Abstract
Compulsive drug abuse has been conceptualized as a behavioral state where behavioral stimuli override normal decision making. Clinical studies of methamphetamine users have detailed decision making changes and imaging studies have found altered metabolism and activation in the parietal cortex. To examine the molecular effects of amphetamine on the parietal cortex, gene expression responses to amphetamine challenge (7.5mg/kg) were examined in the parietal cortex of rats pretreated for nine days with either saline, non-neurotoxic AMPH, or neurotoxic AMPH dosing regimens. The neurotoxic AMPH exposure [3 doses of 7.5 mg/kg/day AMPH (6 hr between doses), for nine days] produced histological signs of neurotoxicity in the parietal cortex while a non-neurotoxic dosing regimen (2.0 mg/kg/day × 3) did not. Neurotoxic AMPH pretreatment resulted in significantly diminished AMPH challenge-induced mRNA increases of activity-regulated cytoskeletal protein (ARC), nerve growth-factor inducible protein A (NGFI-A), and nerve growth-factor inducible protein B (NGFI-B) in the parietal cortex while neither saline pretreatment nor non-neurotoxic AMPH pretreatment did. This effect was specific to these genes as tissue plasminogen activator (t-PA), neuropeptide Y (NPY) and c-jun expression in response to AMPH challenge was unaltered or enhanced by amphetamine pretreatements. In the striatum, there were no differences between saline, neurotoxic AMPH, and non-neurotoxic AMPH pretreatments on ARC, NGFI-A or NGFI-B expression elicited by the AMPH challenge. These data indicate that the responsiveness of synaptic plasticity related genes are sensitive to disruption specifically in the parietal cortex by threshold neurotoxic AMPH exposures.
Keywords: Activity-related cytoskeletal protein, Amphetamine, Nerve growth-factor inducible proteins, Neurotoxicity, Gene expression, Challenge
Introduction
The effects of amphetamine (AMPH) and methamphetamine (METH) in laboratory animals range from catastrophic neurodegeneration to neuroplastic changes such as psychomotor sensitization, that have been extensively used to model human addictive behaviors (Robinson and Berridge, 1993; Nestler and Aghajanian, 1997; White and Kalivas, 1998). The “classic” neurotoxicities produced by AMPH and METH include extensive neurodegeneration within the basal ganglia, thalamus, sensory cortex and limbic system (Commins and Seiden, 1986; Schmued et al., 2005; Bowyer et al., 1998b; Eisch et al., 1998; Davidson et al., 2001; Jayanthi et al., 2002), or dopamine terminal damage (Seiden and Sabol, 1995). The magnitude and regional specificity of either AMPH or METH neurotoxicity is dependent on dose and environmental conditions during exposure (such as environmental temperature), as well as the physiological effects that are produced during exposure (such as body temperature, stress and seizures) (Bowyer et al., 1992; Bowyer et al., 1994; Miller and O'Callaghan, 1994; O'Callaghan and Miller, 1994; Bowyer et al., 1998a).
These findings in laboratory animals are commensurate with the neurotoxicities seen in clinical studies of METH abusers that have described deficits in striatal and cortical function (McCann et al., 1998; Volkow et al., 2001b; Volkow et al., 2001a). Specifically, decreased activation of the parietal cortex has been described in human METH users during decision making tasks (Paulus et al., 2002; Paulus et al., 2003). As well, increased parietal cortex metabolism has been observed in METH users (Volkow et al., 2001a).
Gene expression studies have identified commonalities and differences between METH-and AMPH-induced neurotoxicity with different exposure paradigms and within the various brain regions where they produce damage (Jayanthi et al., 2002; Xie et al., 2002; Bowyer et al., 2004). We have previously demonstrated gene expression changes (NGFI-A, NGFI-B , and NPY) that are specifically observed in parietal cortex, and not in the limbic cortex or striatum, 16 hr following a 2-day AMPH exposure regimen (Bowyer et al., 2004). This 2-day exposure paradigm, which did not produce hyperthermia, resulted in significant neurotoxicity within layer IV of the parietal cortex.
The present study was undertaken to compare AMPH challenge responsive gene expression changes in the parietal cortex after neurotoxic AMPH, non-neurotoxic AMPH, or saline pretreatment. It was anticipated that neurotoxic AMPH pretreatment would either alter the gene expression response to AMPH challenge and to a greater extent than the non-neurotoxic dosing. A greater knowledge of the molecular effects of neurotoxic and non-neurotoxic AMPH administration in the parietal cortex may provide insight into the persistent changes in parietal cortex function and human behavior observed in clinical studies.
Methods
Animal housing conditions and experimental design
Male Sprague-Dawley rats (Crl:COBS CD [SD] BR), 4 to 5 months of age, were obtained from the National Center for Toxicological Research (NCTR). Studies were carried out in accordance with the declaration of Helsinki and the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. Rats were pair-housed in polycarbonate cages on wood shaving bedding until the day before AMPH exposure, at which time each rat was individually housed until sacrifice. Rats were dosed three times per day at 7:00am, 1:00pm and 7:00pm for 9-days with either 1ml/kg normal saline, 2.0 mg/kg (free base) AMPH or 7.5 mg/kg (free base) AMPH and then on the 10th day with either 1ml/kg normal saline or 7.5 mg/kg AMPH. The AMPH (d-Amphetamine sulfate, Sigma Chemical Company, St. Louis, MO) was dissolved in normal saline, and injections were delivered subcutaneously.
24 animals were histologically evaluated for neurotoxicity. AMPH exposed rats (3 × 7.5 mg/kg/day AMPH) were evaluated after either 5 (n = 7) or 9 (n=11) days of AMPH at 10:00 am on the day following their last dose. These were compared to saline exposed rats [(n = 3), 5 days; (n = 3), 9 days). Also, 10 rats were given the non-neurotoxic (3 × 2 mg/kg/day) AMPH exposure for either 5 (n = 5) or 9 (n= 5) days and histologically evaluated to ensure that this lower AMPH exposure did not produce neurotoxicity.
Two sets of animals were generated for gene expression experiments as described in Tables 1 & 2. During dosing, access to food was restricted for the animals receiving 3 × 1ml/kg saline/day or 3 × 2.0 mg/kg AMPH/day to 15 grams of food per day for the first 4 days and 25 grams for the final 5 days to mimic the food consumption of the animals receiving 3 × 7.5 mg/kg AMPH/ day. While food-restriction can effect c-fos expression in the striatum (Carr and Kutchukhidze, 2000), we did not observe any significant effects on the AMPH-induced changes in gene expression produced by the moderate food restriction used in our study (see Results). The body temperatures and behavior were monitored at 1 and 2 hr after every dose for the entire 9-day exposure. The core body temperatures were determined using a rectal thermistor as described by Bowyer et al. [1994]. To avoid AMPH-induced hyperthermia the environmental temperature was kept between 17 and 18°C. Animals having a body temperature above 39.6°C at any time point during the 9-day exposure were removed from the studies. Behaviors and body temperatures were also monitored at 1, 2, and 3 hr after either AMPH or saline challenge.
Table 1.
Treatment groups for first set of gene expression experiments
| Treatment Group | Treatment Days 1-9 | Exposure on Day 10 | n |
|---|---|---|---|
| Neurotoxic AMPH + AMPH |
3 × 7.5 mg/kg/day AMPH | 7.5 mg/kg AMPH | 5 |
| Saline + Saline | 3 × 1ml/kg/day Saline | 1ml/kg saline | 5 |
| Naïve + AMPH | No Treatment | 7.5 mg/kg AMPH | 5 |
| Naïve + Saline | No Treatment | 1ml/kg saline | 5 |
Table 2.
Treatment groups for second set of gene expression experiments
| Treatment Group | Treatment Days 1-9 | Exposure on Day 10 | n |
|---|---|---|---|
| Neurotoxic AMPH + AMPH |
3 × 7.5 mg/kg/day AMPH | 7.5 mg/kg AMPH | 8 |
| Neurotoxic AMPH + Saline |
3 × 7.5 mg/kg/day AMPH | 1ml/kg saline | 6 |
| Saline + AMPH | 3 × 1ml/kg/day Saline | 7.5 mg/kg AMPH | 6 |
| Saline + Saline | 3 × 1ml/kg/day Saline | 1ml/kg saline | 6 |
| Non-Neurotoxic AMPH + AMPH |
3 × 2.0 mg/kg/day AMPH | 7.5 mg/kg AMPH | 7 |
Sacrifice and tissue harvest for cDNA array data collection
Animals were sacrificed at 3 hr after either saline or AMPH challenge by decapitation, and their brains were rapidly removed and chilled in 4°C normal saline. The parietal cortex (50 to 65 mg per hemisphere) and striatum (40 to 50 mg per hemisphere) were dissected on ice, immediately frozen on dry ice, and then transferred to −70°C storage as previously described (Bowyer et al., 2004). Parietal cortex was excised between −0.0 to −2.5 anterior-posterior coordinates (Paxinos and Watson, 1995).
RNA isolation and cDNA array hybridization and imaging
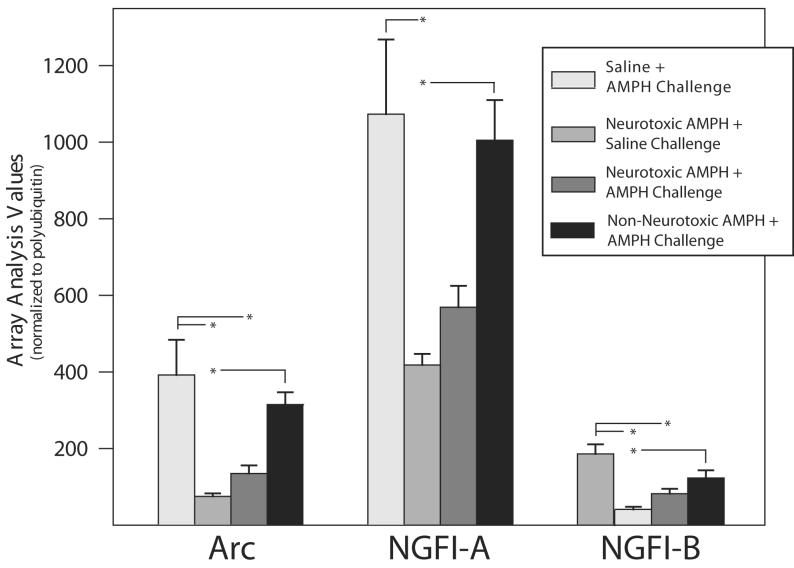
Methods similar to those described by previously (Freeman et al., 2001a; Freeman et al., 2001b) as modified by Bowyer et al. [2004] were used to isolate total RNA from brain tissue and P32-cDNA generated from this total RNA to subsequently hybridize with cDNA macroarray screens. Total cellular RNA was isolated using Tri Reagent (Molecular Research Center Inc., Cincinnati, OH) (Chomczynski and Mackey, 1995). RNA quantity and quality were checked using the Agilent 2100 Bioanalyzer using the RNA 6000 Nano Assay (Agilent, Palo Alto, CA). Gene expression was evaluated using the Atlas 1.2K rat array (Clontech, Palo Alto, CA, cat # 7854) per the manufacturer's protocol with slight modifications (Bowyer et al., 2004). As technical limitations limit array analysis to four samples per batch, one from each of four treatments was included in each batch of arrays. An analysis of variance for treatment and batch effects was performed ‘by probe’ on log2-transformed and normalized intensities. A median-within-subsets normalization was used to normalize across regions of the array (Delongchamp et al., 2004b). All genes were classified as ‘expressed’ or ‘unexpressed’ in the sample based on the magnitude of average-over-arrays log2-intensities (Delongchamp et al., 2005). Within the ‘expressed’ genes, the false discovery rates and false non-discovery rates were computed to generate differentially expressed gene lists (Allison et al., 2002; Delongchamp et al., 2004a). Selection of a gene for QRT-PCR confirmation considered the false discovery rate from the array data as well as biological relevance. In the analysis of the parietal cortex cDNA array data (Figure 3) significance among the 4 treatments is based on Tukey's Studentized Range Test applied to the least squares means of the treatments adjusted for batch effects. These genes were initially selected based on a low false discovery rate associated with the overall F-test for a treatment effect.
Figure 3.
cDNA Array analysis of changes in ARC, NGFI-A & B in the parietal cortex of the treatment groups after AMPH challenge.
Phosphor image levels of 32P-labeling of targets hybridizing to array probes were determined by AtlasImage 2.0 software (units arbitrary) and were normalized to polyubiquitin. The bars represent the means and the error bars the S.E.M. of ARC, NGFI-A and NGFI-B. Significant differences (* p< 0.05) among the 4 groups were determined using Tukey's Studentized Range Test applied to the least squares means of the treatments adjusted for batch effects. For details of statistical analysis see Methods.
QRT-PCR analysis of gene expression
cDNA synthesis was performed on total RNA using Superscript III Reverse Transcriptase (Invitrogen, Carlsbad, CA). 1μg RNA, 500ng Oligo (dT)12-18, and 10mM each dNTP, were incubated for 5 minutes at 65° C and then chilled on ice for 2 minutes. 5X First Strand Buffer (250mM Tris-HCL (pH8.3), 375mM KCL, and 15mM MgCl2), 0.1M DTT, 40 U RNaseOut, and 200 U Superscript III RT were then added. The 20ul reaction was incubated for 60 minutes at 50° C followed by a final incubation at 70° C for 15 minutes for termination.
Quantitative PCR was carried out on a real-time detection instrument (ABI 7900HT Sequence Detection System) in 384-well optical plates using TaqMan Universal PCR Master Mix and Assay on Demand primers and probes (Applied Biosystems, Foster City, CA). Primer/probe sets used included: NGFI-A (nerve growth factor-induced protein A (aka, egr1): Rn00561138_m1), NGFI-B (nerve growth factor-induced protein B (aka, Nr4a1): Rn00561138_m1) and ARC (activity regulated cytoskeletal-associated protein: Rn00571208_g1). Reaction components included: 2X TaqMan Universal Master Mix with UNG, 450nM unlabeled PCR primers, 125nM FAM dye-labeled TaqMan MGB probe, and one μL cDNA reaction product in a 10 uL total reaction volume. PCR conditions were 2 minutes at 50°C, 10 minutes at 95°C and 40 cycles of 15 seconds at 95°C and 1 minute at 60°C. Relative quantities were calculated using ABI SDS 2.0 RQ software and the 2ΔΔCt analysis method (Livak and Schmittgen, 2001) with GAPDH (glyceraldehyde-3-phosphate dehydrogenase: Rn99999916_s1) as the endogenous control. GAPDH levels had been determined in preliminary absolute quantitation experiments to be unchanged with AMPH treatment (data not shown). For the QRT-PCR data (Figures 4 and 5) significance among the 4 treatments is based on Ryan-Einot-Gabriel-Welsch Multiple Range Test applied to the means of the treatments. When applicable, it is more powerful than Tukey's Studentized Range Test, which was used in Figure 3.
Figure 4.
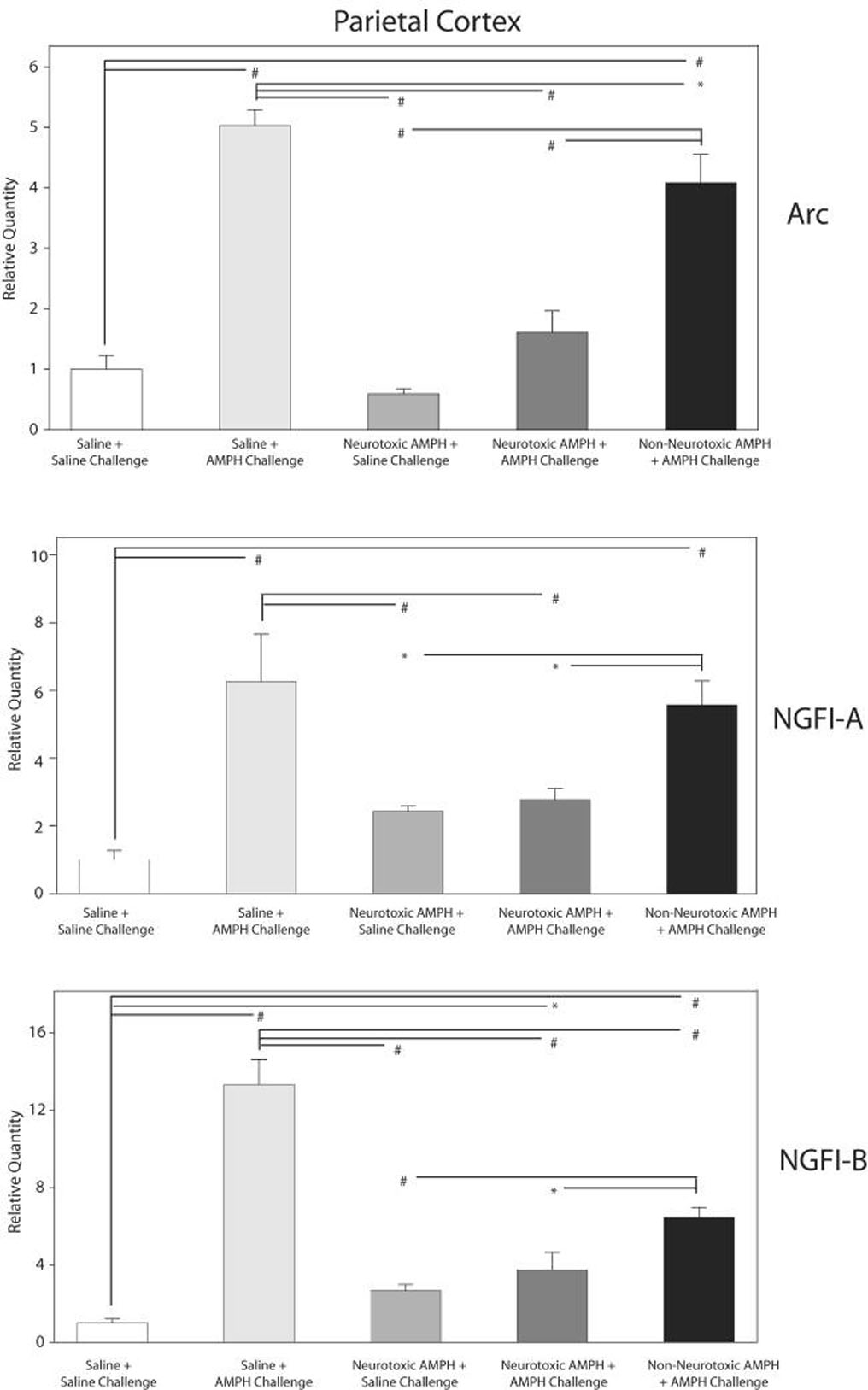
QRT-PCR analysis of changes in ARC, NGFI-A & B in the parietal cortex of the treatment groups after saline or AMPH challenge.
The relative quantities of ARC, NGFI-A and NGFI-B mRNA in parietal cortex of the various treatment groups on the 10th day are shown normalized to the saline group challenged with saline. GAPDH was used as the endogenous control. All three genes demonstrated statistically significant changes in gene expression (ANOVA, p<0.01). Significance (* p< 0.05, # p< 0.01) among the 4 treatments is based on Ryan-Einot-Gabriel-Welsch Multiple Range Test applied to the means of the treatments
Figure 5.
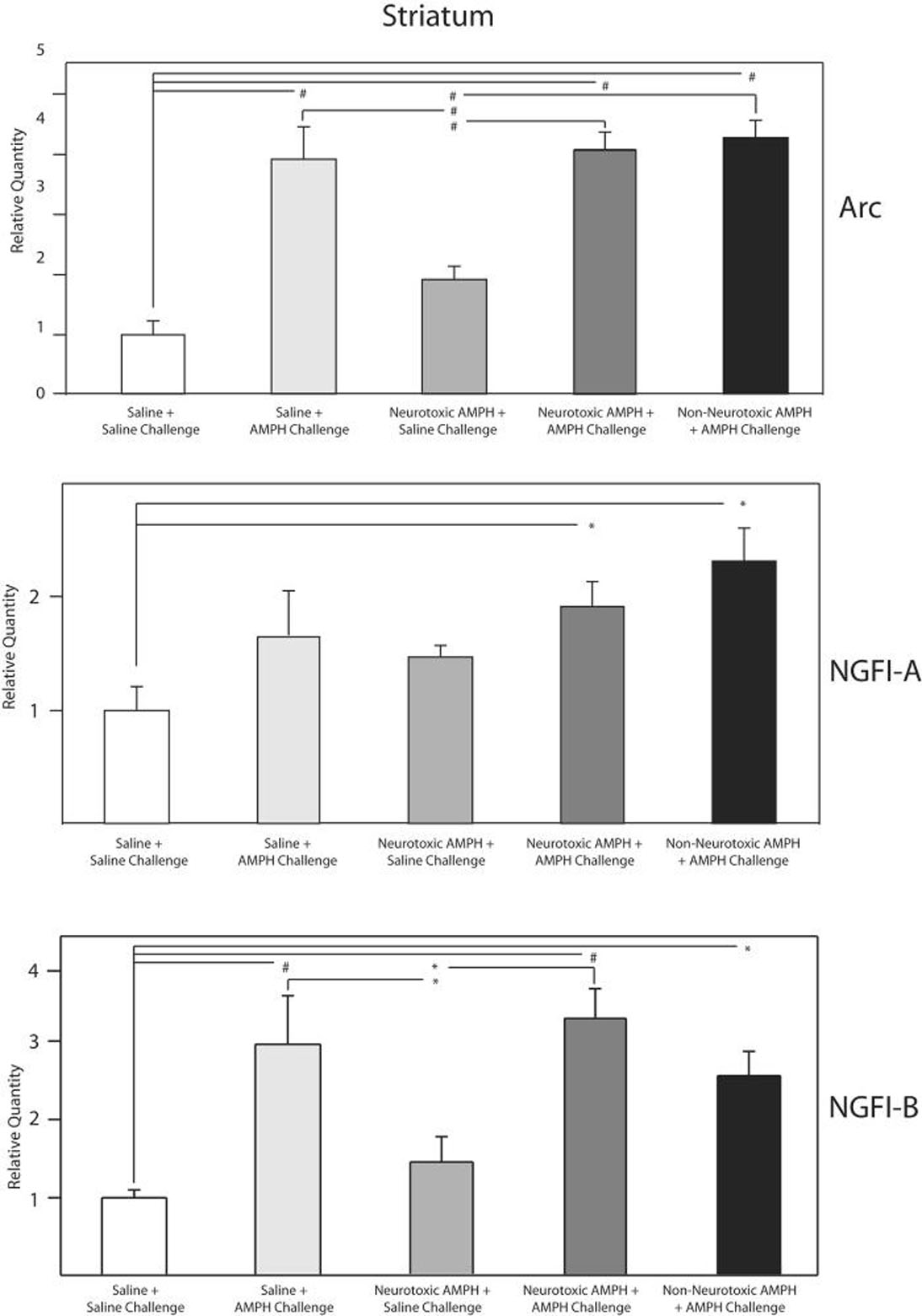
QRT-PCR analysis of changes in ARC, NGFI-A & B in the striatum of the treatment groups after saline or AMPH challenge.
The relative quantities of ARC, NGFI-A and NGFI-B mRNA in parietal cortex of the various treatment groups on the 10th day are shown normalized to the saline group challenged with saline. GAPDH was used as the endogenous control. All three genes demonstrated statistically significant changes in gene expression (ANOVA, p<0.01). Significance (* p< 0.05, # p< 0.01) among the 4 treatments is based on Ryan-Einot-Gabriel-Welsch Multiple Range Test applied to the means of the treatments.
Perfusion and histological processing
For histological evaluation of neurotoxicity, the rats were anesthetized with 150 mg/kg pentobarbital and perfused with 50 ml saline followed by 250 ml of 4% formaldehyde in 0.1M sodium phosphate buffer (pH 7.4). Brains were postfixed for at least 2 days, and then coronal sections 40 μm thick were cut and collected in 2% formaldehyde with 0.1M phosphate (pH7.4) and stored at 4°C until processing. The Fluoro-Jade C (FJ-C) labeling procedure was performed according to Schmued et al.[2005]. The FJ-C fluorescent labeling was examined under an epifluorescent microscope with a filter system designed for visualizing fluorescein (FITC). The isolectin B4-procedure was used to label microglia and identify those that were activated and phagocytic (Streit, 1990). Sections were incubated overnight at 4°C in a solution of B4 isolectin from Griffonia simplicifolia (10ug/ml; Sigma) coupled to horseradish peroxidase and the binding sites were visualized with 3,3'-diaminobenzidine and H2O2.
Results
Body Temperature & Weight
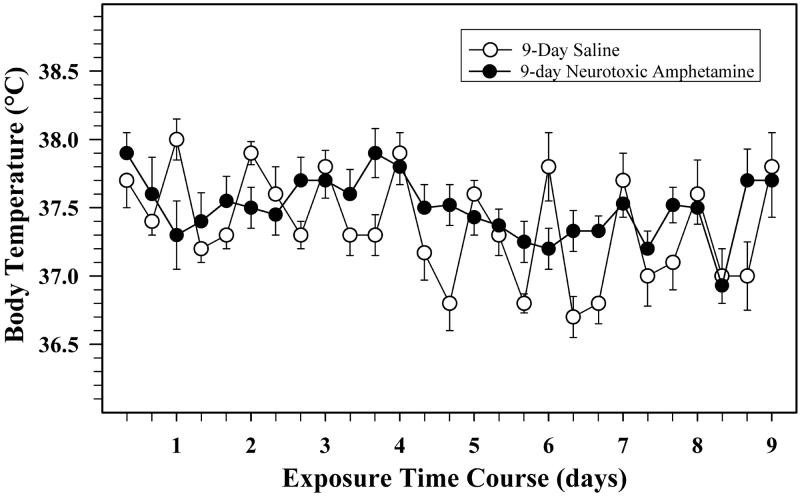
The body temperatures of animals exposed to either saline or 3 × 7.5 mg/kg per day AMPH for 9-days (neurotoxic exposure)and used for gene expression experiments are shown in Figure 1. Animals exposed to the neurotoxic doses of AMPH that had a body temperature above 39.6°C at any time point, (approximately 5% of all the neurotoxic AMPH-treated animals) were removed from the study. A repeated measures analysis of variance shows that the average body temperatures over the time course did not differ significantly between AMPH and saline treated groups (p = 0.77). Temperatures differed over time (p < 0.0001) and there was a significant interaction with treatment (p < 0.0001), which appears to reflect a disruption of the circadian temperature variation in AMPH treated rats. The dose of 3 × 2.0 mg/kg per day AMPH (non-neurotoxic exposure) also did not cause hyperthermia over the 9-day period (data not shown).
Figure 1.
Effects of AMPH on body temperature over the 9-day dosing regimen.
The profile of the temperatures of groups treated with either 3 × 1ml/kg normal saline (open circles, n = 15) or 3 × 7.5 mg/kg AMPH (solid circles, n = 15) are shown over the entire 9-day exposure. The highest temperature obtained over the first two hours after each dose is shown. A two-way ANOVA indicated there was no overall significant effect of time or dose for the two groups (see Results).
Due to food restriction in all groups other than the neurotoxic AMPH, there were no differences in weights prior to sacrifice. A One-way ANOVA indicated there were no statistical differences in the modest weight losses between the 3 groups (F 2, 37 = 2.58, p = 0.13). A 14% weight loss (511 ± 15g to 442 ± 12g, n = 15) occurred from the 9-day neurotoxic AMPH exposure, an 11% loss in the 9-day saline group (89.0 ± 1.5% of starting weight), and a 13% loss (87.0 ± 1.5% of starting weight) in the 9-day non-neurotoxic exposure AMPH group.
Neurodegeneration Immunohistochemistry
FJ-C+ neurons and dendritic processes in the parietal cortex were observed in 80% of the animals sacrificed after either 5 or 9-days of neurotoxic (3×7.5 mg/kg/day) AMPH exposure. However, the FJ-C+ labeling (Figure 2) was relatively sparse and only a few (1 to 6) neurons per section were observed in all the animals. The number of isolectin labeled phagocytic microglia were more prominent (1 to 12 per section) than the number of FJ-C labeled neurons in the animals sacrificed after the 9-day neurotoxic AMPH exposure, and occurred in the parietal cortex in all the animals evaluated. No evidence of neurodegeneration was seen in any of the animals given 3 × 2.0 mg/kg per day AMPH after either 5 (n = 5) or 9 days (n= 5).
Figure 2.
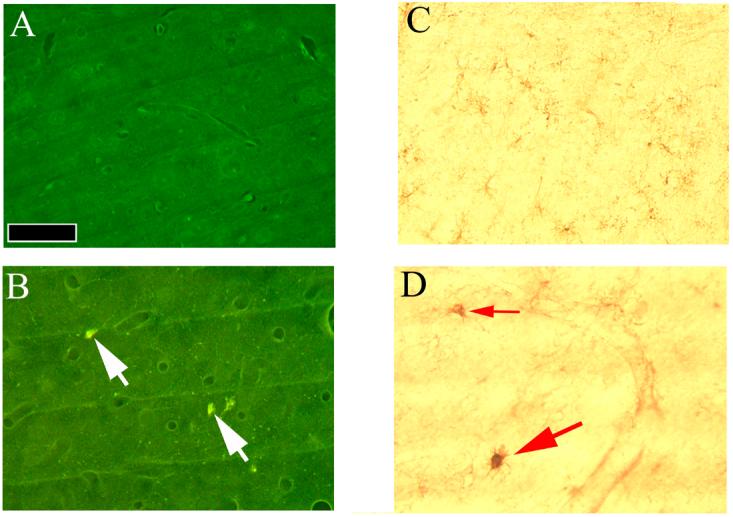
Histological changes observed in the parietal cortex after exposure to a 9-day neurotoxic exposure to AMPH.
FJ-C labeling did not detect any evidence of neurodegeneration in the parietal cortex of any animals given 3 × 2.0 mg/kg amphetamine for either 5 or 9 days, an example of which is seen in panel A. Also, there was no evidence of the presence of either phagocytic or activated microglia in the parietal cortex any of these animals (see panel C). In contrast, FJ-C labeling was seen at low levels in the parietal cortex of animals given 3 × 7.5 mg/kg amphetamine for both 5 and 9 days, as exemplified in panel B (white arrows show the location of FJ-C labeled neurons). The numerous smaller FJ-C labeled puncta indicate the location degenerating dendrites, axons and terminals in the region. Phagocytic (larger red arrow) and activated microglia (smaller red arrow) labeled by isolectin B4 were also present in all the animals receiving the higher dose of amphetamine (panel D). The solid bar shown in the lower left-hand corner of panel A indicates 100 μm. Magnification was the same in all 4 panels
1st Gene Expression Experiment
In the initial experiment gene expression analysis was conducted on four groups of animals (Table 1). Naïve rats challenged with 7.5 mg/kg AMPH were compared with naïve rats challenged with saline 3 hours after injection. These two naïve groups had not been subjected to food restriction nor injected with saline prior to challenge. Rats exposed to neurotoxic AMPH for 9-days and challenged with AMPH were compared to rats exposed to saline for 9 days and challenged with saline. Previously, we and others have demonstrated Arc mRNA induction by psychomotor stimulants (Tan et al., 2000; Freeman et al., 2002; Gonzalez-Nicolini and McGinty, 2002; Bowyer et al., 2004). To establish a baseline expression difference with AMPH challenge, Arc mRNA levels were measured in the parietal cortex by QRT-PCR. Using this method, naïve animals challenged with AMPH showed an 8.0±1.8-fold (n=5/group) increase in ARC mRNA relative to their saline challenged naïve controls. Only a 3.2±0.7-fold (n=5/group) increase in ARC mRNA was observed with the AMPH challenge in the neurotoxic AMPH exposure group relative to their saline challenged saline treated controls.
2nd Gene Expression Experiment
A second set of gene expression experiments was conducted on the parietal cortex and striatum from five groups of animals. In these experiments, the changes in gene expression after either a saline or a 7.5 mg/kg AMPH challenge on the 10th day were determined in animals pretreated with a 9-day exposure to saline, 9-day non-neurotoxic AMPH exposure, or 9-day neurotoxic AMPH exposure (see Table 2 for specifics on the dosing paradigms and numbers of animals). The second set of gene expression experiments were conducted with saline treated animals rather than the naïve animals in the first experiment to obviate any effects of handling and injection. cDNA array analysis was conducted on the parietal cortex and QRT-PCR was performed on the parietal cortex and striatum.
cDNA array analysis was conducted to examine a large number of genes simultaneously. Array analysis was performed on the saline+AMPH, neurotoxic AMPH+saline, neurotoxic AMPH+AMPH, and non-neurotoxic AMPH+AMPH groups. Genes were considered to be ‘expressed’ if their average log2-expression exceeded 5. This criterion partitioned the genes into 582 ‘expressed’ genes and 594 ‘not expressed’ genes. The p-value distribution of the 594 not expressed genes did not differ significantly from a uniform distribution (p = 0.26), which indicates little evidence of treatment effects. The p-value distribution of the 582 expressed genes differed from a uniform distribution (p < 0.0001) indicating the presence of treatment effects. To determine statistically relevant changes in gene expression resulting from cDNA array analysis, p-values for treatment effects were computed in ‘by gene’ analyses of covariance. A set of 6 genes [NGFI-A, NGFI-B, Arc, neuropeptide Y (NPY), DNA binding protein inhibitor I, and tissue-type plasminogen activator protein (t-PA)] had p-values less than 0.0005, and an estimated false discovery rate less than 0.028.
AMPH challenge did not produce significant differences in parietal cortex expression levels of Arc, NGFI-A, or NGFI-A in the neurotoxic AMPH pretreatment group (Figure 3). However, in the non-neurotoxic AMPH and saline pretreated groups, AMPH challenge induced significant increases in gene expression of ARC, NGFI-A and NGFI-B. The cDNA array expression levels of t-PA and four other genes [c-jun, Igfr2, GAP-43, and NPY] mRNA species proposed to be involved in either synaptic plasticity or learning are shown in Table 3. The expressions of these genes have been reported to be important in the processes of either synaptogenesis or learning.
Table 3.
Other genes related to synaptic formation or learning with altered expression in the parietal cortex.
| Gene Expression in the Parietal Cortex | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tissue-Type Plasminogen Activator |
c-JUN | Insulin-Like Growth Factor Receptor 2 |
GAP-43 | NPY | ||||||
| cDNA array |
QRT-PCR | cDNA array |
QRT-PCR | cDNA array |
QRT-PCR | cDNA array |
QRT- PCR |
cDNA array |
QRT-PCR | |
| Saline + Saline Challenge |
NM | 1.0±0.2 | NM | 1.0±0.13 | NM | 1.0±0.1 | NM | 1.0±0.1 | NM | 1.0±0.2 |
| Saline + AMPH Challenge |
82±14 | 1.7±0.5a,b | 66±17 | 1.3±0.3 | 88±17 | 0.8±0.1 | 491±75 | 1.0±0.2 | 159±16 | 0.9±0.1 |
| Neurotoxic AMPH + Saline Challenge |
28±4 | 0.8±0.1 | 40±3 | 0.8±0.2 | 46±9 | 0.8±0.1 | 402±61 | 1.0±0.1 | 171±7 | 1.3±0.1a,c |
| Neurotoxic AMPH + AMPH Challenge |
54±5 | 1.4±0.6b | 44±5 | 0.6±0.2c | 57±5 | 0.8±0.1 | 387±38 | 0.9±0.1 | 207±22 | 1.4±0.3a,c |
| Non-Neurotoxic AMPH + AMPH Challenge |
69±10 | 1.4±0.2b | 54±4 | 1.2±0.6 | 61±6 | 1.0±0.1 | 434±48 | 0.9±0.1 | 197±19 | 1.4±0.2a,c |
Phosphor image levels of hybridization to array probes were determined by AtlasImage 2.0 software (arbitrary units) and normalized to polyubiquitin. Values shown are mean ± SEM. Mean QRT-PCR values are normalized to a value of 1 for the Saline + Saline Challenge ± SEM, with GAPDH used as the endogenous control.
significant difference from saline+saline
significant difference from neurotoxic AMPH+saline
significant difference from saline+AMPH. (p<0.05, one-way ANOVA, Student-Newman-Keuls pairwise post hoc test), NM – not measured.
To confirm the changes observed by array analysis, quantitative RT-PCR analysis of the parietal cortex was performed (Figure 4). An additional control group of saline treated and challenged animals (saline+saline) was included in the qPCR analysis. The saline+saline group could not be included in array analysis for technical reasons but was added to the qPCR confirmation to determine the relative transcript levels of non-AMPH exposed animals. ARC, NGFI-A, and NGFI-B were evaluated by QRT-PCR based on their statistical significance and relationship to synaptic formation and learning processes. QRT-PCR analysis identified significantly lower levels of ARC, NGFI-A, and NGFI-B in the neurotoxic AMPH+AMPH group than the non-neurotoxic AMPH+AMPH and saline+AMPH groups. No significant differences were observed after saline challenge between the neurotoxic AMPH+saline and saline+saline groups. The induction of Arc between saline+AMPH and saline+saline groups was similar to that observed between the naïve+saline and naïve+AMPH groups in the 1st gene expression experiment. This suggests that neither the moderate food restriction, nor daily saline injections significantly altered gene expression changes.
Several additional genes were also examined post-hoc in the parietal cortex by QRT-PCR (Table 3). These genes demonstrated potential differences in expression with array analysis. c-jun expression was significantly higher in the saline+AMPH as compared to the neurotoxic AMPH+AMPH. t-PA mRNA levels were significantly increased by AMPH challenge regardless of the 9-day pretreatment. For t-PA, there was no effect of neurotoxic AMPH exposure on responsiveness. NPY demonstrated a different response profile with AMPH pretreatment (non-neurotoxic and neurotoxic) resulting in significantly higher mRNA levels than with saline pretreatment regardless of the challenge. c-jun AMPH challenge responsiveness was blunted in the neurotoxic AMPH+AMPH group as compared to the saline+AMPH group. No significant differences in the levels of GAP-43 and Igfr2 were observed by QRT-PCR. mRNA levels of glial fibrillary acidic protein (GFAP) were unchanged between neurotoxic AMPH+AMPH and saline+saline groups (Relative Quantities, 1.19 ± 0.13, n=8 versus 1.00± 0.15, n=6).
The mRNA levels of ARC, NGFI-A & B were also determined by QRT-PCR in the striatum to determine anatomical specificity of the observed changes (Figure 5). Unlike the parietal cortex, the increased expression of all three genes produced by the AMPH challenge was equivalent regardless of the pretreatment. NPY mRNA levels were not significantly different among the treatment groups in the striatum (data not shown).
Discussion
The results of these studies demonstrate that administration of 3 × 7.5 mg/kg AMPH per day for 9-days is at or just above the threshold for neurodegeneration in the parietal cortex while 3 × 2.0mg/kg AMPH administration for 9-days does not produce signs of neurotoxicity. The neurotoxic AMPH exposure but not the non-neurotoxic AMPH exposure results in a significantly diminished AMPH challenge induced upregulation of the early-immediate genes ARC, NGFI-A, and NGFI- B in the parietal cortex. This diminished response was not observed in the striatum. t-PA AMPH challenge induction was unaltered in the parietal cortex and NPY increases with AMPH challenge were augmented by AMPH pretreatement. The inhibited upregulation of NGFI-A, NGFI-B, and ARC may indicate that a lessened capacity for new synaptic formation occurs within the parietal cortex but not the striatum after the neurotoxic exposure to AMPH. As well, this diminished responsiveness in the parietal cortex could serve a neuroprotective effect for further damage due to AMPH exposure or other neurotoxic insults.
The 9-day AMPH neurotoxic regimen employed produced neurodegeneration with respect to histological endpoints. The number of degenerating neurons (FJ-C labeled) and phagocytic microglia detected at the end of the 9-day neurotoxic AMPH exposure were only ¼ of that observed in previous studies with higher doses of AMPH (Jakab and Bowyer, 2002; Bowyer et al., 2004). Approximately 30% of the animals evaluated given the 3 × 7.5 mg/kg AMPH dose had evidence of only one or two neurons degenerating per hemisphere. The lower dose of 3 × 2.0 mg/kg AMPH produced no histological signs of neurodegeneration. Therefore, it is likely that the 3 × 7.5 mg/kg AMPH is near the minimal dose (less than 2-fold) necessary to produce neurotoxicity in the 9-day time frame.
GFAP is used as a classical molecular marker for detecting the neurotoxicity produced by amphetamines in the presence or absence of hyperthermia (O'Callaghan and Miller, 1994; Jakab and Bowyer, 2002; Bowyer et al., 2004). In the present study, GFAP increases in the parietal cortex and striatum were not sufficient to reach statistical significance. However, this may also in part be due to the timing of sacrifice and the fact that mRNA levels were determined and not actual GFAP protein levels within regions of the parietal cortex.
ARC, NGFI-A, and NGFI-B are known to be significantly upregulated in the striatum, and to a lesser extent in the cortex after AMPH exposure (Nguyen et al., 1992; Wang et al., 1994; Kodama et al., 1998). In our previous work (Bowyer et al., 2004), we proposed that the decreases in the levels of NGFI-A and NGFI-B 16 hr after a 2-day neurotoxic AMPH insult are a homeostatic-type response involved in returning expression levels back to a pre-AMPH exposure stature. While array analysis was performed in this study to discover novel changes in gene expression in response to AMPH challenge the most salient responses found by array analysis were ARC, NGFI-A, and NGFI-B, which have been previously described by us and others. The present primary finding of this study is that neurotoxic AMPH exposure inhibits ARC, NGFI-A, and NGFI-B induction by AMPH challenge. One commonality to the gene expression changes found in the present study is their relation to synaptic plasticity. ARC induction, in particular, has been implicated in the formation and strengthening of synaptic formation after NMDA receptor stimulation and learned behavior (Lyford et al., 1995; Guzowski et al., 2001; Steward and Worley, 2001; Ramirez-Amaya et al., 2005). In addition, NGFI-A (egr1, zif/268; (Cole et al., 1989; Wisden et al., 1990)) has been implicated in synaptic formation and learning (Wang et al., 1994; Wang et al., 1995), although it may not be as closely linked as ARC (Guzowski et al., 2001).
NGFI-B (Nr4a1, nurr77) upregulation by the AMPH challenge was also diminished by the 9-day neurotoxic AMPH exposure. However, the 9-day non-neurotoxic AMPH exposure did diminish NGFI-B upregulation by the AMPH challenge as compared to saline pretreated animals challenged with AMPH. Thus, the relationship of NGFI-B to histological neurotoxicity is not as clear as ARC and NGFI-A, but NGFI-B alterations remain important considering the role of NGFI-B in memory consolidation (von Hertzen and Giese, 2005). The reduced ability to upregulate these three genes after a neurotoxic AMPH exposure may indicate that the parietal cortex, and not the striatum, has a reduced capability of forming new synapses and strengthening existing synapses. Further experiments will be required to determine if protein levels are altered in a similar manner.
Several other genes examined demonstrated alternations with the different pretreatments. t-PA induction with AMPH challenge was unaffected by pretreatment, while NPY induction was only observed with AMPH pretreatment. Increased c-jun expression with AMPH challenge was inhibited by neurotoxic AMPH pretreatment. These changes are of interest as c-jun (Tischmeyer and Grimm, 1999) and t-PA expression (Madani et al., 1999), have been implicated in learning. In cortical regions, NPY has been postulated to play a protective role against neurodegeneration (Cheung and Cechetto, 1995; Kopp et al., 1999).
Unlike NPY, it is not known whether these decreases in AMPH-induced upregulation of Arc, NGFI-A, and NGFI-B expression after the threshold neurotoxic exposure subsequently serves a neuroprotective effect. Decreased neuronal NMDA receptor density due to ARC decreases would be expected to be protective against NMDA-mediated neurotoxicity. The loss of gene induction responsiveness may also be a desensitization phenomenon resulting from the continual and excessive vibrissae/barrel field and somatosensory forelimb region stimulation occurring with the AMPH-induced stereotypic grooming (O'Dell and Marshall, 2002). This may limit the output from this area of the cortex to the striatum and thalamus (Tracey and Waite, 1995) which are also damaged by AMPH and METH (Commins and Seiden, 1986; Stephans and Yamamoto, 1994; Seiden and Sabol, 1995; Bowyer et al., 1998a; Eisch et al., 1998).
The decreased AMPH-induced upregulation of Arc, NGFI-A, and NGFI-B expression might be considered to be a tolerance effect (Kuczenski and Segal, 1997; Shilling et al., 2000). While this is possible, rats challenged with 7.5mg/kg AMPH after twice daily injections of 7.5mg/kg AMPH do not exhibit locomotor behavioral tolerance (Persico et al., 1993). As well, the diminished responsiveness to AMPH challenge is specific to Arc, NGFI-A, and NGFI-B as t-PA and NPY did not exhibit this profile in the parietal cortex. Furthermore, the loss of gene induction was not associated with the non-neurotoxic AMPH exposure. This effect is also anatomically specific, as none of the pretreatments (including neurotoxic AMPH exposure) affected the increases in Arc, NGFI-A, and NGFI-B expression with AMPH challenge in the striatum. Together, these observations appear to rule out a role of tolerance in the data obtained in our study.
Further studies will be necessary to determine whether these changes relate to learning and decision making deficits seen in humans abusing amphetamines (Paulus et al., 2003; Paulus et al., 2002; Volkow et al., 2001b; Volkow et al., 2001a). Deficits in learning related to object recognition and object placement memory have been reported after methamphetamine (METH) neurotoxicity (Bisagno et al., 2002; Schroder et al., 2003). While such learning deficits are most commonly ascribed to altered hippocampal and limbic function (Steckler et al., 1998), tactile function's interaction with memory may serve a role in the behavioral tests involving object recognition that are disrupted by METH (Bisagno et al., 2002; Schroder et al., 2003). Decision making in the face of uncertainty has been found to be altered in METH users and to correlate to parietal cortex activation levels (Paulus et al., 2002; Paulus et al., 2003). As the parietal cortex has been shown to be critical, in addition to a number of other brain areas, for decision making gene expression changes related to synaptic plasticity may relate to these behavioral outcomes (Paulus et al., 2001). Future studies localizing these changes to specific neurons characterized for their specific inputs and projections are needed to conclusively determine their role in behavior.
In summary, our data indicate that the upregulation of ARC, NGFI-A, and NGFI-B response to AMPH challenge is diminished in the parietal cortex by a 9-day threshold neurotoxic exposure to AMPH. This effect is not produced by a 9-day non-neurotoxic exposure to AMPH that does not produce histological evidence of neurodegeneration. In the striatum, ARC and NGFI-A & B induction with AMPH challenge was unaffected by neurotoxic or non-neurotoxic AMPH exposure. The loss of responsiveness of ARC and NGFI-A, NGFI-B induction may indicate a diminished capability of this region to form new synaptic connections and subsequently may have a protective effect on parietal cortex neurons against either AMPH- or NMDA-mediated neurotoxicity.
Acknowledgments
This work supported by National Institute on Drug Abuse R01DA013770 (to KEV).
Abbreviations
- ARC
activity-related cytoskeletal protein
- AMPH
amphetamine
- FJ-C
Fluoro Jade C
- GFAP
glial fibrillary acidic protein
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- METH
insulin-like growth factor II receptor, Igf2r, methamphetamine
- NGFI-A (aka, Egr1, ZIF/268, KROX24)
nerve growth factor inducible protein A
- NGFI-B (aka, Nurr77, Nr4a1)
nerve growth factor inducible protein B
- NMDA
N-methyl-D-aspartate
- NPY
neuropeptide Y protein
- (t-PA)
tissue plasminogen activator
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The views presented in this article do not necessarily reflect those of the U.S. Food and Drug Administration.
References
- Allison DB, Gadbury GL, Heo M, Fernandez JR, Lee C-K, Prolla TA, Weindruch R. A mixture model approach for the analysis of microarray gene expression data. Computational Statistics and Data Anlysis. 2002;39:20. [Google Scholar]
- Bisagno V, Ferguson D, Luine VN. Short toxic methamphetamine schedule impairs object recognition task in male rats. Brain Res. 2002;940:95–101. doi: 10.1016/s0006-8993(02)02599-4. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Davies DL, Schmued L, Broening HW, Newport GD, Slikker W, Jr., Holson RR. Further studies of the role of hyperthermia in methamphetamine neurotoxicity. J. Pharmacol. Exp. Ther. 1994;268:1571–1580. [PubMed] [Google Scholar]
- Bowyer JF, Frame LT, Clausing P, Nagamoto-Combs K, Osterhout CA, Sterling CR, Tank AW. Long-term effects of amphetamine neurotoxicity on tyrosine hydroxylase mRNA and protein in aged rats. J. Pharmacol. Exp. Ther. 1998a;286:1074–1085. [PubMed] [Google Scholar]
- Bowyer JF, Harris AJ, Delongchamp RR, Jakab RL, Miller DB, Little AR, O'Callaghan JP. Selective changes in gene expression in cortical regions sensitive to amphetamine during the neurodegenerative process. Neurotoxicology. 2004;25:555–572. doi: 10.1016/j.neuro.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Peterson SL, Rountree RL, Tor-Agbidye J, Wang GJ. Neuronal degeneration in rat forebrain resulting from D-amphetamine-induced convulsions is dependent on seizure severity and age. Brain Res. 1998b;809:77–90. doi: 10.1016/s0006-8993(98)00846-4. [DOI] [PubMed] [Google Scholar]
- Bowyer JF, Tank AW, Newport GD, Slikker W, Jr., Ali SF, Holson RR. The influence of environmental temperature on the transient effects of methamphetamine on dopamine levels and dopamine release in rat striatum. J. Pharmacol. Exp. Ther. 1992;260:817–824. [PubMed] [Google Scholar]
- Carr KD, Kutchukhidze N. Chronic food restriction increases fos-like immunoreactivity (FLI) induced in rat forebrain by intraventricular amphetamine. Brain Res. 2000;861:88–96. doi: 10.1016/s0006-8993(00)02018-7. [DOI] [PubMed] [Google Scholar]
- Cheung RT, Cechetto DF. Neuropeptide changes following excitotoxic lesion of the insular cortex in rats. J. Comp Neurol. 1995;362:535–550. doi: 10.1002/cne.903620408. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Mackey K. Short technical reports. Modification of the TRI reagent procedure for isolation of RNA from polysaccharide- and proteoglycan-rich sources. Biotechniques. 1995;19:942–945. [PubMed] [Google Scholar]
- Cole AJ, Saffen DW, Baraban JM, Worley PF. Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature. 1989;340:474–476. doi: 10.1038/340474a0. [DOI] [PubMed] [Google Scholar]
- Commins DL, Seiden LS. alpha-Methyltyrosine blocks methylamphetamine-induced degeneration in the rat somatosensory cortex. Brain Res. 1986;365:15–20. doi: 10.1016/0006-8993(86)90717-1. [DOI] [PubMed] [Google Scholar]
- Davidson C, Gow AJ, Lee TH, Ellinwood EH. Methamphetamine neurotoxicity: necrotic and apoptotic mechanisms and relevance to human abuse and treatment. Brain Res. Brain Res. Rev. 2001;36:1–22. doi: 10.1016/s0165-0173(01)00054-6. [DOI] [PubMed] [Google Scholar]
- Delongchamp RR, Bowyer JF, Chen JJ, Kodell RL. Multiple-testing strategy for analyzing cDNA array data on gene expression. Biometrics. 2004a;60:774–782. doi: 10.1111/j.0006-341X.2004.00228.x. [DOI] [PubMed] [Google Scholar]
- Delongchamp RR, Velasco C, Dial S, Harris AJ. Genome-wide estimation of gender differences in the gene expression of human livers: statistical design and analysis. BMC. Bioinformatics. 2005;6(Suppl 2):S13. doi: 10.1186/1471-2105-6-S2-S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delongchamp RR, Velasco C, Razzaghi M, Harris A, Casciano D. Median-of-subsets normalization of intensities for cDNA array data. DNA Cell Biol. 2004b;23:653–659. doi: 10.1089/dna.2004.23.653. [DOI] [PubMed] [Google Scholar]
- Eisch AJ, Schmued LC, Marshall JF. Characterizing cortical neuron injury with Fluoro-Jade labeling after a neurotoxic regimen of methamphetamine. Synapse. 1998;30:329–333. doi: 10.1002/(SICI)1098-2396(199811)30:3<329::AID-SYN10>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Brebner K, Lynch WJ, Patel KM, Robertson DJ, Roberts DC, Vrana KE. Changes in rat frontal cortex gene expression following chronic cocaine. Brain Res. Mol. Brain Res. 2002;104:11–20. doi: 10.1016/s0169-328x(02)00197-3. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Brebner K, Lynch WJ, Robertson DJ, Roberts DC, Vrana KE. Cocaine-responsive gene expression changes in rat hippocampus. Neuroscience. 2001a;108:371–380. doi: 10.1016/s0306-4522(01)00432-8. [DOI] [PubMed] [Google Scholar]
- Freeman WM, Nader MA, Nader SH, Robertson DJ, Gioia L, Mitchell SM, Daunais JB, Porrino LJ, Friedman DP, Vrana KE. Chronic cocaine-mediated changes in non-human primate nucleus accumbens gene expression. J. Neurochem. 2001b;77:542–549. doi: 10.1046/j.1471-4159.2001.00252.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Nicolini V, McGinty JF. Gene expression profile from the striatum of amphetamine-treated rats: a cDNA array and in situ hybridization histochemical study. Brain Res. Gene Expr. Patterns. 2002;1:193–198. doi: 10.1016/s1567-133x(02)00017-0. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Setlow B, Wagner EK, McGaugh JL. Experience-dependent gene expression in the rat hippocampus after spatial learning: a comparison of the immediate-early genes Arc, c-fos, and zif268. J. Neurosci. 2001;21:5089–5098. doi: 10.1523/JNEUROSCI.21-14-05089.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab RL, Bowyer JF. Parvalbumin neuron circuits and microglia in three dopamine-poor cortical regions remain sensitive to amphetamine exposure in the absence of hyperthermia, seizure and stroke. Brain Res. 2002;958:52–69. doi: 10.1016/s0006-8993(02)03439-x. [DOI] [PubMed] [Google Scholar]
- Jayanthi S, McCoy MT, Ladenheim B, Cadet JL. Methamphetamine causes coordinate regulation of Src, Cas, Crk, and the Jun N-terminal kinase-Jun pathway. Mol. Pharmacol. 2002;61:1124–1131. doi: 10.1124/mol.61.5.1124. [DOI] [PubMed] [Google Scholar]
- Kodama M, Akiyama K, Ujike H, Shimizu Y, Tanaka Y, Kuroda S. A robust increase in expression of arc gene, an effector immediate early gene, in the rat brain after acute and chronic methamphetamine administration. Brain Res. 1998;796:273–283. doi: 10.1016/s0006-8993(98)00349-7. [DOI] [PubMed] [Google Scholar]
- Kopp J, Nanobashvili A, Kokaia Z, Lindvall O, Hokfelt T. Differential regulation of mRNAs for neuropeptide Y and its receptor subtypes in widespread areas of the rat limbic system during kindling epileptogenesis. Brain Res. Mol. Brain Res. 1999;72:17–29. doi: 10.1016/s0169-328x(99)00191-6. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. An escalating dose/multiple high-dose binge pattern of amphetamine administration results in differential changes in the extracellular dopamine response profiles in caudateputamen and nucleus accumbens. J. Neurosci. 1997;17:4441–4447. doi: 10.1523/JNEUROSCI.17-11-04441.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- Madani R, Hulo S, Toni N, Madani H, Steimer T, Muller D, Vassalli JD. Enhanced hippocampal long-term potentiation and learning by increased neuronal expression of tissue-type plasminogen activator in transgenic mice. EMBO J. 1999;18:3007–3012. doi: 10.1093/emboj/18.11.3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Wong DF, Yokoi F, Villemagne V, Dannals RF, Ricaurte GA. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J. Neurosci. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DB, O'Callaghan JP. Environment-, drug- and stress-induced alterations in body temperature affect the neurotoxicity of substituted amphetamines in the C57BL/6J mouse. J. Pharmacol. Exp. Ther. 1994;270:752–760. [PubMed] [Google Scholar]
- Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- Nguyen TV, Kosofsky BE, Birnbaum R, Cohen BM, Hyman SE. Differential expression of c-fos and zif268 in rat striatum after haloperidol, clozapine, and amphetamine. Proc. Natl. Acad Sci U. S. A. 1992;89:4270–4274. doi: 10.1073/pnas.89.10.4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan JP, Miller DB. Neurotoxicity profiles of substituted amphetamines in the C57BL/6J mouse. J. Pharmacol. Exp. Ther. 1994;270:741–751. [PubMed] [Google Scholar]
- O'Dell SJ, Marshall JF. Effects of vibrissae removal on methamphetamine-induced damage to rat somatosensory cortical neurons. Synapse. 2002;43:122–130. doi: 10.1002/syn.10016. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Frank L, Brown GG, Schuckit MA. Decision making by methamphetamine-dependent subjects is associated with error-rate-independent decrease in prefrontal and parietal activation. Biol. Psychiatry. 2003;53:65–74. doi: 10.1016/s0006-3223(02)01442-7. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack N, Zauscher B, McDowell JE, Frank L, Brown GG, Braff DL. Prefrontal, parietal, and temporal cortex networks underlie decision-making in the presence of uncertainty. Neuroimage. 2001;13:91–100. doi: 10.1006/nimg.2000.0667. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL, Schuckit MA. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26:53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotexic Coordinates. Academic Press; Sand Diego, CA: 1995. [Google Scholar]
- Persico AM, Schindler CW, O'Hara BF, Brannock MT, Uhl GR. Brain transcription factor expression: effects of acute and chronic amphetamine and injection stress. Brain Res. Mol. Brain Res. 1993;20:91–100. doi: 10.1016/0169-328x(93)90113-4. [DOI] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Vazdarjanova A, Mikhael D, Rosi S, Worley PF, Barnes CA. Spatial exploration-induced Arc mRNA and protein expression: evidence for selective, network-specific reactivation. J. Neurosci. 2005;25:1761–1768. doi: 10.1523/JNEUROSCI.4342-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res. Brain Res. Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Schmued LC, Stowers CC, Scallet AC, Xu L. Fluoro-Jade C results in ultra high resolution and contrast labeling of degenerating neurons. Brain Res. 2005;1035:24–31. doi: 10.1016/j.brainres.2004.11.054. [DOI] [PubMed] [Google Scholar]
- Schroder N, O'Dell SJ, Marshall JF. Neurotoxic methamphetamine regimen severely impairs recognition memory in rats. Synapse. 2003;49:89–96. doi: 10.1002/syn.10210. [DOI] [PubMed] [Google Scholar]
- Seiden LS, Sabol KE. Handbook of Neurotoxicology. Marcel Dekker, Inc.; 1995. Neurotxicity of Methamphetamine-Related Drugs and Cocaine; pp. 824–844. [Google Scholar]
- Shilling PD, Kelsoe JR, Kuczenski R, Segal DS. Differential regional zif268 messenger RNA expression in an escalating dose/binge model of amphetamine-induced psychosis. Neuroscience. 2000;96:83–90. doi: 10.1016/s0306-4522(99)00510-2. [DOI] [PubMed] [Google Scholar]
- Steckler T, Drinkenburg WH, Sahgal A, Aggleton JP. Recognition memory in rats--II. Neuroanatomical substrates. Prog. Neurobiol. 1998;54:313–332. doi: 10.1016/s0301-0082(97)00061-0. [DOI] [PubMed] [Google Scholar]
- Stephans SE, Yamamoto BK. Methamphetamine-induced neurotoxicity: roles for glutamate and dopamine efflux. Synapse. 1994;17:203–209. doi: 10.1002/syn.890170310. [DOI] [PubMed] [Google Scholar]
- Steward O, Worley PF. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron. 2001;30:227–240. doi: 10.1016/s0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]
- Streit WJ. An improved staining method for rat microglial cells using the lectin from Griffonia simplicifolia (GSA I-B4) J. Histochem. Cytochem. 1990;38:1683–1686. doi: 10.1177/38.11.2212623. [DOI] [PubMed] [Google Scholar]
- Tan A, Moratalla R, Lyford GL, Worley P, Graybiel AM. The activity-regulated cytoskeletal-associated protein arc is expressed in different striosome-matrix patterns following exposure to amphetamine and cocaine. J. Neurochem. 2000;74:2074–2078. doi: 10.1046/j.1471-4159.2000.0742074.x. [DOI] [PubMed] [Google Scholar]
- Tischmeyer W, Grimm R. Activation of immediate early genes and memory formation. Cell Mol. Life Sci. 1999;55:564–574. doi: 10.1007/s000180050315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey DJ, Waite PME. Somatosensory System. In: Paxinos G, editor. The Rat Nervous System. Academic Press; San Diego, CA: 1995. pp. 689–704. [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Wong C, Logan J. Higher cortical and lower subcortical metabolism in detoxified methamphetamine abusers. Am. J. Psychiatry. 2001a;158:383–389. doi: 10.1176/appi.ajp.158.3.383. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN. Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. Am. J. Psychiatry. 2001b;158:377–382. doi: 10.1176/appi.ajp.158.3.377. [DOI] [PubMed] [Google Scholar]
- von Hertzen LS, Giese KP. Memory reconsolidation engages only a subset of immediate-early genes induced during consolidation. J. Neurosci. 2005;25:1935–1942. doi: 10.1523/JNEUROSCI.4707-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JQ, Daunais JB, McGinty JF. NMDA receptors mediate amphetamine-induced upregulation of zif/268 and preprodynorphin mRNA expression in rat striatum. Synapse. 1994;18:343–353. doi: 10.1002/syn.890180410. [DOI] [PubMed] [Google Scholar]
- Wang JQ, Smith AJ, McGinty JF. A single injection of amphetamine or methamphetamine induces dynamic alterations in c-fos, zif/268 and preprodynorphin messenger RNA expression in rat forebrain. Neuroscience. 1995;68:83–95. doi: 10.1016/0306-4522(95)00100-w. [DOI] [PubMed] [Google Scholar]
- White FJ, Kalivas PW. Neuroadaptations involved in amphetamine and cocaine addiction. Drug Alcohol Depend. 1998;51:141–153. doi: 10.1016/s0376-8716(98)00072-6. [DOI] [PubMed] [Google Scholar]
- Wisden W, Errington ML, Williams S, Dunnett SB, Waters C, Hitchcock D, Evan G, Bliss TV, Hunt SP. Differential expression of immediate early genes in the hippocampus and spinal cord. Neuron. 1990;4:603–614. doi: 10.1016/0896-6273(90)90118-y. [DOI] [PubMed] [Google Scholar]
- Xie T, Tong L, Barrett T, Yuan J, Hatzidimitriou G, McCann UD, Becker KG, Donovan DM, Ricaurte GA. Changes in gene expression linked to methamphetamine-induced dopaminergic neurotoxicity. J. Neurosci. 2002;22:274–283. doi: 10.1523/JNEUROSCI.22-01-00274.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]