Summary
Background
Valproate is widely accepted as a drug of first choice for patients with generalised onset seizures, and its broad spectrum of efficacy means it is recommended for patients with seizures that are difficult to classify. Lamotrigine and topiramate are also thought to possess broad spectrum activity. The SANAD study aimed to compare the longer-term effects of these drugs in patients with generalised onset seizures or seizures that are difficult to classify.
Methods
SANAD was an unblinded randomised controlled trial in hospital-based outpatient clinics in the UK. Arm B of the study recruited 716 patients for whom valproate was considered to be standard treatment. Patients were randomly assigned to valproate, lamotrigine, or topiramate between Jan 12, 1999, and Aug 31, 2004, and follow-up data were obtained up to Jan 13, 2006. Primary outcomes were time to treatment failure, and time to 1-year remission, and analysis was by both intention to treat and per protocol. This study is registered as an International Standard Randomised Controlled Trial, number ISRCTN38354748.
Findings
For time to treatment failure, valproate was significantly better than topiramate (hazard ratio 1·57 [95% CI 1·19–2·08]), but there was no significant difference between valproate and lamotrigine (1·25 [0·94–1·68]). For patients with an idiopathic generalised epilepsy, valproate was significantly better than both lamotrigine (1·55 [1·07–2·24] and topiramate (1·89 [1·32–2·70]). For time to 12-month remission valproate was significantly better than lamotrigine overall (0·76 [0·62–0·94]), and for the subgroup with an idiopathic generalised epilepsy 0·68 (0·53–0·89). But there was no significant difference between valproate and topiramate in either the analysis overall or for the subgroup with an idiopathic generalised epilepsy.
Interpretation
Valproate is better tolerated than topiramate and more efficacious than lamotrigine, and should remain the drug of first choice for many patients with generalised and unclassified epilepsies. However, because of known potential adverse effects of valproate during pregnancy, the benefits for seizure control in women of childbearing years should be considered.
Introduction
Epilepsy is a common disorder (50 per 100 000 people; 0·5–1%).1 Rather than being one condition, epilepsies are a heterogeneous group of disorders that have been classified by the International League Against Epilepsy.2,3 Around 30–40% of patients have seizures that are generalised at onset, such as generalised onset tonic clonic seizures, absence seizures, and myoclonic seizures, most of whom are thought to have a genetic predisposition and have an idiopathic generalised epilepsy. Such epilepsies tend to present in childhood and adolescence and have generalised spike-wave abnormalities in an electroencephalogram. Common syndromes include childhood absence epilepsy, juvenile absence epilepsy, juvenile myoclonic epilepsy, and generalised epilepsy with tonic clonic seizures on waking.
Compared with the partial epilepsies, the comparative effects of antiepileptic drugs for patients with generalised onset seizures are poorly studied. Guidelines recommend valproate as a treatment of first choice for patients with generalised onset seizures,4,5 although evidence to support this from randomised controlled trials is scarce. Meta-analyses of randomised controlled trials that recruited patients with generalised onset tonic clonic seizures reported no difference between valproate and either carbamazepine or phenytoin6,7 for time to treatment failure, 12-month remission, or first seizure. However, results were potentially confounded by errors in seizure classification and failure to measure seizures other than tonic clonic during follow-up. A systematic review of small randomised trials that assessed treatments for absence seizures showed no evidence of a difference between valproate and either ethosuximide or lamotrigine.8 Thus, evidence to support valproate as a first line treatment comes mostly from observational studies that suggest efficacy of valproate compared with other treatment,9 or suggest worsening of seizures with treatments such as carbamazepine and phenytoin.10,11 Valproate is also suggested as a first line treatment for patients whose seizures are difficult to classify as either focal or generalised in onset at the time of diagnosis, because of its assumed broad spectrum of activity. To our knowledge, no randomised controlled trials have specifically examined treatment effects in this subgroup of patients.
The past decade and a half has seen the licensing and introduction of several new antiepileptic drugs. These have all been licensed initially on the basis of placebo-controlled add-on randomised trials in patients with refractory partial epilepsy, with few studies examining these drugs in patients with refractory generalised onset seizures. Similarly, few randomised controlled trials have assessed the effects of these new drugs as monotherapy for patients with generalised onset seizures. However, lamotrigine and topiramate have been licensed in the UK as treatments for patients with generalised onset tonic clonic seizures.
Lamotrigine has been suggested as an alternative to valproate, particularly for women of childbearing age, because of concerns about higher rates of teratogenicity and delayed cognitive development in children exposed to valproate in utero.12,13 Although there are randomised trials of add-on lamotrigine indicating efficacy compared with placebo,14-17 apart from the small trials in absence epilepsy outlined above, we are aware of none that have directly compared valproate and lamotrigine monotherapy. We therefore have no reliable evidence about the relative effectiveness of valproate and lamotrigine to inform clinical decisions. There is also little evidence about topiramate, which has been compared with valproate in a randomised trial that reported no difference between the two drugs for short-term outcomes of efficacy, although CIs were wide, and longer-term outcomes were not examined.18 Despite this lack of evidence, there has been a steady rise in the prescribing of new antiepileptic drugs from 0·1% of total antiepileptic drug prescriptions in 1991 to 20% in 2002. New drugs accounted for 69% of the total costs of antiepileptic drugs to the UK National Health Service (NHS, £99 million of £142 million).19
Since most patients who develop epilepsy are treated with one drug and might be on medication for many years, standard and new drugs need to be compared so as to establish which should, in the future, be first choice for appropriate groups of patients. We have therefore undertaken two concurrent pragmatic parallel-group unblinded randomised trials comparing Standard and New Antiepileptic Drugs (SANAD), which examined seizure control, tolerability, quality of life, and health economic outcomes. Arm B of SANAD is reported here and compares valproate, lamotrigine, and topiramate in patients for whom valproate was viewed as the optimum first-line treatment when compared with carbamazepine.
Methods
Patients and procedures
Patients were included in arm B of SANAD if they had a history of two or more clinically definite unprovoked epileptic seizures in the previous year and if the recruiting clinician regarded valproate the better standard treatment option than carbamazepine. This criteria allowed inclusion of patients with newly diagnosed epilepsy, patients who had failed treatment with previous monotherapy (as long as the drug failure did not include one of the drugs present in the randomisation), and patients in remission of epilepsy who had relapsed after withdrawal of treatment. Patients were excluded if the clinician or patient felt that treatment was contraindicated, if all their seizures had been acute symptomatic seizures (including febrile seizures), they were aged 4 years or younger, or if there was a history of progressive neurological disease.
Information recorded at study entry included patient demographics, a history of learning disability or developmental delay, neurological history including head injury, stroke, intracerebral infection, or acute symptomatic seizures, and a history of epilepsy in a first-degree family member. Clinicians were asked to identify seizures and epilepsy syndromes by International League Against Epilepsy classifications20,21 as far as was possible, at least to differentiate between partial onset (focal) or generalised onset seizures. However, where there was uncertainty, patients were recorded as having unclassified convulsive or other unclassified seizures. Results of any electroencephalogram or brain imaging around the time of randomisation were recorded.
Participating patients in arm B were randomly allocated in a 1:1:1 ratio to valproate, lamotrigine, or topiramate. To randomise a patient, the clinician telephoned a central randomisation service, and provided patient identifying information and the clinical factors used for stratification of randomisation, which were centre, sex, and treatment history (newly diagnosed and untreated, treated with ineffective monotherapy, relapse after remission of epilepsy). The central randomisation service then allocated patients with a computer programme using a minimisation procedure. Although choice of drug was randomised, drug dose and preparation was that used by the clinician in their everyday practice. The rate of titration, initial maintenance dose, and any subsequent increments or decrements were decided by the clinician, who was aided by guidelines (webtable 1). The aim of treatment was to control seizures with a minimum effective dose of drug, which necessitated dose increments if further seizures took place (as is usual clinical practice).
There were two primary outcome measures: (1) the time from randomisation to treatment failure (stopping the randomised drug because of inadequate seizure control, intolerable side-effects, or both; or the addition of other antiepileptic drugs, whichever was the earliest); and (2) the time from randomisation to a 1-year period of remission of seizures. Secondary clinical outcomes were: the time from randomisation to a first seizure; time to achieve a 2-year remission; and the frequency of clinically important adverse events and side-effects emerging after randomisation. Quality of life outcomes and cost-effectiveness were also assessed. A detailed description of methods used is given in the first SANAD trial paper.22 For both adults and children, the quality of life assessment used a battery of previously validated generic and epilepsy-specific measures. For adults, we used the NEWQOL (Newly Diagnosed Epilepsy Quality of Life) battery. For the health economic assessment, patients' use of resources were classified under three general headings: consumption of antiepileptic drugs; resource use associated with the management of adverse events needing hospitalisation; and use of other health care and social services resources.
Statistical analysis
The calculations of sample size were based on the two primary outcomes and informed by a meta-analysis of individual patient data comparing valproate and carbamazepine.6 We wished to establish that the lower 95% confidence limit for the old-new treatment comparisons exceeded −10% (non-inferiority), rather than establishing equivalence within 10%. With α=0·05, and β=10%, giving a 95% confidence limit of 10% around an overall 1-year remission rate of 70% and a retention rate of 70% (ie, treatment failure rate of 30%) at a median of 2·5 years follow-up with power 90% (β=0·10) needed 445 patients per treatment group.
SANAD was commissioned by the National Health Service Health Technology Assessment Programme in the UK. The study received appropriate multicentre and local ethics and research committee approvals, and was managed according to Medical Research Council Good Clinical Practice Guidelines.23 Patients gave informed written consent to inclusion and to long-term follow-up.
This study is registered as an International Standard Randomised Controlled Trial, number ISRCTN38354748.
Role of the funding source
SANAD was funded by the Health Technology Assessment Programme, with an additional 20% of resource coming from companies with products assessed. The funding sources had no role in study design, data collection, analysis, and interpretation of data or in writing this report. All authors had full access to the data. The corresponding author had full access to the data and had final responsibility for the decision to submit for publication.
Results
The first patient was randomised into the study on Jan 12, 1999, and randomisation continued up to Aug 31, 2004. Attempts were made to follow-up all patients to, at the latest, a point in time between May 1, 2005, and Aug 31, 2005, although some follow-up data were obtained up to Jan 13, 2006. 716 patients were randomised to arm B, 239 to lamotrigine, 239 to topiramate and 238 to valproate (figure 1). The treatment groups were well balanced for demographic and clinical factors (table 1). 91 patients were aged between 5 years and 9 years at randomisation and 100 patients between 10 years and 15 years. Most patients had an idiopathic generalised epilepsy (450, 63%) or unclassified epilepsy (191, 27%). Of the patients with an idiopathic generalised epilepsy, 66 (15%) had childhood absence epilepsy, 45 (10%) had juvenile absence epilepsy, 119 (26%) had juvenile myoclonic epilepsy, 42 (9%) had generalised epilepsy with tonic clonic seizures on waking, and 168 (37%) had an unspecified idiopathic generalised epilepsy (webtable 2). The ratio of men to women indicates that there might have been some reluctance on the part of clinicians to randomise younger women to arm B, where they could have been randomised to valproate. 17 patients were excluded from all analyses, of whom three had no follow up data, and 14 had a subsequent diagnosis other than epilepsy. A further 16 declined further follow-up during the study and another two were lost to follow-up for other reasons, and data for these 18 patients were included in the analyses up to the date of their last follow-up. Follow-up was 93% complete with 2333 patient years of follow-up, compared to 2504 that could have been expected.
Figure 1.
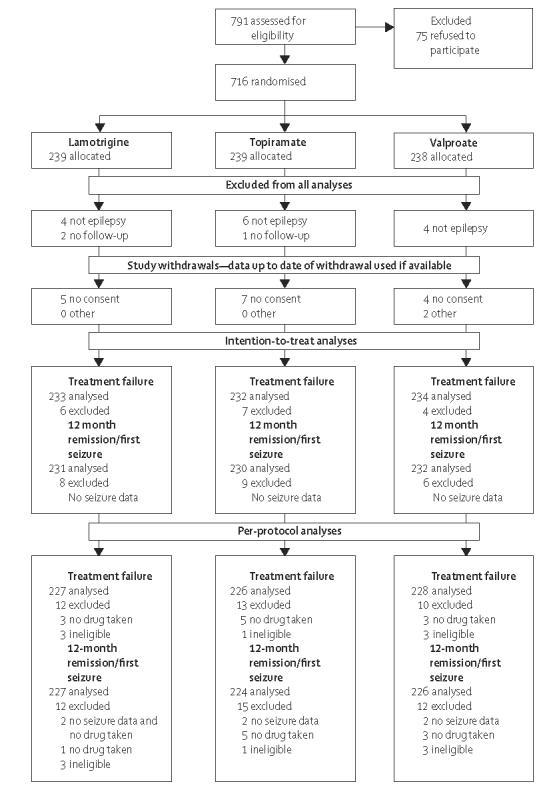
Trial profile
Table 1.
Baseline demographic and clinical characteristics for arm B
| Lamotrigine (n=239) |
Topiramate (n=239) |
Valproate (n=238) |
Total (n=716) |
|
|---|---|---|---|---|
| Sex, n (%) | ||||
| Men | 142 (59·4) | 142 (59·4) | 143 (60·1) | 427 (59·6) |
| Women | 97 (40·6) | 97 (40·6) | 95 (39·9) | 289 (40·4) |
| Treatment history, n (%) | ||||
| Untreated | 210 (87·9) | 209 (87·5) | 209 (87·8) | 628 (87·7) |
| Monotherapy (not optimum treatment) | 19 (8·0) | 20 (8·4) | 21 (8·8) | 60 (8·4) |
| Recent seizures after remission | 10 (4·2) | 10 (4·2) | 8 (3·4) | 28 (3·9) |
| History, n (%) | ||||
| Learning disability | 24 (10·0) | 26 (10·9) | 19 (8·0) | 69 (9·6) |
| Neurological deficit | 5 (2·1) | 3 (1·3) | 8 (3·4) | 16 (2·2) |
| Neurological disorder, n (%) | ||||
| Stroke/cerebrovascular | 0 (0) | 0 (0) | 1 (0·4) | 1 (0·1) |
| Intracranial surgery | 1 (0·4) | 0 (0) | 2 (0·8) | 3 (0·4) |
| Head injury | 3 (1·3) | 2 (0·8) | 6 (2·5) | 11 (1·5) |
| Meningitis/encephalitis | 6 (2·5) | 3 (1·3) | 1 (0·4) | 10 (1·4) |
| Other | 12 (5·0) | 9 (3·8) | 8 (3·4) | 29 (4·1) |
| History of seizures, n (%) | ||||
| Febrile convulsions | 16 (6·7) | 22 (9·2) | 21 (8·8) | 59 (8·2) |
| Any other acute symptomatic seizures | 9 (3·8) | 6 (2·5) | 6 (2·5) | 21 (2·9) |
| Epilepsy in first degree relatives | 53 (22·2) | 38 (15·9) | 38 (16·0) | 129 (18·0) |
| Epilepsy syndrome, n (%)* | ||||
| Idiopathic partial | 1 (0·4) | 2 (0·8) | 0 (0) | 3 (0·4) |
| Symptomatic or cryptogenic partial | 18 (7·5) | 11 (4·6) | 20 (8·4) | 49 (6·9) |
| Idiopathic generalised | 145 (60·7) | 151 (63·5) | 154 (64·7) | 450 (62·9) |
| Other syndrome | 9 (3·8) | 8 (3·4) | 5 (2·1) | 22 (3·1) |
| Unclassified | 66 (27·6) | 66 (27·7) | 59 (24·8) | 191 (26·7) |
| Median interval between first and most recent seizure (25th–75th centile), days | 492 (162–1510) | 401 (105–1702) | 384 (126–1402) | 414 (128–1561) |
| Median interval between most recent seizure and randomisation (25th–75th centile), days | 11 (1–49) | 13 (2–41) | 13 (1–42) | 13 (1·5–44) |
| Median number of seizures (25th–75th centile) | 10 (3–101) | 8 (3–100) | 8·5 (3–100) | 8 (3–100) |
| Mean age at first seizure (SD), years | 17·5 (12·1) | 17·6 (11·5) | 18·3 (13·7) | 17·8 (12·5) |
| Mean age (SD), years | 22·8 (14·3) | 22·3 (13·3) | 22·5 (14·5) | 22·5 (14·0) |
Missing data for epilepsy syndrome for one individual on topiramate.
Because of the pragmatic nature of the trial design and the absence of blinding, the doses of drugs used needed to be assessed and the degree to which the full dose ranges were explored before treatment failure events took place needed to be considered (table 2). There is satisfactory evidence that clinicians did explore a full dose range before accepting treatment failure due to inadequate seizure control. As would be expected, doses associated with treatment failure due to unacceptable adverse events were consistently lower than doses associated with treatment failure due to inadequate seizure control.
Table 2.
Dose taken by adults at withdrawal or last follow-up
| Reason for withdrawal | Lamotrigine | Topiramate | Valproate |
|---|---|---|---|
| Inadequate seizure control | n=24; 341 (169), 75–600 |
n=3; 367 (225), 150–600 |
n=9; 1600 (896), 500–3000 |
| Unacceptable adverse events | n=9; 119 (99), 25–300 |
n=23; 172 (110), 50–500 |
n=13; 838 (240), 500–1200 |
| Inadequate seizure control and unacceptable adverse events |
n=2; 200 (0), 200–200 |
n=11; 177 (109), 50–400 |
n=8; 1325 (568), 700–2000 |
| Other reason for withdrawal | n=10; 150 (47), 50–200 |
n=8; 169 (53), 100–250 |
n=12; 958 (462), 400–2000 |
| Remission of seizures | n=5; 120 (45), 100–200 |
n=5; 130 (27), 100–150 |
n=9; 944 (336), 200–1500 |
| Still on randomised drug | n=77; 203 (101), 50–500 |
n=63; 171 (86), 25–400 |
n=72; 1081 (463), 300–3000 |
Data are mean (SD), range.
The treatment failure events are summarised in webtable 3. Treatment failure for unacceptable adverse events is mostly limited to the early post randomisation period, whereas the timing of treatment failure for inadequate seizure control, with or without unacceptable adverse events takes place later. The median number of days to failure (25th–75th centile) for unacceptable adverse effects was 90 (28–245) and inadequate seizure control was 234 (136–481). Results are presented in figure 2, table 3, and webfigures 1 and 2.
Figure 2.
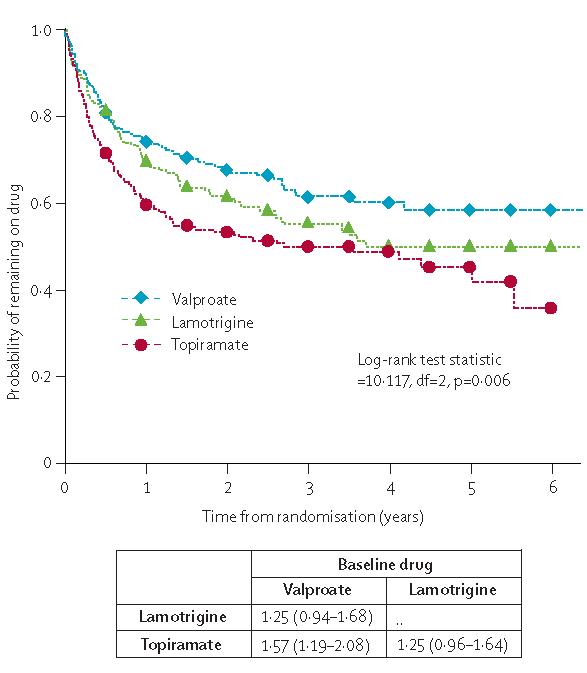
Time to treatment failure
Data are HR (95% CI). HR>1 indicates that failure occurs more rapidly on drug compared with baseline.
Table 3.
Treatment failure
| Year |
||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Any reason | ||||||
| Valproate | ||||||
| Number at risk | 165 | 114 | 61 | 40 | 14 | 4 |
| Percentage still on drug (95% CI) | 74 (68 to 79) | 67 (61 to 73) | 61 (54 to 68) | 60 (52 to 67) | 58 (50 to 66) | 58 (50 to 66) |
| Lamotrigine | ||||||
| Number at risk | 152 | 106 | 60 | 29 | 10 | 3 |
| Difference in percentage without failure compared with valproate (95% CI) | −5 (−13 to 3) | −6 (−15 to 3) | −6 (−16 to 4) | −10 (−21 to 1) | −8 (−20 to 3) | −8 (20 to 3) |
| Topiramate | ||||||
| Number at risk | 129 | 91 | 55 | 35 | 13 | 1 |
| Difference in percentage without failure compared with valproate (95% CI) | −14 (−23 to −6) | −14 (−23 to −5) | −11 (−21 to −2) | −11 (−21 to −1) | −13 (−24 to −2) | −16 (−29 to −4) |
| For adverse events | ||||||
| Valproate | ||||||
| Percentage without failure (95% CI) | 84 (79 to 89) | 82 (77 to 87) | 79 (73 to 85) | 79 (73 to 85) | 79 (73 to 85) | 79 (73 to 85) |
| Lamotrigine | ||||||
| Difference in percentage without failure compared with valproate (95% CI) | 3 (−3 to 10) | 5 (−2 to 11) | 7 (−1 to 14) | 7 (−1 to 14) | 7 (−1 to 14) | 7 (−1 to 14) |
| Topiramate | ||||||
| Difference in percentage without failure compared with valproate (95% CI) | −9 (−16 to −1) | −10 (−18 to −2) | −8 (−16 to 1) | −8 (−16 to 1) | −8 (−16 to 1) | −14 (−30 to 1) |
| For inadequate seizure control | ||||||
| Valproate | ||||||
| Percentage without failure (95% CI) | 90 (87 to 94) | 87 (82 to 91) | 84 (79 to 89) | 84 (79 to 89) | 84 (79 to 89) | 84 (79 to 89) |
| Lamotrigine | ||||||
| Difference in percentage without failure compared with valproate (95% CI) | −7 (−13 to −1) | −10 (−17 to −3) | −13 (−21 to −4) | −15 (−24 to −6) | −15 (−24 to −6) | −15 (−24 to −6) |
| Topiramate | ||||||
| Difference in percentage without failure compared with valproate (95% CI) | −6 (−12 to 0) | −5 (−12 to 2) | −5 (−12 to 3) | −5 (−12 to 3) | −5 (−12 to 3) | −9 (−19 to 2) |
For time to treatment failure for any reason, there were significant differences between drugs, and valproate was the best option. Pair-wise comparisons showed that valproate is statistically better than topiramate (topiramate:valproate 1·57 [1·19–2·08]), with lamotrigine intermediate (lamotrigine:valproate 1·25 [0·94, 1·68]). Cumulative incidence analysis of treatment failure for unacceptable adverse events (webfigure 1) indicates that lamotrigine is least likely to be associated with unacceptable adverse events and topiramate most likely, and that topiramate is significantly inferior to both valproate (topiramate:valproate 1·55 [1·07–2·26]) and lamotrigine (topiramate:lamotrigine 2·15 [1·41–3·30]). However, lamotrigine is significantly inferior to valproate for treatment failure due to inadequate seizure control (webfigure 2) with almost twice the failure rate of valproate (lamotrigine:valproate 1·95 [1·28–2·98]); whereas for topiramate the estimate suggests a higher failure rate than valproate, but the result is not significant (topiramate:valproate 1·45 [0·92–2·27]).
Notably, when the analyses were restricted to patients who at the time of randomisation were identified as having a generalised epilepsy syndrome, the efficacy of valproate for time to treatment failure for any reason was more marked and was significantly better than both topiramate (valproate:topiramate 0·53 [0·37–0·76]) and lamotrigine (valproate:lamotrigine 0·65 [0·45–0·93]) for this outcome.
Results for time to 12-month remission are shown in table 4, and figure 3, and webfigure 3.
Table 4.
Seizure outcomes by drug
| Events/total | Year 1 | Year 2 | Year 3 | Year 4 | Year 5 | |
|---|---|---|---|---|---|---|
| Time to 12-month remission—intention to treat | ||||||
| Number at risk | 180/232 | |||||
| Valproate | 221 | 54 | 28 | 15 | 4 | |
| Lamotrigine | 168/231 | 222 | 74 | 43 | 25 | 8 |
| Topiramate | 178/230 | 219 | 59 | 32 | 14 | 2 |
| Percentage 12-month remission (95% CI) | ||||||
| Valproate | 43 (37 to 50) | 69 (63 to 76) | 81 (75 to 87) | 87 (81 to 92) | 92 (87 to 98) | |
| Difference in percentage of 12-month remission compared with valproate (95% CI) | ||||||
| Lamotrigine | −11 (−20 to −2) | −7 (−16 to 2) | −7 (−15 to 1) | −8 (−16 to 0) | −9 (−17 to 0) | |
| Topiramate | −4 (−13 to 5) | 0 (−9 to 9) | −1 (−9 to 7) | 0 (−8 to 7) | 0 (−8 to 9) | |
| Time to 12-month remission—per protocol | ||||||
| Number at risk | ||||||
| Valproate | 129/226 | 161 | 25 | 6 | 4 | 1 |
| Lamotrigine | 105/227 | 151 | 34 | 12 | 4 | 1 |
| Topiramate | 104/224 | 127 | 16 | 8 | 3 | 1 |
| Percentage 12-month remission (95% CI) | ||||||
| Valproate | 36 (29 to 42) | 55 (48 to 62) | 63 (57 to 70) | 64 (57 to 71) | 66 (59 to 74) | |
| Difference in percentage of 12-month remission compared with valproate (95% CI) | ||||||
| Lamotrigine | −10 (−19 to −1) | −9 (−19 to 0) | −12 (−22 to −2) | −11 (−21 to −1) | −13 (−24 to −3) | |
| Topiramate | −4 (−13 to 5) | −7 (−17 to 2) | −14 (−23 to −4) | −13 (−23 to −3) | −15 (−25 to −5) | |
| Time to 24 month-remission—intention to treat | ||||||
| Number at risk | ||||||
| Valproate | 124/232 | 187 | 61 | 31 | 11 | 1 |
| Lamotrigine | 102/231 | 185 | 80 | 42 | 16 | 1 |
| Topiramate | 108/230 | 183 | 71 | 34 | 10 | 3 |
| Percentage of 24-month remission (95% CI) | ||||||
| Valproate | 39 (32 to 46) | 60 (53 to 67) | 69 (62 to 76) | 71 (63 to 79) | 86 (65 to 106) | |
| Difference in percentage of 24-month remission compared with valproate (95% CI) | ||||||
| Lamotrigine | −16 (−25 to −6) | −13 (−23 to −2) | −13 (−24 to −3) | −11 (−22 to 1) | 3 (−24 to 30) | |
| Topiramate | −8 (−18 to 1) | −8 (−19 to 2) | −8 (−18 to 3) | −1 (−13 to 11) | −15 (−37 to 7) | |
| Time to first seizure—intention to treat | ||||||
| Number at risk | ||||||
| Valproate | 152/232 | 96 | 71 | 43 | 23 | 9 |
| Lamotrigine | 181/231 | 72 | 43 | 23 | 13 | 5 |
| Topiramate | 163/230 | 86 | 55 | 35 | 22 | 14 |
| Percentage still on drug (95% CI) | ||||||
| Valproate | 57 (50 to 63) | 62 (56 to 69) | 66 (59 to 72) | 70 (63 to 77) | 70 (63 to 77) | |
| Difference in percentage still on drug compared with valproate (95% CI) | ||||||
| Lamotrigine | 11 (3 to 20) | 13 (5 to 22) | 14 (6 to 23) | 12 (3 to 21) | 12 (3 to 21) | |
| Topiramate | 4 (−5 to 13) | 7 (−2 to 16) | 5 (−4 to 14) | 4 (−5 to 13) | 4 (−5 to 13) | |
Figure 3.
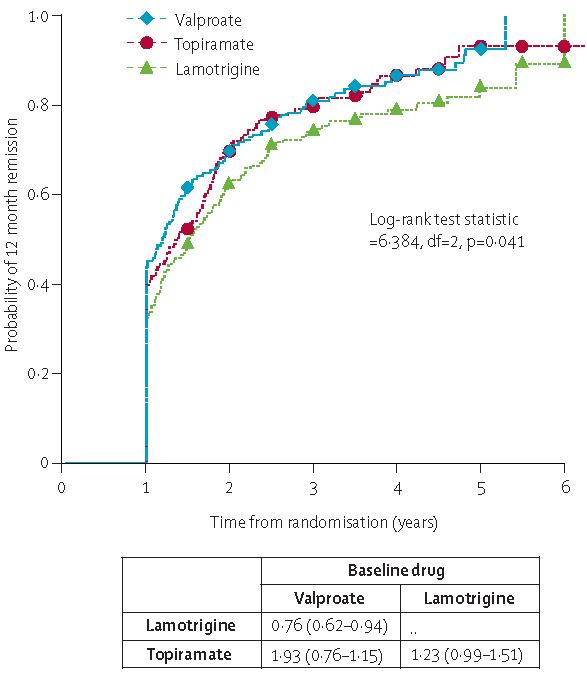
Time to 12-month remission
Data are HR (95% CI). HR>1 indicates that 12-month remission occurs more rapidly on drug compared to baseline.
A high proportion (more than 80% by 4 years) of patients achieved a 1-year remission. Pair-wise comparisons for the intention-to-treat analysis22 indicate that valproate is the preferred option and is statistically better than lamotrigine (lamotrigine:valproate 0·76 [0·62–0·94]). Topiramate seems intermediate between the two (topiramate:valproate 0·93 [0·76–1·15]). However, the survival curves for topiramate and valproate overlap notably from a point about 700 days after randomisation. Once again the difference between valproate and comparator drugs is larger when the analysis is restricted to patients with idiopathic generalised epilepsy (lamotrigine:valproate 0·68 [0·53–0·89], topiramate:valproate 0·82 [0·64–1·06]).
Because intention-to-treat analysis includes seizure data after treatment failure events, a per protocol analysis has been undertaken (table 4). This analysis confirms that valproate is more effective than lamotrigine (lamotrigine:valproate 0·76 [0·60–0·95]) and topiramate (topiramate:valproate 0·77 [0·61–0·97]). The comparisons between the intention-to-treat and per-protocol analyses indicate that the similarity for the outcome between valproate and topiramate for the intention-to-treat analysis is probably because patients who had treatment failure on topiramate were switched to valproate (webtable 4).
Data for the clinically important 24-month outcome are consistent with those for the 12-month remission outcome (table 4). For time to first seizure, valproate was the most effective, lamotrigine the least effective, and topiramate intermediate between the two but nevertheless significantly better than lamotrigine.
As noted for all analyses, valproate was more effective than lamotrigine and topiramate, an effect that seemed greater when analysis was restricted to patients classified as having idiopathic generalised epilepsy compared with the overall analysis. This finding was further explored by testing for an interaction between treatment and epilepsy syndrome in a Cox regression model. Comparisons of outcomes were made between the 441 patients with idiopathic generalised epilepsy, 186 unclassified patients, and 52 classified as partial or other syndromes (numbers included in analyses might deviate from these if outcome data are not available).
Tests for an interaction were done to assess any differences in treatment effects in the subgroup with an idiopathic generalised epilepsy compared with the subgroup with difficult to classify seizures. There was no evidence of an interaction (4 df, p=0·12) for time to treatment failure, which suggested that treatment effects were similar in these subgroups for this outcome. There was some evidence of an interaction for 12 month remission (4 df, p=0·04), 24 month remission (df=4, p=0·007), and first seizure (4 df, p=0·001). For these seizure outcomes, the overall analysis suggested that valproate was the better treatment, but the advantage of valproate was more extreme in the subgroup with an idiopathic generalised epilepsy than in the subgroup with difficult to classify seizure.
Table 5 summarises adverse events deemed clinically important by the reporting clinician. An intention-to-treat approach summarises adverse events associated with the randomised policy, but as patients could have had their treatment changed during follow up, this approach does not clearly present adverse events attributable to specific drugs. In table 5 therefore we present adverse event rates for both intention to treat and per protocol.
Table 5.
Frequency of clinically important adverse events
| Lamotrigine | Topiramate | Valproate | Total | |
|---|---|---|---|---|
| Number of patients randomised | 239 | 239 | 238 | 716 |
| Total number (%) of patients with at least one adverse event | 88 (37%) | 107 (45%) | 85 (36%) | 280 (39%) |
| Tiredness/drowsiness/fatigue/lethargy | 15 [9] | 25 [20] | 18 [12] | 58 [41] |
| Other psychiatric | 7 [4] | 19 [15] | 8 [7] | 34 [26] |
| Weight gain | 8 [5] | 7 [2] | 17 [16] | 32 [23] |
| Behaviour/personality change/aggression | 6 [4] | 20 [18] | 4 [4] | 30 [26] |
| Worsening of seizures | 10 [6] | 13 [9] | 7 [3] | 30 [18] |
| Accidental injury | 11 [7] | 5 [3] | 4 [2] | 20 [12] |
| Other neurological | 4 [3] | 7 [4] | 10 [5] | 21 [12] |
| Headache | 6 [4] | 7 [4] | 5 [4] | 18 [12] |
| Memory problems | 2 [2] | 12 [10] | 3 [0] | 17 [12] |
| Weight loss | 3 [0] | 14 [12] | 0 [0] | 17 [12] |
| Allergic rash | 13 [12] | 1 [1] | 2 [0] | 16 [13] |
| Tremor | 4 [2] | 1 [0] | 8 [6] | 13 [8] |
| Depression | 1 [1] | 9 [6] | 3 [3] | 13 [10] |
| Confusion/difficulty thinking/disoriented | 3 [2] | 7 [7] | 3 [2] | 13 [11] |
| Dizziness/vertigo | 3 [2] | 6 [3] | 1 [1] | 10 [6] |
| Anxiety/agitation/nervousness | 7 [6] | 2 [2] | 1 [1] | 10 [9] |
| Nausea | 4 [4] | 2 [1] | 4 [3] | 10 [8] |
| Other renal tract/genital | 4 [3] | 4 [2] | 3 [2] | 11 [7] |
| Pins and needles/dysaesthesia | 0 [0] | 8 [6] | 2 [0] | 10 [6] |
| Ataxia | 4 [3] | 3 [2] | 2 [2] | 9 [7] |
| Other skin and appendages | 1 [1] | 5 [4] | 5 [3] | 11 [8] |
| Mouth/gum problem | 1 [1] | 2 [1] | 3 [3] | 6 [5] |
| Sleep disturbance | 3 [3] | 4 [3] | 1 [1] | 8 [7] |
| Other* | 30 [21] | 40 [25] | 36 [25] | 106 [71] |
For adverse effects, intention-to-treat analysis outside brackets, per-protocol analysis inside brackets.
Sorted by descending total frequency: abdominal pain, dyspepsia; alopecia; other general; other visual disturbance; word finding difficulty; vomiting; aches and pains; other gastrointestinal; other musculoskeletal; other respiratory or pulmonary; diarrhoea; psychosis; anorexia; bruising; constipation; diplopia; renal or bladder stones; influenza-like symptoms; hallucinations; infection; vaginal bleeding; arthritis; asthma; chest infection; child birth; faints; hypertension; ischaemic heart disease or myocardial infarct; other cardiac or vascular; other haematological; psoriasis; short of breath; status epilepticus; urinary tract infection; urinary retention.
Between 36% (valproate) and 45% (topiramate) of patients reported adverse events at some point in the intention-to-treat study. Estimates for the per-protocol population were between 30% for valproate and 41% for topiramate.
For the individual symptoms reported, tiredness and fatigue, psychiatric symptoms (most frequently for topiramate), and weight gain (most frequently associated with valproate) were the most common. Rash was a prominent non-CNS symptom, especially with lamotrigine. These adverse event profiles were consistent across intention-to-treat and per-protocol summaries. The adverse events associated with treatment failure were most commonly psychiatric and cognitive symptoms and tiredness and fatigue, all of which were more common with topiramate. For lamotrigine, rash was the most common symptom associated with treatment failure (4% of patients randomised), whereas for valproate weight-gain was the most common symptom (4% of patients randomised). We should note that in the study neither patients nor clinicians were masked to drug treatment, which might have affected the symptoms reported to the clinicians and their assessment of the clinical importance.
Response rates for quality of life outcomes in arm B were 80% at baseline and 67% at 2-year follow-up. There were no significant differences in response rates between treatment groups (webtable 5), although, like in arm A,22 there was evidence of response bias, with patients with a poorer quality of life at baseline less likely to return quality of life questionnaires at 2 years.
There were no significant differences for the outcomes assessed (webtable 6). However, data from questionnaires completed by patients do not indicate an increase in anxiety or depression associated with topiramate, compared with adverse event data recorded by clinicians.
There were differences for quality of life between patients who had a positive (ie, remission of seizures) clinical outcome and those who did not; and between patients who had a negative (ie, treatment failure) clinical outcome and those who did not (webtable 7); though for some comparisons, the differences did not reach significance and the CIs were fairly wide. Nonetheless, the direction of effects indicates better quality of life for those who achieved remission or had not been withdrawn from the randomised drug.
The cost per QALY analysis is based on the 165 adult patients who provided complete EQ-5D responses at 2 years. Since the estimate of quality adjusted life years (QALYs) and resource use were dependent on patients returning completed quality of life questionnaires, results might have a response bias as outlined above. Tables 6 and 7 show the point estimates of the incremental cost effectiveness ratios for lamotrigine and topiramate, which were estimated using the lowest costs for valproate and lamotrigine. Disaggregated costs are presented in webtable 8.
Table 6.
Incremental cost-effectiveness ratios—cost per QALY
| Cost (£) | QALYs | Incremental cost (£) |
Incremental QALYs |
Incremental cost-effectiveness ratio (£ per QALY) |
|
|---|---|---|---|---|---|
| Valproate | 1390 | 1·648 | .. | .. | .. |
| Topiramate | 1568 | 1·809 | 178 | 0·161 | 1106 |
| Lamotrigine | 1906 | 1·701 | 338 | −0·108 | Dominated |
Table 7.
Incremental cost-effectiveness ratios—cost per seizure avoided
| Cost (£) | Seizures | Incremental cost (£) | Incremental seizures avoided |
Incremental cost-effectiveness ratio (£ per seizure avoided) |
|
|---|---|---|---|---|---|
| Valproate | 1136 | 44·1 | .. | .. | .. |
| Topiramate | 1568 | 75·1 | 432 | −31·0 | Dominated |
| Lamotrigine | 1761 | 120·9 | 193 | −45·8 | Dominated |
Lamotrigine has a positive incremental cost and a negative incremental QALY gain and is therefore dominated by topiramate—ie, it is more expensive and less effective than topiramate. The same pattern of results is seen when using different combinations of high and low costs for valproate and lamotrigine. The lowest value of the incremental cost-effectiveness ratios for topiramate is when high costs are used for valproate and lamotrigine and is equal to £692. The highest value is £1106 when low costs for valproate and lamotrigine are used. Bootstrapping methods22 were used to generate cost-effectiveness acceptability curves, and table 8 summarises the probabilities that lamotrigine and topiramate are cost effective at ceiling ratios of £10 000, £30 000, and £50 000 per QALY.
Table 8.
Probabilities that the new antiepileptic drugs are cost-effective relative to valproate across a range of ceiling ratios (λ)
| Lamotrigine | Topiramate | |
|---|---|---|
| Cost per QALY | ||
| £10 000 | 0·53 | 0·91 |
| £30 000 | 0·68 | 0·97 |
| £50 000 | 0·70 | 0·98 |
| Cost per seizure avoided | ||
| £160 | 0·01 | 0·14 |
| £400 | 0·01 | 0·15 |
| £800 | 0·01 | 0·16 |
| £1600 | 0·01 | 0·16 |
The cost per seizure avoided analysis is based on 299 adults and children for whom we have data on seizures and resource use. Tables 6 and 7 show the point estimates of the incremental cost effectiveness ratios for lamotrigine and topiramate, which have been estimated using low costs for valproate and lamotrigine. Topiramate and lamotrigine have positive incremental costs and negative incremental seizures avoided and are therefore both dominated by valproate. The same pattern of results is noted when using different combinations of high and low costs for valproate and lamotrigine. Bootstrapping methods were used to generate cost-effectiveness acceptability curves, and table 8 summarises the prob abilities that topiramate and lamotrigine are cost effective at ceiling ratios of £160, £400, £800, and £1600 per seizure avoided.
Discussion
For patients with generalised onset seizures or seizures that are difficult to classify, valproate is significantly more effective than topiramate for treatment failure and significantly more effective than lamotrigine for 12-month remission. Thus valproate should remain a first line treatment for such patients.
SANAD was designed as a pragmatic trial to assess whether any of the newly licensed antiepileptic drugs should become first-line treatment and thereby replace the existing first-line agents, carbamazepine and valproate. Here we have reported results for arm B, which compared valproate, lamotrigine, and topiramate. Although arm B failed to achieve the desired recruitment, we were fortunate in that differences between drugs were larger than expected and there were sufficient events during protracted follow-up to allow robust conclusions. One factor that could have reduced recruitment was a reluctance by clinicians to randomise women of child-bearing age into a study in which they could be allocated to treatment with valproate, a drug that is associated with a relative high fetal malformation rate13 and a risk of neurodevelopmental delay.12 Therefore, 60% of patients randomised to this arm were men, although we believe that these results are still applicable to women.
Because SANAD was a pragmatic trial, patients starting on one drug might switch to another. However, in the intention-to-treat analyses of clinical, quality of life, and health economic outcomes, patients were analysed in the treatment groups to which they had been allocated, and were followed up, even if the allocated treatment had been withdrawn and switched to another. Thus, our analyses take into account the clinical and cost-effectiveness of the differing policies and associated treatment switches.
The clinical results identify valproate as first choice treatment. 63% of patients in arm B of the study were identified at randomisation as having an idiopathic generalised epilepsy, thereby providing the only known randomised trial data for treatment in these syndromes. 27% of patients were unclassified at randomisation and could therefore have been patients with either partial or generalised onset seizures. For time to treatment failure, valproate was the most effective drug and topiramate was least effective. The factors affecting this outcome were the better tolerability of lamotrigine compared with valproate (intermediate for failure for unacceptable adverse events) and topiramate (worst). By contrast, valproate was least likely to be associated with treatment failure for inadequate seizure control, followed by topiramate, with lamotrigine being most likely. There was a similar ordering of drugs when analysis was restricted to patients with idiopathic generalised epilepsy syndromes, but valproate was significantly better than both comparator drugs.
Valproate was therefore the preferred drug for time to 12-month and 24-month remission, being significantly better than lamotrigine for this outcome, with topiramate intermediate. Although the differences were small in the intention-to-treat analysis, the efficacy of valproate was enhanced in the per-protocol analysis, indicating that the switching from lamotrigine for inadequate seizure control, and from topiramate for unacceptable adverse events, to valproate was largely responsible for obscuring the superiority of valproate for this outcome in intention-to-treat analyses. A similar ordering of drugs for time to first seizure was evident, with both valproate and topiramate significantly better than lamotrigine.
Although lamotrigine was the poorest option for seizure control in arm B, it was the overall preferred option in arm A.22 Arm B was designed as a trial of broad-spectrum antiepileptic drugs so as to encourage the randomisation of patients with generalised and unclassified epilepsy. The claims for lamotrigine to be regarded as a broad-spectrum antiepileptic drug are based on limited randomised study data in patients with generalised seizures.8,14,16,17 However, the best identified mechanism of its anti-seizure effect is that of an inhibitor of voltage-sensitive Na+ channels, a mechanism that it shares with drugs with restricted spectrums of efficacy, such as carbamazepine and phenytoin. Results from SANAD could be interpreted as indicating that lamotrigine should not be regarded as a broad spectrum antiepileptic drug, but as a first line treatment that should be reserved for treatment of partial onset seizures and localisation-related epilepsy syndromes.
The differences between drugs were greater in the subgroup of patients with idiopathic generalised epilepsy than in the entire group of patients randomised to this arm, and interaction testing indicates that valproate might be the least effective drug for patients with partial and other epilepsy syndromes. This interpretation has implications for industry-sponsored comparative monotherapy studies of new antiepileptic drugs, which have been used to show non-inferiority of a new drug compared to a standard drug to support a licensing application for monotherapy indications in Europe. Such studies have tended to compare a new antiepileptic drug with carbamazepine, and have recruited a heterogeneous population (typically both patients with partial onset seizures and patients with generalised onset tonic-clonic seizures).24 Accurate identification of patients with generalised onset tonic clonic seizures has been difficult, although most patients were probably more likely to have had an idiopathic generalised epilepsy. SANAD shows that valproate has the greatest efficacy for patients with idiopathic generalised epilepsy. Thus, a study comparing a new antiepileptic drug with a standard such as carbamazepine (or perhaps lamotrigine in the future) that recruits both patients with partial onset seizure and generalised onset seizures will not be exposing those with generalised onset seizures to the optimum treatment. An overall analysis, ignoring epilepsy type, might lead to an erroneous conclusion that a new drug is not inferior to a standard. Therefore, in future monotherapy studies patients should be classified by epilepsy syndrome (and where this is impossible, as unclassified), testing for interactions between epilepsy classification and treatment are undertaken, and that studies are adequately powered to do so.
There were no differences between treatment groups in quality of life outcomes that would detract from the conclusions drawn from clinical outcomes. Possible reasons for this have been discussed elsewhere.22 The health economics analysis based on cost per seizure avoided supports the recommendation of the clinical results that valproate should remain the first choice drug for idiopathic generalised or unclassified epilepsy. However, the cost per QALY analysis suggests that there is a high probability that topiramate is a cost-effective alternative to valproate throughout the full range of values of the ceiling ratio (λ). This apparently conflicting result might be due to the QALY picking up effects on health-related quality of life besides those attributable to seizures alone, or could be due to some other event such as the unrepresentative patient sample on which the cost per QALY analysis is based.
In conclusion, results of SANAD show that valproate should remain the first line treatment for most patients with an idiopathic generalised epilepsy or seizures that are difficult to classify, whereas lamotrigine should be generally avoided because of its inferior efficacy, and topiramate because of inferior tolerability. However, there will always be some individual circumstances that would favour the choice of an alternative drug (drug interactions, family planning). There is insufficient power for us to make definite statements about the relative efficacy and effectiveness of the drugs for individual seizure types and sub-syndromes within the idiopathic generalised epilepsies. For women of child-bearing age SANAD does provide estimates of the relative efficacy and tolerability of valproate, lamotrigine and topiramate that can be used whilst counselling women. The study was not designed or powered to examine pregnancy outcomes, something of concern, when valproate is used in women of child-bearing potential.25 Unfortunately, evidence for safety of topiramate during pregnancy remains sparse, so that there will be persisting difficulty in optimising treatment for women with idiopathic generalised epilepsy during their child-bearing years. Improvements here will await further obser vational data on pregnancy outcomes from registries.13
Two further antiepileptic drugs have been licensed in the UK since this study was designed (levetiracetam and zonisamide), both of which are said to be effective in generalised epilepsies. The same questions that applied to lamotrigine and topiramate now apply to these drugs, for which we need similarly robust comparative trials against valproate in similar populations of patients.
Acknowledgments
The study was supported by a grant from the Health Technology Assessment Programme. There were further contributions from GlaxoSmithKline, Janssen-Cilag, Novartis Pfizer, Sanofi-Synthelabo, and the Wellcome Trust that supported related studies.
Footnotes
Conflict of interest statement
AGM has received fees and reimbursement for attending conferences from Jannsen Cilag, GlaxoSmithKline, Novartis, Pfizer, and Sanofi Synthelabo, and research funding from Pfizer; AMA-K has received speaker fees and reimbursement for attending conferences from Jannsen Cilag, GlaxoSmithKline, Novartis, Pfizer, and Sanofi Synthelabo; RA has received consultancy fees, speaker fees, and reimbursement for attending conferences from Jannsen Cilag, GlaxoSmithKline, and Sanofi Synthelabo; GAB has received research funding, speaker fees, and reimbursement for attending conferences from Jannsen Cilag, Glaxo SmithKline, Novartis, Pfizer, and Sanofi Synthelabo; DWC has received consultancy fees, speaker fees, and reimbursement for attending conferences from Jannsen Cilag, GlaxoSmithKline, and Sanofi Synthelabo; PNC has received speaker fees from Jannsen Cilag, reimbursement for attending conferences from Jannsen Cilag, GlaxoSmithKline, Novartis, Pfizer, and Sanofi Synthelabo, and research funding from Sanofi Synthelabo; PJG has received speaker fees and reimbursement for attending conferences from Eisai, GlaxoSmithKline, Jannsen Cilag, Pfizer, Cyberonics, and UCB; SJLH has received reimbursement for attending conferences from Jannsen Cilag, GlaxoSmithKline, Novartis, Pfizer, and Sanofi Synthelabo, and payment for research from Jannsen Cilag and GlaxoSmithKline; AH has received reimbursement for attending conferences from Jannsen Cilag and GlaxoSmithKline; MJ has received speaker fees and reimbursement for attending conferences from GlaxoSmithKline, Pfizer, and Jannsen Cilag; AJ has received funding from Sanofi Synthelabo, GlaxoSmithKline, and Jannsen Cilag, and has acted as a research consultant to Johnson and Johnson Pharmaceuticals; MK has received speaker fees and reimbursement for attending conferences from Jannsen Cilag, GlaxoSmithKline, and Pfizer; GRL has received speaker fees and reimbursement for attending conferences from UCB and Jannsen Cilag; JPL has received speaker fees and reimbursement for attending conferences from Jannsen Cilag, GlaxoSmithKline, Pfizer, Eisai, UCB, and Sanofi Synthelabo, and research funding from GlaxoSmithKline; PN has received speaker fees and reimbursement for attending conferences from Jannsen Cilag, GlaxoSmithKline, Pfizer, and Sanofi Synthelabo; RR has received consultancy fees, speaker fees, and reimbursement for attending conferences from GlaxoSmithKline, Jannsen Cilag, Novartis, and Pfizer; DFS has received speaker fees and reimbursement for attending conferences from Jannsen Cilag, GlaxoSmithKline, Novartis, Pfizer, and Sanofi Synthelabo, and research funding from GlaxoSmithKline; PEMS has received speaker fees, consultancy fees, and reimbursement for attending conferences from UCB Pharma, Pfizer, Eisai, Novartis, and GlaxoSmithKline, and research funding from UCB Pharma and GlaxoSmithKline; the other authors declare no conflict of interest.
Contributor Information
Anthony G Marson, Division of Neurological Science, University of Liverpool, UK.
Asya M Al-Kharusi, Southport and Formby District General Hospital, Merseyside, UK.
Muna Alwaidh, St Helens and Knowsley Hospital NHS Trust, Whiston Hospital, Liverpool, UK.
Richard Appleton, Roald Dahl EEG Unit, Department of Neurology, Royal Liverpool Children's NHS Trust (Alder Hey), Liverpool, UK.
Gus A Baker, Division of Neurological Science, University of Liverpool, UK.
David W Chadwick, Division of Neurological Science, University of Liverpool, UK.
Celia Cramp, Royal Shrewsbury Hospital, Shrewsbury, UK.
Oliver C Cockerell, Royal London Hospital, London, UK.
Paul N Cooper, Royal Bolton Hospital, Bolton, UK.
Julie Doughty, School of Population and Health Sciences, University of Newcastle, UK.
Barbara Eaton, Division of Neurological Science, University of Liverpool, UK.
Carrol Gamble, Centre for Medical Statistics and Health Evaluation, University of Liverpool, UK.
Peter J Goulding, Leeds General Infirmary, Leeds, UK.
Stephen J L Howell, Royal Hallamshire Hospital, Sheffield, UK.
Adrian Hughes, Arrowe Park Hospital, Wirral, UK.
Margaret Jackson, Royal Victoria Infirmary, Newcastle-Upon-Tyne, UK.
Ann Jacoby, Division of Public Health, University of Liverpool, UK.
Mark Kellett, Royal Bolton Hospital, Bolton, UK.
Geoffrey R Lawson, Sunderland Royal Hospital, Sunderland, UK.
John Paul Leach, Southern General Hospital, Glasgow, UK.
Paola Nicolaides, Great Ormond Street Hospital, London, UK.
Richard Roberts, Ninewells Hospital, Dundee, UK.
Phil Shackley, School of Population and Health Sciences, University of Newcastle, UK.
Jing Shen, School of Population and Health Sciences, University of Newcastle, UK.
David F Smith, The Walton Centre for Neurology and Neurosurgery NHS Trust, Liverpool, UK.
Philip E M Smith, University Hospital of Wales, Cardiff , UK.
Catrin Tudur Smith, Centre for Medical Statistics and Health Evaluation, University of Liverpool, UK.
Alessandr a Vanoli, School of Population and Health Sciences, University of Newcastle, UK.
Paula R Williamson, Centre for Medical Statistics and Health Evaluation, University of Liverpool, UK.
References
- 1.Hauser WA, Hesdorffer DC. Epilepsy: frequency, causes and consequences. New York: Demos Publications; 1990. [Google Scholar]
- 2.Anon Proposal for revised classification of epilepsies and epileptic syndromes. Commission on Classification and Terminology of the International League Against Epilepsy. Epilepsia. 1989;30:389–99. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 3.Commission on Classification and Terminology of the International League Against Epilepsy Proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia. 1981;22:489–501. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- 4.The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care. London: National Institute for Clinical Excellence; 2004. (Clinical Guideline 20). [Google Scholar]
- 5.French JA, Kanner AM, Bautista J, et al. Efficacy and tolerability of the new antiepileptic drugs i: treatment of new onset epilepsy: report of the therapeutics and technology assessment subcommittee and quality standards subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2004;62:1252–60. doi: 10.1212/01.wnl.0000123693.82339.fc. [DOI] [PubMed] [Google Scholar]
- 6.Marson AG, Williamson PR, Clough H, Hutton JL, Chadwick DW. Carbamazepine versus valproate monotherapy for epilepsy: a meta-analysis. Epilepsia. 2002;43:505–13. doi: 10.1046/j.1528-1157.2002.20801.x. [DOI] [PubMed] [Google Scholar]
- 7.Tudur Smith C, Marson AG, Williamson PR. Phenytoin versus valproate monotherapy for partial onset seizures and generalized onset tonic-clonic seizures. Cochrane Database Syst Rev. 2001;4:CD001769. doi: 10.1002/14651858.CD001769. [DOI] [PubMed] [Google Scholar]
- 8.Posner E, Mohamed K, Marson AG. Ethosuximide, sodium valproate or lamotrigine for absence seizures in children and adolescents. Cochrane Database Syst Rev. 2005;4:CD003032. doi: 10.1002/14651858.CD003032.pub2. [DOI] [PubMed] [Google Scholar]
- 9.Nicolson A, Appleton RE, Chadwick DW, Smith DF. The relationship between treatment with valproate, lamotrigine, and topiramate and the prognosis of the idiopathic generalised epilepsies. J Neurol Neurosurg Psychiatry. 2004;75:75–79. [PMC free article] [PubMed] [Google Scholar]
- 10.Shields W, Saslow E. Myoclonic, atonic, and absence seizures following institution of carbamazepine therapy in children. Neurology. 1983;33:1487–89. doi: 10.1212/wnl.33.11.1487. [DOI] [PubMed] [Google Scholar]
- 11.Snead O, Hosey L. Exacerbation of seizures in children by carbamazepine. N Engl J Med. 1985;313:916–21. doi: 10.1056/NEJM198510103131503. [DOI] [PubMed] [Google Scholar]
- 12.Adab N, Kini U, Vinten J, et al. The longer term outcome of children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry. 2004;75:1575–83. doi: 10.1136/jnnp.2003.029132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrow J, Russell A, Guthrie E, et al. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry. 2006;77:193–98. doi: 10.1136/jnnp.2005.074203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biton V, Sackellares JC, Vuong A, Hammer AE, Barrett PS, Messenheimer JA. Double-blind, placebo-controlled study of lamotrigine in primary generalized tonic-clonic seizures. Neurology. 2005;65:1737–43. doi: 10.1212/01.wnl.0000187118.19221.e4. [DOI] [PubMed] [Google Scholar]
- 15.Beran RG, Berkovic SF, Dunagan FM, et al. Double-blind, placebo-controlled, crossover study of lamotrigine in treatment-resistant generalised epilepsy. Epilepsia. 1998;39:1329–33. doi: 10.1111/j.1528-1157.1998.tb01332.x. [DOI] [PubMed] [Google Scholar]
- 16.Eriksson AS, Nergardh A, Hoppu K. The efficacy of lamotrigine in children and adolescents with refractory generalized epilepsy: a randomized, double-blind, crossover study. Epilepsia. 1998;39:495–501. doi: 10.1111/j.1528-1157.1998.tb01411.x. [DOI] [PubMed] [Google Scholar]
- 17.Motte J, Trevathan E, Arvidsson JF, Barrera MN, Mullens EL, Manasco P. Lamotrigine for generalized seizures associated with the Lennox-Gastaut syndrome. Lamictal Lennox-Gastaut Study Group. N Engl J Med. 1997;337:1807–12. doi: 10.1056/NEJM199712183372504. [DOI] [PubMed] [Google Scholar]
- 18.Privitera MD, Brodie MJ, Mattson RH, et al. Topiramate, carbamazepine and valproate monotherapy: double-blind comparison in newly diagnosed epilepsy. Acta Neurol Scand. 2003;107:165–75. doi: 10.1034/j.1600-0404.2003.00093.x. [DOI] [PubMed] [Google Scholar]
- 19.Newer drugs for epilepsy in adults. London: National Institute for Clinical Excellence; 2004. (Technology Appraisal Guidance 76). [Google Scholar]
- 20.Commission on Classification and Terminology of the International League Against Epilepsy Proposal for revised classification of epilepsies and epileptic syndromes. Epilepsia. 1989;30:389–99. doi: 10.1111/j.1528-1157.1989.tb05316.x. [DOI] [PubMed] [Google Scholar]
- 21.Commission on Classification and Terminology of the International League Against Epilepsy Proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia. 1981;22:489–501. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- 22.Marson AG, Al-Kharusi AM, Alwaidh M, et al. The SANAD study of effectiveness of carbamazepine, gabapentin, lamotrigine, oxcarbazepine, or topiramate for treatment of partial epilepsy: an unblinded randomised controlled trial. Lancet. 2007;369:1000–15. doi: 10.1016/S0140-6736(07)60460-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Medical Research Council . Guidelines for good clinical practice in clinical trials. London: MRC; 1998. [Google Scholar]
- 24.Brodie MJ, Richens A, Yuen AWC. Double-blind comparison of lamotrigine and carbamazepine in newly diagnosed epilepsy. UK Lamotrigine/Carbamazepine Monotherapy Trial Group. Lancet. 1995;345:476–79. doi: 10.1016/s0140-6736(95)90581-2. [DOI] [PubMed] [Google Scholar]
- 25.Adab N, Kini U, Vinten J, et al. The longer term outcome of children born to mothers with epilepsy. J Neurol Neurosurg Psychiatry. 2004;75:1575–83. doi: 10.1136/jnnp.2003.029132. [DOI] [PMC free article] [PubMed] [Google Scholar]


