Abstract
Much of what is known of the activities of polycystin-1 has been inferred from the effects of the isolated cytoplasmic COOH terminal domain, but it is not clear whether the truncation acts like polycystin-1, as a dominant negative, or in unrelated pathways. To address this question, we have examined functional interactions between the intact and truncated forms of polycystin-1 in one cell system. In cells expressing only native polycystin-1, introduction of the truncation replicated the activity of the full-length protein. Conversely, when background levels of polycystin-1 were modestly elevated, the truncation acted as a dominant negative. Hence, the truncation acts in the polycystin pathway, but with effects that depend upon the background level of polycystin-1 expression. Our data raise the possibility that the cytoplasmic carboxyl terminus, either through cleavage products or intramolecular interactions, might feed back to modulate the activity of parent or intact polycystin-1.
Keywords: Polycystin-1, Proliferation; Apoptosis; Cell Calcium; Endoplasmic Reticulum; Autosomal Dominant Polycystic Kidney Disease
Introduction
Loss of functional polycystin-1 (Pc-1) or polycystin-2 causes Autosomal Dominant Polycystic Kidney Disease (ADPKD), in which the development of multiple epithelial lined cysts leads to progressive renal impairment [1]. Since heterologous expression of full-length Pc-1 has been technically difficult, many have inferred Pc-1 functions from studies of the effects of the isolated cytoplasmic COOH terminal of this protein. In more recent studies, the function of Pc-1 has been addressed by heterologous expression of the full-length protein in transfected cell lines and from genetic manipulation of mice and other model organisms. In comparing data from studies of the isolated carboxyl terminal (truncated) versus intact forms of Pc-1, it appears that the two molecules do not always act in the same pathways and when they do, often appear to have opposing effects. Furthermore, studies of the truncation are sometimes inconsistent, suggesting dependence on the cell system utilized or other factors. For example: full-length Pc-1 inhibits cell proliferation via activation of JAK-STAT [2], whereas the truncation has been found to enhance proliferation, probably through modulation of cell calcium [3-5]. The capacity of the pc-1 truncation to activate wnt signaling appears to be inconsistent [6, 7] and altered wnt signaling has not been observed in human cystic disease [7]. Although the truncation activates AP-1 [7, 8], full-length Pc-1 might have the opposite effect, since disruption of the PKD-1 gene in mouse and human ADPKD seems to cause increased AP-1 activity [9]. In fact one group observed exactly opposing effects of the truncation and heterologously expressed Pc-1 on Jun:ATF dependent AP-1 activity in the same cell line [7]. A dominant negative potential for the truncation is also indicated by studies in zebrafish [10]. Since it is likely (although never determined) that native Pc-1 is expressed in the cell lines used in the studies of the function of the truncated Pc-1, it is conceivable that the truncation may augment, modulate or interrupt endogenous polycystin signaling. This question has not been experimentally addressed. The fact that the effects of truncated and intact Pc-1 do not necessarily impinge on the same pathways raises significant concerns that the truncation has non-specific effects unrelated to the physiological functions of the intact protein. Even if the truncation operates in polycystin pathways, the extensive literature based on the activity of the truncation can, at best, only identify phenomena that are in some way associated with polycystin signaling. These studies cannot provide direct information about the direction of the effect of Pc-1 in these pathways, and hence cannot easily be integrated into an understanding of the links between gene mutation and disease. In this study, we establish for the first time that: (1) the Pc-1 cytoplasmic COOH terminus truncation has the potential to either augment or interrupt several validated functions of the full length protein in a single cell system, and (2) the effects of the truncation are critically dependent upon the background level of expression of the full length protein. The interactions between truncated and full-length proteins we have observed suggest the possibility that the carboxyl terminus might be a physiological regulator of Pc-1 signaling from the parent protein.
Experimental procedures
Generation of stable cell lines, figure 1
Figure 1. Characterization of cell lines.

A: The HA-tagged pkd193 fusion protein. B: Cotransfected cell lines showed dexamethasone inducible expression of the HA-tagged pkd193 fusion protein, but also appreciable background leak of fusion protein expression in the absence of induction. Prg=progenitor. C: Non-quantitative RT-PCR showed persistence of expression of heterologous polycystin-1 mRNA in co-transfected cells.
As described previously [11], a series of cell lines (herewith referred to as progenitor cell lines) derived from Madine Darby Canine Kidney (MDCK) cells, were stably transfected with either empty vector (progenitor control lines F6 and F8) or heterologous full-length human Pc-1 (progenitor Pc-1 expressing lines C8/68 and G7/36). These progenitor clones were subjected to a further round of transfection with a dexamethasone inducible plasmid [12] encoding the (HA-tagged) c-terminal 193 amino acids of pc-1 (pkd193) linked to a membrane localization cassette (provided by Dr. G. Walz, Freiberg, Germany) comprising the CH2 and CH3 domains of human IgG and the transmembrane domain of CD7. The non-tagged version of this construct has been previously described [13]. A hygromycin resistant plasmid was introduced for second round selection. Two dexamethazone (dex) inducible stable clones were selected for each progenitor line on the basis of the lowest background and greatest dexamethasone stimulated expression of pkd193. These totaled 8 independently derived co-transfected lines: 4 control pkd193 lines (F6pkd193-1 and -2 and F8pkd193-1 and -2), and 4 pc-1 pkd-193 lines (C8pkd193-1 and -2 and G7pkd193-1 and -2). Using these cell lines, we examined the interaction between pkd193 and pc-1 within a single cell-type system. The original progenitor lines (F6, F8, C8/68 and G7/36) were used to determine the effects of heterologous full-length Pc-1 in the absence of pkd193 in the various assays used in these studies. Supplementary figure 1 summarizes the generation of the cell lines described in this study.
Cell Culture Conditions
Cells were propagated in DMEM/F12 supplemented with 5% fetal calf serum under selection with G418, Zeocin and (where appropriate) Hygromycin. For calcium measurements, cells were sparsely seeded onto matrigel-coated glass coverslips. For assessment of cell growth rate, one million cells were seeded and trypsinized two days later to be counted using a hemacytometer. For assessment of resistance to apoptosis, one million cells were seeded and grown for two days in the absence of serum before being assayed for caspase-3 activity using the caspase 3-colorimetric assay kit from Sigma.
Western Blot analysis of pkd193 expression and AKT phosphorylation
Where specified, cells were induced for 18 hours with 1μm dexamethasone, harvested and immunoprecipitated (IP) with anti-HA Affinity matrix (Roche Applied Science). IP sample was subjected to SDS-PAGE and immunoprobed using Rat anti-HA antibody (clone 3F10, Roche Applied Science). Prior to determination of AKT phosphorylation, cells were serum starved for 5 hours, harvested in a phosphatase inhibitor buffer and subjected to SDS PAGE. The same blots were sequentially probed with anti Phospho-AKT (Thr308) and total AKT antibodies (AKT12&3), both from Cell Signaling.
Immunofluorescent cytochemistry
Cells were grown on Matrigel (Becton-Dickenson)-coated glass slides and then treated with dexamethasone for 24h. To demonstrate expression of pkd193, cells were fixed in 4% (v/v) paraformaldehyde, washed in PBS and then incubated sequentially with anti-HA rat monoclonal primary, anti-rat alexa 488 secondary and DAPI.
RT-PCR Analysis of Human PKD1 mRNA expression
Total RNA was prepared using Trizol reagent (GIBCO-BRL) and incubated either in the presence RT(+) or absence (RT-) of M-MLV Superscript II Reverse Trancriptase (Invitrogen, Life Technologies). Ten percent of the resulting cDNA template, or negative control, was used for PCR amplification. A plasmid containing the entire human pc-1 cDNA was used as template for the positive control. The primer sequences used were as follows.
Human PKD1 fwd: 5′-CCGCTTCAAGTACGAGATCC-3′
Human PKD1 rev: 5′-GAAGATGTCCAGGCTGTTGC-3′
Cell calcium measurements
The methods for detecting cytoplasmic and endoplasmic reticulum (ER) calcium responses to 10μM ATP and 10μM Inositol triphosphate (IP3) using fura 2am and mag-fura 2am have been described previously [14, 15]. In the latter, digitonin was used to allow release of cytoplasmic dye to reveal signal from residual dye in the ER lumen. The decay rates of responses were described numerically as the time, in seconds, taken to proceed from peak to 20% of peak.
Buffers and reagents
Buffers were composed as follows: extracellular calcium buffer (140mM NaCl, 5mM KCl, 2mM CaCl2, 1.8mM MgCl2, 10mM HEPES, 5mM probenecid, pH 7.4 with NaOH), 0 calcium EGTA extracellular buffer (140mM NaCl, 5mM KCl, 1.8mM MgCl2, 10mM HEPES, 0.1mM EGTA, 5mM probenecid, pH 7.4 with NaOH and intracellular buffer (19mM NaCl, 125mM KCl, 10mM HEPES, 1mM EGTA, 0.33mM CaCl2, 1.4mM MgCl2, 3mM ATP, pH 7.2 with KOH). Fluorescent dyes were obtained from Molecular Probes, all other chemicals from Sigma.
Analysis
The significance of effects was determined by t test or ANOVA using Graph Pad Prism software. Data are presented as mean [SEM].
Results
Characterization of cell lines, figure 1
A flow chart of the derivation of cell lines is shown in supplemental figure 1. As shown in manuscript figure 1, although dexamethasone induced robust pkd193 expression, detectable fusion protein signal was evident even in the absence of induction. The continued expression of heterologous full-length human Pc-1 mRNA was confirmed in the pc-1/pkd193 cell lines (derived from C8/68 and G7/36) by non-quantitative RT-PCR using human specific primers. In most of the cell lines studied in the cell calcium protocols, the effect of pkd193 was equally evident in the presence or absence of induction with dexamethasone, indicating that background levels of expression were generally sufficient for the full development of activity. Since responses in un-induced cells did not differ from those of dexamethasone treated cells (with one exception, described below) only the dexamethasone-induced data are shown in the results section. Immunofluoresence studies (supplemental figure 2) showed that the PKD193 fusion protein was expressed with a predominantly cytoplasmic location that appeared to be concentrated in the region of the golgi apparatus. This pattern was not influenced by co-expressed heterologous Pc-1. There was no obvious nuclear signal in any of the co-transfected cell lines.
Cytoplasmic and ER calcium responses, Figures 2 and 3
Figure 2. ATP-induced cytoplasmic calcium responses.
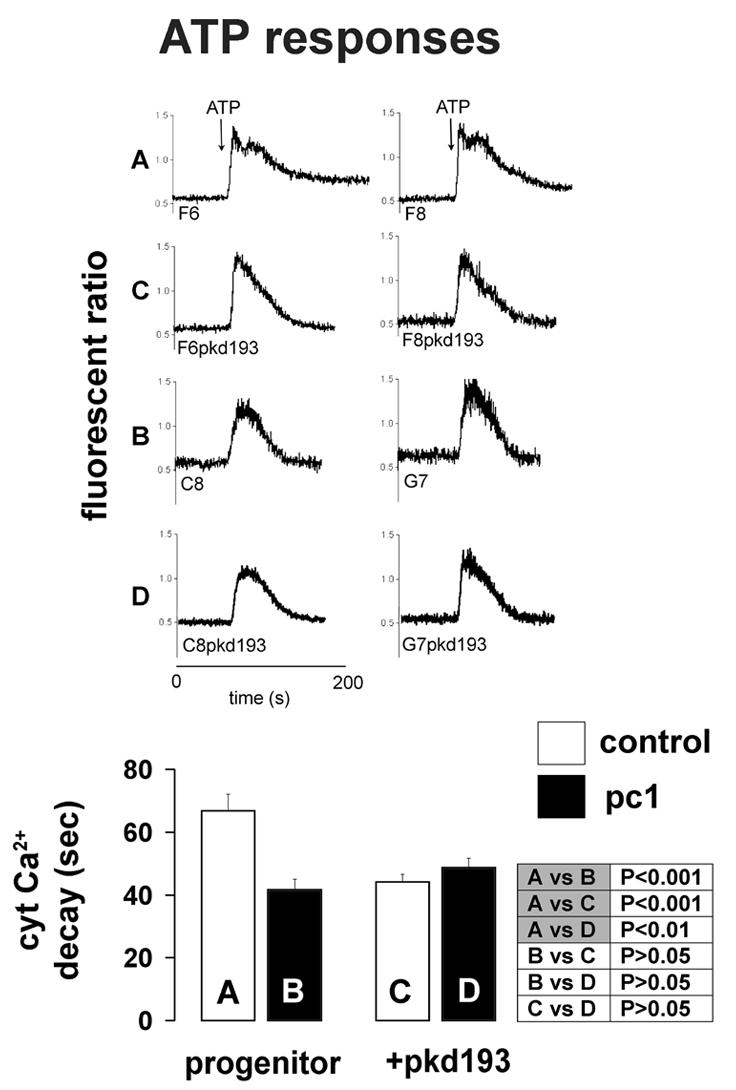
Representative traces (top) and summary data (bar charts) are shown. Labels A through D identify corresponding representative traces, bars and “p” values (by ANOVA). Heterologous expression of intact polycystin-1 accelerated the decay of the ATP response (A vs B). Expression of the pkd193 fusion protein accelerated decay in control progenitor lines (A vs C) but tended to prolong decay when introduced into the pc-1 progenitor lines (B vs D); decay times became similar in co-transfected cells (C vs D).
Figure 3. IP3-induced ER calcium depletion.

The effects of heterologous polycystin-1 and the truncation on ER luminal calcium paralleled those seen in the cytoplasmic calcium responses. Intact polycystin-1 accelerated the recovery of ER calcium following IP3 induced calcium release (A vs B). The truncation mimicked the effect of intact protein in the control progenitor cells (A vs C) but, paradoxically, significantly prolonged recovery times when co-expressed with heterologous polycystin-1 (B vs D).
As previously described [16], the decay phase of the ATP response was significantly accelerated by heterologously expressed pc-1 (progenitor pc-1 cell lines C8/68 and G7/36) relative to transfection control lines (progenitor control cell lines F6 and F8). Introduction of pkd193 into the progenitor control lines F6 and F8 resulted in a similar acceleration of decay of the ATP response. Hence pkd193 converted the original progenitor control phenotype to that of the original progenitor pc-1 phenotype with respect to the ATP-stimulated calcium response, mimicking the effect of the full-length protein. By contrast, the truncation tended to cause a prolongation of decay when co-expressed in lines derived from pc-1 expressing progenitor cells. However, the effect of the truncation on calcium decay time in the pc-1 cells only achieved significance when the t80-20% (rather than the tpeak-20%) was compared between groups (24.6[2.0] to 32.1[1.9] sec, p=.02). In one of the control-pkd193 cell lines, treatment with dexamethasone accelerated the decay time from 66.1 [2.6] to 41.7 [2.8] sec (p=0.0001), clearly transforming the response from that of a progenitor control line (F6 or F8: prolonged decay) to that of a progenitor Pc-1 expressing line (C8/68 or G7/36: accelerated decay). In the remaining cell lines, the effect of the truncation was generally equally evident at the induced and un-induced level of expression. ATP peak responses were not influenced by full-length or truncated constructs.
The effects of the pkd193 fusion protein on ER calcium responses to IP3 were also dependent upon the presence or absence of heterologously expressed pc-1. We have previously shown [16] that full-length Pc-1 accelerated the decay of the ATP-stimulated cell calcium response through augmentation of calcium uptake by the ER. In the current experiments, following calcium release induced by IP3, calcium re-uptake into the ER was activated by the truncation in control cells, but was slowed down in pc-1 expressing cell lines. The same control-pkd193 cell line that showed a dex inducible acceleration of cytoplasmic calcium decay showed a corresponding dex inducible acceleration of the IP3-recovery time from 117 [6.5] to 89.9 [3.0] sec, p=0.0008. In the other cell lines, the effect of the truncation was generally equally evident at the induced and un-induced level of expression.
Proliferation, apoptosis and AKT activation, Figure 4
Figure 4. The effect of the pkd193 fusion protein on cell proliferation, resistance to apoptosis and activation of AKT.
A: proliferation B: serum starvation apoptosis, C: AKT activation. Expression of intact heterologous polycystin-1 resulted in inhibition of proliferation and conferred resistance to apoptosis. The truncation similarly reduced proliferation and conferred protection from apoptosis in control progenitor cells, but, conversely, antagonized the effects of heterologous polycystin-1 in progenitor cells expressing the intact protein (* p values <0.005). Panel C shows representative western blots of phospho- and total AKT and corresponding bar charts of normalized band density, n=4. In the absence of heterologous intact protein, expression of the truncation resulted in a modest decline in phospho-AKT levels (p=0.013). Inhibition of AKT phosphorylation was more marked in cells expressing heterologous pc-1, (p=0.005).
The previously described effect of intact Pc-1 to retard proliferation [11] was evident in the progenitor cell lines C8/68 and G7/36. In cell lines derived from the control progenitors F6 and F8, the polycystin truncation similarly inhibited proliferation. By contrast, when expressed in the pc-1 expressing progenitor lines C8/68 and G7/36, the truncation had the opposite effect, to accelerate proliferation. With respect to serum starvation-induced apoptosis, the truncation again behaved like Pc-1 in the cell lines derived from control progenitor lines F6 and F8, causing pronounced resistance to serum starvation induced apoptosis. The truncation acted in the opposite way in the progenitor pc-1 cell lines (C8/68 and G7/36), rendering these normally highly resistant cells sensitive to serum starvation.
Since Pc-1 has been linked to activation of AKT [17], we determined the level of phospho-AKT relative to total AKT expression in each of the progenitor and truncation transfected cell lines. Heterologous expression of Pc-1 in the progenitor cell lines caused activation of AKT, as previously described [17]. Introduction of the truncation in the context of expression of heterologous Pc-1 resulted in significant inhibition of AKT phosphorylation, as might have been predicted from its effects on apoptosis. However, the relationship between AKT activation and apoptosis broke down in the control progenitor cells, in which the effect of the truncation to confer apoptotic resistance was not obviously linked to activation of AKT.
Discussion
In these studies, we have directly shown that a fusion protein containing the cytoplasmic COOH terminal 193 amino acids of Pc-1 was active in modulating several phenotypes linked to the activity of full-length Pc-1. In the cell calcium experiments, the truncation abolished the original differences between the progenitor cell lines because of opposing effects in the presence and absence of heterologous pc-1. In the case of proliferation and apoptosis, the truncation effectively reversed the pc-1 and control progenitor phenotypes. To our knowledge, this is the first time that functional interactions between full-length Pc-1 and the COOH terminal truncation have been directly studied in one cell type employing multiple readouts for polycystin signaling. The effects we describe were almost certainly due to the fusion protein rather than random clonal variation because multiple co-transfected cell lines were studied for each level of background expression of intact Pc-1. The assertion that the effect of the truncation was specific to Pc-1 signaling seems reasonable because this activity was evident in various Pc-1 associated phenotypes, and the capacity of the truncation to evoke or to block these phenotypes depended upon the background level of expression of intact protein. Although others have suggested that over-expression of Pc-1 can invoke a cystic phenotype [18], it is unlikely that the antagonistic effect of the truncation arose from elevation of polycystin signaling above a specific threshold in our experiments because treatment with dexamethasone did not generally influence the effect of the fusion protein over a substantial range of expression levels. The fact that the effects we observed were generally equally evident in the presence or absence of induction is not surprising since even in the absence of induction the truncation was probably still present at molar excess relative to heterologous pc-1 in the pc-1/pkd193 cells and at levels sufficient to activate pc-1 pathways in the control/pkd193 cells. Previous studies of these progenitor cell lines indicated that the anti-apoptotic activity of Pc-1 was related to the capacity of this protein to activate AKT [17]. The fact that the fusion protein conferred resistance to apoptosis in the control progenitors with no associated increase in AKT activity suggested that Pc-1 might influence apoptosis through alternate pathways. Our data raise the possibility that the effect of the Pc-1 truncation may also be dependent upon native expression levels or activity of full-length Pc-1, which are likely to vary between different types of cell lines and under different culture conditions [19-21].
Recent studies have suggested that the cytoplasmic COOH terminus of Pc-1 might be physiologically cleaved within the last membrane-spanning domain [22] or in the cytoplasmic COOH tail [10], and that the resultant fragments might exhibit independent activity. The pkd193 fusion protein we have used in the present studies had a non-Pc-1 derived transmembrane domain, and was not therefore a target for the proposed intramembranous cleavage event. Also, we were not able to demonstrate nuclear signal with an antibody directed to a C terminal epitope tag, and never saw the previously described 17Kd fragment, even after incubation with proteosome inhibitors (data not shown). None the less, the pkd-193 truncation we studied might still have acted to some extent like the putative physiological cleavage products. Alternatively, the isolated carboxyl truncation we studied might have replicated or interfered with intramolecular interactions between the COOH tail and other cytoplasmic Pc-1 domains.
Supplementary Material
Derivation and characterization of cell lines. Four MDCK derived cell lines served as the source for the subsequent rounds of transfection described in the present study. Two of these progenitor lines, C8/68 and G7/36 expressed intact heterologous polycystin-1. The other two, F6 and F8, were developed by transfection with control vector. In the present study, the progenitor lines were subjected to a second stable transfection to introduce the isolated cytoplasmic carboxyl terminal 193 amino acids of murine polycystin-1 linked to a membrane expression cassette comprising the signal peptide, human IgG domain and transmembrane domain of CD7.
Numbers of observations:
Cytoplasmic calcium responses: F6 n=7, F6pkd193 clones 1&2 n=16, F8 n=8, F8pkd193 clones 1&2 n=15, C8 n=7, C8pkd193 clones 1&2 n=19, G7 n=10, G7pkd193 clones 1&2 n=14. Number of observations ER calcium responses, F6 n=8, F6pkd193 n=8, F8 n=11, F8pkd193 n=6, C8 n=10, C8pkd193 n=9, G7 n=9, G7pkd193 n=7.
Proliferation assay, F6 n=4, F6pkd193 n=4, F8 n=6, F8pkd193 n=6, C8 n=4, C8pkd193 n=4, G7 n=6, G7pkd193 n=6. Number of observations in apoptosis study, F8 n=6, F8pkd193 n=6, F6 n=6, F6pkd193 n=6, C8 n=6, C8pkd193 n=6, G7 n=6, G7pkd193 n=6.
The subcellular pattern of expression of the pkd193 fusion protein was not influenced by intact polycystin-1. Immunolocalization of the polycystin-1 truncation was determined using an anti-HA antibody directed to the C terminal HA tag. Similar patterns were observed irrespective of the presence or absence of heterologous polycystin-1 (compare F8pkd193 with G7pkd193). Specificity of signal was confirmed by the absence of staining in the progenitor cell lines F8 and G7/36.
Acknowledgements
Supported by NIH-DK066323-01, the PKD Foundation and the National Kidney Foundation, Maryland Chapter.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sutters M, Germino GG. Autosomal dominant polycystic kidney disease: Molecular genetics and pathophysiology. Journal of Laboratory and Clinical Medicine. 2003;141:91–101. doi: 10.1067/mlc.2003.13. [DOI] [PubMed] [Google Scholar]
- 2.Bhunia AK, Piontek K, Boletta A, Liu L, Qian F, Xu P-N, Germino FJ, Germino GG. PKD1 Induces p21 (WAF1) and Regulation of the Cell Cycle Via Direct Activation of the JAK-STAT Signaling Pathway in a Process Requiring PKD2. Cell. 2002;109:157–168. doi: 10.1016/s0092-8674(02)00716-x. [DOI] [PubMed] [Google Scholar]
- 3.Yamaguchi T, Wallace DP, Magenheimer BS, Hempson SJ, Grantham JJ, Calvet JP. Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J. Biol. Chem. 2004;279:40419–40430. doi: 10.1074/jbc.M405079200. [DOI] [PubMed] [Google Scholar]
- 4.Yamaguchi T, Hempson SJ, Reif GA, Hedge A-M, Wallace DP. Calcium Restores a Normal Proliferation Phenotype in Human Polycystic Kidney Disease Epithelial Cells. J Am Soc Nephrol. 2006;17:178–187. doi: 10.1681/ASN.2005060645. [DOI] [PubMed] [Google Scholar]
- 5.Manzati E, Aguiari G, Banzi M, Manzati M, Selvatici R, Falzarano S, Maestri I, Pinton P, Rizzuto R, del Senno L. The cytoplasmic C-terminus of polycystin-1 increases cell proliferation in kidney epithelial cells through serum-activated and Ca(2+)-dependent pathway(s) Exp Cell Res. 2005;304:391–406. doi: 10.1016/j.yexcr.2004.10.023. [DOI] [PubMed] [Google Scholar]
- 6.Kim E, Arnould T, Sellin LK, Benzing T, Fan MJ, Gruning W, Sokol SY, Drummond I, Walz G. The Polycystic Kidney Disease 1 Gene Product Modulates Wnt Signaling. J. Biol. Chem. 1999;274:4947–4953. doi: 10.1074/jbc.274.8.4947. [DOI] [PubMed] [Google Scholar]
- 7.Le NH, van der Bent P, Huls G, van de Wetering M, Loghman-Adham M, Ong ACM, Calvet JP, Clevers H, Breuning MH, van Dam H, Peters DJM. Aberrant polycystin-1 expression results in modification of AP-1 activity, while Wnt signaling remains unaffected. J. Biol. Chem. 2004;279:27472–27481. doi: 10.1074/jbc.M312183200. [DOI] [PubMed] [Google Scholar]
- 8.Arnould T, Sellin L, Benzing T, Tsiokas L, Cohen HT, Kim E, Walz G. Cellular Activation Triggered by the Autosomal Dominant Polycystic Kidney Disease Gene Product PKD2. Mol. Cell. Biol. 1999;19:3423–3434. doi: 10.1128/mcb.19.5.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Le NH, van der Wal A, van der Bent P, Lantinga-van Leeuwen IS, Breuning MH, van Dam H, de Heer E, Peters DJM. Increased Activity of Activator Protein-1 Transcription Factor Components ATF2, c-Jun, and c-Fos in Human and Mouse Autosomal Dominant Polycystic Kidney Disease. J Am Soc Nephrol. 2005;16:2724–31. doi: 10.1681/ASN.2004110913. [DOI] [PubMed] [Google Scholar]
- 10.Low SH, Vasanth S, Larson CH, Mukherjee S, Sharma N, Kinter MT, Kane ME, Obara T, Weimbs T. Polycystin-1, STAT6, and P100 Function in a Pathway that Transduces Ciliary Mechanosensation and Is Activated in Polycystic Kidney Disease. Developmental Cell. 2006;10:57–69. doi: 10.1016/j.devcel.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 11.Boletta A, Qian F, Onuchic LF, Bhunia AK, Phakdeekitcharoen B, Hanaoka K, Guggino W, Monaco L, Germino G. Polycystin-1, The Gene Product of PKD1, Induces Resistance to Apoptosis and Spontaneous Tubulogenesis in MDCK Cells. Mol Cell. 2000;6:1267–1273. doi: 10.1016/s1097-2765(00)00123-4. [DOI] [PubMed] [Google Scholar]
- 12.Hirt R, Poulain-Godefroy O, Billotte J, Kraehenbuhl J, Fasel N. Highly inducible synthesis of heterologous proteins in epithelial cells carrying a glucocorticoid-responsive vector. Gene. 1992;111:199–206. doi: 10.1016/0378-1119(92)90687-k. [DOI] [PubMed] [Google Scholar]
- 13.Sutters M, Yamaguchi T, Maser RL, Magenheimer BS, St. John PL, Abrahamson DR, Grantham JJ, Calvet JP. Polycystin-1 Transforms the cAMP Growth-Responsive Phenotype of M-1 Cells. Kidney Int. 2001;60:484–494. doi: 10.1046/j.1523-1755.2001.060002484.x. [DOI] [PubMed] [Google Scholar]
- 14.Wildman SS, Hooper KM, Turner CM, Sham JSK, Lakatta EG, King BF, Unwin RJ, Sutters M. The isolated polycystin-1 cytoplasmic COOH terminus prolongs ATP-stimulated Cl- conductance through increased Ca2+ entry. Am J Physiol Renal Physiol. 2003;285:F1168–1178. doi: 10.1152/ajprenal.00171.2003. [DOI] [PubMed] [Google Scholar]
- 15.Hooper KM, Boletta A, Germino GG, Hu Q, Ziegelstein RC, Sutters M. Expression of polycystin-1 enhances endoplasmic reticulum calcium uptake and decreases capacitative calcium entry in ATP-stimulated MDCK cells. Am J Physiol Renal Physiol. 2005;289:F521–530. doi: 10.1152/ajprenal.00355.2004. [DOI] [PubMed] [Google Scholar]
- 16.Hooper K, Boletta A, Germino G, Hu Q, Ziegelstein R, Sutters M. Expression of polycystin-1 enhances endoplasmic reticulum calcium uptake and decreases capacitative calcium entry in ATP-stimulated MDCK cells. Am J Physiol Renal Physiol. 2005;289:F521–530. doi: 10.1152/ajprenal.00355.2004. [DOI] [PubMed] [Google Scholar]
- 17.Boca M, Distefano G, Qian F, Bhunia AK, Germino GG, Boletta A. Polycystin-1 Induces Resistance to Apoptosis through the Phosphatidylinositol 3-Kinase/Akt Signaling Pathway. J Am Soc Nephrol. 2006;17:637–647. doi: 10.1681/ASN.2005050534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pritchard L, Sloane-Stanley JA, Sharpe JA, Aspinwall R, Lu W, Buckle V, Strmecki L, Walker D, Ward CJ, Alpers CE, Zhou J, Wood WG, Harris PC. A human PKD1 transgene generates functional polycystin-1 in mice and is associated with a cystic phenotype. Hum. Mol. Genet. 2000;9:2617–2627. doi: 10.1093/hmg/9.18.2617. [DOI] [PubMed] [Google Scholar]
- 19.Weston BS, Bagneris C, Price RG, Stirling JL. The Polycystin-1 C-type Lectin Domain Binds Carbohydrate in a Calcium-Dependent Manner, and Interacts with Extracellular Matrix Proteins In Vitro. Biochem Biophys Acta. 2001;1536:161–176. doi: 10.1016/s0925-4439(01)00046-1. [DOI] [PubMed] [Google Scholar]
- 20.Ibraghimov-Beskrovnaya O, Bukanov NO, Donohue LC, Dackowski WR, Klinger KW, Landes GM. Strong homophilic interactions of the Ig-like domains of polycystin-1, the protein product of an autosomal dominant polycystic kidney disease gene, PKD1. Hum. Mol. Genet. 2000;9:1641–1649. doi: 10.1093/hmg/9.11.1641. [DOI] [PubMed] [Google Scholar]
- 21.Forman JR, Qamar S, Paci E, Sandford RN, Clarke J. The Remarkable Mechanical Strength of Polycystin-1 Supports a Direct Role in Mechanotransduction. Journal of Molecular Biology. 2005;349:861–871. doi: 10.1016/j.jmb.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Chauvet V, Tian X, Husson H, Grimm DH, Wang T, Hieseberger T, Igarashi P, Bennett AM, Ibraghimov-Beskrovnaya O, Somlo S, Caplan MJ. Mechanical stimuli induce cleavage and nuclear translocation of the polycystin-1 C terminus. J. Clin. Invest. 2004;114:1433–1443. doi: 10.1172/JCI21753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Derivation and characterization of cell lines. Four MDCK derived cell lines served as the source for the subsequent rounds of transfection described in the present study. Two of these progenitor lines, C8/68 and G7/36 expressed intact heterologous polycystin-1. The other two, F6 and F8, were developed by transfection with control vector. In the present study, the progenitor lines were subjected to a second stable transfection to introduce the isolated cytoplasmic carboxyl terminal 193 amino acids of murine polycystin-1 linked to a membrane expression cassette comprising the signal peptide, human IgG domain and transmembrane domain of CD7.
Numbers of observations:
Cytoplasmic calcium responses: F6 n=7, F6pkd193 clones 1&2 n=16, F8 n=8, F8pkd193 clones 1&2 n=15, C8 n=7, C8pkd193 clones 1&2 n=19, G7 n=10, G7pkd193 clones 1&2 n=14. Number of observations ER calcium responses, F6 n=8, F6pkd193 n=8, F8 n=11, F8pkd193 n=6, C8 n=10, C8pkd193 n=9, G7 n=9, G7pkd193 n=7.
Proliferation assay, F6 n=4, F6pkd193 n=4, F8 n=6, F8pkd193 n=6, C8 n=4, C8pkd193 n=4, G7 n=6, G7pkd193 n=6. Number of observations in apoptosis study, F8 n=6, F8pkd193 n=6, F6 n=6, F6pkd193 n=6, C8 n=6, C8pkd193 n=6, G7 n=6, G7pkd193 n=6.
The subcellular pattern of expression of the pkd193 fusion protein was not influenced by intact polycystin-1. Immunolocalization of the polycystin-1 truncation was determined using an anti-HA antibody directed to the C terminal HA tag. Similar patterns were observed irrespective of the presence or absence of heterologous polycystin-1 (compare F8pkd193 with G7pkd193). Specificity of signal was confirmed by the absence of staining in the progenitor cell lines F8 and G7/36.



