Abstract
Purpose
This study was conducted to examine the bone and body composition effects of S-4, an arylpropionamide derived Selective Androgen Receptor Modulator (SARM) in an ovariectomy induced model of accelerated bone loss.
Methods
One hundred twenty female Sprague-Dawley rats aged to twenty-three weeks were randomly assigned to twelve treatment groups. Drug treatment was initiated immediately following ovariectomy and continued for one hundred twenty days. Whole body bone mineral density (BMD), body composition, and lumbar vertebrae BMD were measured by dual energy x-ray absorptiometry. More stringent regional pQCT and biomechanical strength testing was performed on excised femurs.
Results
We found that S-4 treatment maintained whole body and trabecular BMD, cortical content, and increased bone strength while decreasing body fat in these animals.
Conclusions
The data presented herein show the protective skeletal effects of S-4. Our previous reports have shown the tissue selectivity and muscle anabolic activity of S-4. Together these data suggest that S-4 could reduce the incidence of fracture via two different mechanisms (i.e., via direct effects in bone and reducing the incidence of falls through increased muscle strength). This approach to fracture reduction would be advantageous over current therapies in these patients which are primarily antiresorptive in nature.
Keywords: androgen, bone, osteoporosis, rat model, SARM
INTRODUCTION
Osteoporosis is a significant health problem. Bone fractures commonly cause persistent pain, functional impairment, and death in elderly populations (1-3). Current projections indicate that the elderly population will increase nine fold by 2050 (4), underscoring the need for new therapeutic interventions.
Although the protective skeletal effects of androgens are well documented (5-10), clinical use of androgens as a therapeutic intervention for osteoporosis has been limited due to side effects, especially in women. Virtually all of the current available androgen preparations have severe limitations (11). Unmodified testosterone demonstrates little pharmacologic activity after oral administration resulting from its rapid hepatic elimination. To prolong pharmacologic effects, testosterone implants and longer acting esters were developed. With the exception of testosterone undecanoate, testosterone esters must be administered by intramuscular injection, surgical implantation for implants and pellets, or transdermal delivery, such as patches and gels. As such, testosterone and structurally-related anabolic steroids have been demoted to the therapy of final resort for anemia, endometriosis, and metastatic breast cancer.
Recent interest in using testosterone as hormone replacement in aging men or in age-related frailty has been slowed because of widespread concerns related to the effects of testosterone on the prostate, serum lipids, and cardiovascular system. However, a tissue-selective (i.e., agonistic in bone and muscle) androgen would be a good candidate to fill this current therapeutic void. This androgen which lacks the virilizing side-effects seen with endogenous androgens could be used in male and female patient populations. The therapeutic novelty of these new tissue-selective androgens resides in their potential for use in patient populations where androgens have been historically contraindicated (i.e., women, benign prostatic hypertophy, prostate cancer, etc.) and improved side-effect profile.
S-3-(4-acetylamino-phenoxy)-2-hydroxy-2-methyl-N-(4-nitro-3-trifluoromethyl-phenyl)-propionamide (S-4), a model aryl propionamide SARM (12), shows no cross-reactivity with the other steroid hormone receptors (13) and is not a substrate for aromatase. Additionally, S-4 has a high affinity for the AR, exhibits tissue selective anabolic action (14), and is orally bioavailable (15). We recently showed that S-4 restored skeletal muscle strength, body composition and bone mineral density in castrated, male rats (16). However, female animals were not included in this study, and drug treatment was started twelve weeks after castration in order to assess the anabolic ability of this SARM to rebuild muscle and bone in males with long-term testosterone deficiency. As cross reactivity with the estrogen receptor (ER) and peripheral conversion of endogenous androgens to estradiol have complicated studies to examine the effects of testosterone on bone in females, we conducted an exploratory study to determine the protective effects, if any, of S-4 on bone loss in a female, animal model of accelerated bone loss or postmenopausal osteoporosis. These studies provide the first evidence of the ability of the aryl propionamide SARM, S-4, to prevent bone loss and improve bone strength in females, and suggest that SARMs hold great promise for the treatment of osteoporosis in men and women.
MATERIALS AND METHODS
All cell culture materials used in this study were purchased from Invitrogen (Carlsbad, CA). Experiments were performed to analyze the development of, alkaline phosphatase-positive colonies (ALP+ve) and Tartarate Resistant Acid Phosphatase (TRAP)-positive multinucleated osteoclasts as previously described (17) with the following modifications. Bone marrow cells isolated from femurs were plated in MEM supplemented with 15% charcoal stripped FBS (csFBS), antibiotic (penicillin and streptomycin, 100 U/ml), Fungizone (0.3 μg/ml), 10 mM β-glycerophosphate and 280 μM ascorbic acid at 1.5 million cells per well in 6-well plates to measure colony forming units-fibroblast (CFU-F). Osteoclast cells were plated in 24-well plates at a density of 2.5 million cells/well in MEM supplemented with 10% csFBS, antibiotic and Fungizone. Osteoclasts development was induced by 30 ng/ml RANK-Ligand and 10 ng/ml Granulocyte-macrophage colony-stimulating factor (GM-CSF). Medium was replenished every third day. After 10 days, the fibroblast cultures were stained for ALP+ve colonies (17,18) and osteoclasts were stained for TRAP-positive multinucleated cells (17,18).
One hundred twenty female Sprague–Dawley rats were purchased from Harlan (Indianapolis, IN). The animals were housed with three animals per cage and were allowed free access to tap water and commercial rat chow (Harlan Teklad 22/5 rodent diet-8640). During the course of the study, the animals were maintained on a 12 hr light:dark cycle. This study was reviewed and approved by the Institutional Laboratory Animal Care and Use Committee of The Ohio State University. At 23 weeks of age, the animals were ovariectomized (OVX) or sham-operated and then assigned to one of 12 treatment groups (Table I) of 10 animals as follows: (1) OVX+S-4 (0.1 mg/day); (2) OVX+S-4 (0.3 mg/day); (3) OVX+S-4 (0.5 mg/day); (4) OVX+S-4 (0.75 mg/day); (5) OVX+S-4 (1.0 mg/day); (6) OVX+S-4 (3.0 mg/day); (7) OVX+DHT (1 mg/day); (8) OVX+S-4(0.5 mg/day)+Bicalutamide (1.0 mg/day); (9) OVX+Vehicle; (10) intact+S-4 (1 mg/day); (11) intact+DHT (1 mg/day); (12) intact+ Vehicle. The doses of S-4 were chosen based on a pilot study (data not published) and prior pharmacologic studies performed in our laboratory (14). Since DHT cannot be aromatized to estradiol, DHT treatment was included in both intact and OVX animals to serve as a positive control group to evaluate the skeletal effects of a pure steroidal androgen. Intact and OVX animals receiving vehicle alone served as negative controls. Another control group received an antiandrogen along with S-4 in an effort to delineate the AR-mediated versus AR-independent effects of S-4. During the course of the study, five animals died from non-drug related causes. Therefore, groups 1, 6, and 10 were composed of nine animals each and group 4 was composed of eight animals. Dosing solutions were prepared daily by dissolving drug in dimethyl sulfoxide (DMSO) and diluting in polyethylene glycol 300 (PEG 300). All doses were administered for 120 days via daily subcutaneous injections in a volume of 0.20 ml. At the time of sacrifice, OVX was confirmed by the absence of ovarian tissue. The molecular weights of S-4 and DHT were 441.3 and 290.4, respectively.
Table I.
Summary of Treatment Groups
| Group number |
Gonadal status |
S-4 (mg/day) |
DHT (mg/day) |
Bicalutamide (mg/day) |
|---|---|---|---|---|
| 1 | OVX | 0.1 | – | – |
| 2 | OVX | 0.3 | – | – |
| 3 | OVX | 0.5 | – | – |
| 4 | OVX | 0.75 | – | – |
| 5 | OVX | 1.0 | – | – |
| 6 | OVX | 3.0 | – | – |
| 7 | OVX | – | 1.0 | – |
| 8 | OVX | 0.5 | – | 1.0 |
| 9 | OVX | – | – | – |
| 10 | Intact | 1.0 | – | – |
| 11 | Intact | – | 1.0 | – |
| 12 | Intact | – | – | – |
Groups of animals (n = 10/group) were randomly assigned to receive the individual treatment(s) for 120 days. Doses (0.2 ml) were administered subcutaneously in a vehicle of DMSO and PEG300. Ovariectomy (OVX) was performed one day prior to the initiation of treatment.
Total body bone mineral density (BMD), percent fat mass (FM), body weight (BW), bone mineral content (BMC), bone mineral area (BMA), and lean mass (LM) were determined by dual energy x-ray absorptiometry (DEXA) (GE, Lunar Prodigy™) using the small animal software (Lunar enCORE, version 6.60.041) on days 0 and 120. Animal body weight was also determined by standard gravimetric methods using a 700 series Ohaus triple beam animal balance (Florham Park, NJ). For DEXA scanning, the animals were anesthetized with ketamine:xylazine (87:13 mg/kg) and positioned in a prone position. Total body data was obtained by selecting an area encompassing the entire animal as the region of interest during data processing.
Immediately following the whole body DEXA scan on day 120, groups 1 through 11 were sacrificed, and the lumbar vertebra, femurs, and tibia were excised and cleared of soft tissue. The intact control group for this study (Group 12) also served as a control group for a concurrent delayed treatment study. Therefore, Group 12 was sacrificed at day 210 and excised bone parameters were evaluated. The excised bones were scanned through a 3-inch deep room temperature water bath to simulate soft tissue. The proximal femur, distal femur, proximal tibia, L2–L4 vertebra, and L5–L6 vertebra were selected as regions of interest from the DEXA scan and analyzed for BMD.
The right femurs from the OVX+1.0 mg/day S-4 (Group 5), OVX+3.0 mg/day S-4 (Group 6), OVX+1.0 mg/day DHT (Group 7), OVX control (Group 9), intact+1 mg/day S-4 (Group 10), and intact control (Group 12) were sent to Skeletech, Inc. (Bothell, WA) for peripheral quantitative computed tomography (pQCT) analysis and biomechanical testing. The femur was subjected to pQCT scanning using a Stratec XCT RM and associated software (Stratec Medizintechnik GmbH, Pforzheim, Germany. Software version 5.40 C). The femur was analyzed at both the mid-shaft and distal regions. The length of the femur was determined using scout scan views, and the mid-shaft region was chosen at 50% of the length of the femur. The distal region was chosen at 20% of the length of the femur starting at the distal end. One 0.5 mm slice perpendicular to the long axis of the femur was used for analysis. Total bone mineral content, total bone area, total bone mineral density, cortical bone mineral content, cortical bone area, cortical bone mineral density, cortical thickness, periosteal perimeter (circumference) and endosteal perimeter were determined at the mid-shaft of the femur. At the distal femur total bone mineral content, total bone area, total bone mineral density, trabecular bone mineral content, trabecular bone area and trabecular bone mineral density were determined. After pQCT analysis, the de-fleshed whole femur was used in the three-point bending test. The anterior to posterior diameter (APD) (unit: mm) at the midpoint of the femoral shaft was measured with an electronic caliper. The femur was placed on the lower supports of a three-point bending fixture with the anterior side of the femur facing downward in an Instron Mechanical Testing Machine (Instron 4465 retrofitted to 5500)(Canton, MA). The length (L) between the lower supports was set to 14 mm. The upper loading device was aligned to the center of the femoral shaft. The load was applied at a constant displacement rate of 6 mm/min until the femur broke. The mechanical testing machine directly measured the maximum load (Fu) (unit: N), stiffness (S) (units: N/mm), and energy absorbed (W) (unit: mJ). The axial area moment of inertia (I) (unit: mm4) was calculated by the software during the pQCT analysis of the femoral mid-shaft. Stress (σ) (units: N/mm2), elastic modulus (E) (unit: Mpa), and toughness (T) (units: mJ/m3) were calculated by the following formulas (19): stress: σ = (Fu*L*(a/2))/(4*I); elastic modulus: E = S*L3/(48*I); and toughness: T = 3*W*(APD/2)2/(L*I). The parameters determined to be the most sensitive to estrogen withdrawal are reported herein.
Statistical analyses were performed by Fisher's Protected Least Significant Difference test for multiple comparisons. P-values of less than 0.05 were considered as statistically significant differences.
RESULTS
S-4 Reduces Ovariectomy Induced Bone Loss
Whole body BMD measured by DEXA at day 120 is presented in Table II. As expected, the BMD in OVX animals (0.197 g/cm2) was significantly less than that observed in intact controls (0.215 g/cm2) at day 120. S-4 treatment either partially (i.e., BMD significantly greater than OVX controls) or fully (i.e., BMD not significantly different than intact controls) prevented the loss of skeletal BMD in OVX animals at doses greater than 0.1 mg/day. DHT fully maintained BMD in the OVX animals. However, in intact animals, DHT caused a significant decrease in BMD. S-4 treatment in intact animals maintained BMD at the level of intact controls. Co-administration of bicalutamide, a pure antiandrogen, partially prevented the effects of S-4, suggesting that the AR was important for regulating the bone response to the drug.
Table II.
Whole Body Parameters as Measured by DEXA
| Whole body bone mineral density (g/cm2) |
Body weight (g) | Body fat (%) | Lean mass (%) | ||
|---|---|---|---|---|---|
| Intact | Vehicle | 0.2146 ± 0.0026a | 301 ± 5a | 28.0 ± 1.1a | 72.0 ± 1.1a |
| DHT | 0.2030 ± 0.0031a,b | 339 ± 6b | 33.5 ± 1.3a,b | 66.5 ± 1.3a,b | |
| S-4 | 0.2110 ± 0.0016a | 282 ± 6a,b | 24.6 ± 0.7a,b | 75.4 ± 0.7a,b | |
| Ovariectomized | Vehicle | 0.1973 ± 0.0020b | 334 ± 6b | 41.0 ± 1.9b | 59.0 ± 1.9b |
| DHT | 0.2060 ± 0.0014a | 335 ± 6b | 31.7 ± 1.2a,b | 68.4 ± 1.2a,b | |
| S-4 + Bicalutamide | 0.2057 ± 0.0022a | 335 ± 4b | 38.2 ± 1.4b | 61.8 ± 1.4b | |
| S-4 | |||||
| 0.1 mg/day | 0.2005 ± 0.0020b | 334 ± 6b | 34.8 ± 1.7a,b | 65.2 ± 1.7a,b | |
| 0.3 mg/day | 0.2097 ± 0.0027a | 337 ± 4b | 34.7 ± 1.2a,b | 65.3 ± 1.2a,b | |
| 0.5 mg/day | 0.2115 ± 0.0022a | 337 ± 7b | 33.7 ± 1.0a,b | 66.3 ± 1.0a,b | |
| 0.75 mg/day | 0.2033 ± 0.0019a,b | 338 ± 7b | 34.3 ± 2.2a,b | 65.7 ± 2.2a,b | |
| 1.0 mg/day | 0.2054 ± 0.0019a,b | 339 ± 7b | 32.8 ± 1.1a,b | 67.2 ± 1.1a,b | |
| 3.0 mg/day | 0.2095 ± 0.0023a | 340 ± 7a,b | 29.5 ± 1.5a | 70.5 ± 1.5a |
Data presented as mean ± S.E.M.
denotes P < 0.05 versus OVX controls.
denotes P < 0.05 versus intact controls.
S-4 Dose-Dependently Decreased Body Fat and Increased Lean Mass in Ovariectomized and Intact Rats
Animal body weight and body composition were determined by DEXA after 120 days of treatment and are presented along with the body weight data in Table II. The average body weight for all groups at the beginning of the study was 267 ± 17 g (Mean ± S.D., n = 120). All animals gained a significant amount of weight over the course of the study. Body weight was greater in all ovariectomized groups as compared to the intact control, indicating the influence of estrogen-deprivation on animal growth. Additionally, we observed a further increase in body weight for the 3 mg/day S-4 dose group as compared to other groups in the study. In intact animals, DHT treatment resulted in further increases in body weight when compared with intact controls. However, S-4 administration to intact animals resulted in a significant decrease in body weight when compared to both OVX and intact controls. Percent fat mass (FM) at day 120 was also measured by DEXA. The OVX control group showed a significantly higher FM than that observed in intact controls. We observed a dose-dependent decrease in FM with S-4 therapy, with 3 mg/day S-4 treatment returning FM to the level of intact controls. Co-administration of bicalutamide prevented the decrease in FM observed with S-4 treatment alone. DHT treatment in both intact and OVX animals increased FM to values higher than those observed in intact controls but lower than those observed in OVX controls. Intact animals receiving S-4 showed a decrease in FM compared to intact controls. Corresponding changes in percentage lean mass were observed in all groups.
S-4 Decreased Vertebral Bone Loss Measured by DEXA
Figure 1 summarizes the results of the DEXA analysis of the excised L5YL6 vertebra. OVX animals receiving vehicle alone lost a significant amount of vertebral BMD over the course of the study. We observed a dose-dependent bone-sparing effect with S-4 treatment. The 3 mg/day dose of S-4 completely prevented bone loss caused by OVX. The 0.5 and 1 mg/day doses of S-4 partially prevented the bone loss in the L5–L6 region. We observed positive trends in the data from the 0.1, 0.3, and 0.75 mg/day doses, but statistical significance was not reached. Co-administration of bicalutamide with S-4 partially abolished the effects seen with S-4 alone. DHT treatment in intact animals resulted in a significant decrease in BMD to a level not different from OVX controls. DHT treatment in OVX animals did not prevent bone loss in the L5–L6 vertebra. BMD in intact animals receiving S-4 was not different from intact controls. However, it is important to note that the intact control group was sacrificed at day 210.
Fig. 1.
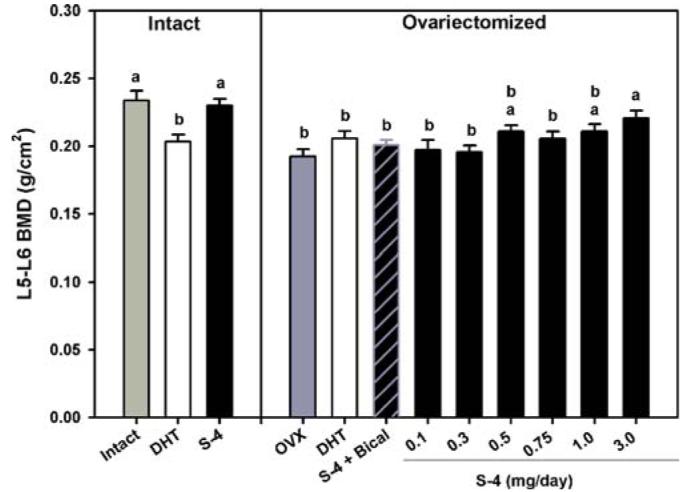
Bone mineral density as measured by DEXA in excised bones. Lumbar vertebrae (L5–L6). Data presented as mean ± S.E.M. adenotes P < 0.05 versus OVX controls.bdenotes P < 0.05 versus intact controls.
S-4 Positively Influences Cortical Bone
Excised femurs were analyzed by pQCT at the mid-shaft for cortical thickness (CT), periosteal circumference (PC), cortical content (CC), and cortical density (CD) and at the distal femur for trabecular BMD. DHT and S-4 prevented the decrease in CT following OVX (Fig. 2A). However, CT in S-4 treated groups was greater than that observed with DHT treatment. Additionally, intact animals (1 mg/day) and OVX animals (3 mg/day) receiving S-4 showed significant increases in CT above the level of intact controls. We observed a significant loss in CC following OVX, with values of 10.3 and 8.8 mg/mm observed in intact and OVX animals, respectively (Fig. 2B). DHT only partially prevented the loss in CC in OVX animals. S-4 completely blocked the loss in CC at the femoral mid-shaft following OVX. The 3 mg/day dose of S-4 caused a non-significant increase in CC over intact control levels. S-4 treatment in OVX animals completely prevented the decrease in PC observed between intact and OVX control groups (Fig. 2C). Although CT was increased in intact animals receiving S-4, PC was decreased in these animals. The DHT treated animals were not different from either intact or OVX control animals. Cortical bone mineral density (CD) of the femoral mid-shaft was also measured by pQCT (Fig. 2D). S-4 completely prevented the loss in CD caused by OVX, while DHT only partially prevented the loss in CD. Intact animals receiving S-4 (1 mg/day) showed an increased CD compared to OVX and intact control animals. Trabecular BMD was measured by pQCT at the distal femur (Fig. 2E). Profound trabecular bone loss was evident in the distal femur following OVX, with values of 735 and 609 mg/cm3 observed in intact and OVX animals, respectively. S-4 and DHT partially prevented the loss of trabecular bone in the distal femur. Additionally, S-4 treatment in intact animals resulted in an increase of trabecular BMD to a level significantly higher than intact controls, suggesting an anabolic action of S-4 in trabecular bone.
Fig. 2.
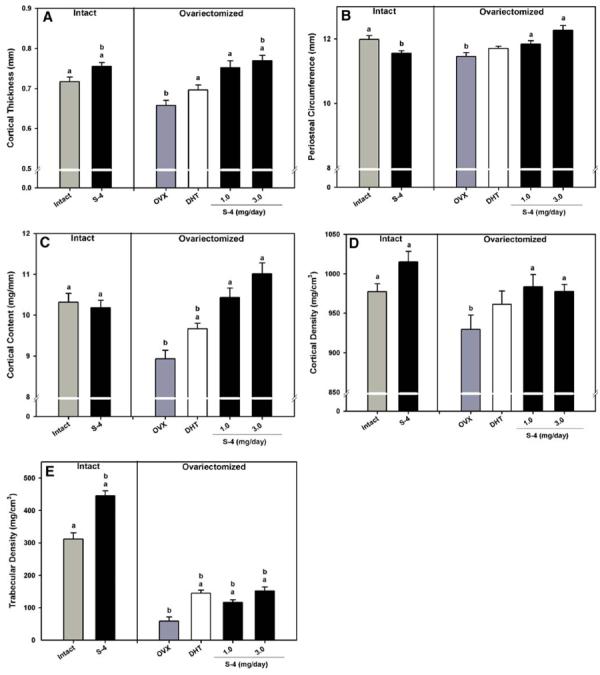
pQCT analysis of the mid-shaft and distal femur. (A) Cortical thickness at the femoral mid-shaft. (B) Periosteal circumference at the femoral mid-shaft. (C) Cortical content at the femoral mid-shaft. (D) Cortical bone density at the femoral mid-shaft. (E) Trabecular density of the distal femur. Data presented as mean ± S.E.M. adenotes P < 0.05 versus OVX controls. bdenotes P < 0.05 versus intact controls.
S-4 Improves Bone Strength
Biomechanical strength of the femur was determined by three-point bending analysis. Results from biomechanical strength testing are shown in Fig. 3. S-4 treatment in OVX animals completely prevented the loss of femoral biomechanical strength that was observed in OVX control animals. DHT also prevented the decrease in femoral biomechanical strength. Intact animals treated with S-4 were not different from intact controls.
Fig. 3.
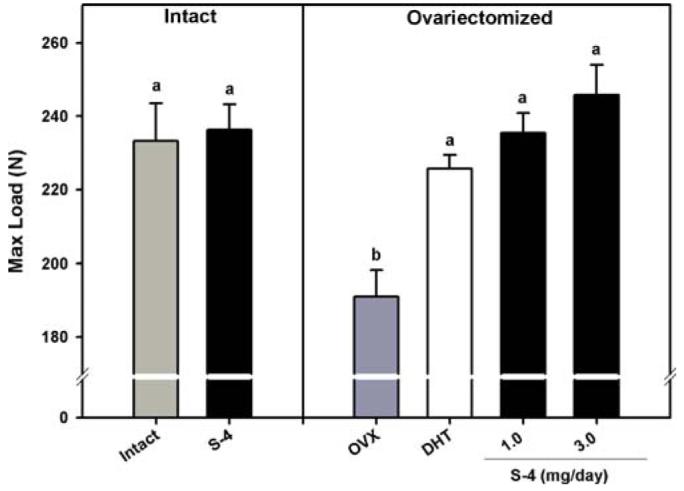
Femoral maximum load determined by 3-point bending. Data presented as mean ± S.E.M. adenotes P < 0.05 versus OVX controls. bdenotes P < 0.05 versus intact controls.
S-4 Affects Osteoblast and Osteoclast Differentiation
To determine the mechanism of this bone sparing effects of S-4, bone marrow osteoprogenitor cells were cultured to differentiate towards the osteoblast or osteoclast lineage in the presence or absence of DHT or S-4. As shown in Fig. 4a, both S-4 and DHT dose-dependently increased the differentiation of bone marrow cells towards the osteoblast lineage as measured by the % ALP forming CFU-F. Figure 4b shows the effect of DHT and S-4 on the formation of multinucleated osteoclasts. Treatment of cells with S-4 or DHT significantly decreased the number of multinucleated osteoclast induced by RANK-L and GM-CSF stimulation. A comparison of the effects of DHT and S-4 on fibroblasts and osteoclasts indicate that S-4 is more anabolic than DHT, as shown by the increase in the ALP forming CFU-F. On the other hand, DHT was a more potent inhibitor of osteoclast formation than S-4 as indicated by the number of osteoclasts.
Fig. 4.
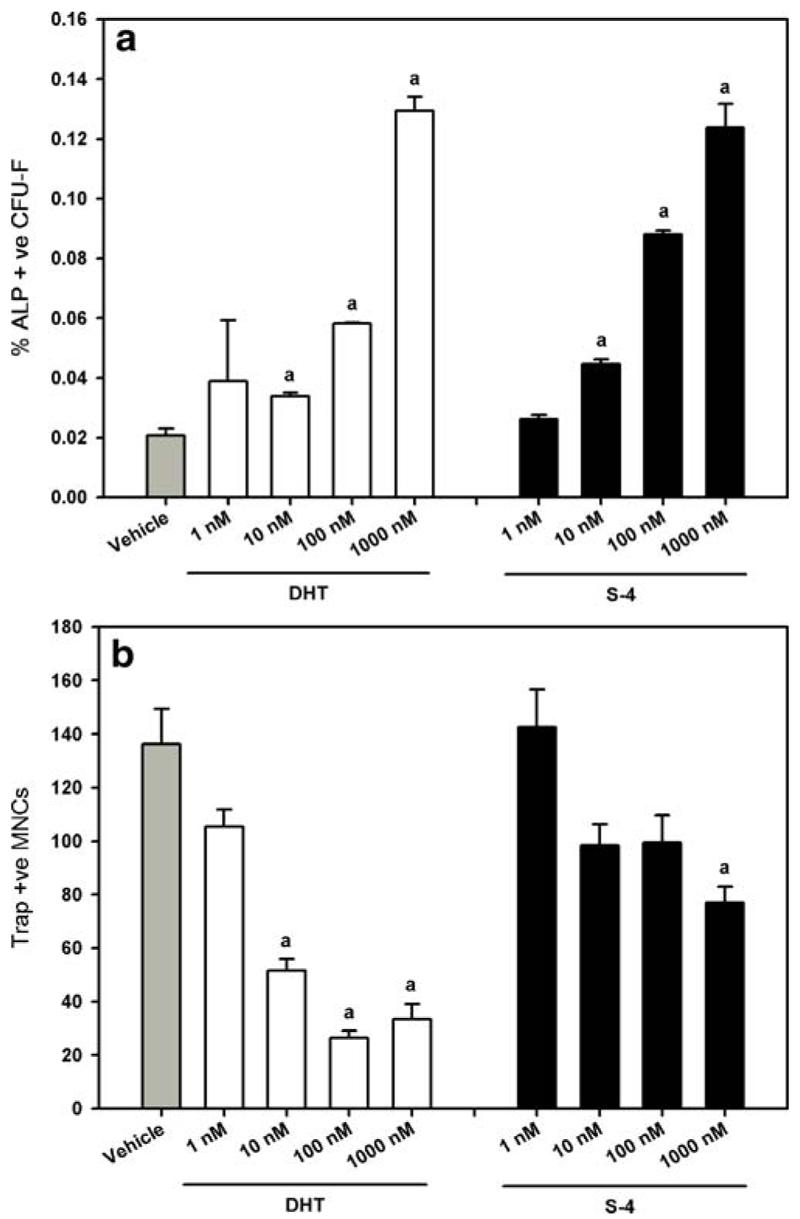
Primary bone marrow osteoprogenitor cell cultures. (a) Percent ALP+ve CFU-F. (b) Trap+ve multinucleated osteoclasts. Data presented as mean ± S.E.M.
DISCUSSION
Previous studies in our laboratory characterized the pharmacokinetics (15), anabolic, androgenic, and tissue-selective effects of S-4 in rats (14), as well as the lack of cross-reactivity with the other steroid hormone receptors in vitro (13). Since androgens are known to exert skeletal effects, we hypothesized that a nonsteroidal SARM would reduce bone loss in the ovariectomized rat. To this end, we conducted an exploratory study to evaluate the bone sparing effects of S-4 in an animal model of post-menopausal bone loss. The aged rat model was chosen for its robustness in predicting clinical outcomes (i.e., every clinically used therapy that has been shown to modulate bone loss (e.g., estrogens, bisphosphonates, PTH, etc.) either partially or completely prevented OVX-induced bone loss in this model) (20).
Our studies clearly show that OVX-induced changes in whole body BMD, body weight, percent fat mass, percent lean mass, L5–L6 BMD, femoral CT, femoral CC, femoral PC, femoral CD, trabecular density, and biomechanical strength were modulated by a nonsteroidal SARM. We observed positive therapeutic outcomes in nearly all of the parameters that were examined. Taken together, these data suggest that S-4 could provide a novel pharmacological intervention in the prevention of bone loss in postmenopausal women.
Most of the parameters we measured appeared to respond in a dose-dependent manner. However, some of the whole body DEXA measurements showed variability between similar doses. The variability could be a result of all doses producing maximal effect in the measured parameter, pharmacokinetic variability, or DEXA variability. However, we did not measure S-4 plasma concentrations or perform more stringent analyses on the excised bones of each group. Therefore, we are unable to speculate as to the contributions of the factors listed above.
The primary circulating and the most significant biologically active androgen in women is testosterone (21). However, aromatization to estradiol makes it difficult to delineate the androgenic versus estrogenic action of testosterone. Therefore, we included DHT, which cannot be aromatized, as a positive control group for the AR-mediated actions in this model. While DHT partially prevented some of the OVX-induced changes, DHT treatment in intact animals caused some detrimental effects. These effects were most likely due to the ability of DHT to inhibit luteinizing hormone (LH) and follicle stimulating hormone (FSH) release from the pituitary. Therefore, DHT indirectly inhibited production of ovarian hormones, in effect, pharmacologically ovariectomizing the intact animals. Further studies are needed to validate this hypothesis as estrogen levels were not measured in this study. Other studies in our laboratory have shown that S-4 does not affect LH or FSH levels (data not shown), highlighting an important difference between steroidal AR agonists and nonsteroidal SARMs.
Our results show that S-4 decreases the percentage of fat mass and increase the percentage of lean mass in OVX animals. Lean mass is important to fracture risk for two reasons. First, increases in muscle mass are indirectly responsible for increases in BMD (22). Secondly, increasing muscle mass may improve balance and muscle strength, thereby reducing the risk of falling (23), which is a primary cause of fracture in the elderly. Although, the primary endpoints in this study were BMD and biomechanical strength, increases in muscle mass may be clinically relevant to reduce fractures. More detailed studies focused on delineating the effects of SARMs on muscle mass and muscle strength have been reported elsewhere by Gao et al. (16).
DEXA is currently the “gold-standard” for measuring BMD in human populations (24). However, instrument sensitivity and animal positioning effects necessitate the validation of small animal DEXA results by more rigorous analysis. We accomplished this by analysis of excised bones using pQCT and biomechanical strength testing. Biomechanical testing was employed to verify that the changes in bone parameters measured by DEXA and pQCT were physiologically relevant to bone quality.
DEXA, pQCT, and biomechanical strength testing on excised bones further validated the whole body DEXA results and support our hypothesis that SARMs inhibit bone loss. The L5–L6 BMD and the distal femur trabecular BMD data suggest that S-4 is a modest inhibitor of trabecular bone loss. We showed that the 0.5, 1.0, and 3.0 mg/day doses of S-4 were able to significantly increase BMD in the L5–L6 region and both 1.0 and 3.0 mg/day dose groups exhibited higher BMD in the distal femur. Our data are in agreement with previous studies published by Lea et al. (7,25) and Tobias et al. (10), which have shown that androgens reduce cancellous bone loss in OVX rats. Recent studies reported by Hanada et al. (26) failed to observe significant anti-resorptive effects in the cancellous bone of the distal femur with S-40503, a tetrahydroquinolin-based SARM. Differences in study duration and method of BMD detection may explain the apparent contradictory results.
Cortical bone at the femoral mid-shaft was analyzed by pQCT. These results demonstrated a dose-dependent effect of S-4 on CT, PC, CC, and CD. BMD was completely maintained by S-4 although we did not observe dose-dependent effects. Three-point bending analysis showed that the increases in bone parameters observed by pQCT were important to bone quality. DHT and S-4 significantly increased the maximum load required to break the femur compared to OVX controls. Further, S-4-treated groups showed a non-significant increase over DHT-treated controls. The effects of S-4 treatment on cortical bone were similar to those reported by Hanada et al. (26) during their evaluation of the anabolic effects of S-40503, in a rat model of osteoporosis. They concluded that S-40503 was anabolic in cortical bone (30 mg/kg dose). Although direct comparisons with our study are not possible due to the differences in study design, their data supports our conclusions that nonsteroidal SARMs exert protective effects in cortical bone. Importantly, the dose at which they observed significant bone effects was approximately 3-fold higher than the highest dose reported in this study. These data, in addition to the data that we have previously published, demonstrate that S-4 is more potent than S-40503 in bone and levator ani muscle and more tissue selective (i.e., S-4 fully restored levator ani muscle weight at a dose that only restored the prostate to 34% of intact (14)).
Although this study was not designed to address the mechanism by which S-4 affects bone, a brief in vitro study suggests that the improvement in the bone quality by S-4 may be due to both anabolic and anti-resorptive effects (Fig. 4a and b). S-4 was better than DHT in promoting osteoblast differentiation, as shown by the % ALP forming CFU-F, whereas, DHT was a more potent inhibitor of osteoclastogenesis than S-4. This is not the first study to indicate that androgens act both by inhibiting osteoclastogenesis and inducing osteoblastogenesis. Treatment of elderly men with estradiol or testosterone or combination to study the mechanism of bone remodeling by sex steroids, indicated that estradiol and testosterone inhibited the bone resorption parameters by 70% and 30% respectively whereas, both estradiol and testosterone were equipotent in increasing the bone formation parameters (27). It is known that AR is expressed in the osteoblasts and osteoclasts suggesting that, in concordance with our study, the effects seen with androgens on bone could be a direct effect on the bone forming and resorbing cells (28-30). It is important to note that the antiandrogen hydroxyflutamide has been shown to inhibit Il-6 production in an osteoclastic cell line (31), suggesting that anti-androgens may have at least a basal level of antiresorptive activity. This could explain why bicalutamide did not completely abrogate the positive effects of S-4 in this study. Additional studies incorporating histomorphometric analyses, hind limb suspension, and/or muscle strength analyses should be considered a high priority to accurately determine the effects of S-4 and other SARMs on the skeleton.
These studies show that S-4 is a promising candidate for further development as a treatment for osteoporosis. Ongoing studies in our laboratory are investigating the effects of S-4 in rats that are allowed to become osteopenic before drug therapy begins and are of similar design to the study reported herein. These studies will provide support for the mechanism of action (i.e., anti-resorptive versus anabolic) of S-4 in bone. Separation of the anabolic and androgenic effects of AR ligands may provide a unique mechanism by which clinicians could exploit the anabolic potential of androgens in muscle and bone without the virilizing side effects of current clinically available androgens.
Although many studies have attempted to delineate the androgenic versus estrogenic regulation of the skeleton the mechanism of action of AR ligands is still being debated. Steroidal AR ligands, even in the presence of aromatase inhibitors, exhibit some cross reactivity with other steroid hormone receptors and feedback at the level of the pituitary complicating the interpretation of the results. Since S-4 cannot be aromatized to an estrogen, does not affect the pituitary hormone release, and shows no cross-reactivity with any of the other steroid hormone receptors, it may be used in future studies as a model compound to elucidate some of the debated effects of androgens in bone.
CONCLUSION
This study is the first to show that an orally bioavailable SARM (15), S-4, can improve body composition, increase BMD, and enhance bone quality in an ovariectomized rat model of post-menopausal osteoporosis. Although further studies are needed to delineate the effects of S-4 on bone, serum lipids, uterine tissue, and circulating hormones, this study demonstrates the potential for SARMs in the treatment of osteoporosis in both men and women.
ACKNOWLEDGMENTS
The outstanding technical assistance and advice of Juhyun Kim, Dr. Jun Yang, Dr. Victor Shen, and Dr. Mitch Steiner are gratefully acknowledged. Supported by a grant from GTx, Inc., Memphis, TN.
REFERENCES
- 1.Dennison E, Cooper C. Epidemiology of osteoporotic fractures. Horm. Res. 2000;54(Suppl 1):58–63. doi: 10.1159/000063449. [DOI] [PubMed] [Google Scholar]
- 2.Kannus P, Niemi S, Parkkari J, Palvanen M, Heinonen A, Sievanen H, Jarvinen T, Khan K, Jarvinen M. Why is the age-standardized incidence of low-trauma fractures rising in many elderly populations? J. Bone Miner. Res. 2002;17:1363–1367. doi: 10.1359/jbmr.2002.17.8.1363. [DOI] [PubMed] [Google Scholar]
- 3.Melton LJ, 3rd, Crowson CS, O'Fallon WM. Fracture incidence in Olmsted County, Minnesota: comparison of urban with rural rates and changes in urban rates over time. Osteoporos. Int. 1999;9:29–37. doi: 10.1007/s001980050113. [DOI] [PubMed] [Google Scholar]
- 4.United Nations. Dept. of Economic and Social Affairs. United Nations. Dept. of International Economic and Social Affairs. United Nations. Dept. of Economic and Social Affairs . Population Division. United Nations; New York: 1998. [Google Scholar]
- 5.Anderson FH, Francis RM, Faulkner K. Androgen supplementation in eugonadal men with osteoporosis—effects of 6 months of treatment on bone mineral density and cardiovascular risk factors. Bone. 1996;18:171–177. doi: 10.1016/8756-3282(95)00441-6. [DOI] [PubMed] [Google Scholar]
- 6.Goulding A, Gold E. Flutamide-mediated androgen blockade evokes osteopenia in the female rat. J. Bone Miner. Res. 1993;8:763–769. doi: 10.1002/jbmr.5650080615. [DOI] [PubMed] [Google Scholar]
- 7.Lea C, Kendall N, Flanagan AM. Casodex (a nonsteroidal antiandrogen) reduces cancellous, endosteal, and periosteal bone formation in estrogen-replete female rats. Calcif. Tissue Int. 1996;58:268–272. doi: 10.1007/BF02508647. [DOI] [PubMed] [Google Scholar]
- 8.Lea CK, Flanagan AM. Physiological plasma levels of androgens reduce bone loss in the ovariectomized rat. Am. J. Physiol. 1998;274:E328–E335. doi: 10.1152/ajpendo.1998.274.2.E328. [DOI] [PubMed] [Google Scholar]
- 9.Lea CK, Moxham V, Reed MJ, Flanagan AM. Androstenedione treatment reduces loss of cancellous bone volume in ovariectomised rats in a dose-responsive manner and the effect is not mediated by oestrogen. J. Endocrinol. 1998;156:331–339. doi: 10.1677/joe.0.1560331. [DOI] [PubMed] [Google Scholar]
- 10.Tobias JH, Gallagher A, Chambers TJ. 5 alpha-Dihydrotestosterone partially restores cancellous bone volume in osteopenic ovariectomized rats. Am. J. Physiol. 1994;267:E853–E859. doi: 10.1152/ajpendo.1994.267.6.E853. [DOI] [PubMed] [Google Scholar]
- 11.Bhasin S, Bremner WJ. Clinical review 85: emerging issues in androgen replacement therapy. J. Clin. Endocrinol. Metab. 1997;82:3–8. doi: 10.1210/jcem.82.1.3640. [DOI] [PubMed] [Google Scholar]
- 12.Gao W, Bohl CE, Dalton JT. Chemistry and structural biology of androgen receptor. Chem. Rev. 2005;105:3352–3370. doi: 10.1021/cr020456u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yin D, He Y, Perera MA, Hong SS, Marhefka C, Stourman N, Kirkovsky L, Miller DD, Dalton JT. Key structural features of nonsteroidal ligands for binding and activation of the androgen receptor. Mol. Pharmacol. 2003;63:211–223. doi: 10.1124/mol.63.1.211. [DOI] [PubMed] [Google Scholar]
- 14.Yin D, Gao W, Kearbey JD, Xu H, Chung K, He Y, Marhefka CA, Veverka KA, Miller DD, Dalton JT. Pharmacodynamics of selective androgen receptor modulators. J. Pharmacol. Exp. Ther. 2003;304:1334–1340. doi: 10.1124/jpet.102.040840. [DOI] [PubMed] [Google Scholar]
- 15.Kearbey JD, Wu D, Gao W, Miller DD, Dalton JT. Pharmacokinetics of S-3-(4-acetylamino-phenoxy)-2-hydroxy-2-methyl-N-(4-nitro-3-trifluoromethyl-phenyl)-propionamide, a nonsteroidal selective androgen receptor modulator. Xenobiotica. 2004;34:273–280. doi: 10.1080/0049825041008962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao W, Reiser PJ, Coss CC, Phelps MA, Kearbey JD, Miller DD, Dalton JT. Selective Androgen Receptor Modulator (SARM) treatment improves muscle strength and body composition, and prevents bone loss in orchidectomized rats. Endocrinology. 2005;146:4887–4897. doi: 10.1210/en.2005-0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaddy-Kurten D, Coker JK, Abe E, Jilka RL, Manolagas SC. Inhibin suppresses and activin stimulates osteoblastogenesis and osteoclastogenesis in murine bone marrow cultures. Endocrinology. 2002;143:74–83. doi: 10.1210/endo.143.1.8580. [DOI] [PubMed] [Google Scholar]
- 18.Narayanan R, Allen MR, Gaddy D, Bloomfield SA, Smith CL, Weigel NL. Differential skeletal responses of hindlimb unloaded rats on a vitamin D-deficient diet to 1,25-dihydroxyvitamin D3 and its analog, seocalcitol (EB1089) Bone. 2004;35:134–143. doi: 10.1016/j.bone.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 19.Turner CH, Burr DB. Basic biomechanical measurements of bone: a tutorial. Bone. 1993;14:595–608. doi: 10.1016/8756-3282(93)90081-k. [DOI] [PubMed] [Google Scholar]
- 20.Kalu DN, Hardin RR. Evaluation of the role of calcitonin deficiency in ovariectomy-induced osteopenia. Life Sci. 1984;34:2393–2398. doi: 10.1016/0024-3205(84)90427-2. [DOI] [PubMed] [Google Scholar]
- 21.Burger HG, Davis SR. The role of androgen therapy. Best Pract. Res. Clin. Obstet. Gynaecol. 2002;16:383–393. doi: 10.1053/beog.2001.0281. [DOI] [PubMed] [Google Scholar]
- 22.Ott S. Osteoporosis and Bone Physiology. 1998 [Google Scholar]
- 23.Lipsitz LA, Nakajima I, Gagnon M, Hirayama T, Connelly CM, Izumo H. Muscle strength and fall rates among residents of Japanese and American nursing homes: an international cross-cultural study. J. Am. Geriatr. Soc. 1994;42:953–959. doi: 10.1111/j.1532-5415.1994.tb06586.x. [DOI] [PubMed] [Google Scholar]
- 24.Newton JL, Kenny RA, Frearson R, Francis RM. A prospective evaluation of bone mineral density measurement in females who have fallen. Age Ageing. 2003;32:497–502. doi: 10.1093/ageing/afg062. [DOI] [PubMed] [Google Scholar]
- 25.Lea CK, Flanagan AM. Ovarian androgens protect against bone loss in rats made oestrogen deficient by treatment with ICI 182,780. J. Endocrinol. 1999;160:111–117. doi: 10.1677/joe.0.1600111. [DOI] [PubMed] [Google Scholar]
- 26.Hanada K, Furuya K, Yamamoto N, Nejishima H, Ichikawa K, Nakamura T, Miyakawa M, Amano S, Sumita Y, Oguro N. Bone anabolic effects of S-40503, a novel nonsteroidal selective androgen receptor modulator (SARM), in rat models of osteoporosis. Biol. Pharm. Bull. 2003;26:1563–1569. doi: 10.1248/bpb.26.1563. [DOI] [PubMed] [Google Scholar]
- 27.Falahati-Nini A, Riggs BL, Atkinson EJ, O'Fallon WM, Eastell R, Khosla S. Relative contributions of testosterone and estrogen in regulating bone resorption and formation in normal elderly men. J. Clin. Invest. 2000;106:1553–1560. doi: 10.1172/JCI10942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.EerdenVan Der BC, Gevers EF, Lowik CW, Karperien M, Wit JM. Expression of estrogen receptor alpha and beta in the epiphyseal plate of the rat. Bone. 2002;30:478–485. doi: 10.1016/s8756-3282(01)00703-7. [DOI] [PubMed] [Google Scholar]
- 29.EerdenVan Der BC, VenVan De J, Lowik CW, Wit JM, Karperien M. Sex steroid metabolism in the tibial growth plate of the rat. Endocrinology. 2002;143:4048–4055. doi: 10.1210/en.2002-220093. [DOI] [PubMed] [Google Scholar]
- 30.Wiren KM, Chapman Evans A, Zhang XW. Osteoblast differentiation influences androgen and estrogen receptor-alpha and -beta expression. J. Endocrinol. 2002;175:683–694. doi: 10.1677/joe.0.1750683. [DOI] [PubMed] [Google Scholar]
- 31.Hofbauer LC, Ten RM, Khosla S. The anti-androgen hydroxyflutamide and androgens inhibit interleukin-6 production by an androgen-responsive human osteoblastic cell line. J. Bone Miner. Res. 1999;14:1330–1337. doi: 10.1359/jbmr.1999.14.8.1330. [DOI] [PubMed] [Google Scholar]


