Abstract
Hand preference for tool use was assessed in a sample of captive chimpanzees (Pan troglodytes). Whether the subjects solved the tool task using either a unimanual or coordinated bimanual strategy was manipulated in the chimpanzees. No population-level hand preference was found for tool use when unimanual strategies were used by the chimpanzees. However, a population-level right-hand bias was found when coordinated bimanual actions were required of the chimpanzees. A significant correlation was found in hand use for the two hand preference testing conditions. Neither sex nor rearing was found to significantly affect the direction or strength in hand preference. These results may explain discrepancies in hand preference reported in captive and wild chimpanzees with regard to tool use and other manual activities.
INTRODUCTION
There is a growing body of evidence indicating the presence of population-level hand preference in nonhuman primates (Bradshaw & Rogers, 1993; Fagot & Vauclair, 1991; Hopkins, 1996; Hopkins & Morris, 1993; MacNeilage, Studdert-Kennedy, & Lindblom, 1987; Ward & Hopkins, 1993). Population-level hand preference is inferred when a significant proportion of subjects within a sample exhibit the same directional hand preferences for one or more specific tasks. Although directions in hand preferences have been reported to vary among species (Bradshaw & Rogers, 1993), the data nonetheless challenge the long-held belief that population-level hand preferences are unique to humans (Warren, 1980). What remains unknown is the degree to which handedness in nonhuman primates is related to manifestations of human handedness (see Corballis, 1991; Ettlinger, 1988; Marchant & McGrew, 1991; McGrew & Marchant, 1996).
Recently, Hopkins and colleagues have revealed population-level right-handedness in a sample of captive chimpanzees (Pan troglodytes) for bipedal reaching (Hopkins, 1993), bimanual feeding (Hopkins, 1994), throwing (Hopkins, Bard, Jones, & Bales, 1993), and a coordinated bimanual task (Hopkins, 1995). Other investigators have similarly reported population-level handedness in captive chimpanzees (Aruguete, Ely, & King, 1992; Colell, Segarra, & Pi, 1995a,b). In contrast, several historical (Finch, 1941) as well as recent reports of hand preference in wild chimpanzees have failed to document population-level hand preference. For instance, McGrew and Marchant (1992) did not find population-level hand preference for termite fishing in wild chimpanzees. Similarly, both Boesch (1991) and Sugiyama, Fushimi, Sakura, and Matsuzawa (1993) did not find population-level hand preference for a nutcracking task in wild chimpanzees. The apparent discrepancies in findings between captive and wild chimpanzees have led some to suggest that captive rearing may induce population-level hand preferences in chimpanzees (McGrew & Marchant, 1995). Alternatively, the captive environment results in the animals adopting certain behavioural patterns that reveal hand preferences under conditions that are not frequently observed in wild subjects. For example, captive chimpanzees often feed with one hand while simultaneously holding on to extra food items with the opposite hand (Hopkins, 1994). This type of feeding posture is seldom observed in wild chimpanzees (Marchant & McGrew, 1996).
In contrast to the aforementioned arguments, Hopkins (1995) has argued that differences in hand preference findings between captive and wild chimpanzees could reflect either (a) the type of task or (b) the motor and cognitive demands of the task (see Fagot & Vauclair, 1991, for discussion). Most of the recent studies in wild chimpanzees have measured hand preferences for tool use tasks, probably because of the theoretical links that have been proposed between the evolution of handedness and tool use and/or language functions (Corballis, 1991; Frost, 1980). In contrast, few studies in captive apes have examined tool use. Thus, there is a need for studies on hand preference and tool use in captive chimpanzees for comparison with findings in wild chimpanzees. If the rearing environment is the causal factor in accounting for differences in captive and wild chimpanzees, then a different pattern of results should be evident in the captive chimpanzees. One purpose of this study was to examine hand preference in captive chimpanzees for a tool-use task that was designed to model similar tool-use tasks in wild chimpanzees.
A second issue that pertains to the differences in findings between captive and wild chimpanzees is the motor complexity involved in any given hand preference task. Tool-use tasks studied in wild chimpanzees are defined or described as being complex, bimanual tasks. Although tool use is arguably a complex task, whether it involves one or two hands (i.e. bimanual), and whether or not the hands are used independently or in a coordinated fashion, is not clear from the existing studies in wild chimpanzees (e.g. Boesch, 1991). This is a critical distinction because certain tool-use tasks can be solved using either bimanual or unimanual strategies which may or may not influence the distribution of hand preference (see Morris, Hopkins, & Bolser-Gilmore, 1993). Hopkins (1995) has argued that motor tasks, including tool use, that require coordinated bimanual actions would be more likely to elicit population-level hand preferences than tasks involving unimanal actions (see Fragaszy & Adams-Curtis, 1993). Therefore, a second purpose of this study was to compare hand preference distributions in a sample of chimpanzees that were required to solve a tool-use task using either unimanual or bimanual motor actions. It was hypothesised that bimanual actions would elicit stronger and more consistent hand preferences than unimanual actions in solving a tool-use task.
METHOD
Subjects
There were a total of 37 chimpanzees (Pan troglodytes), including 14 males and 23 females. All subjects were adolescents or adults and ranged in age from 9 to 57 years. Of these, 9 chimpanzees (4 males, 5 females) were mother-reared, 19 were nursery-reared (8 males, 10 females), and 9 were of unknown origin (8 females, 1 male). Mother-reared subjects were those who were raised by their biological mothers, whereas nursery-reared subjects were those raised by humans in a nursery setting (see Bard, 1994 for description).
Procedure
The basic tool-use task used in this study was a dipping task designed to require subjects to extract food from containers using some type of stick or metal rod. This task was designed to simulate termite fishing in wild chimpanzees (see Goodall, 1986). The independent variable of interest in this study was hand use when performing the dipping task using either a unimanual (UNI) or bimanual (BIM) strategy. To elicit unimanual responses, one or more polyvinylchloride (PVC) pipes were fixed to the inside portion of the chimpanzees' home cage. The PVC pipes measured between 42 and 44cm in length and either 10 or 19cm in diameter, and were capped at one end. For simplicity, this device was referred to as the “honey pot”. Two different-sized honey pots were used because smaller chimpanzees could reach into the device and extract the food without use of a tool. The honey pot was positioned approximately .5m above the floor, and the number attached to the cage and the size of the honey pots varied depending on the number of subjects in the cage and the size of the subjects.
At the start of each observation period, the subjects were locked in either the inside or outside portion of their home cages. The experimenter attached the honey pot(s) to the cage mesh and placed a small stick (53 to 64cm in length, 3 to 5cm diameter) made of either PVC or aluminium inside the honey pot. In those cases for which more than one honey pot was needed, they were placed .5 to 1 metre apart. Approximately 1 cup of food was placed at the bottom of each honey pot. (For the most part, honey was used as the food incentive because it stuck to the dipping stick. However, some animals disliked honey so peanut butter was substituted as the food incentive.) The animals were then released into the observation area. Once the chimpanzee removed the stick, the observer began recording the hand with which animals probed the container for the food, and the hand with which they took the stick to the mouth. Only those observations in which the subjects dipped and extracted the stick with the same hand were included in the analyses.
To elicit coordinated bimanual responses, the experimenters modified the motor demands and materials used for the tool-use task. Peanut butter was smeared on the inside, middle portion of uncapped PVC pipes measuring approximately 62cm in length. Placing the peanut butter in this location precluded subjects from extracting the peanut butter using their digits. A smaller plastic stick or aluminium rod (between 53 and 64cm in length, 3 to 5cm in diameter) was placed inside the larger PVC pipe and the combined tubes were handed to the subjects through the cage mesh. In order to extract the peanut butter, the subjects had to hold the larger PVC pipe with one hand and dip and feed with the smaller tube held by the opposite hand. The hand used for dipping was defined as the dominant hand and the experimenter recorded the frequency of left and right hand dipping responses within an observation period.
All testing occurred between the hours of 12:00 and 3:00 pm. No attempt was made to isolate animals for the purposes of testing. Rather, small groups of two to three animals were tested concurrently, with one animal the focal subject within an observation period. Observation periods lasted between one and two hours, depending on the level of tool-use skill for each focal animal. For both tasks, data collection ended when the chimpanzees had removed all of the food and/or lost interest in the device. The order of task presentation (i.e. unimanual vs bimanual) was counterbalanced across subjects. At the end of each testing period, the number of responses by each hand was tallied. Not all subjects performed these tasks within the first session, and so subsequent testing was necessary. Subjects were given as many as four test sessions. If the subjects failed to perform these tasks after four test sessions, then testing stopped and the subject was dropped from the study.
RESULTS
A total of 36 subjects successfully performed at least 10 responses on the UNI version of the tool-use task (Mean = 87); 28 chimpanzees performed at least 10 responses on the BIM version of task (Mean = 110); 26 chimpanzees performed at least 10 responses on both tasks. The individual data for each subject can be seen in Table 1. Each subject's lateral bias for each task was characterised using three measures. First, a z-score based on the number of left and right hand responses was calculated for each subject. Chimpanzees with z-scores that exceeded 1.64 were classified as right-handed: left-handed chimpanzees were those with z-scores less than −1.64. Subjects with z-scores between −1.64 and 1.64 were classified as ambiguously handed. The second measure was a handedness index (HI) which was calculated by subtracting the number of left hand responses from the number of right hand responses and dividing by the total number of responses (#R−#L) /(#R+#L). The HI values varied from −1.0 to 1.0 which corresponded to being strongly left-handed to strongly right-handed. Finally, strength in hand preference was determined by taking the absolute value of the HI score (referred to ABS–HI). This value reflected the subject's degree of preferred hand use.
Table 1.
Individual Data for Each Measure of Hand Preference
| Unimanual | Bimanual | |||
|---|---|---|---|---|
| Subject | z-score | Hand | z-score | Hand |
| Females | ||||
| Angela | 8.16 | R | 3.81 | R |
| Anna | 1.38 | A | ||
| Brandy | 4.76 | R | 0.63 | A |
| Brodie | −4.38 | L | −4.16 | L |
| Cheetah | 6.58 | R | 2.42 | R |
| Dara | 2.45 | R | 1.73 | R |
| Ellie | −10.55 | L | 3.99 | R |
| Flora | 8.94 | R | 11.53 | R |
| Garbo | 4.00 | R | ||
| Gay | 0.38 | A | 9.11 | R |
| Jaqueline | 1.50 | A | ||
| Jenifere | 3.36 | R | 7.39 | R |
| Jenny | −0.17 | A | −0.88 | A |
| Kasey | 3.74 | R | ||
| Lee | −1.78 | L | 7.21 | R |
| Leslie | −7.75 | L | −1.98 | L |
| Lilone | 2.50 | R | ||
| Lucy | −0.87 | A | 6.19 | R |
| Marilyne | 3.10 | R | −1.90 | L |
| Reba | 5.74 | R | 6.55 | R |
| Sheena | −5.69 | L | ||
| Shirley | −3.49 | L | ||
| Sparkle | 2.29 | R | ||
| Males | ||||
| Clint | −0.05 | A | 4.14 | R |
| Columbus | −5.43 | L | −1.62 | A |
| Gelb | −1.45 | L | 2.18 | R |
| Hoboh | −5.33 | L | −8.25 | L |
| J.Carter | −17.24 | L | −2.52 | L |
| Justin | 1.89 | R | 5.48 | R |
| Les | 1.41 | A | 5.16 | R |
| Lux | 0.58 | A | 5.29 | R |
| Merv | 4.16 | R | 7.48 | R |
| Ossobaw | 1.07 | A | ||
| Puddin | 6.80 | R | −7.57 | L |
| Rufus | −7.49 | L | 2.96 | R |
| Storer | 10.63 | R | −0.07 | A |
| Scott | 3.13 | R | ||
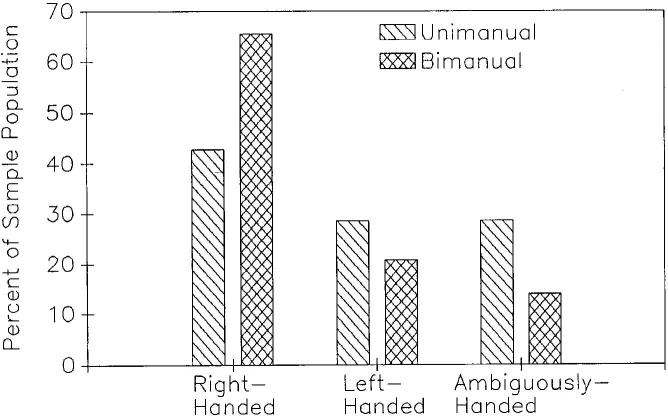
In the initial analysis, the numbers of left-, right-, and ambiguously handed subjects were compared for the UNI and BIM versions of the task. For the UNI version, there were 11 left-handed, 9 ambiguously handed, and 16 right-handed subjects. This distribution did not differ significantly from chance using a chi-square goodness-of-fit test. In contrast, there were 6 left-handed, 4 ambiguously handed, and 18 right-handed subjects for the BIM version of task. This distribution differed significantly from chance as determined by chi-square analysis χ2(2,28) = 12.29, P < .05. Subsequent chi-square analyses indicated that the number of right-handed chimpanzees was significantly greater than the number of left-handed χ2(1,24) = 6.00, P < .05 and ambiguously handed chimpanzees χ2(1,22) = 8.90, P < .05. The relative proportion of left-, ambiguously, and right-handed subjects for the UNI and BIM versions of the tasks can be seen in Fig. 1. One-sample t-tests using the HI scores confirmed the chi-square effects. For the UNI version of the task, no significant population bias was found t(35) = .71, P > .10; however, a significant right-hand population bias was found for the BIM version t(27) = 3.16, P < .01.
FIG. 1.
Relative proportion of left-, ambiguously, and right-handed subjects for the unimanual and bimanual versions of the tool task.
Sex differences in direction and strength of hand preference were evaluated separately for the UNI and BIM version of the tool-use task. Mann-Whitney tests using the HI score failed to reveal significant differences in hand preference between males and females. Kruskal-Wallis tests comparing rearing environments also failed to reveal differences in the HI scores. Independent sample t-tests using the ABS–HI score failed to reveal sex differences in strength of hand preference for either version of the task. A one-way ANOVA comparing the ABS–HI scores between rearing conditions was not significant. In addition, a mixed design ANOVA comparing strength in hand preference between the UNI and BIM versions was not significant. The average ABS–HI scores for the UNI and BIM versions of the task were .562 and .528 respectively. Finally, Pearson Product–Moment correlations between the UNI and BIM versions of the task for the HI and ABS–HI scores were conducted. A significant positive correlation was found for the HI scores (r = .435, df = 25, P < .05) but not for the ABS–HI scores (r = −.067, df = 25, ns). Additionally, the total number of responses for each measure was correlated with the HI and ABS–HI scores to assess whether the variation in the number of responses influenced either strength or direction of hand preference. No significant correlations were found.
DISCUSSION
The results of this study indicate that hand preferences for tool use in chimpanzees are influenced by the motor and cognitive demands of the task. When the two hands had to work in a coordinated manner, a significant population-level right hand bias was found. In contrast, when the tool-use task could be solved using a unimanual strategy, no population-level hand preferences were evident in the chimpanzees. Note that there were no differences in strength of hand preference between the two variations of this task. Thus, it is the direction not the strength of lateral bias that was largely influenced by unimanual and bimanual strategies in solving a tool-use task. This finding is consistent with the prediction that bimanual tasks are more sensitive in detecting population-level hand preference in chimpanzees.
The lack of population-level hand preference for the unimanual tool-use task is consistent with the data reported in wild chimpanzees. For example, McGrew and Marchant (1992) reported approximately equal numbers of left- and right-handed chimpanzees for termite fishing. The motor demands of the unimanual version of our tool-use task were similar to those for termite fishing, in that the chimpanzees had to insert a stick into a hole to extract a food source and did not require any coordination between the hands. The lack of population-level hand preference in this task is also consistent with findings in capuchin monkeys. Westergaard and Suomi (1994) measured hand use for a dipping task in a sample of 13 capuchin monkeys and found no population-level hand preference. Taken together, these data strongly suggest that tool-use tasks involving unimanual actions do not elicit population-level hand preference in nonhuman primates. In contrast, performing a tool-use task using coordinated bimanual actions results in a population-level right hand bias. In our opinion, until a distinction is made between unimanual and bimanual solutions to tool-use tasks by investigators studying wild apes, it is premature to infer differences in captive and wild apes as an artifact of the captive environment.
Although this study focused on tool use, we do not believe that a tool-use task requiring bimanual actions is necessary to elicit population-level hand preferences in great apes and at least some monkey species. For example, chimpanzees required to hold a tube and extract peanut butter with fingers of the opposite hand exhibited a population right hand bias (Hopkins, 1995). Westergaard and Suomi (1996) have reported a similar population right hand bias in rhesus monkeys but not capuchin monkeys (also see Beck & Barton, 1972) for the same tube task. Finally, Byrne and Byrne (1991) found a slight but significant right hand population bias in bimanual feeding in wild gorillas (also see Fagot & Vauclair, 1988). These cumulative results highlight the significance of bimanual contrasted with unimanual forms of hand use in solving behavioural tasks, be it in wild or captive primates (see Wundrum, 1986). Additionally, these findings raise serious questions about the role of tool use in the evolution of lateralised behaviour in humans (e.g. Frost, 1980).
We did not distinguish between laterality in bouts of tool use contrasted with individual tool-use events, whereas McGrew and Marchant (1992) did so in their study of wild chimpanzees. Recently, several authors have proposed that recording bouts of behavioural events is a better approach to the assessment of hand preference than recording individual acts (Byrne & Byrne, 1991; McGrew & Marchant, 1992). For example, McGrew and Marchant (1996) argue that a key issue in nonhum an primate handedness research is independence of data points and that recording individual, repetitive actions as independent events artificially increases sample size and increases the likelihood of finding significant z-scores. Although this could be the case, at the same time considering the frequency bouts of actions as the sole measure of hand preference can potentially remove important information regarding hand use. For example, it is conceivable that chimpanzees could use their left and right hands to termite-fish for 50 bouts but spend 120 minutes using their left hand and 600 minutes using their right hand. Dividing the time by the frequency of bouts would reveal a larger asymmetry in bout length. Thus, solely measuring bouts can be meaningless in the absence of the global aspects of the behaviour.
In addition to removing important information about motor behaviour and asymmetries in hand use, it is not clear what is confounded by the use of recording events contrasted with bouts. Presumably, recording events artificially inflates z-scores, which results in a greater number of subjects being classified as right- or left-handed rather than ambiguously handed. However, there is no reason to assume that the inflated z-scores would necessarily bias the sample towards either population-level right or left handedness. Presumably, this confound would be randomly distributed among the subjects and testing conditions. So, at a minimum, all that is gained by using bouts is a more conservative estimate of whether the measure reliably elicits significant hand use but not direction of hand use.
In conclusion, the results of this study indicate that investigators studying hand preference in nonhum an primates need to pay special attention to the motor and cognitive demands of different tasks in their assessment of laterality. In particular, whether the two hands perform actions in a coordinated or uncoordinated manner in solving different tasks appears to be a critical distinction. Enhancing and refining the motor and cognitive demands of different hand preference measures will hopefully clarify differences between field and captive studies and, perhaps, species differences in laterality.
Acknowledgments
This investigation was supported in part by National Institutes of Health Grant RR-00165 from the National Center for Research Resources to the Yerkes Regional Primate Research Center. Additional support was provided by National Institutes of Neurological Disorders and Stroke Grant NS-29574 to W.D. Hopkins. The Yerkes Primate Center is fully accredited by the American Association for Accreditation of Laboratory Animal Care. We would like to extend our appreciation to the supportive services provided by the animal resources staff of the Yerkes Primate Center.
Contributor Information
William D. Hopkins, Berry College, and Yerkes Regional Primate Research Center, Emory University, Atlanta, USA
Deborah M. Rabinowitz, Yerkes Regional Primate Research Center, Emory University, Atlanta, USA
REFERENCES
- Aruguete MS, Ely EA, King JE. Laterality in spontaneous motor activity of chimpanzees and squirrel monkeys. American Journal of Primatology. 1992;27:177–178. doi: 10.1002/ajp.1350270303. [DOI] [PubMed] [Google Scholar]
- Bard KA. Evolutionary foundations of intuitive parenting: A special case of maternal competence in chimpanzees. Early Development and Parenting. 1994;3:19–28. [Google Scholar]
- Beck CHM, Barton RL. Deviation and laterality of hand preference in monkeys. Cortex. 1972;8:339–363. doi: 10.1016/s0010-9452(72)80001-7. [DOI] [PubMed] [Google Scholar]
- Boesch C. Handedness in wild chimpanzees. International Journal of Primatology. 1991;6:541–558. [Google Scholar]
- Bradshaw JL, Rogers L. The evolution of lateral asymmetries, language, tool use and intellect. Academic Press; San Diego, CA: 1993. [Google Scholar]
- Byrne RW, Byrne JM. Hand preferences in the skilled gathering tasks of mountain gorillas (Gorilla gorilla berengei) Cortex. 1991;27:521–536. doi: 10.1016/s0010-9452(13)80003-2. [DOI] [PubMed] [Google Scholar]
- Colell M, Segarra MD, Pi JS. Hand preferences in chimpanzees (Pan troglodytes), bonobos (Pan paniscus) and orangutans (Pongo pygmaeus) in food-reaching and other daily activities. International Journal of Primatology. 1995a;16:413–434. [Google Scholar]
- Colell M, Segarra MD, Pi JS. Manual laterality in chimpanzees (Pan troglodytes) in complex tasks. Journal of Comparative Psychology. 1995b;109:298–307. doi: 10.1037/0735-7036.109.3.298. [DOI] [PubMed] [Google Scholar]
- Corballis MC. The lopsided ape: Evolution of the generative mind. Oxford University Press; New York: 1991. [Google Scholar]
- Ettlinger G. Hand preference, ability, and hemispheric specialization: How far are these factors related in the monkey? Cortex. 1988;24:389–398. doi: 10.1016/s0010-9452(88)80002-9. [DOI] [PubMed] [Google Scholar]
- Fagot J, Vauclair J. Handedness and bimanual coordination in Gorilla gorilla. Brain Behavior and Evolution. 1988;32:89–95. doi: 10.1159/000116536. [DOI] [PubMed] [Google Scholar]
- Fagot J, Vauclair J. Manual laterality in nonhuman primates: A distinction between handedness and manual specialization. Psychological Bulletin. 1991;109:76–89. doi: 10.1037/0033-2909.109.1.76. [DOI] [PubMed] [Google Scholar]
- Finch G. Chimpanzee handedness. Science. 1941;94:117–118. doi: 10.1126/science.94.2431.117. [DOI] [PubMed] [Google Scholar]
- Fragaszy DM, Adams-Curtis LE. An exploration of manual preference and performance in crab-eating macaques. In: Ward JP, Hopkins WD, editors. Primate laterality: Current behavioral evidence of primate asymmetries. Springer-Verlag; New York: 1993. pp. 75–105. [Google Scholar]
- Frost GT. Tool behavior and the origins of laterality. Journal of Human Evolution. 1980;9:447–459. [Google Scholar]
- Goodall J. The chimpanzees of Gombe: Patterns in adaptation. Harvard University Press; Cambridge, M A: 1986. [Google Scholar]
- Hopkins WD. Posture and reaching in chimpanzees (Pan) and orangutans (Pongo) Journal of Comparative Psychology. 1993;17:162–168. doi: 10.1037/0735-7036.107.2.162. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hand preference for bimanual feeding in 140 captive chimpanzees (Pan troglodytes): Rearing and ontogenetic factors. Developmental Psychobiology. 1994;27:395–407. doi: 10.1002/dev.420270607. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Hand preferences for a coordinated bimanual task in 110 chimpanzees (Pan troglodytes): Cross-sectional analysis. Journal of Comparative Psychology. 1995;109:291–297. doi: 10.1037/0735-7036.109.3.291. [DOI] [PubMed] [Google Scholar]
- Hopkins WD. Chimpanzee handedness revisited: 55 years since Finch (1941) Psychonomic Bulletin and Review. 1996;3:449–457. doi: 10.3758/BF03214548. [DOI] [PubMed] [Google Scholar]
- Hopkins WD, Bard KA, Jones A, Bales S. Chimpanzee hand preference for throwing and infant cradling: Implications for the origin of human handedness. Current Anthropology. 1993;34:786–790. [Google Scholar]
- Hopkins WD, Morris RD. Handedness in great apes. A review of findings. International Journal of Primatology. 1993;14:1–25. [Google Scholar]
- MacNeilage PF, Studdert-Kennedy MG, Lindblom B. Primate handedness reconsidered. Behavioral and Brain Sciences. 1987;10:247–303. [Google Scholar]
- Marchant LF, McGrew WC. Laterality of function in apes: A meta-analysis of methods. Journal of Human Evolution. 1991;21:425–438. [Google Scholar]
- Marchant LF, McGrew WC. Laterality of limb function in wild chimpanzees of Gombe National Park: Comprehensive study of spontaneous activities. Journal of Human Evolution. 1996;30:427–443. [Google Scholar]
- McGrew WC, Marchant LF. Chimpanzees, tools, and termites: Hand preference or handedness? Current Anthropology. 1992;33:114–119. [Google Scholar]
- McGrew WC, Marchant LF. On which side of the apes? Ethological study of laterality of hand use. In: McGrew WC, Marchant LF, Nishida T, editors. Great ape societies. Cambridge University Press; Cambridge, M A: 1996. pp. 255–272. [Google Scholar]
- Morris RD, Hopkins WD, Bolser-Gilmore L. Assessment of hand preference in two language-trained chimpanzees (Pan troglodytes): A multimethod analysis. Journal of Clinical and Experimental Neuropsychology. 1993;15:487–502. doi: 10.1080/01688639308402573. [DOI] [PubMed] [Google Scholar]
- Sugiyama Y, Fushimi T, Sakura O, Matsuzawa T. Hand preference and tool use in wild chimpanzees. Primates. 1993;34:151–159. [Google Scholar]
- Ward JP, Hopkins WD. Primate laterality: Current behavioral evidence of primate asymmetries. Springer-Verlag; New York: 1993. [Google Scholar]
- Warren JM. Handedness and laterality in humans and other animals. Physiological Psychology. 1980;8:351–359. [Google Scholar]
- Westergaard GC, Suomi SJ. The use of probing tools by tufted capuchins (Cebus apella): Evidence for increased right-hand preference with age. International Journal of Primatology. 1994;15:521–529. [Google Scholar]
- Westergaard GC, Suomi SJ. Hand preference for a bimanual task in tufted capuchins (Cebus apella) and rhesus macaques (Macaca mulatta) Journal of Comparative Psychology. 1996;110:406–411. doi: 10.1037/0735-7036.110.4.406. [DOI] [PubMed] [Google Scholar]
- Wundrum IJ. Cortical motor asymmetries and Hominid feeding strategies. Human Evolution. 1986;1:183–188. [Google Scholar]