 (-.12) | -.25 (-.14) | -.30 (-.16) | -.54 (-.46) |
| 3rd ventricle | .29 (.11) | .18 (-.03) | -.26 (-.08) | -.23 (-.00) | -.42 (-.37) |
| 4th ventricle | .09 (-.23) | .07 (-.30) | -.19 (.10) | -.24 (-.12) | -.36 (-.17) |
Discussion
The present findings suggest that a number of neuroanatomical volumes are smaller in children prenatally exposed to opiates and other substances compared to controls. There are smaller brain volumes, including smaller amygdala, putamen, pallidum, brainstem, cerebellar white matter, inferior lateral ventricles, and smaller overall cortical volume, specifically including thinner cortex in the right rostral anterior cingulate and a region of the right lateral orbitofrontal cortex. Trends towards smaller volumes are also observed for other structures. On one hand, then, the effects seem to be of a rather general nature, with somewhat smaller regional volumes naturally being associated with somewhat smaller total brain and intracranial volumes., Indeed, many of the regional neuroanatomical effects can be explained by differences in intracranial volume across exposed children and controls. However, there may also be region-specific effects beyond those that can be accounted for by general differences. Cerebellar cortical volume was smaller in the total group of substance-exposed children independently of intracranial volume, and there was a similar trend for smaller accumbens area. In the opiate-exposed group, there appeared to be further region-specific effects, as discussed below.
Specificity of effects of opiate exposure
The present sample is too small to allow for secure conclusions, but it seems that opiate exposure may be especially associated with a reduction of the pallidum, and also to some degree the putamen and the inferior lateral ventricle. These are the strongest group effects observed in the present study, they are observed more strongly when excluding poly-substance exposure cases not involving opiates, and remain significant also when excluding cases of other potential CNS pathology. Further, these volumes are significantly smaller independent of intracranial volume in the opiate exposed group only. The smaller volume of the inferior lateral ventricle, that is, the temporal horn of the lateral ventricle, could to some degree be due to a joint effect of the structures surrounding it being smaller in the exposed group. These include the amygdala, hippocampal formation, and caudate tail. The present results partly concur with, and expand those previously reported on prenatal substance exposure. The putamen, and especially the pallidum, has also been found to be reduced in children prenatally exposed to methamphetamine (Chang et al., 2004). Morphological abnormalities in the anterior cingulate cortex have been identified in a rabbit model of prenatal cocaine exposure (Harvey, 2004), but the findings of thinner cortical thickness in the right anterior cingulate and lateral orbitofrontal cortex in substance-exposed humans are novel.
Functional significance of effects
What functional significance may these structural reductions have? The anterior cingulate and lateral orbitofrontal cortex, parts of which were found to be thinner in the right hemisphere for the exposed group, and the pallidum and putamen, which were among the most reduced subcortical structures across the smaller subgroups, are critical parts of different frontal-striatal circuits. First, the anterior cingulate cortex is considered to play a critical role in complex cognitive processsing, and its role in optimal performance may be that of reward-guided response selection or modulation of autonomic tone (for a review, see Fellows and Farah, 2005). Based on lesion and functional imaging studies, the lateral orbitofrontal cortex appears to have a specific role when appropriate action requires inhibition of previously rewarded responses (for a review, see Elliott et al, 2000). These functions are in part achieved through the interconnections of these frontal areas with subcortical structures, including - even though not by means of direct projections - the pallidum and putamen (Alexander et al., 1986).
The present set of cerebral volume reductions appear meaningful when one considers the set of attentional and hyperactivity difficulties typically experienced by many children exposed to opiates and other substances (Moe and Slinning, 2002; Slinning, 2004). As reviewed by Seidman et al. (2005), a number of investigators have hypothesized that ADHD involves structural and functional brain abnormalities in frontal-striatal circuitry, and there is substantial empirical support for this hypothesis (although other brain areas including the corpus callossum and cerebellum, are also involved). Frontal-striatal circuits, of which the anterior cingulate, parts of the prefrontal cortex, and the pallidum and putamen have different roles, have in functional and/or structural neuroimaging and lesion studies repeatedly been targeted as implicated in ADHD (for reviews, see Seidman et al., 2005; Bush et al., 2005). For instance, compared to other focal lesions, putamen strokes have been associated with an increased risk of attention-deficit/hyperactivity symptomatology (Max et al., 2002) and all four structural imaging studies of pallidal volume in ADHD have found smaller volumes in affected children (Seidman et al., 2005). As mentioned, ADHD and related problems are highly prevalent in the present sample of largely opiate exposed children as indicated by the heightened diagnosis rate and elevated CBCL scores relative to controls.
There are certainly a number of other brain characteristics than volume that may influence cognition and behavior. Still, volume of neuroanatomical structures may relate to size or amount of neurons and synaptic connections, so some association with cognition and behavior may be expected. The correlations between neuroanatomical volumes and behavioral variables are, however, generally not strong. This may to some degree be related to noise in the material, since the behavioral assessments were done at a mean age of 8.6 years, while mean age at scan was 10.6 years, but at least for the ability measures, quite some temporal stability would be expected (Wechsler, 1974; Canivez and Watkins, 1999). However, the volumetric differences are, even though statistically significant, still small in absolute terms, so it may not be very surprising that only rather subtle possible effects on behavioral measures are found. The sample is very small, and multiple correlations were performed. The few relationships that were observed would therefore not withstand strict corrections for multiple comparisons, and should be interpreted with great caution. However, there is a slight hint that prenatal substance exposure may exert an effect on distractibility, or in Wielkiewicz’s (1990) terms “executive” and short term memory processes involved in planning, monitoring and evaluating task performance, via the right lateral orbitofrontal cortex. This is only one possibility, but one that is theoretically meaningful. The lateral orbitofrontal cortex is involved in response inhibition, and inhibition has been targeted as a particular executive process that may constitute a core deficit in ADHD. In addition, accumbens volume was related to general cognitive ability. The accumbens has long been known to have an important role in learning and motivation (for a review, see Kelley, 1999), but we are not aware of previous studies reporting similar volume-ability relationships, and the significance of this finding is not clear.
Limitations and conclusion
There are a number of limitations to the present study, many of which cannot be avoided when studying effects of substance exposure in human beings. First, what constitutes a cause and what constitutes an effect can be questioned: the cerebral differences found could be a matter of comparing a group of healthy children to a group with a high prevalence of ADHD and related problems. We cannot rule out the possibility of genetic effects on brain volumes, and perhaps also ADHD-related problems in the exposed sample (see e.g. Faraone et al., 2005; Posthuma et al., 2002). Further, prenatal poly-substance exposure, and especially opiate exposure, is naturally associated with a number of risk factors that may also influence neuroanatomical volumes to varying degrees. Besides genetic predisposition to some diseases and cognitive difficulties, these may include poor maternal nutrition and health and increased probability of premature birth.
Hans and Jeremy (2001) studied children of methadone-using mothers compared to a control group very carefully matched on maternal demographic and general ability factors. They found consistent, but rather small effects of prenatal opiate exposure on mental and psychomotor function throughout infancy as measured by the Bayley Scales and Infant Behavior Record ratings. They argued that much of the effect of maternal opioid drug use on the mental development of infants is attributable to the experience of more social-environmental risk factors by children in substance-abusing families. However, the control group of the present study is matched with the effective caregiving environment of the exposed children (i.e. the SES of the adoptive parents). This was done in order to eliminate possible effects of a non-optimal rearing environment. Hence, it is noteworthy that the present effects on brain volumes were found in exposed children who were placed in supportive homes at an early age, adopted and growing up under conditions of minimal postnatal risk. These children constitute a very rare and therefore very small sample, so conclusions cannot be drawn with certainty. However, exposure effects did persist when statistically controlling for gestational age at birth, gender and age at scan. As discussed above, cell culture and animal data do support a direct effect of prenatal opiate and substance exposure on cerebral characteristics, including basal ganglia, limbic and frontal areas (Bhat et al., 2006; Harlan and Song, 1994; Harvey, 2004; Hu et al., 2002; Malanga and Kosofsky, 2004). Thus, the present pattern of volumetric reductions in children prenatally exposed to opiates may to some degree be a result of cerebral developmental changes brought on by substance exposure in utero.
Supplementary Material
Supplementary Figure 1 The automated segmentation seen, anchored at the red cross in the images, from the coronal, horizontal and saggital views respectively.
Supplementary Figure 2 With a cluster-wise p-value of less than .050 and a smoothing level of 10 mm FWHM, permutation testing of cortical thickness effects comparing exposed and control children indicated at the lateral pial surface the cluster shown here. This cluster corresponds to an area of the effect observed in the right ACC, was 523 mm2 / 847 vertices and had a clusterwise p-value of .032. When multiplied by 2 to correct for 2 smoothing levels used (see Supplementary Figure 3), the clusterwise p-value is .064
Supplementary Figure 3 With a cluster-wise p-value of less than .050 and a smoothing level of 20 mm FWHM, permutation testing of cortical thickness effects comparing exposed and control children indicated at the medial pial surface the cluster shown here. This cluster corresponds to an area of the effect observed in the right LOFC, was 1744 mm2 / 2321 vertices, and had a clusterwise p-value of .021. When multiplied by 2 to correct for 2 smoothing levels used (see Supplementary Figure 2), the clusterwise p-value is .043.
Acknowledgements
Support for this research was provided by the Institute of Psychology at the University of Oslo, the National Institutes of Health (R01-NS39581, R01-RR16594, P41-RR14075, and R01-RR13609), the Mental Illness and Neuroscience Discovery (MIND) Institute, and in part by the Biomedical Informatics Research Network Project (BIRN, http://www.nbirn.net), which is funded by the National Center for Research Resources at the National Institutes of Health (NCRR BIRN Morphometric Project BIRN002).
Footnotes
Please note that full McCarthy data at 4 years was not collected for 1 of the refusing adoptees and 2 of the refusing controls.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach T,M. Manual for the Child Behavior Checklist/4-18 and 1991 Profile. University of Vermont Department of Psychiatry; Burlington: 1991. [Google Scholar]
- Alexander G,E, Delong M,R, Strick P,L. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Bhat R, Chari G, Rao R. Effects of prenatal cocaine, morphine, or both on postnatal opioid (μ) receptor development. Life Sci. 2006;78:1478–1482. doi: 10.1016/j.lfs.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Bush G, Valera E,M, Seidman L,E. Functional neuroimaging of attention-deficit/hyperactivity disorder: a review and suggested future directions. Biol Psychiatry. 2005;57:1273–1284. doi: 10.1016/j.biopsych.2005.01.034. [DOI] [PubMed] [Google Scholar]
- Canivez GL, Watkins MW. Long term stability of the Wechsler Intelligence Scale for Children-Third Edition among Demographic Subgroups: Gender, Race/Ethnicity, and Age. Journal of Psychoeducational Assessment. 1999;17:300–313. [Google Scholar]
- Caviness V,,S, Filipek P,A, Kennedy D,N. Magnetic resonance technology in human brain science: blueprint for a program based upon morphometry. Brain Dev. 1989;11:1–13. doi: 10.1016/s0387-7604(89)80002-6. [DOI] [PubMed] [Google Scholar]
- Chang L, Smith L,M, LoPresti C, Yonekura M,L, Kuo J, Walot I, Ernst T. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res Neuroimaging. 2004;132:95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Dale A,M, Fischl B, Sereno M,I. Cortical surface-based analysis I: Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dale A,M, Sereno M,I. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Elliott R, Dolan R,J, Frith C,D. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cereb Cortex. 2000;10:308–317. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Faraone S,V, Perlis R,H, Doyle A,E, Smoller J,W, Goralnick J,J, Holmgren M,A, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Fellows L,K, Farah M,J. Is anterior cingulate cortex necessary for cognitive control? Brain. 2005;128:788–796. doi: 10.1093/brain/awh405. [DOI] [PubMed] [Google Scholar]
- Finnegan L,P, Kron R,E, Connaughton J,F, Emich J,P. Neonatal abstinence syndrome: Assessment and management. In: Harbison R,D, editor. Perinatal Addiction. Spectrum Publications; New York: 1975. pp. 141–58. [PubMed] [Google Scholar]
- Fischl B, Salat D,H, van der Kouwe A,J,W, Makris N, Ségonne F, Dale A,M. Sequence-Independent Segmentation of Magnetic Resonance Images. NeuroImage. 2004a;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Ségonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004b;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat D,H, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale A,M. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Dale A,M. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Sereno M,I, Dale A,M. Cortical surface-based analysis: II. Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Gillberg C, Cederlund M. Asperger Syndrome: Familial and Pre- and Perinatal Factors. J Autism Dev Disord. 2005;35:159–166. doi: 10.1007/s10803-004-1993-7. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd J,N, Lusk L, Hayashi K,M, Greenstein D, Vaituzis A,C, Nugent T,F, 3rd., Herman D,H, Clasen L,S, Toga A,W, Rapoport J,L, Thompson P,M. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield S,F, Manwani S,G, Nargiso J,E. Epidemiology of substance use disorders in women. Obstet Gynecol Clin North Am. 2003;30:413–446. doi: 10.1016/s0889-8545(03)00072-x. [DOI] [PubMed] [Google Scholar]
- Hans S,L, Jeremy R,J. Postneonatal mental and motor development of infants exposed in utero to opioid drugs. Infant Mental Health Journal. 2001;22:300–315. [Google Scholar]
- Hans S,L. Maternal opioid drug use and child development. In: Zagon I, Slotkin T, editors. Maternal Substance Abuse and the Developing Nervous System. Academic Press; New York: 1992. pp. 177–213. [Google Scholar]
- Harlan R,E, Song D,D. Prenatal morphine treatment and the development of the striatum. Regul Pept. 1994;54:117–118. [Google Scholar]
- Harvey J,A. Cocaine effects on the developing brain: current status. Neurosci Biobehav Rev. 2004;27:751–764. doi: 10.1016/j.neubiorev.2003.11.006. [DOI] [PubMed] [Google Scholar]
- Hu S, Sheng W,S, Lokensgard J,R, Peterson P,K. Morphine induces apoptosis of human microglia and neurons. Neuropharmacology. 2002;42:829–836. doi: 10.1016/s0028-3908(02)00030-8. [DOI] [PubMed] [Google Scholar]
- Kadesjø C, Kadesjø B, Haggløf B, Gillberg C. ADHD in Swedish 3-to-7-year-old children. J Am Acad Child Adoles Psychiatry. 2001;40:1021–1028. doi: 10.1097/00004583-200109000-00010. [DOI] [PubMed] [Google Scholar]
- Kelley AE. Neural integrative activities of nucleus accumbens subregions in relation to learning and memory. Psychobiology. 1999;27:198–213. [Google Scholar]
- Kennedy D,N, Filipek P,A, Caviness V,S. Anatomic segmentation and volumetric calculations in nuclear magnetic resonance imaging. IEEE Trans Med Imaging. 1989;8:1–7. doi: 10.1109/42.20356. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SCR, van der Kouwe AJW, Salat DH, Dale AM, Fischl B. Regionally Localized Thinning of the Cerebral Cortex in Schizophrenia. Arch Gen Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Malanga C,J, Kosofsky B,E. Effects of drugs of abuse on brain development. In: Charney D,S, Nestler E,J, editors. Neurobiology of mental illness. 2nd edition Oxford University Press; New York: 2004. pp. 720–739. [Google Scholar]
- Max J,E, Fox P,T, Lancaster J,L, Kochunov P, Mathews K, Manes F,F, Robertson B,A, Arndt S, Robin D,A, Lansing A,E. Putamen lesions and the development of attention-deficit/hyperactivity symptomatology. J Am Acad Child Adolesc Psychiatry. 2002;41:563–571. doi: 10.1097/00004583-200205000-00014. [DOI] [PubMed] [Google Scholar]
- McCarthy D. Manual for the McCarthy Scales of Children’s Abilities. The Psychological Corporation; New York, NY: 1971. [Google Scholar]
- Mitchell L,E, Adzick N,S, Melchionne J, Pasquariello P,S, Sutton L,N, Whitehead A,S. Spina befida. Lancet. 2004;364:1885–1895. doi: 10.1016/S0140-6736(04)17445-X. [DOI] [PubMed] [Google Scholar]
- Moe V. Foster-placed and adopted children exposed in utero to opiates and other substances: Prediction and outcome at four and a half years. Developmental and behavioral pediatrics. 2002;23:330–339. doi: 10.1097/00004703-200210000-00006. [DOI] [PubMed] [Google Scholar]
- Moe V, Slinning K. Prenatal drug exposure and the conceptualization of long-term effects. Scand J Psychol. 2002;43:41–47. doi: 10.1111/1467-9450.00267. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. Nonparametric Permutation Tests For Functional Neuroimaging: A Primer with Examples. Hum Brain Mapp. 2001;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nøvik T,S. Validity of the Child Behavior Checklist in a Norwegian sample. Europ Child Adolesc Psychiatry. 1999;8:247–254. doi: 10.1007/s007870050098. [DOI] [PubMed] [Google Scholar]
- Ornoy A, Segal J, Bar-Hamburger R, Greenbaum C. Developmental outcome of school-age children born to mothers with heroin dependency: importance of environmental factors. Dev Med Child Neurol. 2001;43:668–675. doi: 10.1017/s0012162201001219. [DOI] [PubMed] [Google Scholar]
- Posthuma D, De Geus E,J, Baaré W,F,C, Pol H,E,H, Kahn R,S, Boomsma D,I. The association between brain volume and intelligence is of genetic origin. Nat Neurosci. 2002;5:83–84. doi: 10.1038/nn0202-83. [DOI] [PubMed] [Google Scholar]
- Riley E,P, McGee C,L. Fetal alcohol spectrum disorders: an overview with emphasis on changes in brain and behavior. Exp Biol Med. 2005;230:357–365. doi: 10.1177/15353702-0323006-03. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Salat D,H, Buckner R,L, Snyder A,Z, Greve D,N, Desikan R,S,R, Busa E, Morris J,C, Dale A,M, Fischl B. Thinning of the cerebral cortex in aging. Cereb Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Seidman L,J, Valera E,M, Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1263–1272. doi: 10.1016/j.biopsych.2004.11.019. [DOI] [PubMed] [Google Scholar]
- Seidman L,J, Faraone S,V, Goldstein J,M, Goodman J,M, Kremen W,S, Toomey R, Tourville J, Kennedy D, Makris N, Caviness V,S, Tsuang M,T. Thalamic and amygdala-hippocampal volume reductions in first-degree relatives of patients with schizophrenia: an MRI-based morphometric analysis. Biol Psychiatry. 1999;46:941–954. doi: 10.1016/s0006-3223(99)00075-x. [DOI] [PubMed] [Google Scholar]
- Sgouros S, Goldin JH, Hockley AD, Wake MJC, Natarajan K. Intracranial volume change in childhood. J Neurosurg. 1999;91:610–616. doi: 10.3171/jns.1999.91.4.0610. [DOI] [PubMed] [Google Scholar]
- Slinning K. Foster placed children prenatally exposed to poly-substances--attention-related problems at ages 2 and 4 1/2. Eur Child Adolesc Psychiatry. 2004;13:19–27. doi: 10.1007/s00787-004-0350-x. [DOI] [PubMed] [Google Scholar]
- Smith L,M, Chang L, Yonekura M,L, Grob C, Osborn D, Ernst T. Brain proton magnetic resonance spectroscopy and imaging in children exposed to cocaine in utero. Pediatrics. 2001;107:227–231. doi: 10.1542/peds.107.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suess P,E, Newlin D,B. Motivation, sustained attention, and autonomic regulation in school-age boys exposed in utero to opiates and alcohol. Exp Clin Psychopharmacol. 1997;5:375–387. doi: 10.1037//1064-1297.5.4.375. [DOI] [PubMed] [Google Scholar]
- Vathy I. Prenatal opiate exposure: long-term CNS consequences in the stress system of the offspring. Psychoneuroendocrinology. 2002;27:273–283. doi: 10.1016/s0306-4530(01)00049-x. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children - Revised. The Psychological Corporation; New York: 1974. [Google Scholar]
- Wielkiewicz R,M. Interpreting Low Scores on the WISC-R Third Factor: It’s More Than Distractibility. Psychological Assess. 1990;2:91–97. [Google Scholar]
- Wilke M, Schmithorst VJ, Holland SK. Assessment of Spatial Normalization of Whole-Brain Magnetic Resonance Images in Children. Hum Brain Mapp. 2002;17:48–60. doi: 10.1002/hbm.10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanai J, Huleihel R, Izrael M, Metsuyanim M, Shahak H, Vatury O, Yaniv SP. Functional changes after prenatal opiate exposure related to opiate receptors’ regulated alterations in cholinergic innervation. Int J Neuropsychopharmacol. 2003;6:253–265. doi: 10.1017/S1461145703003523. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 The automated segmentation seen, anchored at the red cross in the images, from the coronal, horizontal and saggital views respectively.
Supplementary Figure 2 With a cluster-wise p-value of less than .050 and a smoothing level of 10 mm FWHM, permutation testing of cortical thickness effects comparing exposed and control children indicated at the lateral pial surface the cluster shown here. This cluster corresponds to an area of the effect observed in the right ACC, was 523 mm2 / 847 vertices and had a clusterwise p-value of .032. When multiplied by 2 to correct for 2 smoothing levels used (see Supplementary Figure 3), the clusterwise p-value is .064
Supplementary Figure 3 With a cluster-wise p-value of less than .050 and a smoothing level of 20 mm FWHM, permutation testing of cortical thickness effects comparing exposed and control children indicated at the medial pial surface the cluster shown here. This cluster corresponds to an area of the effect observed in the right LOFC, was 1744 mm2 / 2321 vertices, and had a clusterwise p-value of .021. When multiplied by 2 to correct for 2 smoothing levels used (see Supplementary Figure 2), the clusterwise p-value is .043.