Abstract
The IrgA homologue adhesin (Iha) is an adherence-conferring outer membrane protein of Escherichia coli associated with enterohemorrhagic and uropathogenic strains. Here, we used primer extension analysis to identify iha promoters in O157:H7 and uropathogenic E. coli strains. Transcriptional fusions demonstrated that iha transcription is repressed by iron. Gel shifts using purified ferric uptake regulator protein (Fur) demonstrated that repression involves a direct interaction between Fur and the iha promoter. We identified strain-dependent differences in iha expression and determined that single nucleotide polymorphisms upstream of the iha promoter, in particular position −85, contribute to differences in expression levels.
Keywords: Iha, enterohemorrhagic E. coli, uropathogenic E. coli, Fur
1. Introduction
Escherichia coli O157:H7 is a Shiga toxin-producing organism that causes diarrhea, bloody diarrhea, and the hemolytic uremic syndrome. A homologue of the locus of enterocyte effacement (LEE) pathogenicity island of enteropathogenic E. coli encodes proteins associated with the attaching and effacing lesions on intestinal epithelial cells colonized by enterohemorrhagic E. coli (EHEC) such as serotype O157:H7 [reviewed in 1]. However, the mechanism of initial adherence of these organisms is not well understood. Candidate EHEC adherence factors include long polar fimbriae (LPF) related to those of Salmonella enterica [2, 3]; ToxB, encoded on plasmid pO157, which positively influences the adherence of O157:H7 [4]; EHEC factor for adherence (efa1) [5] in sorbitol-fermenting O157:H- (but which is not found in the closely related E. coli O157:H7); an sfp operon homologous to the P-fimbrial operon of uropathogenic E. coli also found in sorbitol-fermenting O157:H- [6]; and F9 fimbriae [7].
The IrgA homologue adhesin (Iha) encoded by iha is an adherence-conferring protein of E. coli O157:H7 [8] that is also a catecholate siderophore recepter [9]. iha is present in 25 of 25 O157:H7 isolates of diverse origins but in only 3 of 20 commensal non-O157 fecal E. coli [8]. Additionally, iha is conserved among EHEC of a variety of serotypes [10]. Interestingly, an iha homologue is present on an island located at selC (where LEE is generally integrated into the chromosome) of LEE-negative STEC that produce activatable Stx2d [11].
iha is frequently found in uropathogenic E. coli (UPEC). Johnson et al. found that iha was present in 55% of urosepsis isolates and was more prevalent in isolates from immunocompetent hosts, suggesting that Iha confers some pathogenic advantage [12]. In a different study, Johnson et al. found that presence of iha predicted multiple same-strain recurrences of urinary tract infections (UTI) [13]. Also, iha was more frequent in UTI than fecal isolates [14]. Finally, iha is required for full virulence of the pyelonephritis isolate CFT073 in a mouse model of ascending UTI, defining it as a virulence factor, at least in this organism [15].
Some data have addressed expression of Iha. Tarr et al. found that a 78 kDa protein, which is the predicted size of Iha and which was reactive with an anti-Iha antibody, was expressed in outer membrane proteins (OMPs) from recombinant strain ORN172 transformed with a plasmid that expressed Iha (pIHA); however, they did not observe a 78 kDa protein in OMPs of iha-containing O157:H7 isolates which were grown in LB [8]. Schmidt et al. noted that Iha of non-O157:H7 STEC was more easily detected in OMPs of bacteria grown in glycerol-DMEM than LB [11]. Likewise, Iha is better expressed in CFT073 when grown in DMEM but not LB [15]. Also, iha was expressed in vivo in a mouse model of peritonitis during infection with CFT073 [16]. Following preparation of this manuscript, Leveille et al. reported that Iha of the uropathogen UCB34 is regulated by iron availability [9]. Finally, we have recently identified iha expression in E. coli O157:H7 in stools that have been freshly shed by infected children and experimentally inoculated cattle[17].
The purpose of this work was to characterize the mechanism of regulation of Iha expression in E. coli O157:H7 strain 86-24 [18] and UPEC O6:K2:H1 strain CFT073 [19], specifically, testing the hypothesis that iha is regulated at the transcriptional level by iron. This hypothesis is based on the presence of a ferric uptake regulator protein (Fur) binding site predicted upstream of the iha coding region [20].
2. Results
2.1. Identification of the iha promoter
To examine the regulation of expression of iha, we first identified its promoter. Primer extensions to determine the 5′ end of iha mRNA in recombinant E. coli strain ORN172 (pIHA) [8] identified an extension product that is not present in the vector control. This product is also present in clinical isolates 86-24 and CFT073 when these organisms are grown in DMEM minimal medium (figure 1A). S1 nuclease protection of ORN172 (pIHA) confirmed that this product represents a true 5′ end rather than a transcriptional pause site (data not shown). Though primer extension analysis identified two 5′ ends, at G and T, only the T was identified using S1 nuclease analysis. Insertion of the omega fragment containing transcriptional terminators [21] in ORN172 (pIHA) upstream of the putative promoter region did not abolish the primer extension product, indicating this product is the result of transcription from the iha, and not a vector, promoter (data not shown). The omega fragment was inserted in the correct orientation to block transcription [22].
Figure 1. Identification of the iha promoter region.

(A): Identification of the 5′ end of the iha transcript.
Primer extension products were analyzed on a 6% acrylamide sequencing gel. A product is observed in ORN172(pIHA) but not in the vector control ORN172(pSK+). A product of the same size is observed in clinical isolates CFT073 and 86-24 when grown in DMEM minimal medium.
(B): Promoter region identified by primer extension analysis.
Putative ribosome binding site, −10 and −35 sequences, and putative Fur binding site are noted. The transcriptional start site identified by primer extensions and nuclease protection assays is underlined.
The −10 and −35 sequences upstream of the transcriptional start sites are identical in strains 86-24 and CFT073 (figure 1B). Homology between strains CFT073 and 86-24 upstream of iha begins 464 bp upstream of the transcriptional start site, and there are 5 single nucleotide polymorphisms in this interval, at positions −460, −414, −214, −85, and −51. The sequence upstream of iha in the genomes of O157:H7 strains EDL933 and Sakai are identical to 86-24 at these five positions [23, 24].
2.2. iha is repressed by iron
We hypothesized that Fur represses iha in the presence of iron, based on the prediction of Panina et. al, who identified a putative Fur binding site upstream of iha that contains 13 of the 19 bases of the consensus site [20] and spans the −35 locus. To test this hypothesis, we first examined iha transcriptional regulation by constructing single copy chromosomal transcriptional fusions of approximately 1.6 kb of iha with the firefly luc reporter gene. We chose to use chromosomal fusions so that any upstream regulatory elements would be included. A constitutively expressed sea pansy Renilla reniformis luc gene was utilized as a control, and luciferase activity was measured as the ratio of firefly:Renilla Relative Light Units (RLU) (figure 2). In both 86-24 and CFT073, the fusion was expressed in cells grown in DMEM minimal medium to a significantly greater extent than in DMEM medium supplemented with 10 μM FeSO4. Expression in CFT073 was approximately 25-fold higher in DMEM than iron-supplemented DMEM, and expression in 86-24 was approximately 2–5-fold higher in DMEM than iron-supplemented DMEM. iha expression in LB was approximately equal to iha expression in iron-supplemented DMEM for both strains. Addition of 200 μM 2,2′dipyridyl, an iron chelator, to LB increased expression.
Figure 2. iha transcription is repressed in the presence of iron.
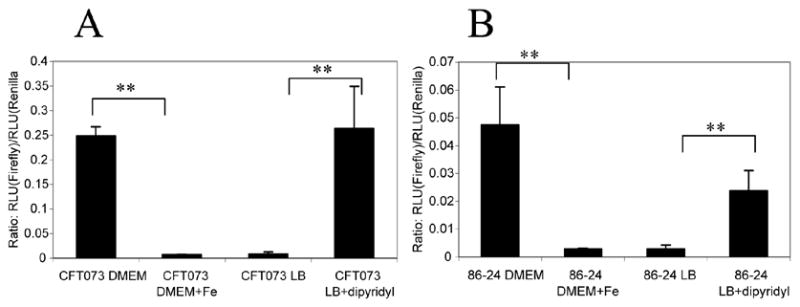
Cells containing single-copy chromosomal transcriptional fusions between iha and firefly luc were grown in DMEM minimal medium with or without addition of 10 μM FeSO4.7H2O. Cells were grown in LB with or without the addition of 200 μM of the iron chelator 2,2′dipyridyl. Results are shown as the ratio of firefly: Renilla RLU at OD600 0.6. (A) CFT073B5 grown in the presence or absence of iron. (B) 86-24A3 grown in the presence or absence of iron.
**p < 0.01
To determine if iha gene expression correlates with levels of protein, Iha was detected by immunoblot analysis in outer membrane protein preparations of 86-24 and CFT073 grown in DMEM with or without added iron, and LB with or without added 2,2′dipyridyl. As predicted, a 78 kDa band representing Iha was detected in cells grown under iron-limiting, but not iron-replete, conditions (figure 3).
Figure 3. Iha is expressed under iron-limiting conditions.

Iha expression was examined in outer membrane preparations of overnight cultures grown in iron-replete or iron-limiting conditions. 2 μg total outer membranes were separated by SDS-PAGE and transferred to nitrocellulose for immunoblot analysis (left) or stained with Coomassie blue (right).
(A): Addition of 10 μM FeSO4.7H2O to DMEM abolishes Iha expression in the outer membrane.
(B): Addition of the iron chelator 2,2′dipyridyl to LB results in expression of Iha in the outer membrane.
2.3. Interaction of the iha promoter with Fur
The observed transcriptional repression of iha in the presence of iron is consistent with our hypothesized role for Fur in iha regulation. To further determine if Fur binds directly to the iha promoter region, Fur was isolated for use in gel-shift analysis.
A 150 bp PCR product surrounding the putative Fur-binding site was used as a probe in gel-shift analysis. The probe’s migration through a polyacrylamide gel was retarded after adding increasing concentrations of Fur (figure 4A), indicating that Fur bound the iha promoter region. Multiple shift products were observed, as has been described for Fur [25, 26]. Abundance of the higher molecular weight complex was decreased when the binding reaction was incubated in increasing (competitive) concentrations of unlabeled probe or of a short double-stranded oligonucleotide consisting of only the putative iha Fur-binding site flanked by 7 bp on either side (figure 4C). This suggests competition for Fur between the binding site on the labeled probe and on the competitor DNA. A second short oligonucleotide containing a 5 bp mutation previously demonstrated to reduce Fur binding to the FepA promoter [27] had diminished ability to inhibit formation of the higher molecular weight complex (figure 4C). Together, these data demonstrate that Fur binds to the iha promoter at the site predicted by Panina et. al [20].
Figure 4. Fur binds the iha promoter region.
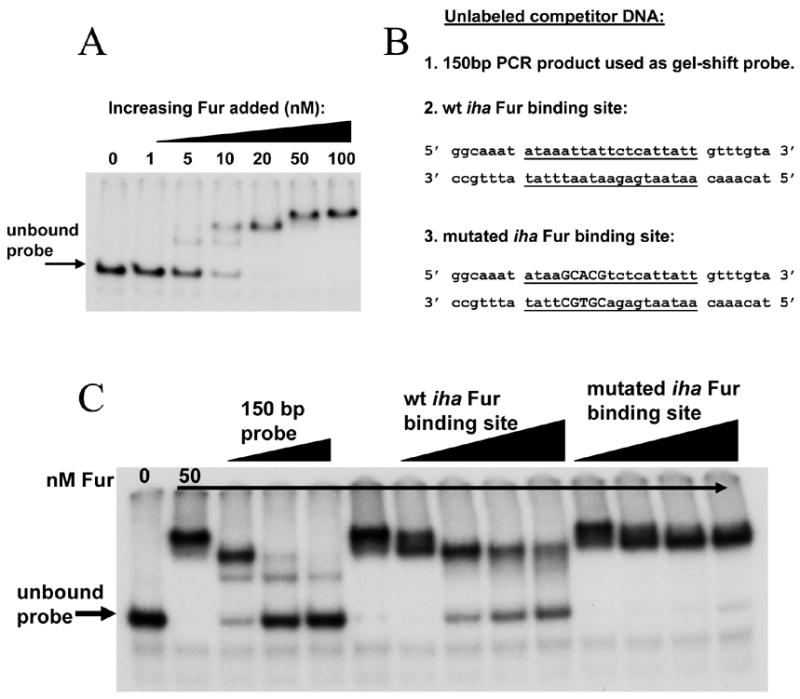
(A): A 150 bp DNA fragment encompassing the putative Fur-binding site was radiolabelled, incubated with increasing concentrations of Fur protein, and separated on a 5% acrylamide gel.
(B): Unlabeled competitor DNA sequences. Center 19 nucleotides represent the putative iha Fur binding site (underlined). Capital letters represent sequence that has been changed to decrease Fur binding.
(C): Fur binding in the presence of unlabeled competitor DNA. Lane 1, probe only; all other lanes, probe with 50 nM Fur. Lanes 3–5 contain 0.5 pMol, 2 pMol, and 5 pMol unlabeled probe, respectively. Lanes 7–10 contain 10 ng, 50 ng, 100 ng, and 150 ng, respectively, of unlabeled wt iha Fur binding site. Lanes 11–14 contain 10 ng, 50 ng, 100 ng, and 150 ng, respectively, of unlabeled mutated iha Fur binding site.
2.4. Additional regulation of iha
During the course of this work, we observed that under iron-limiting conditions, CFT073 expresses iha to a greater extent than does 86-24, as demonstrated by .primer extensions (figure 1A) and luciferase assays (figure 2); these observations correlate with expression of Iha antigen (figure 3). Northern analyses of 86-24Δfur and CFT073Δfur confirm that iha message is more abundant in CFT073 even in the absence of Fur (data not shown). As expected, iha expression in 86-24Δfur and CFT073Δfur is constitutive in iron-replete and iron-poor media; however, we still observe higher iha expression in the CFT073 background, suggesting this difference is independent of Fur-mediated repression.
To test the hypothesis that this difference might be associated with the nucleotide polymorphisms closest to the promoter region (positions −51 and −85), we inserted the promoter regions of 86-24 and CFT073 into plasmid pRS415 to drive expression of the promoterless lacZ gene. β-galactosidase activities for these fusions were measured in strain CC1005, a host in which pRS415 copy number is reduced to approximately 10% [28]. By examining the fusions in identical backgrounds, we were able to determine the effects of these polymorphisms without the complications that might arise from differences in strain background. pCFT073ihaP, containing the promoter from CFT073, yielded approximately 1.3-fold higher activity than pO157ihaP (figure 5) (P = 0.02). This suggests that the nucleotide differences between the two strains at positions −51 and/or −85 contribute to differences in iha expression.
Figure 5. Role of single nucleotide polymorphisms in iha expression.
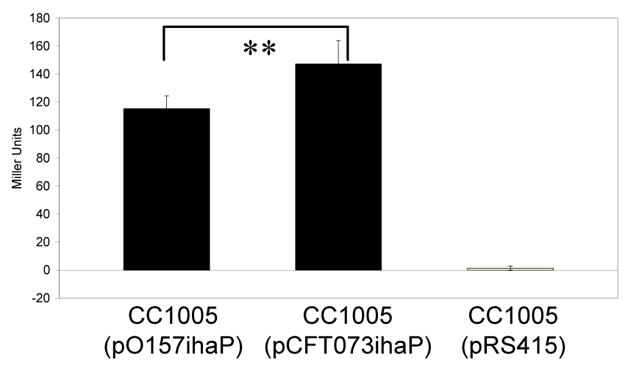
Cells were grown to mid-log phase in LB containing 200 μM 2,2′dipyridyl and assayed for β-galactosidase activity as expressed in Miller Units. CC1005(pO157ihaP) contains the promoter from 86-24 driving lacZ expression. CC1005(pCFT073ihaP) contains the promoter from CFT073 driving lacZ expression. CC1005(pRS415) is the vector-only control. **P = 0.02
To elucidate which position is responsible for differences in iha expression, two additional lacZ promoter fusions were constructed: plasmid pihaP-G/T contains the −85 position G (identical to 86-24) and the −51 position T (identical to CFT073). Plasmid pihaP-A/C contains the −85 position A (identical to CFT073) and the −51 position C (identical to 86-24). CC1005 (pihaP-A/C) had expression similar to CC1005 (pCFT073ihaP), whereas CC1005 (pihaP-G/T) had expression similar to CC1005 (pO157ihaP) (figure 6). These data suggest that the −85 polymorphism most strongly influences iha expression.
Figure 6. Role of individual polymorphisms in iha expression.
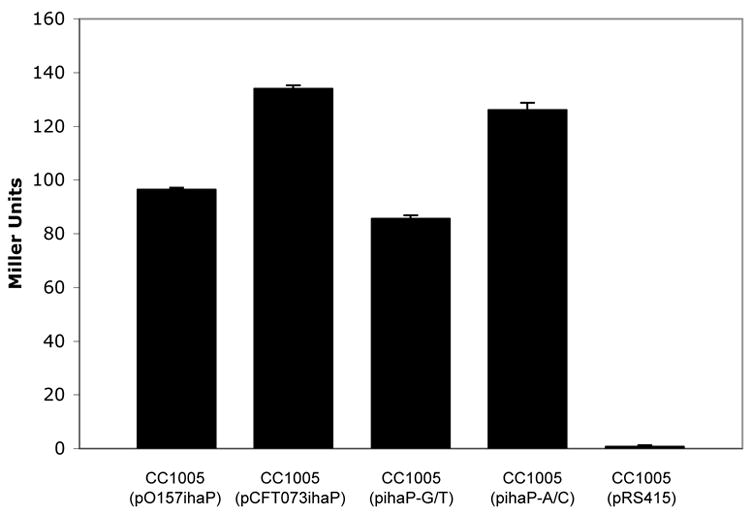
Cells were grown to mid-log phase in LB containing 200 μM 2,2′dipyridyl and assayed for β-galactosidase activity as expressed in Miller Units. CC1005 (pCFT073ihaP) and CC1005 (pihaP-A/C) express significantly more β-galactosidase than CC1005 (pO157ihaP) and CC1005 (pihaP-G/T). P < 0.05
3. Discussion
Iron is vital for bacterial growth; but it is essential that bacteria strictly regulate the amount of iron that enters, because excess iron catalyzes the production of the hydroxy radical (OH−) [29]. One method to monitor and to respond to the iron in the bacterial cell is via the ferric uptake regulation (Fur) protein [30]. In the presence of iron, Fur dimerizes and can bind DNA to repress transcription. In the absence of iron, Fur does not bind DNA, and target genes are expressed. Fur regulates several genes involved in iron uptake, and it also provides the cell with a way to express other genes in an iron-limited environment. Because mucosal surfaces of the mammalian host are iron-poor environments due to the high affinity of lactoferrin for free iron [reviewed in 31], several bacterial virulence factors, including stx1 of E. coli and irgA of V. cholerae, are transcribed under iron-poor conditions [32, 33]. In this study, we demonstrate that iha is repressed by iron via a direct interaction between Fur and the iha promoter. This regulation might allow optimal expression of iha within the host, where its role as an adhesin would be useful to the bacterium. Consistent with this hypothesis, iha was upregulated in the mouse model of peritonitis [16] and is required for full virulence of UPEC [15]. Our results explain why Schmidt et al. [11] and Johnson et al. [15] found Iha to be more readily detected in minimal medium than in LB and why Tarr et al. [8] did not detect Iha in O157 isolates grown in LB.
Iron uptake systems are vital to bacterial survival within the host, and many bacteria secrete siderophores, which bind iron and are brought into the bacterial cell via a specific siderophore receptor. Iha has 33% identity to IrgA of Vibrio cholerae and 27% identity to FepA of E. coli. Both of these proteins are receptors for the siderophore enterobactin and, like Iha, are directly repressed by Fur [27, 34–36]. In fact, Iha from the uropathogen, UCB34, was recently determined to be iron-repressed and to act as both a catecholate siderophore receptor and an adhesin [9].
Interestingly, our results demonstrate that iha is more highly expressed in CFT073 than in 86-24 under iron-limiting conditions. Although the −35 and −10 regions are identical in these strains, it is possible that single nucleotide polymorphisms in other areas of the promoter region might affect iha transcription, in particular those closest to the promoter region, at positions −51 and −85. Our data demonstrate that these nucleotides do contribute to differential expression of iha, and that this is mainly the results of position −85, though it is unlikely to fully account for the observed differences in expression levels. It is likely that additional strain-specific factors play a role, for example a repressor in 86-24, an activator in CFT073, or additional 5′ polymorphisms such as those at −460, −414, and −214.
In conclusion, regulation of Iha occurs at the transcriptional level via a direct interaction with Fur, which represses iha expression in the presence of iron. Though the mechanism of repression is the same, iha is expressed differently in 86-24 and CFT073 under iron-limiting conditions. Single nucleotide polymorphisms near the promoter, in particular that at position −85, contribute to this difference.
4. Materials and Methods
4.1. Media and growth conditions
Bacteria were grown at 37° in Luria-Bertani (LB) medium or in DMEM minimal medium prepared as described [15]. Ampicillin (100μg/ml), chloramphenicol (25 μg/ml), gentamicin (15 μg/ml), and nalidixic acid (15μg/ml) were added, if appropriate. To replete or restrict iron, FeSO4.7H2O was added to DMEM minimal medium to a final concentration of 10 μM, or 2,2′dipyridyl was added to a final concentration of 200 μM to LB, respectively.
4.2. Insertion of omega fragment
To insert the omega fragment upstream of iha, pUC19Ω [21] was digested with SmaI and the omega fragment moved into the SphI site of pIHA that had been blunt-ended using T4 DNA polymerase (NEB). Correct orientation of the omega fragment was confirmed by sequencing with T7 Sequenase v2.0 (USB).
4.3. Primer extension analysis
RNA was extracted from bacteria [37] that had been grown to logarithmic phase in a shaking incubator at 37°C in LB containing ampicillin for recombinant strains, or in DMEM minimal medium for strains 86-24 and CFT073. Primer extensions were performed using primer iha.PE2 (5′CGAGACAGGGAATGACTACGGAAGC3′) as described [38].
4.4. S1 nuclease analysis
End-labeling of probe, hybridization, and S1 nuclease digestion were performed as described [39] using primer iha.s1 (5′CGCATATTACTAATCTCCGATCATGTTAACCGGCAAGACAATACACAAATA CAAACAATAATGAGAATAATTTATATTTGCC3′).
4.5. Construction of chromosomal iha-luciferase transcriptional fusions
pIHA was digested with EcoRI and NaeI, each of which cleaves within the iha open reading frame. A 567 bp fragment was gel-purified and ligated into vector pGPL01 [40], which had been digested with SmaI and EcoRI. pGPL01 is a suicide vector with a lambda/pir-dependent origin of replication that contains the promoterless luc gene encoding firefly luciferase. The resulting 6.5 kb construct, pGPL01R3, containing 567 bp of iha sequence in-frame with a TAA stop codon upstream of luc, was transformed into SM10λpir and used as the donor strain in matings where 86-24nalR [41] or CFT073nalR [15] was the recipient. Matings were performed as described previously [42] and nalidixic acid-resistant, ampicillin-resistant transconjugates, designated 86-24A3 and CFT073B5, respectively, were selected. Integration of the suicide vector into the chromosome in the correct orientation was confirmed by PCR analysis using a forward primer in iha and a reverse primer in luc.
Vector pEM42 (gift of Dr. Samuel Miller) contains the tet promoter from pBR322 driving expression of the sea pansy Renilla reniformis luciferase in pRL-Pnull (Promega). pCRIICam (gift of Dr. Brad Cookson) contains cat in the AgeI site of pCRII (Invitrogen). cat was excised from pCRIICam with AgeI and blunt ends were created using T4 DNA polymerase as directed by the manufacturer (NEB). This product was inserted into the BamHI site of pEM42, which had been blunt-ended with T4 DNA polymerase, to create pRL-PtetCm. This construct was electroporated into 86-24A3 and CFT073B5, where it served as a constitutively expressed control in the luciferase assays.
4.6. Luciferase assays
Overnight cultures of cells were diluted and grown to OD600 = 0.6 in 3 ml of appropriate medium. 90 μl of culture was mixed with 10 μl 1 M K2HP04 (pH 7.8)/20 mM EDTA (pH 8.0) and frozen at −80°C. Samples were thawed and analyzed with the Dual-Luciferase Reporter Assay System (Promega) per the manufacturer’s recommendations. Data reported represent the ratio of firefly to Renilla luciferase activity in relative light units (RLU) for three experiments measured in duplicate.
4.7. Construction of iha-β-galactosidase transcriptional fusions
The iha promoter regions from 86-24 and CFT073, encompassing the −51 and −85 single nucleotide polymorphisms, were amplified using primers iha.BamF (5′GGGATCCAATTCTGGCATGCCGAGGCAGTCG3′) [8] and RR10Bam-2 (5′GGGATCCTCAACATCCACACCCTCTAC3′). The resulting product was digested with BamHI, ligated into the BamHI site of vector pRS415 [43], and transformed into DH5α. pRS415 contains a promoterless lacZ; thus, colonies were selected that expressed β-galactosidase on iron-limiting plates containing X-gal and ampicillin. The correct sequence and orientation of the iha promoter was confirmed via sequence analysis using BigDye v3.1 (Applied Biosystems). Resulting vectors containing the 86-24 or CFT073 iha promoter driving β-galactosidase expression were named pO157ihaP and pCFT073ihaP, respectively. These plasmids were introduced into strain CC1005 (MC4100 pcnBTn10 ΔlacU169 araD139 rpsL thi) (gift of Dr. Colin Manoil). The number of ColE1-related plasmids is reduced in E. coli pcnB mutants to approximately 10% [28]; therefore, pRS415, which has an origin of replication from pBR322, is maintained at low copy number in CC1005.
To construct pihaP-G/T and pihaP-A/C, the iha promoter regions of 86-24 and CFTO73 were amplified using two polymerase chain reactions. Primers RR15 (5′ CGGGATCCCCATTCATGGTTGAC 3′) and RR14 (5′ CTTTTACAAAAAATAAAGATTATTGAC 3′) were used to amplify the region surrounding position −85. Primers RR13 (5′ GTCAATAATCTTTATTTTTTGTAAAAG 3′) and RR10-Bam.2 (5′ GGGATCCTCAACATCCACACCCTCTAC 3′) were used to amplify the region surrounding position −51. The resulting amplicons were column purified using the QIAquick PCR Purification Kit (Qiagen, Maryland), and 1 μL of the upstream and downstream amplicon were used as template to amplify the entire promoter region with primers RR15 and RR10-Bam.2. The program parameters were: (94°C 1 min, 55°C 1 min, 72°C 3 min) x 30 cycles. The resulting 468 bp product was gel-purified using the QIAquick Gel Extraction Kit (Qiagen, Maryland), digested with BamHI, ligated into the BanHI site of pRS415, and transformed into DH5α. Colonies were selected that expressed β-galactosidase on iron-limiting plates containing X-gal and ampicillin. Correct sequence and orientation of the iha promoter was confirmed via sequence analysis using BigDye v3.1 (Applied Biosystems). pihaP-G/T contains the −85 region from 86-24 and the −51 region from CFT073. pihaP-A/C contains the −85 region from CFT073 and the −51 region from 86-24. pihaP-G/T and pihaP-A/C were moved into the CC1005 background for use in β-galactosidase assays.
4.8. β-galactosidase assays
Overnight cultures of CC1005 (pO157ihaP) and CC1005 (pCFT073ihaP) were diluted in LB containing ampicillin and 2, 2′ dipyridyl and grown to OD600 = 0.5 – 0.6. Cells were iced for 20 minutes, then pelleted before resuspension in 1x PBS. β-galactosidase activity was measured [42], and data reported represent the results of four experiments performed in triplicate for Figure 5. For Figure 6, data reported represent the results of one representative experiment performed in triplicate. Three additional experiments were performed with similar results.
4.9. OMP preparation and analysis
OMPs were prepared as previously described [44] from bacteria grown overnight at 37°C with shaking in 100 ml of medium, except that cells were lysed by French press. The protein concentration was determined using the Bradford Assay [45], and 2 μg outer membrane proteins were separated on a 12% SDS-polyacrylamide gel. To observe total protein, gels were Coomassie stained and stored in acetate paper [46].
To observe Iha, proteins were transferred to a nitrocellulose membrane (PROTRAN; Schleicher & Schuell BioScience) for two hours at 7.5 volts/cm in Towbin transfer buffer (25 mM Tris, 192 mM glycine, 20% MeOH, 0.1% SDS). The membranes were blocked in PBS containing 0.05% Tween-20 and 5% (w/v) non-fat dried milk for two hours at room temperature, and then washed in PBS containing 0.05% Tween-20 (PBS-T) and incubated at 4°C overnight in rabbit polyclonal anti-Iha antibody [8] diluted 1:2000 in PBS-T. The membranes were then washed three times in PBS-T and incubated in goat anti-rabbit alkaline phosphatase (AP)-conjugated antibody (Pierce, Rockford, IL), diluted 1:2000 in PBS-T for one hour at room temperature. The membrane was washed three times and bound antibodies were detected in AP assay buffer (0.1 M Tris pH 9.5, 5 mM MgCl2, 100 mM NaCl) by adding nitro blue tetrazolium/5-bromo-4-chloro-3-indolylphosphate (NBT/BCIP).
4.10. Slot blot analysis
86-24Δfur and CFT073Δfur were constructed using the λ/Red system to create in-frame chromosomal deletions [47] using primers FurDelL2 (5′CAGGAGCCGGACAACCATCACGTCAGTGCGTGTGTAGGCTGGAGCTGCTT C3′) and FurDelR2 (5′GTGGTTAGTCAGGCGAATGCCATGTTTTGCCATATGAATATCCTCC3′). Analysis was performed on RNA immobilized by slot blotting [39]. Primers primers ihaL2 (5′GAGGCTGGGGTGTATTACGA3′) and ihaR2 (5′CAGTGACAGCGTGACATCCT3′) were used to amplify iha in 86-24, and the resulting amplicon was used to create a probe using the Random Primers DNA Labeling System (Invitrogen) following instructions of the manufacturer.
4.11. Fur Purification
pET21dFur (gift of Dr. Thomas O’Halloran) contains fur digested from pARC306FUR [48] using HindIII and NcoI and moved into the same sites of pET21d (Novagen). pET21dFur was transformed into BL21(DE3) (Novagen), and the purification strategy was based on previously published methods [48, 25, 26]. An overnight culture was diluted 1:100 and grown to OD600 = 0.6 while shaking at 37°C. 400 μM isopropyl-β–D-thiogalactopyranoside was added to induce Fur expression, and the culture was grown for an additional 2.5 hours. Cells were harvested by centrifugation and washed in 20 mM Tris-HCL, pH 8.0, 50 mM EDTA. The cell pellet was frozen at −80°C overnight. On the next day, the pellet was suspended in an equal volume (to the pellet) of ice-cold 20 mM Tris-HCL, pH 8.0 containing 1 mM of the protease inhibitor Pefabloc (Boehringer Mannheim), and sonicated. Cell debris was removed by centrifugation, and 20% w/v streptomycin sulfate was added to a final concentration of 1% to precipitate nucleic acids. Nucleic acids were removed by centrifugation at 20,000 x g for 15 minutes at 4°C, and ammonium sulfate was added to the supernatant to 80% saturation. The sample was incubated at 4°C overnight to precipitate protein. Protein was harvested by centrifugation at 20,000 x g for 30 minutes at 4°C. The protein pellet was dissolved in 6 ml 20 mM Tris-HCL, pH 8.0, 1 mM DTT and added to an iminodiacetate agarose (IDA) column that had a bed volume of 3 ml. The IDA resin was obtained from Novagen and equilibrated following the recommendation of the manufacturer using 5 bed volumes of 20 mM ZnSO4. The column was then washed with 3 bed volumes of IDA Wash Buffer (50 mM Tris-Acetate, pH 7.9; 500 mM NaCl). The sample was run through the column at a flow rate of approximately 0.5 ml/min. The column was washed with 5 bed volumes of IDA Wash Buffer, and protein was eluted in wash buffer containing increasing amounts of L-histidine. Sample aliquots were mixed with Bradford Reagent, and samples containing protein were pooled and precipitated by adding ammonium sulfate to 80% saturation. Protein was centrifuged as before; the pellet was suspended in 1 ml HEPES buffer (20 mM HEPES, pH 7.0, 0.1 mM NaCl, 2 mM DTT, 5% glycerol) and added to a Sephadex G25 column to de-salt. Final protein concentration was determined using the Bradford Assay [45]. Recovered Fur shows a predominant protein band of 17 kDa on SDS-PAGE, which reacts with polyclonal anti-Fur antibody in immunoblot analysis (data not shown).
4.12. Gel-Shift Analysis
Primers iha.EcoR (5′CGGAATTCCGATCTCCGATCATGTTAACCG3′) and iha.EcoL (5′CGGAATTCCGGCATGCCGAGGCAGTCGTTA3′), containing EcoRI sites at the 5′ ends, were used to amplify an approximately 150 bp PCR product surrounding the putative Fur binding site of the iha promoter. PCR was performed using Amplitaq, buffer, and MgCl2 from Applied Biosystems, and dNTPs from NEB. 4 pmol of this product was digested with EcoRI and end-labeled with 32P using T4 Polynucleotide Kinase as directed by the manufacturer (NEB). Reaction products were cleaned using a Sephadex G50 column (Nick Column; Amersham) per the manufacturer’s recommendations. Approximately 125 fmol/ml of probe was used in a binding reaction containing 40 mM KCl, 50 μg/ml polydIdC, 10 mM BisTris, pH 7.5, 100 μg/ml BSA, 100 μM MnCl2.4H2O, 1 mM MgCl2.6H2O, 5% glycerol, and increasing concentrations of Fur [49]. 20 μl of the binding reaction was electrophoresed on a 5% acrylamide gel containing 20 mM BisTris (pH 7.5), 100 μM MnCl2.4H2O. Running buffer was 20 mM BisTris (pH 7.5), 100 μM MnCl2.4H2O. The gel was run at 5.3 V/cm, and the buffer in the upper chamber was replaced with fresh buffer as it oxidized.
Table 1.
Strains and Plasmids
| E. coli strains | Description | Reference |
|---|---|---|
| 86-24 | EHEC O157:H7 | [18] |
| CFT073 | UPEC O6:K2:H1 | [19] |
| SM10λpir | λ/pir integrate with RP4 mob function | [50] |
| CC1005 | MC4100 pcnBTn10 DlacU169 araD139 rpsL thi | gift of Dr. Colin Manoil |
| BL21(DE3) | F-ompT hsdS gal dcm (DE3) | Novagen |
| 86-24nalR | [41] | |
| CFT073nalR | [15] | |
| ORN172 | [51] | |
| 86-24A3 | contains chromosomal iha-luc transcriptional fusion | this work |
| CFT073B5 | contains chromosomal iha-luc transcriptional fusion | this work |
|
| ||
| plasmids | Description | Reference |
|
| ||
| pIHA | iha in pBluescript | [8] |
| pUFRO47 | IncW replicon | [52] |
| pUC19Ω | omega fragment in pUC19 | [21] |
| pGPL01 | λ/pir-dependent origin of replication and promoterless luc | [40] |
| pRS415 | contains promoterless β-galactosidase and origin of replication from pBR322 | [43] |
| pEM42 | tet promoter drives expression of Renillaluciferase in pRL-Pnull | gift of Dr. Samuel Miller |
| pCRIICam | cat in AgeI site of pCRII | gift of Dr. Brad Cookson |
| pET21dFur | fur in pET21d | gift of Dr. T.V. O’Halloran |
| pGPL01R3 | iha sequence upstream of luc in pGPL01 | this work |
| pRL-PtetCm | cat from pCRIICam in Bam HI site of pEM42 | this work |
| pO157ihaP | 86-24 iha promoter driving β-galactosidase expression in pRS415 | this work |
| pCFTO73ihaP | CFTO73 iha promoter driving β-galactosidase expression in pRS415 | this work |
| pihaP-G/T | same as pO157ihaP but with "T" at −51 | this work |
| pihaP-A/C | same as pO157ihaP but with "A" at −85 | this work |
Acknowledgments
The authors wish to thank Drs. Thomas O’Halloran, Samuel Miller, Brad Cookson, and Colin Manoil for generously sharing strains. The authors also wish to thank members of the Moseley and Sokurenko laboratories and Srdjan Jelacic and Konstantin Korotkov for many helpful discussions and Tami Tabata, Irena Medenica, and Diane Capps for laboratory technical support.
R. Rashid was supported in part by Public Health Service National Research Service award GM07270 and R01 AI47499.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Goosney DL, Gruenheid S, Finlay BB. Gut feelings: enteropathogenic E. coli (EPEC) interactions with the host. Annu Rev Cell Dev Biol. 2000;16:173–89. doi: 10.1146/annurev.cellbio.16.1.173. [DOI] [PubMed] [Google Scholar]
- 2.Doughty S, Sloan J, Bennett-Wood V, Robertson M, Robins-Browne RM, Hartland EL. Identification of a novel fimbrial gene cluster related to long polar fimbriae in locus of enterocyte effacement-negative strains of enterohemorrhagic Escherichia coli. Infect Immun. 2002 Dec;70(12):6761–9. doi: 10.1128/IAI.70.12.6761-6769.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torres AG, Giron JA, Perna NT, Burland V, Blattner FR, Avelino-Flores F, et al. Identification and characterization of lpfABCC’DE, a fimbrial operon of enterohemorrhagic Escherichia coli O157:H7. Infect Immun. 2002 Oct;70(10):5416–27. doi: 10.1128/IAI.70.10.5416-5427.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tatsuno I, Horie M, Abe H, Miki T, Makino K, Shinagawa H, et al. toxB gene on pO157 of enterohemorrhagic Escherichia coli O157:H7 is required for full epithelial cell adherence phenotype. Infect Immun. 2001 Nov;69(11):6660–9. doi: 10.1128/IAI.69.11.6660-6669.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicholls L, Grant TH, Robins-Browne RM. Identification of a novel genetic locus that is required for in vitro adhesion of a clinical isolate of enterohaemorrhagic Escherichia coli to epithelial cells. Mol Microbiol. 2000 Jan;35(2):275–88. doi: 10.1046/j.1365-2958.2000.01690.x. [DOI] [PubMed] [Google Scholar]
- 6.Brunder W, Khan AS, Hacker J, Karch H. Novel type of fimbriae encoded by the large plasmid of sorbitol-fermenting enterohemorrhagic Escherichia coli O157:H(-) Infect Immun. 2001 Jul;69(7):4447–57. doi: 10.1128/IAI.69.7.4447-4457.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Low AS, Dziva F, Torres AG, Martinez JL, Rosser T, Naylor S, et al. Cloning, Expression, and Characterization of Fimbrial Operon F9 from Enterohemorrhagic Escherichia coli O157:H7. Infect Immun. 2006 Apr;74(4):2233–44. doi: 10.1128/IAI.74.4.2233-2244.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarr PI, Bilge SS, Vary JC, Jr, Jelacic S, Habeeb RL, Ward TR, et al. Iha: a novel Escherichia coli O157:H7 adherence-conferring molecule encoded on a recently acquired chromosomal island of conserved structure. Infect Immun. 2000 Apr;68(3):1400–7. doi: 10.1128/iai.68.3.1400-1407.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leveille S, Caza M, Johnson JR, Clabots C, Sabri M, Dozois CM. Iha from an Escherichia coli urinary tract infection outbreak clonal group A strain is expressed in vivo in the mouse urinary tract functions as a catecholate siderophore receptor. Infect Immun. 2006 Jun;74(6):3427–36. doi: 10.1128/IAI.00107-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toma C, Martinez Espinosa E, Song T, Miliwebsky E, Chinen I, Iyoda S, et al. Distribution of Putative Adhesins in Different Seropathotypes of Shiga Toxin-Producing Escherichia coli. J Clin Microbiol. 2004 Dec;42(11):4937–46. doi: 10.1128/JCM.42.11.4937-4946.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schmidt H, Zhang WL, Hemmrich U, Jelacic S, Brunder W, Tarr PI, et al. Identification and characterization of a novel genomic island integrated at selC in locus of enterocyte effacement-negative, Shiga toxin-producing Escherichia coli. Infect Immun. 2001 Dec;69(11):6863–73. doi: 10.1128/IAI.69.11.6863-6873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson JR, Russo TA, Tarr PI, Carlino U, Bilge SS, Vary JC, Jr, et al. Molecular epidemiological and phylogenetic associations of two novel putative virulence genesihairoN(E. coli) among Escherichia coli isolates from patients with urosepsis. Infect Immun. 2000 Jun;68(5):3040–7. doi: 10.1128/iai.68.5.3040-3047.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson JR, O’Bryan TT, Delavari P, Kuskowski M, Stapleton A, Carlino U, et al. Clonal relationships and extended virulence genotypes among Escherichia coli isolates from women with a first or recurrent episode of cystitis. J Infect Dis. 2001 Jun 15;183(10):1508–17. doi: 10.1086/320198. [DOI] [PubMed] [Google Scholar]
- 14.Kanamaru S, Kurazono H, Ishitoya S, Terai A, Habuchi T, Nakano M, et al. Distribution and genetic association of putative uropathogenic virulence factors iroN, iha, kpsMT, ompT and usp in Escherichia coli isolated from urinary tract infections in Japan. J Urol. 2003 Dec;170(6 Pt 1):2490–3. doi: 10.1097/01.ju.0000094185.48467.dc. [DOI] [PubMed] [Google Scholar]
- 15.Johnson JR, Jelacic S, Schoening LM, Clabots C, Shaikh N, Mobley HL, et al. The IrgA homologue adhesin Iha is an Escherichia coli virulence factor in murine urinary tract infection. Infect Immun. 2005 Feb;73(2):965–71. doi: 10.1128/IAI.73.2.965-971.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Redford P, Roesch PL, Welch RA. DegS is necessary for virulence and is among extraintestinal Escherichia coli genes induced in murine peritonitis. Infect Immun. 2003 Jul;71(6):3088–96. doi: 10.1128/IAI.71.6.3088-3096.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rashid RA, Tabata TA, Oatley MJ, Besser TE, Tarr PI, Moseley SL. Expression of putative virulence factors of Escherichia coli O157:H7 differs in bovine human infections. Infect Immun. 2006 Jul;74(7):4142–8. doi: 10.1128/IAI.00299-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tarr PI, Neill MA, Clausen CR, Newland JW, Neill RJ, Moseley SL. Genotypic variation in pathogenic Escherichia coli O157:H7 isolated from patients in Washington, 1984–1987. J Infect Dis. 1989 Feb;159(2):344–7. doi: 10.1093/infdis/159.2.344. [DOI] [PubMed] [Google Scholar]
- 19.Mobley HL, Green DM, Trifillis AL, Johnson DE, Chippendale GR, Lockatell CV, et al. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun. 1990 May;58(5):1281–9. doi: 10.1128/iai.58.5.1281-1289.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Panina EM, Mironov AA, Gelfand MS. Comparative analysis of FUR regulons in gamma-proteobacteria. Nucleic Acids Res. 2001 15;29(24):5195–206. doi: 10.1093/nar/29.24.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prentki P, Krisch HM. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984 Sep;29(3):303–13. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 22.Zeilstra-Ryalls JH, Somerville RL. Orientation of the aadA gene within the omega fragment. Biotechniques. 1990 Sep;9(3):301–2. [PubMed] [Google Scholar]
- 23.Hayashi T, Makino K, Ohnishi M, Kurokawa K, Ishii K, Yokoyama K, et al. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 2001 Feb 28;8(1):11–22. doi: 10.1093/dnares/8.1.11. [DOI] [PubMed] [Google Scholar]
- 24.Perna NT, Plunkett G, 3rd, Burland V, Mau B, Glasner JD, Rose DJ, et al. Nature. 2001 Feb 25;409(6819):529–33. doi: 10.1038/35054089. [DOI] [PubMed] [Google Scholar]
- 25.Lavrrar JL, Christoffersen CA, McIntosh MA. Fur-DNA interactions at the bidirectional fepDGC-entS promoter region in Escherichia coli. J Mol Biol. 2002 Oct 4;322(5):983–95. doi: 10.1016/s0022-2836(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 26.Wee S, Neilands JB, Bittner ML, Hemming BC, Haymore BL, Seetharam R. Expression isolation properties of Fur (ferric uptake regulation) protein of Escherichia coli K 12. Biol Met. 1988;1(1):62–8. doi: 10.1007/BF01128019. [DOI] [PubMed] [Google Scholar]
- 27.Hunt MD, Pettis GS, McIntosh MA. Promoter and operator determinants for fur-mediated iron regulation in the bidirectional fepA-fes control region of the Escherichia coli enterobactin gene system. J Bacteriol. 1994 Jul;176(13):3944–55. doi: 10.1128/jb.176.13.3944-3955.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He L, Soderbom F, Wagner EG, Binnie U, Binns N, Masters M. PcnB is required for the rapid degradation of RNAI the antisense RNA that controls the copy number of ColE1-related plasmids. Mol Microbiol. 1993 Sep;9(6):1131–42. doi: 10.1111/j.1365-2958.1993.tb01243.x. [DOI] [PubMed] [Google Scholar]
- 29.Touati D. Iron and oxidative stress in bacteria. Arch Biochem Biophys. 2000 Jan 1;373(1):1–6. doi: 10.1006/abbi.1999.1518. [DOI] [PubMed] [Google Scholar]
- 30.Braun V, Hantke K, Koster W. Bacterial iron transport: mechanisms, genetics, and regulation. Met Ions Biol Syst. 1998;35:67–145. [PubMed] [Google Scholar]
- 31.Ward PP, Conneely OM. Lactoferrin: role in iron homeostasis and host defense against microbial infection. Biometals. 2004 Jun;17(3):203–8. doi: 10.1023/b:biom.0000027693.60932.26. [DOI] [PubMed] [Google Scholar]
- 32.Calderwood SB, Mekalanos JJ. Iron regulation of Shiga-like toxin expression in Escherichia coli is mediated by the fur locus. J Bacteriol. 1987 Oct;169(10):4759–64. doi: 10.1128/jb.169.10.4759-4764.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldberg MB, DiRita VJ, Calderwood SB. Identification of an iron-regulated virulence determinant in Vibrio cholerae using TnphoA mutagenesis. Infect Immun. 1990 Feb;58(1):55–60. doi: 10.1128/iai.58.1.55-60.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McIntosh MA, Earhart CF. Coordinate regulation by iron of the synthesis of phenolate compounds and three outer membrane proteins in Escherichia coli. J Bacteriol. 1977 Jul;131(1):331–9. doi: 10.1128/jb.131.1.331-339.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mey AR, Wyckoff EE, Oglesby AG, Rab E, Taylor RK, Payne SM. Identification of the Vibrio cholerae enterobactin receptors VctA and IrgA: IrgA is not required for virulence. Infect Immun. 2002 Aug;70(7):3419–26. doi: 10.1128/IAI.70.7.3419-3426.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Watnick PI, Butterton JR, Calderwood SB. The interaction of the Vibrio cholerae transcription factors, Fur and IrgB, with the overlapping promoters of two virulence genes, irgA and irgB. Gene. 1998 Apr 16;209(1–2):65–70. doi: 10.1016/s0378-1119(98)00018-3. [DOI] [PubMed] [Google Scholar]
- 37.Goluszko P, Moseley SL, Truong LD, Kaul A, Williford JR, Selvarangan R, et al. Development of experimental model of chronic pyelonephritis with Escherichia coli O75:K5:H-bearing Dr fimbriae: mutation in the dra region prevented tubulointerstitial nephritis. J Clin Invest. 1997 Apr 1;99(7):1662–72. doi: 10.1172/JCI119329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bilge SS, Apostol JM, Jr, Fullner KJ, Moseley SL. Transcriptional organization of the F1845 fimbrial adhesin determinant of Escherichia coli. Mol Microbiol. 1993 Mar;7(6):993–1006. doi: 10.1111/j.1365-2958.1993.tb01191.x. [DOI] [PubMed] [Google Scholar]
- 39.Ausubel FRBRKDMJSJS, Struhl K. Current Protocols in Molecular Biology. New York: John Wiley and Sons; 1994. [Google Scholar]
- 40.Gunn JS, Miller SI. PhoP-PhoQ activates transcription of pmrAB, encoding a two-component regulatory system involved in Salmonella typhimurium antimicrobial peptide resistance. J Bacteriol. 1996 Dec;178(23):6857–64. doi: 10.1128/jb.178.23.6857-6864.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bilge SS, Vary JC, Jr, Dowell SF, Tarr PI. Role of the Escherichia coli O157:H7 O side chain in adherence analysis of an rfb locus. Infect Immun. 1996 Nov;64(11):4795–801. doi: 10.1128/iai.64.11.4795-4801.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller JH. Experiments in molecular genetics. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 43.Simons RW, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53(1):85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 44.Achtman M, Mercer A, Kusecek B, Pohl A, Heuzenroeder M, Aaronson W, et al. Six widespread bacterial clones among Escherichia coli K1 isolates. Infect Immun. 1983 Jan;39(1):315–35. doi: 10.1128/iai.39.1.315-335.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 46.Blakesley RW, Boezi JA. A new staining technique for proteins in polyacrylamide gels using coomassie brilliant blue G250. Anal Biochem. 1977 Oct;82(2):580–2. doi: 10.1016/0003-2697(77)90197-x. [DOI] [PubMed] [Google Scholar]
- 47.Datsenko KA, Wanner BL. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A. 2000 Jun 6;97(12):6640–5. doi: 10.1073/pnas.120163297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Althaus EW, Outten CE, Olson KE, Cao H, O’Halloran TV. The ferric uptake regulation (Fur) repressor is a zinc metalloprotein. Biochemistry. 1999 May 18;38(20):6559–69. doi: 10.1021/bi982788s. [DOI] [PubMed] [Google Scholar]
- 49.de Lorenzo V, Giovannini F, Herrero M, Neilands JB. Metal ion regulation of gene expression. Fur repressor-operator interaction at the promoter region of the aerobactin system of pColV-K30. J Mol Biol. 1988 Oct 20;203(4):875–84. doi: 10.1016/0022-2836(88)90113-1. [DOI] [PubMed] [Google Scholar]
- 50.Simon R, Priefer U, Puhler A. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in gram negative bacteria. Bio/Technology. 1983;1:784–91. [Google Scholar]
- 51.Woodall LD, Russell PW, Harris SL, Orndorff PE. Rapid synchronous stable induction of type 1 piliation in Escherichia coli by using a chromosomal lac UV5 promoter. J Bacteriol. 1993 May;175(9):2770–8. doi: 10.1128/jb.175.9.2770-2778.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Feyter R, Yang Y, Gabriel DW. Gene-for-genes interactions between cotton R genes and Xanthomonas campestris pv. malvacearum avr genes. Mol Plant Microbe Interact. 1993 Mar–Apr;6(2):225–37. doi: 10.1094/mpmi-6-225. [DOI] [PubMed] [Google Scholar]


