Abstract
The partial agonist activity of a selective androgen receptor modulator (SARM) in the prostate was demonstrated in orchidectomized rats. In the current study, we characterized the full agonist activity of S-3-(4-acetylamino-phenoxy)-2-hydroxy-2-methyl-N-(4-nitro-3-trifluoromethyl-phenyl)-propionamide (a structurally related SARM referred to in other publications and hereafter as S-4) in skeletal muscle, bone, and pituitary of castrated male rats. Twelve weeks after castration, animals were treated with S-4 (3 or 10 mg/kg), dihydrotestosterone (DHT) (3 mg/kg), or vehicle for 8 wk. S-4 (3 and 10 mg/kg) restored soleus muscle mass and strength and levator ani muscle mass to that seen in intact animals. Similar changes were also observed in DHT-treated (3 mg/kg) animals. Compared with the anabolic effects observed in muscle, DHT (3 mg/kg) stimulated prostate and seminal vesicle weights moire than 2-fold greater than that observed in intact controls, whereas S-4 (3 mg/kg) returned these androgenic organs to only 16 and 17%, respectively, of the control levels. S-4 (3 and 10 mg/kg) and DHT (3 mg/kg) restored castration-induced loss in lean body mass. Furthermore, S-4 treatment caused a significantly larger increase in total body bone mineral density than DHT. S-4 (3 and 10 mg/kg) also demonstrated agonist activity in the pituitary and significantly decreased plasma LH and FSH levels in castrated animals in a dose-dependent manner. In summary, the strong anabolic effects of S-4 in skeletal muscle, bone, and pituitary were achieved with minimal pharmacologic effect in the prostate. The tissue-selective pharmacologic activity of SARMs provides obvious advantages over steroidal androgen therapy and demonstrates the promising therapeutic utility that this new class of drugs may hold.
Testosterone therapy results in major improvements in muscle function (1), body composition, and bone mineral density (BMD) (1, 2), but the major concern for testosterone therapy in elderly men is the increased risk of prostate cancer. As shown in our previous study (3), two selective androgen receptor modulators [SARMs; S-1 and S-4, S-3-(4-fluorophenoxy)- and S-3-(4-acetylaminophenoxy)-2-hydroxy-2-methyl-N-(4-nitro-3-trifluoromethylphenyl)-propionamides, respectively] showed strong anabolic effects in levator ani muscle without stimulating prostate growth in orchidectomized (ORX) animals, suggesting that they may serve as better alternatives for hormone replacement therapy in men. In this study, the anabolic effects of SARM on muscle, bone, and body composition and the agonist activity of SARM in the pituitary were characterized after prolonged androgen deprivation. The ability of SARM to stimulate prostate growth under these conditions was also evaluated.
The strong anabolic activity of SARM in muscle can be used to treat disease-related muscle wasting or improve muscle performance in hypogonadal men. Although S-4 showed stronger agonist activity in maintaining levator ani muscle weight in ORX animals (3), the maintenance of the levator ani muscle weight does not provide direct evidence for the improvement in muscle performance. Therefore, the effects of SARM treatment on skeletal muscle strength in ORX animals were measured directly using isolated soleus muscle.
Soleus muscle is a slow twitch muscle in the hind limb of the rat that contains mainly slow muscle fibers that are rich in myosin heavy chain (MHC)-I. The soleus muscle is generally considered as an antigravity muscle. It is also one of the most commonly used models for skeletal muscle function. Orchidectomy significantly decreases soleus muscle weight and strength in male rats (4), and the effect is reversed by dihydrotestosterone (DHT) treatment (5). In this study, the ability of S-4 (stronger anabolic reagent, compared with S-1, as shown in Refs. 3 and 6) and DHT to restore soleus muscle mass and strength were compared in ORX animals. Muscle contractile properties, including the kinetic properties and contractile force, were measured. Peak tetanic tension (P0) was used as the major parameter for muscle strength comparison.
Myosin is a motor protein that interacts with actin to generate the force for muscle contraction. It is a hexameric protein consisting of two MHC subunits (200 kDa) and two pairs of nonidentical light chain subunits (17–23 kDa) (7). MHC is the most abundant myofibrillar protein expressed in muscle. The major MHC isoforms expressed in skeletal muscle include the fast fibers (i.e. MHC-IIa, IIb, and IId) and the slow fiber (i.e. MHC-I, the main form expressed in the soleus muscle) (7). In comparison, cardiac muscle expresses mainly two slow forms: MHCα and −β (MHCβ is identical with MHC-I in the skeletal muscle) (8, 9).
MHC provides both the motor and filament-forming functions of the intact myosin molecule. Changes in whole muscle contractile force are very likely to be related to the MHC isoform expression because the contractile properties of the muscle, including shortening velocity and maximal force, are correlated with MHC composition (10, 11). The expression of certain MHC isoforms appears to be directly regulated by androgen, as demonstrated in cardiac MHCα expression in spontaneously hypertensive rats (12, 13). MHC isoform expression in the soleus muscle samples from different treatment groups was also compared to explore the possible mechanism of action of SARMs.
Besides anabolic effects in muscle, androgen treatment can also improve body composition (i.e. increase lean mass and decrease fat mass) and prevent bone loss in hypogonadal men (14-18). The effects of testosterone in bone may be mediated directly by androgen receptor (AR) or indirectly via aromatization of testosterone into estrogen and subsequent stimulation of the estrogen receptor (ER) because impaired skeletal development and growth was observed in aromatase inhibitor-treated male rats (19), aromatase knockout (20), and ERα knockout mice (21). Furthermore, testosterone treatment, but not estradiol treatment, prevented bone loss in ORX ERα knockout male mice (21). The anti-resorptive effects of AR were confirmed in AR knockout mice (50). The direct action of testosterone in bone via the AR-mediated pathway is essential for its anabolic effects in bone. However, conversion of testosterone to DHT by 5α-reductase is not required for the process because finasteride (a 5α-reductase inhibitor) treatment did not affect BMD in rats (22) or humans (23). In this study, a nonaromatizable androgen, DHT, was used as a positive control to avoid the indirect actions of androgens through conversion to estrogen. S-4 does not interact with the ER and cannot be aromatized. Thus, the effects of S-4 on bone should be mediated only by direct action on the AR, providing a valid and direct comparison to DHT.
Considering the fact that both muscle and bone are DHT-independent tissues, we hypothesized that S-4 would have similar anabolic activity to DHT in these tissues, and both treatments would improve muscle strength and body composition and restore ORX-caused bone loss. Because the decline in both muscle strength and bone remodeling are relatively slow processes (4, 24), androgen treatment was not initiated until 12 wk after ORX to allow significant decreases in muscle strength and BMD to occur, which is different from the immediate treatment design (i.e. treatment started right after ORX) used in previous studies (6). The effects of S-4 on body composition and bone in ORX rats were measured by dual x-ray absorptiometry (DEXA) during the study. Changes in serum markers for bone formation, such as IGF-I and osteocalcin, were also measured after treatment. Additionally, studies using immediate treatment only tested the ability of SARM to maintain the androgen-dependent tissues in ORX animals, whereas the delayed treatment design used in this study allowed us to test the ability of SARM to restore tissue growth in ORX animals for the first time.
Gonadotropins, especially LH, contribute to the regulation of production and secretion of endogenous testosterone. Testosterone, in turn, affects the release of LH and FSH through negative feedback regulation at both pituitary and hypothalamus. At the hypothalamic level, testosterone indirectly regulates LH and FSH secretion via its ability to influence GnRH release. At the pituitary level, testosterone directly inhibits LH release. The regulatory effects of S-4 on LH and FSH release after long-term treatment were also investigated in this study. As a whole, the studies reported herein demonstrate profound and intriguing differences in SARM pharmacology, compared with that of steroidal androgens, and indicate that a unique approach to androgen therapy may soon be realized.
Materials and Methods
Materials
Compound S-4 (5) was synthesized by Dr. Duane Miller's research group at the University of Tennessee. The purities of these compounds were greater than 99%, as determined by HPLC. Polyethylene glycol 300 (reagent grade), dimethylsulfoxide (reagent grade), DHT, and urea were purchased from Sigma Chemical Co. (St. Louis, MO). IGF-I concentrations were determined using a commercially available enzyme immunoassay (EIA) kit purchased from Diagnostic Systems Laboratories Inc. (Webster, TX). Rat osteocalcin concentrations were determined using a commercially available EIA kit purchased from Biomedical Technologies Inc. (Stoughton, MA). Rat plasma LH and FSH concentrations were determined using commercially available EIA kits purchased from Amersham Biosciences (Piscataway, NJ). Acrylamide, bisacrylamide, ammonium persulfate, sodium dodecyl sulfate, tetramethylethylenedia-mine, and dithiothreitol were purchased from Bio-Rad Laboratories (Hercules, CA).
Animals
Male Sprague Dawley rats were purchased from Harlan Biosciences (Indianapolis, IN). The animals were maintained on a 12-h light, 12-h dark cycle with food and water available ad libitum. The animal protocol was reviewed and approved by the Institutional Laboratory Animal Care and Use Committee of The Ohio State University.
Experimental design
Male Sprague Dawley rats (12 wk old) were ORX at the beginning of the study. A group of sham-operated male rats was also included as intact control. The ORX animals (279–324 g) were randomly distributed into groups of seven to eight animals. Animals were maintained for 12 wk after orchidectomy to allow for the maximum decrease in soleus muscle mass and strength (4, 25) and were then treated with S-4 (3 or 10 mg/kg), DHT (3 mg/kg), or vehicle for 8 wk. The intact animals were also treated with vehicle during the treatment period. The dosage was adjusted weekly based on animals' body weights. The drugs were dissolved in dimethylsulfoxide-polyethylene glycol 300 (20:80, vol/vol) and administered via daily sc injections.
At the end of treatment, animals were weighed, anesthetized, and killed within 8 h after the last dose. The soleus muscle from the left hind limb was dissected immediately and was used for muscle strength measurements. After strength measurements, the soleus muscle was frozen in liquid nitrogen and preserved at −80 C for electrophoretic analysis of MHC isoform expression. The heart was also excised at euthanasia, frozen, and preserved to examine the MHC isoform expression in the left ventricles.
The androgenic (ventral prostate and seminal vesicle) and anabolic tissues (levator ani muscle) and the soleus muscle from the right rear leg were removed and weighed. Blood samples were collected and used for the measurement of serum markers, including IGF-I, osteocalcin, LH, and FSH. Statistical analyses of all the parameters were performed by single-factor ANOVA with the alpha value set a priori at P < 0.05.
Soleus muscle strength measurement
The soleus muscle was isolated with care so as not to damage the muscle and its tendons and then mounted in the experimental chamber. The muscle was perfused in oxygenated (95% O2-5% CO2) Krebs-Ringer solution (pH 7.35–7.45 at room temperature, 137 mm NaCl , 5 mm KCl, 13 mm NaHCO3, 1.8 mm KH2PO4, 2mm CaCl2, 1mm MgSO4, and 11 mm glucose) at room temperature (20–25 C). The proximal tendon was attached to a rigid post, and the distal tendon was attached to a Kulite BG1000 transducer (Kulite Semiconductor Products, Inc., Leonia, NJ) with 4.0 silk. The muscle was stimulated using a Grass S48 stimulator (Quincy, MA) through two platinum field electrodes attached to the chamber walls. The output from the transducer was recorded using ASI dynamic muscle control and analysis software from Aurora Scientific Inc. (Aurora, Canada).
Twitch kinetics and amplitude (Pt) were measured before the tetanus amplitude (P0) was measured. Force responses were obtained by stimulating the muscle at supramaximal voltage (2 msec pulse duration) while stretching the muscle between stimuli at increments of 1 mm. Once the optimal length of the muscle (L0, muscle length at which maximal twitch tension was achieved) was determined, maximal twitch (Pt) and tetanic (P0) tensions, time to peak twitch tension (tPt), and time to one half twitch relaxation (t½R) were measured. These parameters are illustrated in Fig. 1.
Fig. 1.
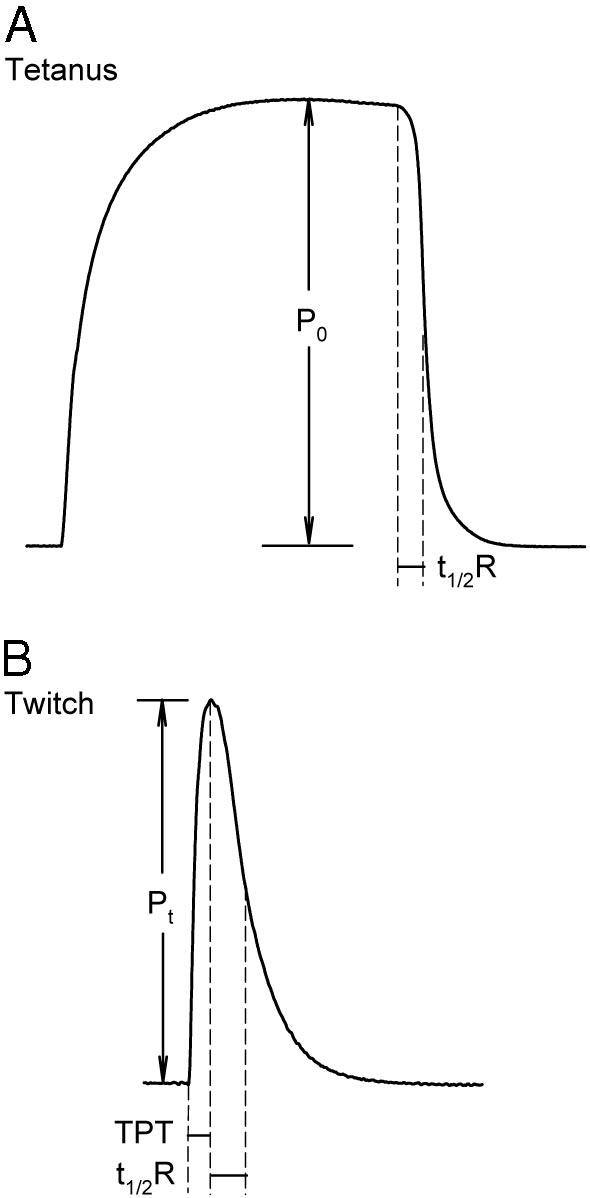
Representative twitch and tetanus measurements in rat soleus muscle. A, Maximal P0 was measured at the plateau of the tetanus. B, Maximal Pt, time to peak twitch tension (TPT), and t1/2R were measured at the L0 of the muscle.
Isometric twitches were elicited at 0.1 Hz. Sixteen continuous twitches were recorded, and one of every three twitches was analyzed. The average of five measurements for each parameter was calculated for each muscle sample. Tetanus was evoked with 3.0-sec trains of stimuli (each pulse at 2 msec duration and 40 Hz frequency). Three tetani were obtained for each muscle, and the average of three measurements of P0 was calculated.
After the measurements, the soleus muscle was weighed, and the cross-sectional area (CSA) of the muscle was estimated using following equation (26):
CSA (square millimeters) = muscle mass (milligrams)/[L0 (millimeters) × muscle density (milligrams/cubic millimeters)].
Muscle density was assumed to be 1 mg/mm3 as previously determined in rat skeletal muscle (27). Contractile force measurements were normalized to the CSA of the muscle before statistical comparison.
Body composition and BMD measurement
Animals were analyzed monthly by DEXA (GE Lunar Prodigy, GE Healthcare, Milwaukee, WI) using the small animal software (Lunar encore, version 6.60.041). The animals were anesthetized with ketaminexylazine (87:13 mg/kg) for the scanning. Total body bone mineral content (BMC), BMD, and body composition (e.g. lean mass and fat mass) were measured. DEXA analyses were completed the same day for all the animals to avoid potential errors associated with interday variability and instrument performance.
Electrophoretic separation of skeletal and cardiac MHC isoforms
Samples of the soleus and left ventricular muscle were homogenized (Pro200 homogenizer, Pro Scientific, Monroe, CT) for 5–10 sec in sample buffer (30 μl per mg tissue) (28, 29). Sample buffer contained 6 m urea, 2 m thiourea, 0.075 m dithiothreitol, 0.05 m Tris base, and 3% sodium dodecyl sulfate, and pH was adjusted to 6.8. Dissolved samples were further diluted [1:10 (vol/vol)] with sample buffer before loading on the gel. An aliquot (3 μl) of each sample was loaded on each lane of the gel.
For soleus muscle sample analysis, the stacking and separating gels (0.75 mm thick) consisted of 4 and 7% acrylamide (wt/vol), respectively, with an acrylamide to bisacrylamide ratio of 50:1 (29). The stacking gel included 5% glycerol, and the separating gel included 30% glycerol. 2-Mercaptomethanol was added to the upper electrode buffer at a final concentration of 10 mm. Gels were run in a Hoefer SE600 unit (Hoefer Scientific, San Francisco, CA) at 8 C, with a constant voltage of 330 V for 23 h. The ventricular sample analyses were conducted in a similar manner, except that the separating gel consisted of 6% acrylamide and 5% glycerol and the gels were run at 200 V for 20 h (28). The gels were then fixed and silver stained. The stained gels were analyzed using a GS 300 scanning densitometer (Hoefer Scientific).
Results
Anabolic effects of S-4 on soleus muscle strength in ORX rats
The body weight and soleus muscle weight of the ORX animals were significantly lower than those of the intact control animals 20 wk after orchidectomy (Table 1). Although S-4 and DHT treatment slightly increased the body weight and the soleus muscle weight in ORX animals, the changes were not significant, compared with the ORX control group. When the soleus muscle weight was normalized by the body weight, no significant change was observed in any of the treatment groups. Besides the observed decreases in muscle size, the L0 of the soleus muscle (the length at which the maximal twitch tension was elicited) also significantly decreased in ORX animals. Both S-4 and DHT treatment returned the L0 back to that observed in intact animals. No significant difference in CSA was observed between treatment groups. Therefore, the decreases in soleus muscle weight and L0 observed in ORX animals were more likely due to the decrease in animal body size. Neither S-4 nor DHT treatment in the ORX animals caused significant changes in the normalized soleus muscle size, consistent with prior reports that the soleus muscle weight was less androgen sensitive (4) than the levator ani muscle weight, with soleus muscle weight decreased by only about 11% after ORX, whereas levator ani muscle weight decreased by approximately 64%.
TABLE 1.
Body weight, soleus muscle weight, L0, CSA, and soleus muscle weight to body weight ratio (n = 7–8) in different treatment groups
| Intact + Veh | ORX + Veh | ORX + S-4 (3 mg/kg) |
ORX + S-4 (10 mg/kg) |
ORX + DHT (3 mg/kg) |
|
|---|---|---|---|---|---|
| Body weight (g) | 437 ± 14a | 380 ± 33b | 406 ± 31b | 406 ± 19b | 403 ± 14b |
| Soleus muscle (mg) | 161 ± 14a | 144 ± 11b | 159 ± 18 | 147 ± 12 | 155 ± 23 |
| Soleus muscle weight/body weight (mg/g) | 0.37 ± 0.03 | 0.38 ± 0.03 | 0.39 ± 0.04 | 0.36 ± 0.03 | 0.38 ± 0.05 |
| Soleus muscle L0 (mm) | 27.9 ± 1.2a | 25.0 ± 1.9b | 27.3 ± 2.1a | 27.9 ± 1.2a | 26.8 ± 1.2a |
| Soleus muscle CSA (mm2) | 7.0 ± 0.8 | 7.0 ± 0.7 | 7.2 ± 0.4 | 6.6 ± 0.7 | 6.9 ± 0.6 |
Data are presented as mean ± SD.
P < 0.05, compared with the vehicle-treated ORX group.
P < 0.05, compared with the intact control group.
The P0 is often used as a measure of the contractile force of the soleus muscle (4). Although S-4 and DHT did not cause significant changes in muscle size, both treatments significantly increased P0 in the soleus muscle in ORX animals (Fig. 2A). P0 of the soleus muscle decreased from 0.85 n in intact animals to 0.57 n in ORX animals, whereas S-4 (3 mg/kg) and DHT (3 mg/kg) increased P0 in the soleus muscle to 0.86 and 0.95 n, respectively. S-4 (10 mg/kg) increased P0 further to 1.02 n, which was significantly higher than P0 in intact animals. Because the CSA of the soleus muscle was not changed by either ORX or any of the treatments (Table 1), identical results were observed (Fig. 2B) when P0 was normalized by the CSA and compared between treatment groups.
Fig. 2.

P0 and normalized P0/CSA in different treatment groups (n = 7–8). Data are presented as mean ± sd. *, P < 0.05, compared with the intact control group. #, P < 0.05, compared with the vehicle (Veh)-treated ORX group.
As summarized in Table 2, other tetanus and twitch parameters, including twitch tPt and tetanus and twitch t1/2R, were not significantly different among ORX, S-4, and DHT treatment groups. Different from our observations of P0 (Fig. 2), the peak twitch tension was not significantly changed by ORX. Only the higher dose of S-4 (10 mg/kg) significantly increased the peak twitch tension in ORX animals.
TABLE 2.
Contractile properties of the soleus muscle (n = 7–8) in different treatment groups
| Intact + Veh | ORX + Veh | ORX + S-4 (3 mg/kg) | ORX + S-4 (10 mg/kg) | ORX + DHT (3 mg/kg) | |
|---|---|---|---|---|---|
| Tetanus | |||||
| P0 (N) | 0.85 ± 0.13a | 0.57 ± 0.17b | 0.86 ± 0.25a | 1.02 ± 0.17a,b | 0.95 ± 0.21a |
| P0/CSA (kN/m2) | 124 ± 27a | 83 ± 29b | 120 ± 37a | 156 ± 22a,b | 138 ± 31a |
| t1/2R (ms) | 256 ± 53 | 300 ± 71 | 286 ± 50 | 294 ± 74 | 308 ± 63 |
| Twitch | |||||
| Pt (N) | 0.21 ± 0.09 | 0.19 ± 0.07 | 0.24 ± 0.07 | 0.28 ± 0.06a | 0.22 ± 0.05 |
| Pt/CSA (kN/m2) | 30 ± 13 | 28 ± 12 | 33 ± 11 | 43 ± 10a,b | 33 ± 8 |
| tPt (ms) | 160 ± 17 | 162 ± 34 | 161 ± 11 | 158 ± 18 | 162 ± 17 |
| t1/2R (ms) | 222 ± 25 | 230 ± 72 | 238 ± 46 | 290 ± 155 | 237 ± 49 |
Data are presented as mean ± SD.
P < 0.05, compared with the vehicle-treated ORX group.
P < 0.05, compared with the intact control group.
In summary, ORX decreased rat body weight, soleus muscle weight, and P0. Both S-4 and DHT treatments significantly increased soleus muscle strength (P0) of ORX animals to that of the intact animals without affecting the contraction kinetics (tPt and t1/2R). S-4 and DHT also tended to increase soleus muscle mass in ORX animals, but the change was not significant due to the larger variability of the data.
Tissue-selective restoration of the androgen-dependent tissues by S-4 in ORX rats
Drug treatment was initiated immediately after ORX in our prior studies (3, 6). In the current study, we examined the ability of S-4 and DHT to restore androgen-dependent tissues after prolonged androgen deprivation. As such, drug treatment was initiated 12 wk after ORX. Prolonged androgen deprivation (i.e. 20 wk) caused significant decreases in the size of the prostate, seminal vesicle, and levator ani muscle, with these organs shrinking to 3.6, 6.7, and 41.4%, respectively, of those observed in intact animals (Fig. 3). Treatment with DHT (3 mg/kg) during wk 13–20 significantly increased the prostate and seminal vesicle weights by more than 2-fold, compared with the intact animals, and increased the levator ani muscle to 131% of that observed in intact controls. S-4 (3 mg/kg) for wk 13–20 selectively restored the levator ani muscle weight to that observed in intact animals but only partially restored the prostate and seminal vesicle weights to less than 20% of that observed in intact animals and less than 10% of that observed in DHT- (3 mg/kg) treated animals. S-4 (10 mg/kg) showed very similar effects in the levator ani muscle, compared with the lower-dose group, but stronger androgenic effects in the prostate and seminal vesicle.
Fig. 3.
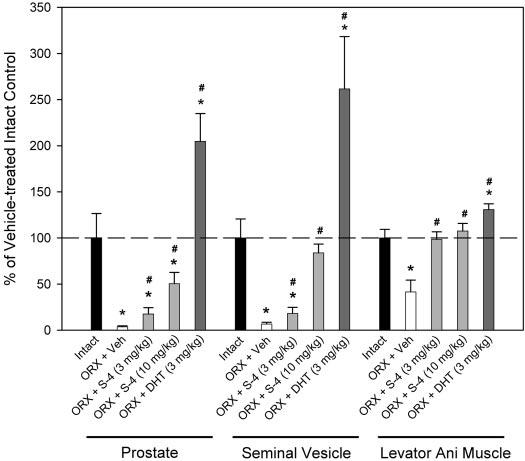
Normalized prostate, seminal vesicle, and levator ani muscle weights (n = 7–8) in different treatment groups. All organ weights were normalized by body weight and are shown as the percentage of the weights in vehicle (Veh)-treated intact control group. Data are presented as mean ± sd. *, P < 0.05, compared with the intact control group. #, P < 0.05, compared with the vehicle-treated ORX group.
These studies demonstrate that: 1) S-4 can restore androgen-dependent tissue mass after prolonged androgen depletion; 2) S-4 (3 mg/kg) and DHT (3 mg/kg) demonstrate similar anabolic effects in increasing soleus muscle strength (Fig. 2) and levator ani muscle weight (Fig. 3); and 3) S-4 demonstrates weak androgenic activity in the prostate at doses capable of maximally promoting anabolic activity.
Effects of S-4 on plasma levels of IGF-I and osteocalcin
Besides their effects in skeletal muscle, androgens may also be anabolic in the skeleton. Plasma levels of IGF-I and osteocalcin (19, 24, 30) are commonly used markers of anabolic activity and bone turnover rate. Twenty weeks after ORX, circulating IGF-I levels in ORX animals (Fig. 4A) were similar to those observed in intact animals; S-4 (3 or 10 mg/kg) treatment did not affect IGF-I levels. However, DHT (3 mg/kg) significantly decreased plasma IGF-I concentrations to 271 ng/ml, approximately 70% of the level observed in intact animals.
Fig. 4.
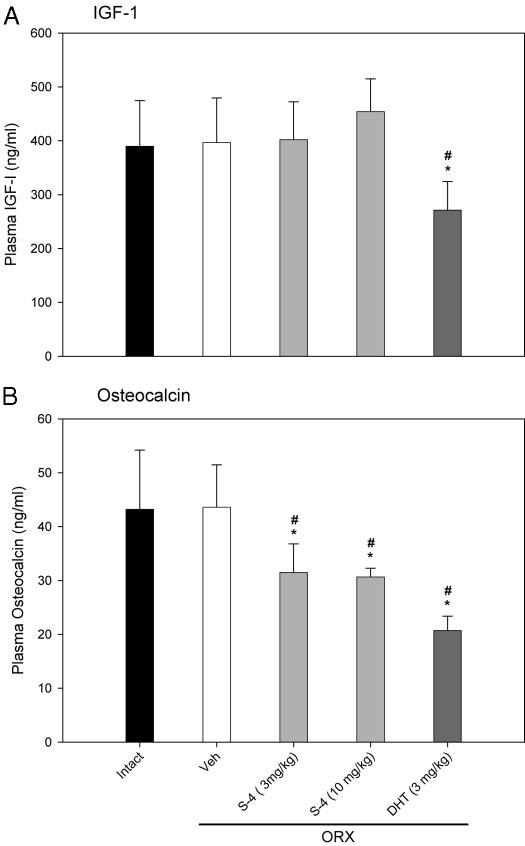
Plasma IGF-I and osteocalcin levels (n = 7–8) in different treatment groups. Data are presented as mean ± sd. *, P < 0.05, compared with the intact control group. #, P < 0.05, compared with the vehicle (Veh)-treated ORX group.
Plasma osteocalcin levels were not significantly different between intact and ORX animals when measured 20 wk after ORX (Fig. 4B). However, 8 wk of treatment with S-4 (3 or 10 mg/kg) or DHT (3 mg/kg) in ORX animals significantly decreased the plasma osteocalcin levels to about 70 and 50%, respectively, of the level in intact animals. Decreases in plasma osteocalcin concentration suggest that S-4 and DHT decreased bone turnover rate in ORX animals.
Effects of S-4 on body composition and BMD in ORX rats
The direct effects of S-4 and DHT on the skeleton were also assessed by monthly DEXA scans. Total body BMD (0.159 g/cm2) and BMC (10.53 g) in ORX animals were significantly lower than that observed in intact animals, 0.166 g/cm2 and 11.47 g, respectively (Fig. 5A) within 12 wk after ORX. Animals were then treated with vehicle, S-4 (3 or 10 mg/kg) or DHT (3 mg/kg) for another 8 wk. By the end of the treatment, the total body BMD and BMC in vehicle-treated intact animals increased by 0.010 g/cm2 and 1.45 g (Fig. 5B), reaching 0.176 g/cm2 and 12.92 g, respectively, whereas the total body BMD and BMC in the ORX animals increased by only 0.006 g/cm2 and 0.65 g (Fig. 5B), reaching 0.165 g/cm2 and 11.18 g, respectively. Although there were no significant differences in body weight among any of the ORX groups (Table 1), S-4-treated (3 and 10 mg/kg) ORX animals showed significantly greater increases in total body BMD, compared with vehicle-treated ORX animals (Fig. 5B). The change in the BMD in S-4-treated ORX animals was similar to that observed in intact animals, with the total body BMD of both dose groups increasing to 0.171 g/cm2 and was significantly higher than that observed in vehicle-treated ORX animals. S-4-treated (10 mg/kg) ORX animals also showed significantly higher increases in total body BMC, compared with the vehicle-treated ORX animals, with the total body BMC increasing to 12.00 g (Fig. 5A) and the change in BMC being similar to that observed in intact animals (Fig. 5B). However, changes in BMD and BMC for DHT-treated animals were smaller than that observed in intact animal and S-4-treated ORX animals and were not significantly different from the changes in ORX animals that received vehicle.
Fig. 5.

Total body BMD and BMC (n = 7–8) in different treatment groups. A, Total body BMD and BMC measured at 12 wk (before S-4 and DHT treatment) and 20 wk (after S-4 and DHT treatment). B, Changes in total body BMD and BMC during 8 wk treatment with S-4 and DHT (between 12 and 20 wk after ORX). Data are presented as mean ± sem. *, P < 0.05, compared with the intact control group. #, P < 0.05, compared with the vehicle (Veh)-treated ORX group.
Body composition of the animals (i.e. total tissue mass, fat mass, and lean mass) was also measured using DEXA. The body weights of all ORX animals were significantly lower than that observed in the intact animals (Table 1 and Fig. 6B) when measured 12 wk after ORX and before drug treatment. Although there was no significant difference in total body weight between the S-4-treated and vehicle-treated ORX animals by the end of the treatment (20 wk after ORX) (Fig. 6B), S-4-treated ORX animals gained more weight than the vehicle-treated ORX animals during the 8-wk treatment (Fig. 6A, gray bars). DHT-treated ORX animals, however, showed similar body weight change during the treatment period, compared with the vehicle-treated control group. The intact animals gained a similar amount of fat mass and lean mass (approximately 15 g for each) during the last 8 wk of the study. However, the vehicle-treated ORX animals lost about 6 g of lean mass and gained the same amount of fat mass as intact controls. DHT treatment significantly increased the lean mass in ORX animals by more than 20 g in 8 wk and decreased the fat mass by more than 10 g. S-4 (3 or 10 mg/kg) treatment restored lean mass in ORX animals, significantly increasing the lean mass by 15 g in 8 wk. However, different from DHT treatment, S-4 treatment did not decrease the fat mass in ORX animals but restored overall body composition to the levels more similar to that observed in intact animals.
Fig. 6.
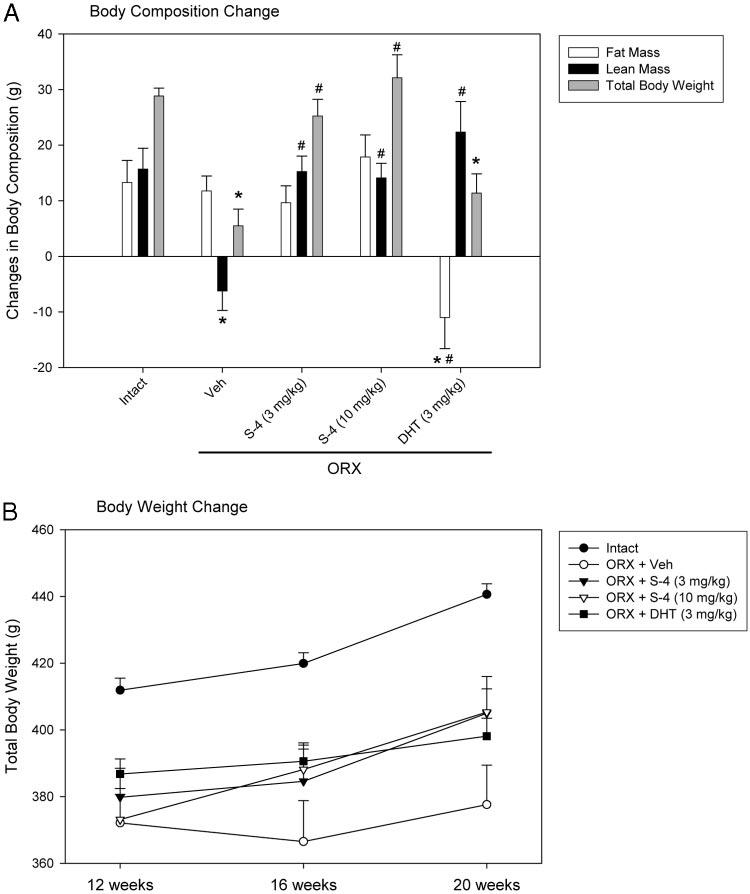
Body composition and body weight change (n = 7–8) in different treatment groups during 8 wk treatment. A, Body composition change between 12 (before S-4 and DHT treatment) and 20 wk (after S-4 and DHT treatment) after ORX. B, Animal body weight measured during 8 wk treatment with S-4 and DHT (between 12 and 20 wk after ORX). Data are presented as mean ± sem. *, P < 0.05, compared with the intact control group. #, P < 0.05, compared with the vehicle (Veh)-treated ORX group.
Effects of S-4 on MHC isoform expression in skeletal and cardiac muscles
As described above, S-4 and DHT fully restored soleus muscle strength (measured as P0, Fig. 2) in ORX animals but only partially restored muscle mass (Table 1), suggesting that the increase in muscle strength was not simply due to an increase in muscle size. Changes in MHC expression provide another possible mechanism for the observed changes in muscle contractile force. MHC isoform expression is different in different types of skeletal muscle. Fast muscles like the extensor digitorum longus express mainly MHC-IIb (fast fiber); whereas slow muscles like the soleus muscle express more MHC-I (slow fiber). The expression of MHC isoforms in both the soleus muscle sample and cardiac ventricular sample were analyzed using modified SDS-PAGE analysis (28, 29).
Similar to literature reports (10), in male Sprague Dawley rats, two isoforms were detected in the soleus muscle samples: MHC-I and MHC-IIa. In most samples analyzed, MHC-I expression accounted for more than 85% of the total MHC expressed (data not shown). In intact animals, only two of the seven samples expressed MHC-IIa, whereas seven of the eight samples in the ORX sample expressed MHC-IIa. Likewise, because no difference was observed between the S-4- and DHT-treated animals and vehicle-treated animals, the slow-to-fast shift observed in ORX animals did not seem to account for the increase in soleus muscle strength.
MHCα and MHCβ are the two major MHC isoforms expressed in cardiac muscle. In intact animals, the expression of MHCα accounted for 65% of the total MHC expressed (Fig. 7), and androgen depletion (ORX) significantly decreased MHCα expression to 44%. S-4 (3 mg/kg) treatment in ORX animals increased MHCα expression to 57% of the total MHC expressed, which was significantly higher than that observed in the vehicle-treated ORX animals but still significantly lower than that observed in intact animals. Both S-4 (10 mg/kg) and DHT (3 mg/kg) increased MHCα expression to a level that was not different from that in intact animals.
Fig. 7.
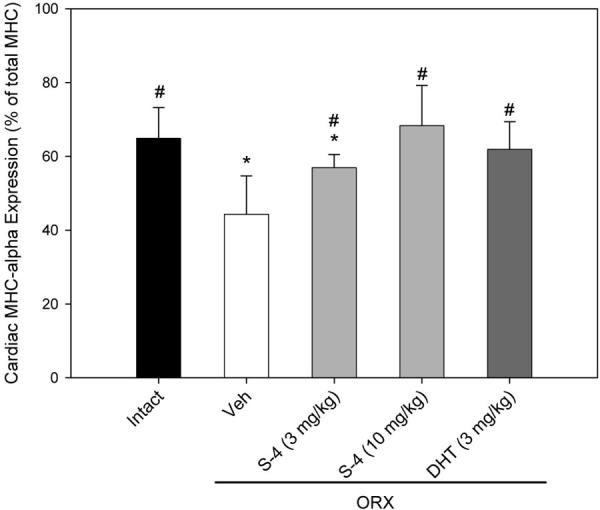
MHCα and MHCβ isoforms expressed in the ventricular muscle samples from different treatment groups (n = 7–8). Data were presented as mean ± sd. *, P < 0.05, compared with the intact control group. #, P < 0.05, compared with the vehicle (Veh)-treated ORX group.
The androgen-regulated β-to-α-shift in cardiac muscle might be related to the function of androgen in the heart (13). However, the slow-to-fast shift observed in the soleus muscle does not seem to be related to the androgen-induced increase in muscle strength in ORX animals.
Effects of S-4 on plasma levels of LH and FSH
The agonist activity of S-4 in the pituitary was also characterized by measuring the plasma concentrations of LH and FSH. Plasma LH and FSH concentrations increased dramatically in ORX animals 20 wk after orchidectomy (Fig. 8). At the 3 mg/kg dose, S-4 significantly decreased LH level to 9.4 ng/ml, which was still higher than the control level in intact animals. With the higher dose of 10 mg/kg, S-4 restored LH to intact levels, similar to what was observed in DHT-treated ORX animals.
Fig. 8.
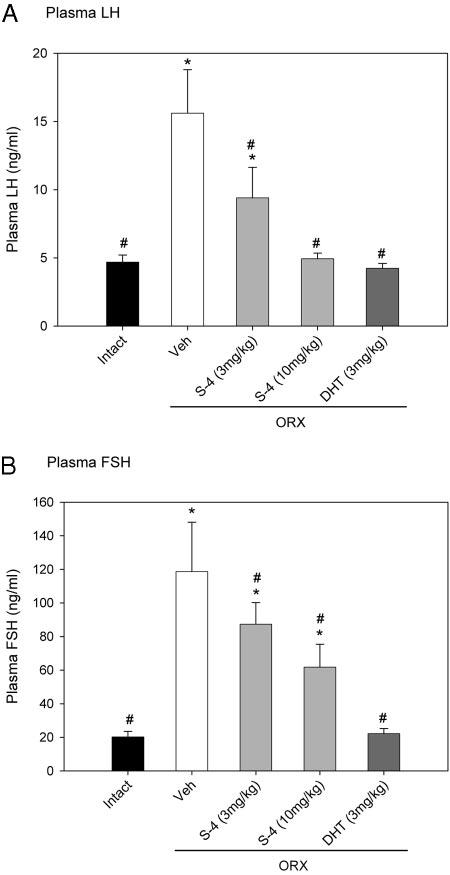
Plasma LH (A) and FSH (B) levels (n = 7–8) in different treatment groups. Data are presented as mean ± sd. *, P < 0.05, compared with the intact control group. #, P < 0.05, compared with the vehicle (Veh)-treated ORX group.
In addition, DHT (3 mg/kg) fully returned FSH level in ORX animals to the intact control level (Fig. 8B). S-4 significantly decreased FSH level in ORX animals in a dose-dependent manner. However, even at the higher dose (10 mg/ kg), S-4 did not fully return the FSH level to the intact control level.
Discussion
Androgen treatment improves skeletal muscle performance in both animal models (5, 31) and men (32, 33). In our study, S-4 and DHT treatment fully restored soleus muscle strength in ORX animals (Fig. 2) without changing the kinetics of muscle contraction (Table 2). Both S-4 (3 mg/kg) and DHT (3 mg/kg) were similarly potent in restoring P0 of the soleus muscle in ORX rats. Some clinical studies have indicated that androgen-induced increases in muscle performance in healthy men are related to muscle fiber hypertrophy (34). However, the S-4- and DHT-induced increase in soleus muscle strength cannot be fully explained by an increase in muscle mass. Although the soleus muscle size was increased in S-4- and DHT-treated ORX animals, compared with that in vehicle-treated ORX animals, the change was not significant and it was still smaller than that observed in intact animals (Table 1). In contrast, muscle strength in S-4-treated ORX animals was similar to (S-4, 3 mg/kg) or higher (10 mg/kg) than that observed in intact animals (Fig. 2). Furthermore, the cross-sectional area of the whole muscle (Table 1), estimated based on muscle mass and optimal length, was not different among any of the treatment groups. Thus, the change in muscle size did not correlate with the change in muscle strength, corroborating other findings that the improvement in muscle strength after androgen treatment cannot be fully explained by the increase in muscle size (5).
Another possible mechanism for the increase in muscle strength that we observed in our study could be related to changes in MHC expression in the soleus muscle. The functional importance of MHC in muscle contraction and direct regulation of certain MHC isoforms expression by androgen is well known (12, 13). Castration significantly decreases MHCα expression in the ventricle of spontaneously hypertensive rats (12), and the change can be reversed by testosterone treatment. Similar changes were observed when rat cardiomyocytes were treated with testosterone (13). Furthermore, computational analysis of the promoter region recognized potential AR binding sites (androgen response element) in both the human and rat MHCα gene, suggesting that certain MHC isoform expression could be directly regulated by androgen (13). Therefore, androgen-regulated MHC expression might also contribute to the increase in muscle strength after androgen treatment.
Regulation of MHCα expression by androgen was also observed in our study (Fig. 7). S-4 and DHT treatment increased MHCα to MHCβ ratio in the hearts of ORX animals. However, no significant change in MHC isoform expression was observed in the soleus muscle samples. Although more animals expressed MHC-IIa in the ORX group, S-4 and DHT treatments did not reverse the change, which suggests that the increase in MHC-IIa expression may not be related to androgen deprivation. As a slow antigravity muscle, the soleus muscle is more sensitive to changes in gravity. Space flight causes a significant slow-to-fast shift in MHC isoform expression in rat soleus muscle (i.e. higher percentage of MHC-IIa and lower percentage of MHC-I) (35-37). Thus, the shift in MHC expression observed in our experiment could have been due to the decreased body weight (30–50 g less, compared with the intact control group, Table 1) in ORX animals. Because androgens did not reduce MHC-IIa expression in the soleus muscle, the change in whole muscle strength that we observed was not related to MHC isoform expression.
Previous studies by Gentile et al. (5) showed similar changes in soleus muscle strength in ORX animals treated with DHT. These earlier studies revealed that DHT treatment stimulated remodeling of the neuromuscular junction by regulating related gene expression. Likewise, animal studies (38) showed that testosterone treatment in male rats decreased diaphragm neuromuscular transmission failure as well. Recent immunohistochemistry study (39) demonstrated the enrichment of AR in myonuclei and fibroblast proximate to neuromuscular junction, suggesting that AR may play an important role in regulating synapse-specific genes important for the survival and growth of motoneurons. Furthermore, AR expression in myonuclei is much higher in levator ani muscle than in skeletal muscle (i.e. extensor digitorum longus), which could explain why levator ani muscle size (Fig. 3) respond to the androgen treatment much more significantly than the skeletal muscles (Table 1). Therefore, the anabolic effects of S-4 treatment in improving muscle strength could also be related to changes in neuromuscular junction communication and remodeling, which needs to be further investigated in future studies.
Besides their strong anabolic effects in skeletal muscle, S-4 and DHT also improved body composition (Fig. 6) in the ORX animals. The anabolic effects of androgens in skeletal muscle and the improvement of body composition might not be completely unrelated (40, 41). Many clinical studies have shown that testosterone treatment causes reciprocal changes in muscle and fat mass (15, 17, 32, 42-44). Similar results were observed with the DHT-treated ORX animals (Fig. 6A) in our study. However, S-4 treatment increased only the muscle mass without changing the fat mass in the ORX animals (Fig. 6A). Recent studies (40, 41) using pluripotent, mesenchymal C3H 10T1/2 cells that are capable of differentiating into muscle, fat, cartilage, and bone cells, a model widely used to study the regulation of myogenic and adipogenic lineage determination, showed that testosterone and DHT promoted the differentiation of these cells to myogenic lineage and inhibited their differentiation into the adipogenic lineage by up-regulating MyoD and MHC (markers for myogenic differentiation) expression and down-regulating peroxisomal proliferator-activated receptor-γ2 and CCAAT/enhancerbinding protein-α (markers for adipogenic differentiation) expression, respectively. Because both testosterone and DHT showed similar effects in both processes, the regulation is believed to be mediated by AR. Gene expression profiling performed in our laboratory (Gao, W., C. C. Coss, V. X. Jin, T. D. Schmittgen, R. Davaluri, and J. T. Dalton, data to be published) revealed differential regulation of gene expression by S-4 and DHT in a prostate cancer cell line. Therefore, the lack of reciprocal changes in muscle and fat mass in S-4-treated ORX animals could be related to the different effects of S-4 and DHT in regulating the expression of the gene markers that are responsible for the differentiation of the mesenchymal cells. More detailed gene expression experiments need to be conducted to confirm this hypothesis.
Another important target organ of androgen is bone. In the mature adult, bone undergoes a continuous remodeling process, consisting of new bone formation by osteoblasts and bone resorption by osteoclasts (23). The remodeling process is also regulated by mechanical factors, systemic hormones (sex steroids, PTH, GH, etc.), and locally produced factors (cytokines, growth factors). Orchidectomy or ovariectomy increases bone turnover rate in animals. Both estrogen and androgen treatment in ORX animals has been shown to have antiresorptive effects in bone by decreasing the bone remodeling turnover rate (23, 45, 46).
Although both S-4 and DHT treatments prevented or restored bone loss (Fig. 5) in the ORX animals, S-4 and DHT treatments showed significant differences in some parameters, indicating possible differences in their mechanism of action. DHT was very effective in improving body composition and muscle strength in ORX animals. However, it was not as effective in restoring ORX-induced bone loss in these animals (Fig. 5). S-4 was more potent than DHT in restoring or preventing bone loss in the ORX animals. Recent studies have shown that ER and AR can act through both genomic (i.e. gene expression regulation) and nongenomic pathways (i.e. cross-talk with other signaling pathways through direct protein-protein interaction without directly regulating gene expression) (46) in regulating bone remodeling. The nongenomic effects appear to be very important to the antiapoptotic activities of estrogen and androgen in the osteoblast (45, 46). More importantly, different ER ligands showed varying ability to stimulate genomic or nongenomic pathways (45). The fact that S-4 is more potent in restoring ORX-induced bone loss could be related to a difference in potency of S-4 and DHT in stimulating the AR nongenomic pathway in bone cells.
In mature rats, ORX-induced bone loss is associated with increased bone turnover in the first few months after ORX, followed by a lower turnover state (24). Osteocalcin is a noncollagenous protein associated with the mineralized matrix and is accepted as a highly specific osteoblastic marker for bone formation. Plasma osteocalcin levels are thought to reflect changes in bone turnover (24, 47). In our study, the plasma osteocalcin level in ORX animals was similar to that observed in intact animals, suggesting that animals had transitioned to a lower bone turnover rate within 5 months after ORX (Fig. 4B). Both S-4 and DHT treatments further decreased the plasma osteocalcin levels in ORX animals, reflecting an even lower turnover rate in these animals. Changes observed in DHT-treated ORX animals were similar to the observations in previous studies (24, 30). These results suggest that S-4 might have antiresorptive activity as well.
IGF-I is a growth factor that increases bone turnover rate by stimulating osteoblast proliferation and osteoclast differentiation, with a net increase in bone accumulation (48). The effects of IGF-I in bone are more related to the local concentration of IGF-I, which is related to both circulating IGF-I and tissue-specific expression of IGF-I and IGF binding proteins (48). Although circulating IGF-I is mainly released from liver (49), which may not reflect the tissue concentration of IGF-I in the bone, changes in plasma IGF-1 could still reflect the effects of S-4 and DHT on IGF-I and IGF binding protein expression. S-4 treatment tends to increase IGF-I expression in ORX animals (Fig. 4A), whereas DHT significantly decreased IGF-I expression in these animals, providing another example for possible differential regulation of gene expression by S-4 and DHT, which could contribute to the tissue-specific pharmacological activities of these AR ligands.
Besides the strong anabolic activities in muscle and bone, S-4 also demonstrated agonist activity in the pituitary by suppressing LH and FSH levels in ORX animals (Fig. 8). Furthermore, the LH level was suppressed more significantly than was the FSH level, suggesting that the observed decreases in LH and FSH levels were mediated through negative feedback effects of S-4 in both hypothalamus and pituitary. Partial repression of circulating LH and FSH levels to values similar to that observed in intact rats corroborates our prior findings in shorter-term (i.e. 9 d) studies that S-4 has minimal effects on endogenous testosterone production.
In summary, S-4 treatment greatly improved the muscle strength and body composition and restored or prevented lost bone in ORX rats. The anabolic effects of S-4 in muscle, bone, and body composition were very similar to those observed in DHT-treated ORX animals. However, at an equi-potent dose that induced similar changes in bone and muscle (3 mg/kg dose), S-4 only restored prostate weight to less than 10% of the level observed in DHT-treated animals, showing minimum stimulation of the prostate, compared with DHT treatment. Significant differences between S-4 and DHT treatments in regulating fat mass change and bone turnover rates in ORX animals were also observed, suggesting that possible differences in the mechanism of action of S-4 and DHT could exist, even though they both work through AR-mediated pathways. Therefore, SARMs with different intrinsic activity provide a novel treatment option for osteoporosis, muscle wasting, and hypogonadism.
Acknowledgments
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK59800-01.
Abbreviations
- AR
Androgen receptor
- BMC
bone mineral content
- BMD
bone mineral density
- CSA
cross-sectional area
- DEXA
dual x-ray absorptiometry
- DHT
dihydrotestosterone
- EIA
enzyme immunoassay
- ER
estrogen receptor
- L0
optimal length of the muscle
- MHC
myosin heavy chain
- ORX
orchidectomized
- P0
peak tetanic tension
- Pt
maximal twitch
- SARM
selective androgen receptor modulator
- tPt
time to peak twitch tension
- t½R
one half twitch relaxation
References
- 1.Slater S, Oliver RT. Testosterone: its role in development of prostate cancer and potential risk from use as hormone replacement therapy. Drugs Aging. 2000;17:431–439. doi: 10.2165/00002512-200017060-00001. [DOI] [PubMed] [Google Scholar]
- 2.Basaria S, Dobs AS. Risks versus benefits of testosterone therapy in elderly men. Drugs Aging. 1999;15:131–142. doi: 10.2165/00002512-199915020-00006. [DOI] [PubMed] [Google Scholar]
- 3.Yin D, Gao W, Kearbey JD, Xu H, Chung K, He Y, Marhefka CA, Veverka KA, Miller DD, Dalton JT. Pharmacodynamics of selective androgen receptor modulators. J Pharmacol Exp Ther. 2003;304:1334–1340. doi: 10.1124/jpet.102.040840. [DOI] [PubMed] [Google Scholar]
- 4.Brown M, Fisher JS, Hasser EM. Gonadectomy and reduced physical activity: effects on skeletal muscle. Arch Phys Med Rehabil. 2001;82:93–97. doi: 10.1053/apmr.2001.18697. [DOI] [PubMed] [Google Scholar]
- 5.Gentile MA, Nantermet P, Phillips R, Holder D, Cheng S, Dai H, Yu Y, Kimmel D, Rodan G, Sachs A, Freedman L, Ray WJ. Mechanisms of androgen receptor mediated increases in muscle mass and strength in hypogonadal rats; Program of the 85th Annual Meeting of The Endocrine Society; Philadelphia, PA. 2003; p. 521. Abstract P3-197. [Google Scholar]
- 6.Gao W, Kearbey JD, Nair VA, Chung K, Parlow AF, Miller DD, Dalton JT. Comparison of the pharmacological effects of a novel selective androgen receptor modulator (SARM), the 5{α}-reductase inhibitor finasteride, and the antiandrogen hydroxyflutamide in intact rats: new approach for benign prostate hyperplasia (BPH) Endocrinology. 2004;145:5420–5428. doi: 10.1210/en.2004-0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weiss A, Leinwand LA. The mammalian myosin heavy chain gene family. Annu Rev Cell Dev Biol. 1996;12:417–439. doi: 10.1146/annurev.cellbio.12.1.417. [DOI] [PubMed] [Google Scholar]
- 8.Lompre AM, Nadal-Ginard B, Mahdavi V. Expression of the cardiac ventricular α- and β-myosin heavy chain genes is developmentally and hormonally regulated. J Biol Chem. 1984;259:6437–6446. [PubMed] [Google Scholar]
- 9.Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- 10.Reiser PJ, Moss RL, Giulian GG, Greaser ML. Shortening velocity in single fibers from adult rabbit soleus muscles is correlated with myosin heavy chain composition. J Biol Chem. 1985;260:9077–9080. [PubMed] [Google Scholar]
- 11.Johnson BD, Wilson LE, Zhan WZ, Watchko JF, Daood MJ, Sieck GC. Contractile properties of the developing diaphragm correlate with myosin heavy chain phenotype. J Appl Physiol. 1994;77:481–487. doi: 10.1152/jappl.1994.77.1.481. [DOI] [PubMed] [Google Scholar]
- 12.Morano I, Gerstner J, Ruegg JC, Ganten U, Ganten D, Vosberg HP. Regulation of myosin heavy chain expression in the hearts of hypertensive rats by testosterone. Circ Res. 1990;66:1585–1590. doi: 10.1161/01.res.66.6.1585. [DOI] [PubMed] [Google Scholar]
- 13.Thum T, Borlak J. Testosterone, cytochrome P450, and cardiac hyper-trophy. FASEB J. 2002;16:1537–1549. doi: 10.1096/fj.02-0138com. [DOI] [PubMed] [Google Scholar]
- 14.Mauras N, Hayes V, Welch S, Rini A, Helgeson K, Dokler M, Veldhuis JD, Urban RJ. Testosterone deficiency in young men: marked alterations in whole body protein kinetics, strength, and adiposity. J Clin Endocrinol Metab. 1998;83:1886–1892. doi: 10.1210/jcem.83.6.4892. [DOI] [PubMed] [Google Scholar]
- 15.Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, Klibanski A. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab. 1996;81:4358–4365. doi: 10.1210/jcem.81.12.8954042. [DOI] [PubMed] [Google Scholar]
- 16.Behre HM, Kliesch S, Leifke E, Link TM, Nieschlag E. Long-term effect of testosterone therapy on bone mineral density in hypogonadal men. J Clin Endocrinol Metab. 1997;82:2386–2390. doi: 10.1210/jcem.82.8.4163. [DOI] [PubMed] [Google Scholar]
- 17.Wang C, Swedloff RS, Iranmanesh A, Dobs A, Snyder PJ, Cunningham G, Matsumoto AM, Weber T, Berman N. Transdermal testosterone gel improves sexual function, mood, muscle strength, and body composition parameters in hypogonadal men. Testosterone Gel Study Group. J Clin Endocrinol Metab. 2000;85:2839–2853. doi: 10.1210/jcem.85.8.6747. [DOI] [PubMed] [Google Scholar]
- 18.Wang C, Eyre DR, Clark R, Kleinberg D, Newman C, Iranmanesh A, Veldhuis J, Dudley RE, Berman N, Davidson T, Barstow TJ, Sinow R, Alexander G, Swerdloff RS. Sublingual testosterone replacement improves muscle mass and strength, decreases bone resorption, and increases bone formation markers in hypogonadal men—a clinical research center study. J Clin Endocrinol Metab. 1996;81:3654–3662. doi: 10.1210/jcem.81.10.8855818. [DOI] [PubMed] [Google Scholar]
- 19.Vanderschueren D, van Herck E, Nijs J, Ederveen AG, De Coster R, Bouillon R. Aromatase inhibition impairs skeletal modeling and decreases bone mineral density in growing male rats. Endocrinology. 1997;138:2301–2307. doi: 10.1210/endo.138.6.5216. [DOI] [PubMed] [Google Scholar]
- 20.Oz OK, Zerwekh JE, Fisher C, Graves K, Nanu L, Millsaps R, Simpson ER. Bone has a sexually dimorphic response to aromatase deficiency. J Bone Miner Res. 2000;15:507–514. doi: 10.1359/jbmr.2000.15.3.507. [DOI] [PubMed] [Google Scholar]
- 21.Vidal O, Lindberg MK, Hollberg K, Baylink DJ, Andersson G, Lubahn DB, Mohan S, Gustafsson JA, Ohlsson C. Estrogen receptor specificity in the regulation of skeletal growth and maturation in male mice. Proc Natl Acad Sci USA. 2000;97:5474–5479. doi: 10.1073/pnas.97.10.5474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen HN, Tollin S, Balena R, Middlebrooks VL, Moses AC, Yamamoto M, Zeind AJ, Greenspan SL. Bone density is normal in male rats treated with finasteride. Endocrinology. 1995;136:1381–1387. doi: 10.1210/endo.136.4.7895648. [DOI] [PubMed] [Google Scholar]
- 23.Compston JE. Sex steroids and bone. Physiol Rev. 2001;81:419–447. doi: 10.1152/physrev.2001.81.1.419. [DOI] [PubMed] [Google Scholar]
- 24.Vanderschueren D, Van Herck E, Suiker AM, Visser WJ, Schot LP, Bouillon R. Bone and mineral metabolism in aged male rats: short and long term effects of androgen deficiency. Endocrinology. 1992;130:2906–2916. doi: 10.1210/endo.130.5.1572302. [DOI] [PubMed] [Google Scholar]
- 25.Fisher JS, Hasser EM, Brown M. Effects of ovariectomy and hindlimb unloading on skeletal muscle. J Appl Physiol. 1998;85:1316–1321. doi: 10.1152/jappl.1998.85.4.1316. [DOI] [PubMed] [Google Scholar]
- 26.Carlson FD, Wilkie DR. Muscle physiology. Prentice-Hall; Englewood Cliffs, NJ: 1974. [Google Scholar]
- 27.Gollnick PD, Timson BF, Moore RL, Riedy M. Muscular enlargement and number of fibers in skeletal muscles of rats. J Appl Physiol. 1981;50:936–943. doi: 10.1152/jappl.1981.50.5.936. [DOI] [PubMed] [Google Scholar]
- 28.Reiser PJ, Kline WO. Electrophoretic separation and quantitation of cardiac myosin heavy chain isoforms in eight mammalian species. Am J Physiol. 1998;274:H1048–H1053. doi: 10.1152/ajpheart.1998.274.3.H1048. [DOI] [PubMed] [Google Scholar]
- 29.Blough ER, Rennie ER, Zhang F, Reiser PJ. Enhanced electrophoretic separation and resolution of myosin heavy chains in mammalian and avian skeletal muscles. Anal Biochem. 1996;233:31–35. doi: 10.1006/abio.1996.0003. [DOI] [PubMed] [Google Scholar]
- 30.Vanderschueren D, Vandenput L, Boonen S, Van Herck E, Swinnen JV, Bouillon R. An aged rat model of partial androgen deficiency: prevention of both loss of bone and lean body mass by low-dose androgen replacement. Endocrinology. 2000;141:1642–1647. doi: 10.1210/endo.141.5.7472. [DOI] [PubMed] [Google Scholar]
- 31.Lewis MI, Fournier M, Yeh AY, Micevych PE, Sieck GC. Alterations in diaphragm contractility after nandrolone administration: an analysis of potential mechanisms. J Appl Physiol. 1999;86:985–992. doi: 10.1152/jappl.1999.86.3.985. [DOI] [PubMed] [Google Scholar]
- 32.Bhasin S, Woodhouse L, Casaburi R, Singh AB, Bhasin D, Berman N, Chen X, Yarasheski KE, Magliano L, Dzekov C, Dzekov J, Bross R, Phillips J, Sinha-Hikim I, Shen R, Storer TW. Testosterone dose-response relationships in healthy young men. Am J Physiol Endocrinol Metab. 2001;281:E1172–E1181. doi: 10.1152/ajpendo.2001.281.6.E1172. [DOI] [PubMed] [Google Scholar]
- 33.Ferrando AA, Sheffield-Moore M, Yeckel CW, Gilkison C, Jiang J, Achacosa A, Lieberman SA, Tipton K, Wolfe RR, Urban RJ. Testosterone administration to older men improves muscle function: molecular and physiological mechanisms. Am J Physiol Endocrinol Metab. 2002;282:E601–E607. doi: 10.1152/ajpendo.00362.2001. [DOI] [PubMed] [Google Scholar]
- 34.Sinha-Hikim I, Artaza J, Woodhouse L, Gonzalez-Cadavid N, Singh AB, Lee MI, Storer TW, Casaburi R, Shen R, Bhasin S. Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am J Physiol Endocrinol Metab. 2002;283:E154–E164. doi: 10.1152/ajpendo.00502.2001. [DOI] [PubMed] [Google Scholar]
- 35.Caiozzo VJ, Baker MJ, Herrick RE, Tao M, Baldwin KM. Effect of spaceflight on skeletal muscle: mechanical properties and myosin isoform content of a slow muscle. J Appl Physiol. 1994;76:1764–1773. doi: 10.1152/jappl.1994.76.4.1764. [DOI] [PubMed] [Google Scholar]
- 36.Caiozzo VJ, Haddad F, Baker MJ, Herrick RE, Prietto N, Baldwin KM. Microgravity-induced transformations of myosin isoforms and contractile properties of skeletal muscle. J Appl Physiol. 1996;81:123–132. doi: 10.1152/jappl.1996.81.1.123. [DOI] [PubMed] [Google Scholar]
- 37.Staron RS, Kraemer WJ, Hikida RS, Reed DW, Murray JD, Campos GE, Gordon SE. Comparison of soleus muscles from rats exposed to micro-gravity for 10 versus 14 days. Histochem Cell Biol. 1998;110:73–80. doi: 10.1007/s004180050267. [DOI] [PubMed] [Google Scholar]
- 38.Blanco CE, Zhan WZ, Fang YH, Sieck GC. Exogenous testosterone treatment decreases diaphragm neuromuscular transmission failure in male rats. J Appl Physiol. 2001;90:850–856. doi: 10.1152/jappl.2001.90.3.850. [DOI] [PubMed] [Google Scholar]
- 39.Monks DA, O'Bryant EL, Jordan CL. Androgen receptor immunoreactivity in skeletal muscle: enrichment at the neuromuscular junction. J Comp Neurol. 2004;473:59–72. doi: 10.1002/cne.20088. [DOI] [PubMed] [Google Scholar]
- 40.Bhasin S, Taylor WE, Singh R, Artaza J, Sinha-Hikim I, Jasuja R, Choi H, Gonzalez-Cadavid NF. The mechanisms of androgen effects on body composition: mesenchymal pluripotent cell as the target of androgen action. J Gerontol A Biol Sci Med Sci. 2003;58:M1103–M1110. doi: 10.1093/gerona/58.12.m1103. [DOI] [PubMed] [Google Scholar]
- 41.Singh R, Artaza JN, Taylor WE, Gonzalez-Cadavid NF, Bhasin S. Androgens stimulate myogenic differentiation and inhibit adipogenesis in C3H 10T1/2 pluripotent cells through an androgen receptor-mediated pathway. Endocrinology. 2003;144:5081–5088. doi: 10.1210/en.2003-0741. [DOI] [PubMed] [Google Scholar]
- 42.Brodsky IG, Balagopal P, Nair KS. Effects of testosterone replacement on muscle mass and muscle protein synthesis in hypogonadal men—a clinical research center study. J Clin Endocrinol Metab. 1996;81:3469–3475. doi: 10.1210/jcem.81.10.8855787. [DOI] [PubMed] [Google Scholar]
- 43.Snyder PJ, Peachey H, Berlin JA, Hannoush P, Haddad G, Dlewati A, Santanna J, Loh L, Lenrow DA, Holmes JH, Kapoor SC, Atkinson LE, Strom BL. Effects of testosterone replacement in hypogonadal men. J Clin Endocrinol Metab. 2000;85:2670–2677. doi: 10.1210/jcem.85.8.6731. [DOI] [PubMed] [Google Scholar]
- 44.Snyder PJ, Peachey H, Hannoush P, Berlin JA, Loh L, Lenrow DA, Holmes JH, Dlewati A, Santanna J, Rosen CJ, Strom BL. Effect of testosterone treatment on body composition and muscle strength in men over 65 years of age. J Clin Endocrinol Metab. 1999;84:2647–2653. doi: 10.1210/jcem.84.8.5885. [DOI] [PubMed] [Google Scholar]
- 45.Kousteni S, Bellido T, Plotkin LI, O'Brien CA, Bodenner DL, Han L, Han K, DiGregorio GB, Katzenellenbogen JA, Katzenellenbogen BS, Roberson PK, Weinstein RS, Jilka RL, Manolagas SC. Nongenotropic, sex-nonspecific signaling through the estrogen or androgen receptors: dissociation from transcriptional activity. Cell. 2001;104:719–730. [PubMed] [Google Scholar]
- 46.Kousteni S, Chen JR, Bellido T, Han L, Ali AA, O'Brien CA, Plotkin L, Fu Q, Mancino AT, Wen Y, Vertino AM, Powers CC, Stewart SA, Ebert R, Parfitt AM, Weinstein RS, Jilka RL, Manolagas SC. Reversal of bone loss in mice by nongenotropic signaling of sex steroids. Science. 2002;298:843–846. doi: 10.1126/science.1074935. [DOI] [PubMed] [Google Scholar]
- 47.Hauschka PV, Lian JB, Cole DE, Gundberg CM. Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev. 1989;69:990–1047. doi: 10.1152/physrev.1989.69.3.990. [DOI] [PubMed] [Google Scholar]
- 48.Olney RC. Regulation of bone mass by growth hormone. Med Pediatr Oncol. 2003;41:228–234. doi: 10.1002/mpo.10342. [DOI] [PubMed] [Google Scholar]
- 49.Butler AA, LeRoith D. Minireview: tissue-specific versus generalized gene targeting of the igf1 and igf1r genes and their roles in insulin-like growth factor physiology. Endocrinology. 2001;142:1685–1688. doi: 10.1210/endo.142.5.8148. [DOI] [PubMed] [Google Scholar]
- 50.Kawano H, Sato T, Yamada T, Matsumoto T, Sekine K, Watanabe T, Nakamura T, Fukuda T, Yoshimura K, Yoshizawa T, Aihara K, Yamamoto Y, Nakamichi Y, Metzger D, Chambon P, Nakamura K, Kawaguchi H, Kato S. Suppressive function of androgen receptor in bone resorption. Proc Natl Acad Sci USA. 2003;100:9416–9421. doi: 10.1073/pnas.1533500100. [DOI] [PMC free article] [PubMed] [Google Scholar]


