INTRODUCTION
Social bonds are made up of numerous subsystems mediating the overall social relationships that develop between infants and caretakers. The effects of an infant’s formation of early life relationships and its social interactions with caretakers are known to last well in to adulthood with both beneficial and harmful effects [28,35,70]. In our laboratory, we study the development of social relationships between infant rats and their dams, sires, and littermates using an isolation, reunion, and re-isolation test paradigm.
Infant rats emit ultrasonic vocalizations (USV) in a variety of situations, including as a response to isolation, physical manipulation, and thermal and olfactory challenge [1–3,6,19,25,46,79,80,82,97]. Many researchers have used the presence and absence of these sounds to make inferences about the internal state of the rodents, e.g., isolation-induced USV’s have often been called “distress vocalizations” [29,76,78].
The isolation-induced vocalization of a rat pup is modulated both by current social interactions and by the pup’s rearing experience. This modulation of USV by social interactions provides a paradigm for investigating the neurobehavioral mechanisms underlying acquisition and expression of infant attachment.
What is maternal potentiation of isolation-induced vocalizations of infant rats?
Maternal potentiation of ultrasonic vocalization occurs when, after a brief contact with the dam, isolated infant rats produce USV at a rate significantly higher than they would during an isolation not preceded by dam contact. Although the definition may seem of narrow interest, there is a growing belief that the potentiation phenomenon has broad applicability for the study of early life social relationships in many species. But before going further, the reader needs to understand the usual test paradigm in our laboratory.
Test procedure
On the day of testing, the dam is removed from the home cage and anesthetized. The litter is allowed to adapt to her removal for ~15–20min in the home cage on a thermoregulated heating pad. Then, each pup is tested in turn. The pup is picked up and placed alone in a clean polycarbonate rectangular box (18 × 21 × 20 cm) at room temperature. The test consists of three periods, which can range from 1–5min in length (3min each in Figure 1). The pup is isolated during the first and final period. During the middle epoch, the anesthetized dam is placed into the novel cage with the experimental pup. The test procedure is illustrated by the cartoon at the top of Figure 1.
Figure 1.
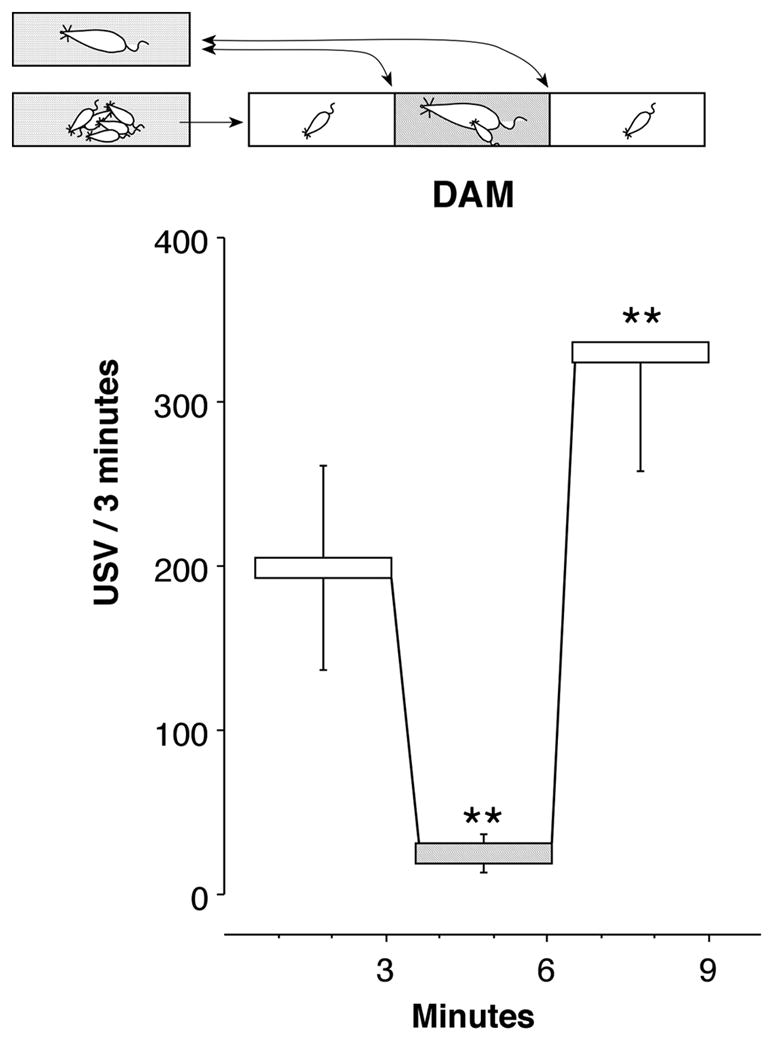
Standard USV test paradigm. The dam is separated from the litter at least 15 minutes before testing. The home cage and litter are placed on a thermoregulated heating pad. At test time, a pup is taken from its littermates and immediately placed into a novel box where USV and other behaviors are recorded throughout three phases, which typically range from 1 to 5min in length (3min in this illustration): Isolation1, Dam Reunion, and Isolation2. The cartoon above the graphs illustrates the sequence of events. The test pup is picked up and put back down at 3 and 6min as the dam is placed into and taken out of the cage. Contact quieting is a significant reduction in calls from Isolation1 to Dam Reunion. Maternal potentiation is a significant increase in calls from Isolation1 to Isolation2. Asterisks mark significant differences from the 1st isolation.
Throughout the test, the behavior of the pup is observed. The time in contact with the dam is recorded. Whether the pup is active or immobile is noted. USVs are counted using a bat detector (Pettersson Elektronik or Ultrasound Advice) with its microphone suspended approximately 10 cm above the test container floor. A variety of other behaviors in the novel cage, body and ambient temperature, weight, and more are also measured [41,42].
Initial isolation period
Isolation-induced USV has been extensively used as a marker for distress in infant rodents [31,68,75,78,116]. The actions of anxiolytic and anxiogenic compounds on USV are generally compatible with this explanation [56,68,83,116,119]. Other behaviors that have been considered indicators of distress accompany USV, e.g., increases in locomotor activity, self grooming, and micturation [68]. The first period in Figure 1 (minutes 0–3) illustrates this response. It is known that isolation-induced USV has communicatory value for an isolated pup, both hastening and directing maternal retrieving behavior [18,98].
Reunion period
In the presence of the dam in the second period, the rate of USV is significantly reduced from the calling rate in the initial isolation (minutes 3–6 in Figure 1). This decrease in vocalizations has been called ‘companion comfort’ or, more neutrally, ‘contact quieting’. The isolated pups also nose the fur of, burrow under, and maintain proximity with their dam. The dam is not the only companion that can induce quieting. Factors with a prima facie appearance of supplying comfort tend to reduce the USV of the isolated animal, e.g., warmth, furry texture of the substrate, home cage shavings, an anesthetized littermate [1,7,17,46].
Re-isolation
Maternal potentiation of USV is exhibited in the third period of Figure 1. Upon re-isolation, pups call at a higher rate than in the first period. The reunion with the dam does not increase all aspects of the isolation response, only the vocal behavior. Thus, it is not part of a generalized increase in arousal [40,42,44,91,95,96]. But more vocal parameters than rate are affected, at least at postnatal day 12 (PND12, the only age at which other parameters have been tested). On average, each potentiated vocalization is louder; tends to be longer; and is emitted in an altered bout structure [74], all of which fits with its hypothesized function as a call to elicit maternal retrieval [40].
There are two forms of potentiation, active and passive, which will be discussed in the Acquisition and Expression sections, below. Active potentiation occurs after a brief interaction with an awake, unanesthetized dam; passive potentiation after a brief reunion with an anesthetized dam. Unless specifically mentioned, the following discussions will relate to the passive potentiation paradigm.
Why study potentiation of isolation-induced vocalizations of infant rats?
The potentiation of vocalization by a pup after contact with its dam reflects, we believe, a filial bond that has been formed by the pup during the first weeks of life. Filial bonds, a subclass of affiliative bonds, are those shown by infants towards parents (or other primary caretakers) but not to siblings, friends, etc. Bowlby’s concept of ‘attachment’ describes one class of filial bonds [11]. Is our hypothesis about USV potentiation true? Although there is no definitive proof, there is a convergence of data that is difficult to explain in any other way (listed in order of importance to the hypothesis):
The intensity of the USV response upon re-isolation depends on the identity of the social companion during reunion.
The valence of the USV response to an adult male is altered by early experience (described in the section on Acquisition, below).
The expression of USV potentiation is regulated by dopamine activity in the nucleus accumbens, a system associated with attention and reward (described in the section on Expression, below).
The development emergence of USV potentiation provides ample time for learning to play a role (described in the section on Acquisition, below).
Vocal potentiation occurs in other species.
Isolation-induced USV is not a by-product of thermal or cardiac mechanisms.
The intensity of the USV response depends on the identity of the social companion during reunion
Figure 2 illustrates the critical impact of the identity of the social companion on the USV response of isolated rat pups. In each of the 4 illustrations, pups are treated exactly the same, except for the type of companion (which do not differ in their behavior due to anesthesia).
Figure 2.
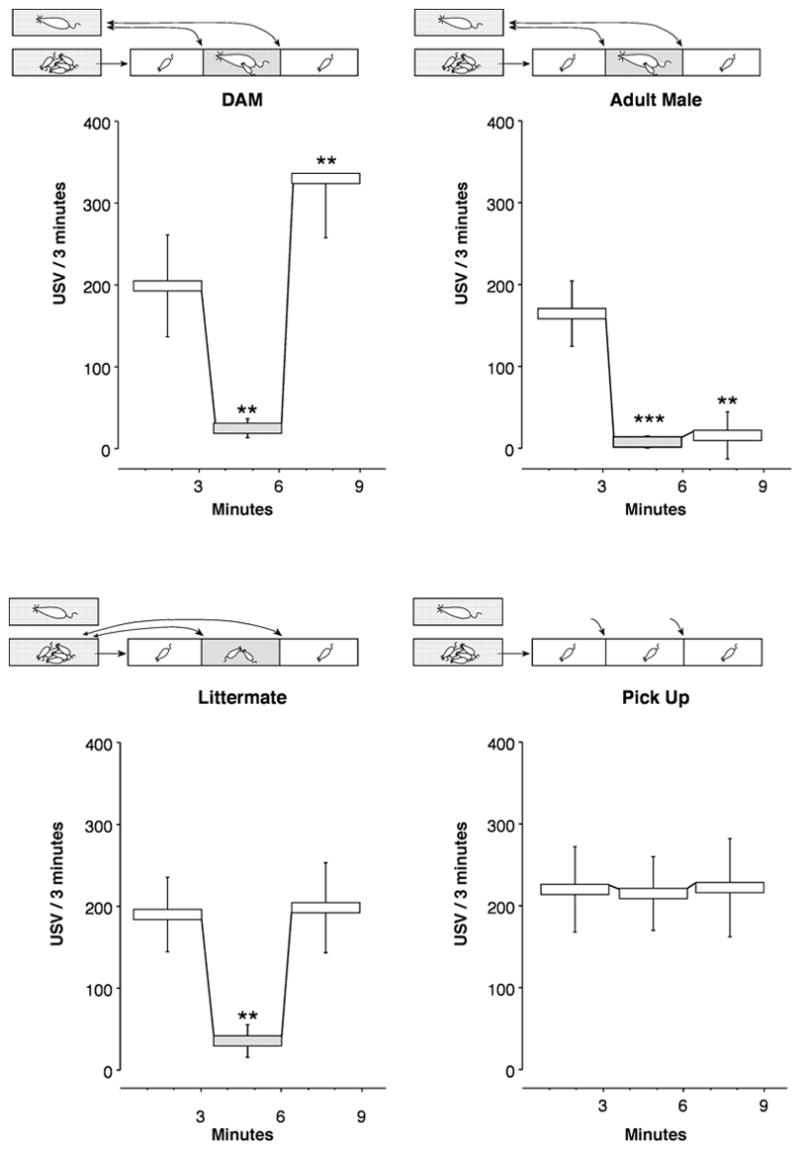
Illustration of responses to different social companions. The test paradigm is the same as illustrated in Figure 1, except that the anesthetized companion is either (1) the dam, (2) an unfamiliar adult male, (3) a littermate, or (4) ‘pick up’ controls. ‘Pick up’ control pups are handled in between test phases to control for the handling necessary for adding and removing the conspecifics. Asterisks mark significant differences from the 1st isolation (composite data from [15,42,93]).
The control condition is illustrated in the ‘Pick up’ graph. These pups are handled in between test phases to control for the handling necessary for adding and removing the companions. The rate of vocalization does not change significantly in response to handling.
All of the social companions have a similar effect on USV rate in their presence (Figure 2 Dam, Littermate, & Adult Male). However, it would be wrong to label them all ‘companion comfort’ and confusing to call them ‘contact quieting’. Although exposure to an anesthetized adult male reduces a dam-reared pup’s rate of USV emission dramatically (Figure 2 Adult Male), the adult male also elicits fear-like behaviors, e.g., immobility, micturation, corticosterone and ACTH release, increased analgesia [95,106,110]. This phenomenon is labeled ‘behavioral inhibition’ and is thought to have evolved because adult males are potential predators of infant rats. Vocal rates in the third period validate differentiation of contact quieting and behavioral inhibition. Pups exposed to the adult male continue to suppress USV after the male is removed [95,111].
The re-isolation period also demonstrates that the response of the pup to its dam differs from its reaction to a littermate. A brief interaction of an isolated pup with its anesthetized dam or another adult female causes maternal potentiation, the increase in USV rate in the subsequent isolation (Figure 2 Dam). A pup exposed to a littermate or several littermates (or home cage shavings which also elicit ‘contact quieting’) shows no such elevated response (Figure 2 Littermate) [17,42,61].
It is important to emphasize that USV potentiation can be elicited by adult females other than the dam. This issue is discussed below in the section ‘Problems with USV potentiation as a model system’.
Vocal potentiation occurs in other species
The fact that the young of most mammalian species increase vocalization rate in response to separation from their caretakers supports the likelihood that these calls have ecological importance [36,55,64,69,85]. A similar assumption can be made about maternal potentiation. In support of its possible ecological significance, maternal potentiation of calling has now been found in two species besides rats.
Infant Swiss-Webster mice show USV potentiation in isolation after a brief interaction with the dam [69], although it must be noted that potentiation has not been found in some other strains [14]. Furthermore, the Moles report links USV potentiation with a standard test of affiliative behavior [69]. Wild type Swiss-Webster mice prefer their dam to clean shavings in a choice test. The μ-opioid receptor knockout mice (Orpm−/−) from the strain, on the other hand, are deficient in both behaviors. Orpm−/− mouse pups emit fewer vocalizations during an initial isolation; do not show a preference for maternal cues; and do not show USV potentiation following brief reunions. As μ-opioid activity does not regulate USV potentiation in rats [91] nor expression of odor learning at PND12 [88,89], we hypothesize that the knockout mice have a deficit in the acquisition of a strong social bond, rather than with the expression.
Guinea pigs produce separation cries in the audible, not ultrasonic range, but show many similarities with rat and mouse pups. For example, the presence of the dam reduces the calling rate [36,38]. In collaboration with Michael Hennessy of Wright State University, we have now shown that 15 day-old guinea pigs show a form of potentiation in response to a brief reunion with their dams [37]. Littermate pairs of guinea pigs were tested in a similar fashion to the rat potentiation test described above. Control pups showed a significant reduction in calls from Isolation 1 to Isolation 2. In fact, 10 of 16 control pups did not call at all in Isolation 2. In the dam-exposed group, 13 of 16 continued to call in Isolation 2. While the rate of calling of dam-exposed pups did not increase, as it does with rat pups, it did not show a significant reduction. Also in contrast to the rat results, the dam-exposed group was also more active than the controls in Isolation 2. Follow up experiments demonstrated that reunion with neither a littermate nor another adult female prevented the decline in vocalization.
The fact that the form of maternal potentiation of vocalizations in guinea pigs does not match precisely the form seen in rats is to be expected. The life histories and environmental patterns of the species are very different, e.g., precocial vs. altricial.
Isolation-induced USV is not a by-product of thermal or cardiac mechanisms
If it is true that maternal potentiation reflects the presence of a social bond, it seems likely that motivational and/or emotional processes are involved in its production. Although, as noted above, the role of anxiolytic and anxiogenic drugs on isolation-induced USV is generally consistent with such a hypothesis, it has been proposed that USV is not at all related to affective state. Rather, the vocalization is thought to be an epiphenomenon of some other physiological process. The first form of the hypothesis argued that all USV results from attempts to increase thermogenesis [6–8]. We tested and disproved that hypothesis in several ways: 1. demonstrating that activating thermogenesis does not alter USV rate [47]; 2. that emitting USV does not influence body temperature except in cases of recovery from hypothermia so intense that the heart stops beating [48]; 3. showing that contact quieting occurs even if the body temperature of the social companion is well below room temperature [43]; and 4. that maternal potentiation occurs in a thermoneutral environment [93].
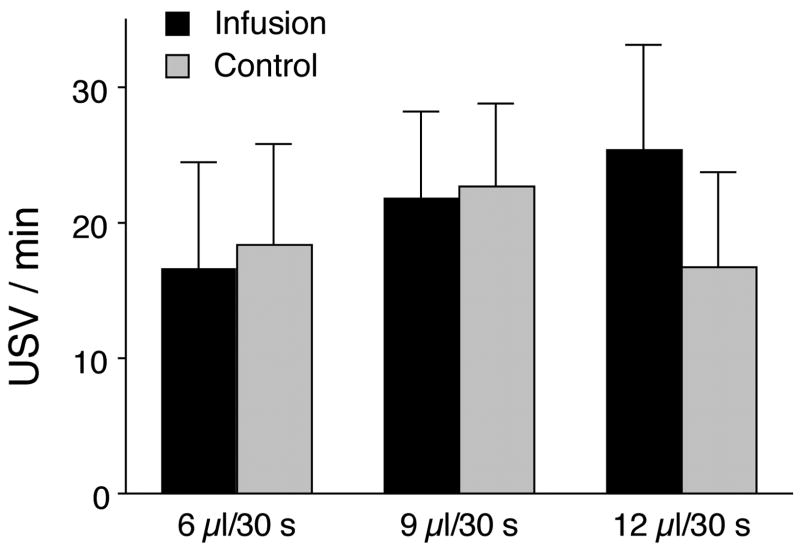
The second version of the epiphenomenon hypothesis suggested that all USV results from a cardiovascular maneuver called the abdominal compression reaction whose function is to increase venous return [9,10,60]. This hypothesis was also disproved [94]. In a series of 7 experiments, we found direct alterations of venous return have no effect on USV. In 5 of the experiments, we artificially reduced venous return in pups that were not vocalizing. For example, we withdrew blood from the superior vena cavas of 2-week-old pups (see Table 1). None of the animals so treated produced any USV, although their response to pick up demonstrated that they were capable of vocalizing. In the other 2 experiments, we artificially increased venous return by infusing either dextrose 5% in water or blood into pups that were vocalizing. In neither case was the rate of USV significantly altered (see Figure 3).
Table 1.
Number of USV produced before, during, and after withdrawal of blood from the superior vena cava.
| Pup | W rate range (μl/30s) | W durations (No. of 30s epochs) | No. of W | USV pre W (30 s) | USV during W (per 30s) | USV post W (30s) | USV in response to pick up stimulation (per 30s) |
|---|---|---|---|---|---|---|---|
| 1 | 9–60 | 1–4 | 5 | 0 | 0 | 0 | 1 |
| 2 | 40–100 | 3 | 1 | 0 | 0 | 0 | 6 |
| 3 | 100–200 | 1–1.2 | 2 | 0 | 0 | 0 | 19 |
| 4 | 5–10 | 4 | 1 | 0 | 0 | 0 | 1 |
| 5 | 70–200 | 1–2 | 2 | 0 | 0 | 0 | 22 |
| 6 | 30–150 | 1–4 | 2 | 0 | 0 | 0 | 11 |
| 7 | 65–250 | 2–4 | 2 | 0 | 0 | 0 | 16 |
Note: Pups were tested in the home cage. When multiple withdrawals (W) were performed on a pup, the blood was reinfused before any subsequent withdrawal. The USV response to being picked up and gently rotated or picked up and placed in a novel cage is also given [94]. Reprinted by permission of Behavioral Neuroscience.
Figure 3.
Mean (±S.E.M.) number of ultrasonic vocalization (USV) responses of Postnatal Day 12 rat pups abruptly isolated in a novel cage for a 3min observation period. During three 30sec epochs, D5W was infused into the pups’ jugular cannulas. Rates of infusion were 6, 9, and 12μl/30sec. The 3 control epochs without infusion were temporally matched to the experimental epochs [92]. Reprinted by permission of Behavioral Neuroscience.
Our results do not imply that temperature has no effect on USV. That it does has been known since the very earliest studies [1,80,82]. But the results do show that the function of USV as frequently tested, e.g., during brief isolations at room temperature, is not part of a thermogenic response. Whether decreased venous return ever has an effect on USV in any test situation is less certain. Venous return has never actually been measured in rats. While this would be a daunting task in pups, it is feasible in adult animals whose vocalizations are presumed to be driven by the same mechanisms [5]. Thus far, evidence supporting a role for the abdominal compression reaction and venous return in any test situation depends on indirect measures and inferences.
Problems with USV potentiation as a model system
Mammals form social bonds with a variety of individuals, e.g., parents, siblings, mates, offspring, and other companions. Rat pups are no exception. Their isolation-induced vocalization and contact quieting can help determine the neurobehavioral processes underlying such social relationships. It is less clear that USV potentiation can serve as a model for human filial bonds. USV potentiation responses by pups are not restricted to specific individuals. Dam-reared pups will potentiate USV to unfamiliar adult females, not just to the dam. Similarly, biparentally-reared pups potentiate USV to unfamiliar adult males [17]. For humans, in Bowlby’s view, such a lack of individual discrimination by human infants would likely indicate severe disturbance [12, p.28]. Others suggest that species like guinea pigs, which show more individual specificity, are better models of human attachment [77]. However, we believe that studies in rats will shed insight on the formation of filial bonds in humans for two reasons.
First, whether or not rat pups will demonstrate individually-specific social responses has not been well tested yet. To our knowledge, all such studies have used rats housed under the same conditions and of unknown relationship, as was true in our work with Wistar rats [17]. Second, even if rats are found to have positive social relationships only to a class of conspecifics, e.g., adult females, that result does not negate rats’ value as a comparative model. Analyses of the differences in behavior and CNS regulation between montaine and prairie voles, for example, has led to important advances in our understanding of the neurobiology of affiliation [52,118]. Similarly, comparisons of rats and guinea pigs may help determine which CNS areas are involved in the individual-maternal attachment of guinea pigs, but not in the class-attachment of rats.
Functional hypothesis
Given the information above, we theorize that isolation-induced USV of an infant rat is a reflection of its internal state, which has evolved to serve a communicative function. Maternal potentiation, as well as predator-induced suppression of USV, evolved through the selective advantage conferred to pups by a capacity to regulate their vocal response according to contextual cues in the environment signaling risk/benefit ratio of high vs. low calling rates. A maximal vocal response is adaptive if the pup has been interacting with its mother just before a sudden isolation (maternal potentiation). Littermates alone predict a lesser likelihood of maternal response before a predator is attracted (standard isolation response), and the scent of an unfamiliar male signals the need to suppress all vocalization (behavioral inhibition).
ACQUISITION
Developmental pattern
The time course of isolation-induced USV and contact quieting by rats during ontogeny have often been described, including by our laboratory [1,16,20,22,25]. Both behaviors appear by PND3 or earlier. Isolation-induced USV peaks around 8–12 days of age (depending on strain, testing conditions, etc) and disappears around the third week of age. Maternal potentiation of USV does not occur until PND7–9, approximately a week later than the initial isolation-induced USV and contact quieting responses are established [45]. Remember that potentiation occurs after a brief interaction with an awake, unanesthetized dam (active potentiation) or after a brief reunion with an anesthetized dam (passive potentiation). Both types of potentiation peak at PND11–13 and then decline between PND17 and 23, along with the isolation response.
Potentiation by an active dam occurs 2 days earlier than passive potentiation. Further, an active dam produces a higher rate of USV responding than passive dam at all ages. At all ages, the potentiation effect is specific to the vocal component of the pups’ response.
This developmental pattern and especially the temporal relationship between active and passive potentiation permits a role for experiential and/or learning processes in the acquisition of the behavior. Although isolation-induced USV and contact quieting to the dam can develop without postnatal interactive experience—pups reared artificially from PND0 show both behaviors at ~PND12 [49]—we do not yet know if experience is critical in potentiation of USV to the dam. Such a role has been demonstrated for the behavioral response to the sire.
Early experience alters the behavioral response to adult males
One of the strongest evidences that USV potentiation is a marker for a social relationship is that early rearing experience can alter the behavioral responses of pups to adult males from fear-like (behavioral inhibition) to ‘paternal’ potentiation. It is well known that when 2-week-old, dam-reared pups are exposed to the presence or even the odor of an adult male rat, they exhibit a fear-related suppression of behavior that continues even after the male is removed including USV inhibition [95,105,111] (see Figure 2 Adult Male). This quieting response is presumed to have evolved due to selection pressure: young rats show similar responses to odors of predators [109]; adult male rats are known to practice infanticide [15,67].
On the other hand, if pups are reared with both dam and sire in the nest cage, 2-week-old pups demonstrate companion comfort and USV potentiation to their sire and other adult males (see Figure 4) [17]. In the presence of the anesthetized sire or other adult male, they nose its fur and try to burrow under its ventrum, behaviors they also show in the presence of their dams. Once the sire is removed, the rate of USV during re-isolation is significantly higher than in an initial isolation, just as is true with the dam. Let me emphasize this latter fact: biparental rearing experience causes the pups to produce USV potentiation in response to their sires and other adult males. Remember that pups do not show potentiation to other familiar social stimuli with which they have been reared, even those that produce contact quieting, e. g., littermates and home cage shavings. Even if pups are reared with a castrated adult male in the home cage, that familiar castrated male will not induce USV potentiation [17]. Given the fact that anosmic, dam-reared pups do not show behavioral inhibition to adult males or potentiation to their anesthetized dams [96], it seems likely that some testosterone-related olfactory cue is a necessary stimulus, either during acquisition or during expression of ‘paternal’ potentiation.
Figure 4.
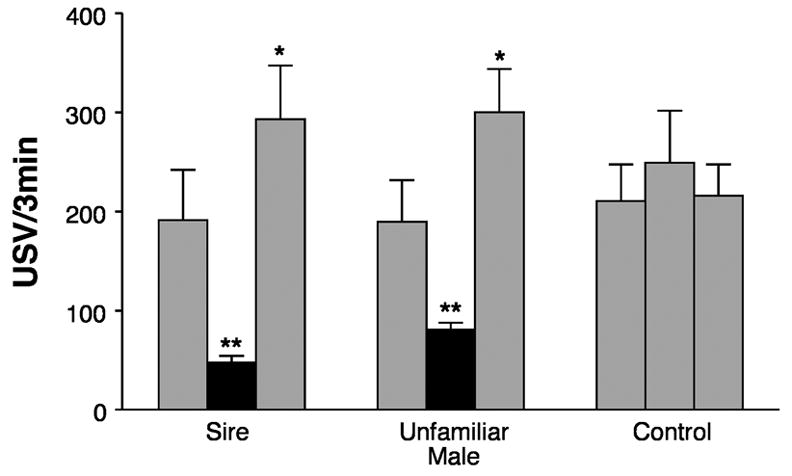
Ultrasonic vocalization (USV) rates shown by pups reared by the dam and sire and exposed to adult males in isolation. The gray bars indicate that the pup is isolated. The filled bars indicate the presence of an anesthetized adult male. The adult male is either the familiar Sire (left set of 3 bars) or an Unfamiliar Adult Male (middle set). In the Control condition (right set of bars, the pup is in isolation throughout, merely picked up and put down between each period. Asterisks mark significant differences from the 1st isolation [15]. Figure redrawn. Reprinted by permission of Journal of Comparative Psychology.
The role of olfactory cues in the development of paternal potentiation
A previously unpublished investigation into the cues necessary to alter the relationship with the sire has demonstrated an important role for olfactory input. With the use of a cage split diagonally with wire mesh, each litter was reared with its dam on one side. The sire was on the other. Thus, pups were exposed postnatally to the smells and sounds of their sire without direct physical contact.
Twenty four breeding pairs of randomly bred N:NIH rats were housed in standard colony cages in our animal facility. One or 2 days before the day of birth, the cages of 12 pairs were split diagonally with stiffened wire screening. The dam was on one side, the male on the other. The other 12 litters were reared biparentally in unmodified cages. Litters were culled to 9 pups within 48hrs of birth. On PND 12–13, each litter was tested in the passive potentiation paradigm with one male and one female in each of 4 reunion conditions: dam, sire, group of 4 littermates, or ‘pick up’ control. All social companions were anesthetized. Overall ANOVAs showed highly significant effects of social companion and test period, but no effect of sex. Thus, USV rates of male and female littermates in each companion condition were averaged. The statistics reported for potentiation are planned, paired t-tests comparing the amount of USV in Isolation2 to the amount in Isolation1 of these average values for each litter and condition. Similar paired t-tests comparing Reunion to Isolation1 assess contact quieting effects. Each experimental condition had an N of 12 such pairs.
The results demonstrate that olfactory cues from the sire are sufficient for it to become a potentiating stimulus, at least when pups experience those cues when being reared by the dam. A role for auditory cues seems extremely unlikely as rat pups’ ears do not open until PND10–12. Split cage reared pups show USV potentiation to their sire (p<.05), even though they have had no previous physical contact (see Figure 5A). During the reunion period with the sire, they not only reduced USV rate significantly, they displayed no ‘freezing’ or other signs of fear. In fact, they nosed their sire’s fur and tried to burrow underneath its body. The littermates tested in the other conditions showed the expected results: potentiation to the dam (p<.01, data not shown), but not to littermates or in the ‘pick up’ control test (p>.50 for both, data not shown).
Figure 5.
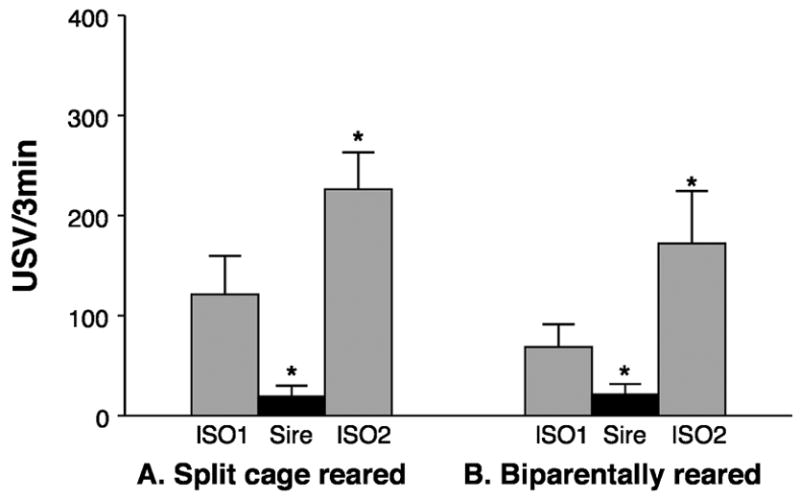
USV rates of pups tested in the potentiation paradigm, previously reared in a split cage and exposed to the odor of their sire without contact (A) or reared biparentally in a single compartmented cage (B). The gray bars indicate that the pup is isolated. The filled bars indicate the presence of the anesthetized, familiar Sire. Asterisks mark significant differences from the 1st isolation.
Because the potentiation to the sire does not look as robust as usually seen to the dam, one might believe that the lack of physical contact may account for this. The data from the matched biparentally-reared animals show that this is not likely the cause as their paternal potentiation effects are no stronger (p<.05, see Figure 5B). In fact, since the original finding in 1998 [17], we have done 6 experiments testing potentiation to the sire. In 4 out of the 6 experiments, potentiation to the sire was less robust than to the dam. Once, the relationship was reversed. In 1, pups did not show significant paternal potentiation (maternal potentiation was not tested). We do not know yet what accounts for the variability.
Thus, at least when experienced in the presence of the dam, odor cues appear to be sufficient for the acquisition of an altered social relationship to the sire.
Hypothesized mechanism for acquisition of paternal potentiation
There is a long history of research into the formation of infantile social bonds in animals, going back to the work of Scott, Harlow, and others [32,33,66]. A possible role for odor cues has often been found, even in human infants [26,65,84,108,113]. It has been proposed that the dam’s manipulations of pups during normal mother/infant interactions may be how rat pups form an attraction to the maternal odor and an attachment to the dam [30]. We suggest that this process underlies the pups’ positive response to the sires.
Over the past 30 years, the factors and physiological processes involved in the development of an odor preference have received considerable study [54,107,120]. Sullivan, Wilson and colleagues have mapped many of the neurobiological mechanisms underlying olfactory learning in the infant rat [102–104,112]. Important roles have been found for alterations in the olfactory bulb, norepinephrine secretion from the locus coeruleus into the bulb, and modulating effects of the amygdala. Studies from their laboratory are beginning to demonstrate a similar role for norepinephrine in somatosensory conditioning [62,63].
We hypothesize that biparental rearing causes an alteration in the pups’ USV response to the male, which is mediated by neural mechanisms similar to that described for the induction of an odor preference [71,99–101]. That is, stimulation of the pups in the presence of the adult male activates the locus coeruleus and norepinephrine secretion to enhance the development of an odor preference and a social bond. This hypothesis does not imply that USV potentiation is simply another manifestation of an odor (or somatosensory) preference. Pups must have a predisposition to respond to specific odors. Pups reared in the presence of lemon odors do not have an augmented separation response after exposure to an otherwise neutral stimulus scented with lemon [92]. Pups show preferences for the odors of siblings [39], but siblings do not elicit USV potentiation.
This hypothesized mechanism may include a role for the opioid system in the acquisition of the potentiation response. Naltrexone administration blocks the formation and consolidation of odor learning in 12 day-old rats, but not its expression [88,89]. Such a role is also suggested by the report that μ-receptor knockout mice do not potentiate USV to their dams [69].
Whether or not our hypothesis is true remains to be tested. Perhaps mere exposure to the scent of the sire without stimulation is enough to alter the social interaction from behavioral inhibition to potentiation. We also cannot be certain if it is the pups that are altered by the postnatal exposure. It is conceivable that exposure to the pups causes the sire to emit a different odor, one to which pups are predisposed to respond positively. It is known that sexual experience and cohabitation with a female through pregnancy and lactation can have important effects. The experience inhibits infanticide in adult male rats, even if previously infanticidal [15]. If the hypothesis is true, the many avenues for future research include: 1) This paradigm will allow us to ask whether the positive relationship with the sire depends on whether or not the pups’ exposure to his odor occurs in the presence of the dam. The results may have broader implications as to how attachments to secondary caretakers are formed. 2) It will remain to be determined if the formation of the social bond to the dam depends on similar processes as those to the sire.
EXPRESSION
Sensory and behavioral mechanisms
Although the usual potentiation test in our laboratory involves an initial isolation, a reunion, and a second isolation, the initial isolation is not necessary for maternal potentiation of USV. Pups taken from the litter pile and placed in contact with their anesthetized dam in a novel cage will vocalize at a potentiated rate upon her removal [43], in comparison with littermates isolated directly in the novel cage (see Figure 6).
Figure 6.
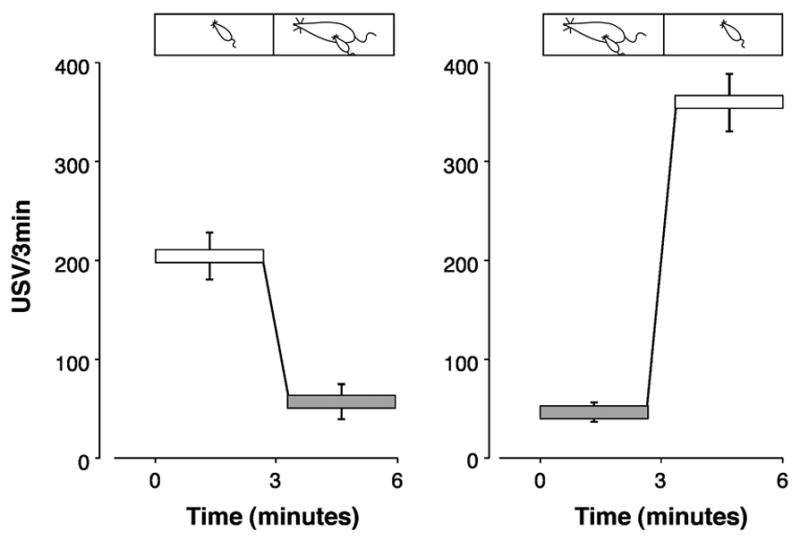
Mean rates of ultrasonic vocalization (USV/3min) of rat pups in response to dams. On the left, the pup was isolated in the novel test chamber for the first 3 min and then the dam was placed next to the pup for the last 3 min. On the right, the pup was with the dam for the first 3min and then separated from her during the last 3 min. The silhouettes of pup and dam at the top of each graph depict the changing conditions. Asterisks mark significant differences from the 1st isolation [41]. Figure redrawn. Reprinted by permission of Developmental Psychobiology.
Thermal cues are not an essential factor in the elicitation of USV potentiation: potentiation will occur to an anesthetized dam with a very reduced body temperature or in a thermoneutral environment [43,61,93]. As potentiation can occur after contact with either an active or anesthetized (passive) dam, movement of the dam cannot be a necessary factor either [41,96]. This does not mean, however, that movement cannot have an important role. A pup placed in contact with its anesthetized dam for 30min does not increase vocalization rate in a subsequent isolation. A pup in contact with its unanesthetized dam for 30min will vocalize more [41].
The pup’s sense of olfaction is necessary for passive potentiation of USV, but not for active potentiation. Anosmic pups show neither potentiation nor suppression of USV after exposure to an anesthetized dam or male. Their vocal response to isolation remains unchanged. Anosmic pups, however, continue to show undiminished levels of USV potentiation after interaction with an active dam [96]. The dam’s manipulations of the pup may be sensed via somatosensory or vestibular mechanisms.
Neurochemical regulation of the expression of potentiation
A number of neurotransmitter systems are known to be capable of regulating isolation-induced USV by rat pups including GABA [51,81,86,119], opioid [4,20,57], serotonergic [53,117], cholinergic [13], corticotrophin-releasing hormone [34,50], vasopressinergic [114], and dopaminergic [27,58,59]. In most cases, it has not yet been determined whether the effects of exogenous administration of neuroactive ligands truly demonstrate roles for endogenous processes.
Opioids do not regulate the expression of potentiation
The endogenous opioid system is not generally thought to control isolation-induced USV in rats, but application of opioid agonists and antagonists can influence the rate of USV. Moreover, opioids are known to have a role in contact quieting [21,23,24,115]. For these reasons, we tested and disproved the hypothesis that the interaction with the dam activates endogenous opioids to regulate the expression of USV potentiation. While exogenous opioid ligands did affect USV rate at some doses, they had no greater effect on potentiated USV [91]. In fact, the effect was usually reduced.
Dopamine activity in the nucleus accumbens does
Given the extensive literature linking dopaminergic processes to reward (e.g., [87,90], the effects of systemic administration of dopamine 2-family ligands (D2) on maternal potentiation were investigated [72]. The D2 family of receptors includes the D2, D3, and D4 subtypes. The agonist quinpirole and antagonist raclopride act on all three. PND12 pups were injected intraperitoneally with quinpirole, raclopride, or both. The D2 agonist quinpirole completely blocked maternal potentiation (see Figure 7), even though quinpirole’s effect on the USV rate in the first isolation period was not significant. Quinpirole increased behavioral activity, so its block of potentiated vocalization was not related to a generalized decrease in arousal. Nor were its effects the result of incapacity to produce high rates of USV: rates more than doubled when quinpirole-injected pups were placed in a cold environment (data not shown). The D2 antagonist had no effect on potentiation, but did block the agonist effect, demonstrating the specificity of the agonist effects.
Figure 7.
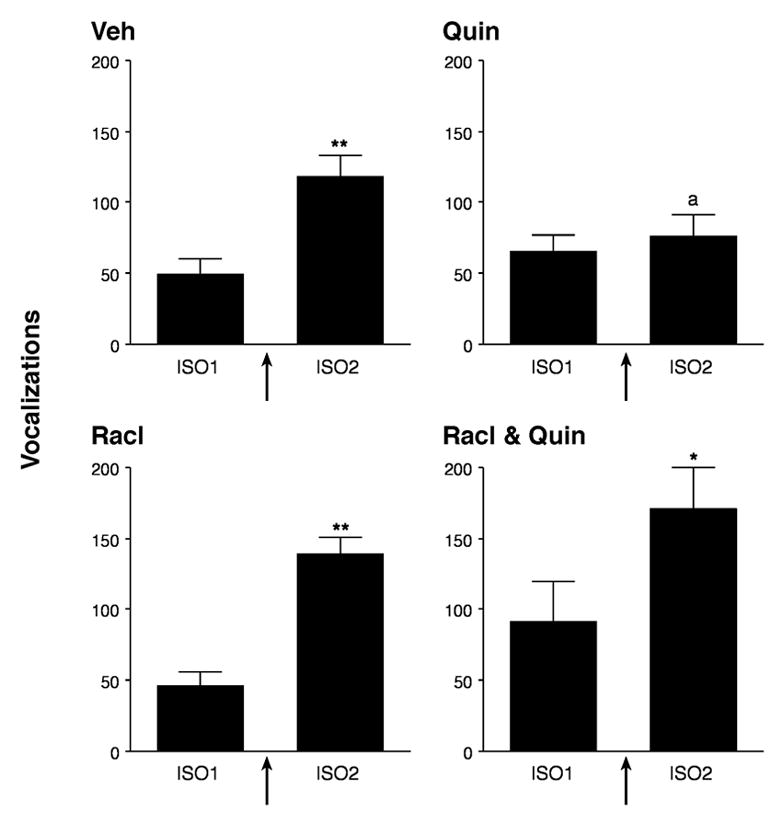
Mean (± SEM) number of vocalizations during a 2-min initial isolation (ISO1) and a 2-min re-isolation (ISO2). Arrows indicate a 2-min reunion with the anesthetized dam between isolations. In the group that received a systemic injection of vehicle (Veh), the pups called more in the second isolation than in the first, a phenomenon called “maternal potentiation.” Injection with the D2 agonist quinpirole (Quin; 1 mg/kg) blocked potentiation without preventing isolation-induced vocalization in either isolation. Injection of the D2 antagonist raclopride (Racl; 1 mg/kg) did not prevent potentiation. Raclopride (1 mg/kg) restored potentiation when injected before quinpirole (1 mg/kg) (Racl and Quin). The ‘a’ indicates that the Quin group’s difference from ISO1 to ISO2 was significantly less than the vehicle group’s (p = .003). Asterisks indicate a significant increase in number of vocalizations from ISO1 to ISO2. *p < .01. **p < .001 [70]. Reprinted by permission of Behavioral Neuroscience.
We believe that these findings suggest that the activation of D2 receptors interferes with normal dopaminergic mediation of the isolation, reunion and re-isolation experiences by generating reward signals continuously. This, in turn, blocks the expression of heightened isolation distress and potentiated vocalization.
If our interpretation is true, then brain areas known to be involved in reward processes should be the site of regulation for the socially mediated responses. One such area is the nucleus accumbens [90]. Infusions of quinpirole directly into the nucleus accumbens block potentiation just as systemic injections do [73]. Infusions of the agonist into the dorsal striatum do not, nor do saline infusions into the accumbens. Finally, and we believe most convincingly, infusions of raclopride into the accumbens completely block the suppression of USV potentiation by systemic quinpirole injections [73].
It is important to note that the results from these studies suggest that the D2 dopamine system alone is unlikely to account fully for the regulation of USV potentiation. Pups showed potentiation with the D2 receptors blocked by raclopride, a permissive effect. But, blockade of D2 receptors did not increase calling rates without a reunion with the dam, e. g., in the first isolation or in ‘pick up’ control pups (data not shown). One feasible explanation is that a very precise pattern or level of D2 activation does regulate potentiation. On the other hand, given the large number of neuroactive compounds known to influence USV, it seems more likely that some other system is altered by the brief interaction with the dam. Which possibility is true remains to be determined.
SUMMATION
When isolated from their mothers and siblings, infant animals including humans express a variety of behaviors that indicate the existence of social attachment, especially vocalizations. The isolation-induced vocalization of a rat pup is modulated by current and prior social interaction. An isolated pup reduces its rate of vocalization when placed in contact with familiar stimuli, particularly social ones such as its dam or littermates, a phenomenon called contact quieting. The isolated pup’s vocalization is greatly increased if the pup has been in contact with its mother immediately before isolation, a phenomenon called maternal potentiation. Other familiar stimuli that can induce contact quieting do not elicit potentiation. Thus, the potentiation of vocalization depends on some degree of social specificity, but not individual recognition. Unrelated adult females can also elicit potentiation.
Research from our laboratory and others has elucidated the developmental time course of maternal potentiation in rats and that vocal potentiation occurs in other species. Early experience can play a role in the acquisition of potentiation. If rat pups are reared by both dam and sire, or even reared by the dam in the presence of the sire’s odor, the pups show potentiation to the sire instead of the fear-related behavioral inhibition. The neurobiological mechanisms of acquisition are unknown, but are hypothesized to depend on the processes underlying development of an odor preference.
Expression of USV potentiation to an inactive dam depends on olfactory cues, but potentiation to an active dam does not. Thermal cues are not critical. Among the many neurochemical systems able to regulate isolation-induced USV, it is dopamine activity that appears to play a decisive role on potentiation. Activation of D2 receptors in the nucleus accumbens blocks maternal potentiation of USV without altering USV rate in an initial isolation.
In conclusion, our research is directed towards understanding the bases of normal and pathological development of animal and human social relations. As such, our investigations focus on how early life affiliative bonds are formed and what processes regulate their expression. The research uses the separation and reunion responses of infant rats: isolation-induced vocalization, contact quieting, and maternal/paternal potentiation of vocalization. The characteristics of USV potentiation, e.g., social specificity and a role for early experience, suggest that potentiation reflects a type of filial attachment, a subclass of affiliative bonds directed by infants towards parents (or other primary caretakers). The modulation of isolation-induced vocalization by social interactions provides a paradigm for investigating the neurobehavioral mechanisms underlying the formation of infant filial and affiliative social relationships.
Acknowledgments
The studies reported here come from the efforts of many investigators, technicians, and students. In particular, I would like to thank Susan Brunelli, Myron Hofer, Holly Moore, Jeff Muller, and Michael Myers. The work was supported by funds from the New York State Office of Mental Health and PHS grant MH40430 from the National Institute of Mental Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Allin JT, Banks EM. Effects of temperature on ultrasound production by infant albino rats. Dev Psychobiol. 1971;4:149–156. doi: 10.1002/dev.420040206. [DOI] [PubMed] [Google Scholar]
- 2.Barron S, Segar TM, Yahr JS, Baseheart BJ, Willford JA. The effects of neonatal ethanol and/or cocaine exposure on isolation- induced ultrasonic vocalizations. Pharmacol Biochem Behav. 2000;67:1–9. doi: 10.1016/s0091-3057(00)00304-x. [DOI] [PubMed] [Google Scholar]
- 3.Bell RW, Nitschke W, Bell NJ, Zachman TA. Early experience, ultrasonic vocalizations, and maternal responsiveness in rats. Dev Psychobiol. 1974;7:235–242. doi: 10.1002/dev.420070307. [DOI] [PubMed] [Google Scholar]
- 4.Blass EM, Fillion TJ, Weller A, Brunson L. Separation of opioid from nonopioid mediation of affect in neonatal rats: nonopioid mechanisms mediate maternal contact influences. Behav Neurosci. 1990;104:625–636. doi: 10.1037//0735-7044.104.4.625. [DOI] [PubMed] [Google Scholar]
- 5.Blumberg MS. Rodent ultrasonic short calls: locomotion, biomechanics, and communication. J Comp Psychol. 1992;106:360–365. doi: 10.1037/0735-7036.106.4.360. [DOI] [PubMed] [Google Scholar]
- 6.Blumberg MS, Alberts JR. Ultrasonic vocalizations by rat pups in the cold: an acoustic by- product of laryngeal braking? Behav Neurosci. 1990;104:808–817. doi: 10.1037//0735-7044.104.5.808. [DOI] [PubMed] [Google Scholar]
- 7.Blumberg MS, Efimova IV, Alberts JR. Ultrasonic vocalizations by rat pups: the primary importance of ambient temperature and the thermal significance of contact comfort. Dev Psychobiol. 1992;25:229–250. doi: 10.1002/dev.420250402. [DOI] [PubMed] [Google Scholar]
- 8.Blumberg MS, Sokoloff G. Thermoregulatory competence and behavioral expression in the young of altricial species--revisited. Dev Psychobiol. 1998;33:107–123. doi: 10.1002/(sici)1098-2302(199809)33:2<107::aid-dev2>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 9.Blumberg MS, Sokoloff G, Kent KJ. Cardiovascular concomitants of ultrasound production during cold exposure in infant rats. Behav Neurosci. 1999;113:1274–1282. doi: 10.1037//0735-7044.113.6.1274. [DOI] [PubMed] [Google Scholar]
- 10.Blumberg MS, Sokoloff G, Kirby RF, Kent KJ. Distress vocalizations in infant rats: what’s all the fuss about? Psychological Science. 2000;11:78–81. doi: 10.1111/1467-9280.00219. [DOI] [PubMed] [Google Scholar]
- 11.Bowlby J. Attachment and loss. New York: Basic Books; 1969. p. 3 . [Google Scholar]
- 12.Bowlby J. A secure base: parent-child attachment and healthy human development. New York: Basic Books; 1988. p. 205. [Google Scholar]
- 13.Branchi I, Campolongo P, Alleva E. Scopolamine effects on ultrasonic vocalization emission and behavior in the neonatal mouse. Behav Brain Res. 2004;151:9–16. doi: 10.1016/S0166-4328(03)00277-8. [DOI] [PubMed] [Google Scholar]
- 14.Branchi I, Santucci D, Alleva E. Ontogeny of behavioral inhibition induced by the odor of an unfamiliar adult male conspecific and absence of maternal potentiation in the mouse. Dev Psychobiol. 2004;45:264. [Google Scholar]
- 15.Brown RE. Social and hormonal factors influencing infanticide and its suppression in adult male Long-Evans rats (Rattus norvegicus) J Comp Psychol. 1986;100:155–161. [PubMed] [Google Scholar]
- 16.Brunelli SA, Keating CC, Hamilton NA, Hofer MA. Development of ultrasonic vocalization responses in genetically heterogeneous National Institute of Health (N:NIH) rats. I. Influence of age, testing experience, and associated factors. Dev Psychobiol. 1996;29:507–516. doi: 10.1002/(SICI)1098-2302(199609)29:6<507::AID-DEV3>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 17.Brunelli SA, Masmela JR, Shair HN, Hofer MA. Effects of biparental rearing on ultrasonic vocalization (USV) responses of rat pups (Rattus norvegicus) J Comp Psychol. 1998;112:331–343. doi: 10.1037/0735-7036.112.4.331. [DOI] [PubMed] [Google Scholar]
- 18.Brunelli SA, Shair HN, Hofer MA. Hypothermic vocalizations of rat pups (Rattus norvegicus) elicit and direct maternal search behavior. J Comp Psychol. 1994;108:298–303. doi: 10.1037/0735-7036.108.3.298. [DOI] [PubMed] [Google Scholar]
- 19.Campbell JO, Fogarty JA, Spear LP. Inhibition of nitric oxide synthesis with L-NAME suppresses isolation- induced ultrasounds in rat pups. Pharmacol Biochem Behav. 1999;63:45–53. doi: 10.1016/s0091-3057(98)00233-0. [DOI] [PubMed] [Google Scholar]
- 20.Carden SE, Davachi L, Hofer MA. U50,488 increases ultrasonic vocalizations in 3-, 10-, and 18-day-old rat pups in isolation and the home cage. Dev Psychobiol. 1994;27:65–83. doi: 10.1002/dev.420270107. [DOI] [PubMed] [Google Scholar]
- 21.Carden SE, Hernandez N, Hofer MA. The isolation and companion comfort responses of 7- and 3-day-old rat pups are modulated by drugs active at the opioid receptor. Behav Neurosci. 1996;110:324–330. doi: 10.1037//0735-7044.110.2.324. [DOI] [PubMed] [Google Scholar]
- 22.Carden SE, Hofer MA. Effect of a social companion on the ultrasonic vocalizations and contact responses of 3-day-old rat pups. Behav Neurosci. 1992;106:421–426. doi: 10.1037//0735-7044.106.2.421. [DOI] [PubMed] [Google Scholar]
- 23.Carden SE, Hofer MA. The effects of opioid and benzodiazepine antagonists on dam-induced reductions in rat pup isolation distress. Dev Psychobiol. 1990;23:797–808. doi: 10.1002/dev.420230804. [DOI] [PubMed] [Google Scholar]
- 24.Carden SE, Hofer MA. Socially mediated reduction of isolation distress in rat pups is blocked by naltrexone but not by Ro 15–1788. Behav Neurosci. 1990;104:457–463. doi: 10.1037//0735-7044.104.3.457. [DOI] [PubMed] [Google Scholar]
- 25.Conely L, Bell RW. Neonatal ultrasounds elicited by odor cues. Dev Psychobiol. 1978;11:193–197. doi: 10.1002/dev.420110302. [DOI] [PubMed] [Google Scholar]
- 26.Coureaud G, Schaal B. Attraction of newborn rabbits to abdominal odors of adult conspecifics differing in sex and physiological state. Dev Psychobiol. 2000;36:271–281. doi: 10.1002/(sici)1098-2302(200005)36:4<271::aid-dev2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 27.Dastur FN, McGregor IS, Brown RE. Dopaminergic modulation of rat pup ultrasonic vocalizations. Eur J Pharmacol. 1999;382:53–67. doi: 10.1016/s0014-2999(99)00590-7. [DOI] [PubMed] [Google Scholar]
- 28.David D, Giron A, Mellman TA. Panic-phobic patients and developmental trauma. J Clin Psychiatry. 1995;56:113–117. [PubMed] [Google Scholar]
- 29.Fish EW, Sekinda M, Ferrari PF, Dirks A, Miczek KA. Distress vocalizations in maternally separated mouse pups: modulation via 5-HT(1A), 5-HT(1B) and GABA(A) receptors. Psychopharmacology (Berl) 2000;149:277–285. doi: 10.1007/s002130000370. [DOI] [PubMed] [Google Scholar]
- 30.Fleming AS, O’Day DH, Kraemer GW. Neurobiology of mother-infant interactions: experience and central nervous system plasticity across development and generations. Neurosci Biobehav Rev. 1999;23:673–685. doi: 10.1016/s0149-7634(99)00011-1. [DOI] [PubMed] [Google Scholar]
- 31.Gardner CR. Distress vocalization in rat pups. A simple screening method for anxiolytic drugs. J Pharmacol Methods. 1985;14:181–187. doi: 10.1016/0160-5402(85)90031-2. [DOI] [PubMed] [Google Scholar]
- 32.Gurski JC, Davis K, Scott JP. Interaction of separation discomfort with contact comfort and discomfort in the dog. Dev Psychobiol. 1980;13:463–467. doi: 10.1002/dev.420130504. [DOI] [PubMed] [Google Scholar]
- 33.Harlow HF, Zimmermann RR. Affectional responses in the infant monkey; orphaned baby monkeys develop a strong and persistent attachment to inanimate surrogate mothers. Science. 1959;130:421–432. doi: 10.1126/science.130.3373.421. [DOI] [PubMed] [Google Scholar]
- 34.Harvey AT, Hennessy MB. Corticotropin-releasing factor modulation of the ultrasonic vocalization rate of isolated rat pups. Brain Res Dev Brain Res. 1995;87:125–134. doi: 10.1016/0165-3806(95)00064-k. [DOI] [PubMed] [Google Scholar]
- 35.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 36.Hennessy MB, Maken DS, Graves FC. Consequences of the presence of the mother or unfamiliar adult female on cortisol, ACTH, testosterone and behavioral responses of periadolescent guinea pigs during exposure to novelty. Psychoneuroendocrinology. 2000;25:619–632. doi: 10.1016/s0306-4530(00)00014-7. [DOI] [PubMed] [Google Scholar]
- 37.Hennessy MB, Miller EE, Shair HN. Brief exposure to the biological mother “potentiates” the isolation behavior of precocial Guinea pig pups. Dev Psychobiol. 2006;48:653–659. doi: 10.1002/dev.20180. [DOI] [PubMed] [Google Scholar]
- 38.Hennessy MB, Ritchey RL. Hormonal and behavioral attachment responses in infant guinea pigs. Dev Psychobiol. 1987;20:613–625. doi: 10.1002/dev.420200607. [DOI] [PubMed] [Google Scholar]
- 39.Hepper PG. Sibling recognition in the rat: Change in stimulus value or individual preference? Anim Behav. 1986;34:607–609. [Google Scholar]
- 40.Hofer MA. Multiple regulators of ultrasonic vocalization in the infant rat. Psychoneuroendocrinology. 1996;21:203–217. doi: 10.1016/0306-4530(95)00042-9. [DOI] [PubMed] [Google Scholar]
- 41.Hofer MA, Brunelli SA, Masmela J, Shair HN. Maternal interactions prior to separation potentiate isolation-induced calling in rat pups. Behav Neurosci. 1996;110:1158–1167. doi: 10.1037//0735-7044.110.5.1158. [DOI] [PubMed] [Google Scholar]
- 42.Hofer MA, Brunelli SA, Shair HN. Potentiation of isolation-induced vocalization by brief exposure of rat pups to maternal cues. Dev Psychobiol. 1994;27:503–517. doi: 10.1002/dev.420270804. [DOI] [PubMed] [Google Scholar]
- 43.Hofer MA, Brunelli SA, Shair HN. Ultrasonic vocalization responses of rat pups to acute separation and contact comfort do not depend on maternal thermal cues. Dev Psychobiol. 1993;26:81–95. doi: 10.1002/dev.420260202. [DOI] [PubMed] [Google Scholar]
- 44.Hofer MA, Masmela JR, Brunelli SA, Shair HN. Behavioral mechanisms for active maternal potentiation of isolation calling in rat pups. Behav Neurosci. 1999;113:51–61. doi: 10.1037//0735-7044.113.1.51. [DOI] [PubMed] [Google Scholar]
- 45.Hofer MA, Masmela JR, Brunelli SA, Shair HN. The ontogeny of maternal potentiation of the infant rats’ isolation call. Dev Psychobiol. 1998;33:189–201. doi: 10.1002/(sici)1098-2302(199811)33:3<189::aid-dev1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 46.Hofer MA, Shair H. Sensory processes in the control of isolation-induced ultrasonic vocalization by 2-week-old rats. J Comp Physiol Psychol. 1980;94:271–279. doi: 10.1037/h0077665. [DOI] [PubMed] [Google Scholar]
- 47.Hofer MA, Shair HN. Independence of ultrasonic vocalization and thermogenic responses in infant rats. Behav Neurosci. 1991;105:41–48. doi: 10.1037//0735-7044.105.1.41. [DOI] [PubMed] [Google Scholar]
- 48.Hofer MA, Shair HN. Ultrasonic vocalization, laryngeal braking, and thermogenesis in rat pups: a reappraisal. Behav Neurosci. 1993;107:354–362. doi: 10.1037//0735-7044.107.2.354. [DOI] [PubMed] [Google Scholar]
- 49.Hofer MA, Shair HN, Murowchick E. Isolation distress and maternal comfort responses of two-week-old rat pups reared in social isolation. Dev Psychobiol. 1989;22:553–566. doi: 10.1002/dev.420220603. [DOI] [PubMed] [Google Scholar]
- 50.Insel TR, Harbaugh CR. Central administration of corticotropin releasing factor alters rat pup isolation calls. Pharmacol Biochem Behav. 1989;32:197–201. doi: 10.1016/0091-3057(89)90233-5. [DOI] [PubMed] [Google Scholar]
- 51.Insel TR, Hill JL, Mayor RB. Rat pup ultrasonic isolation calls: possible mediation by the benzodiazepine receptor complex. Pharmacol Biochem Behav. 1986;24:1263–1267. doi: 10.1016/0091-3057(86)90182-6. [DOI] [PubMed] [Google Scholar]
- 52.Insel TR, Shapiro LE. Oxytocin receptor distribution reflects social organization in monogamous and polygamous voles. Proc Natl Acad Sci U S A. 1992;89:5981–5985. doi: 10.1073/pnas.89.13.5981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Joyce MP, Carden SE. The effects of 8-OH-DPAT and (+/−)-pindolol on isolation-induced ultrasonic vocalizations in 3-, 10-, and 14-day-old rats. Dev Psychobiol. 1999;34:109–117. [PubMed] [Google Scholar]
- 54.Jubilan BM, Nyby JG. The intrauterine position phenomenon and precopulatory behaviors of house mice. Physiol Behav. 1992;51:857–872. doi: 10.1016/0031-9384(92)90127-n. [DOI] [PubMed] [Google Scholar]
- 55.Kalin NH, Shelton SE, Snowdon CT. Affiliative vocalizations in infant rhesus macaques (Macaca mulatta) J Comp Psychol. 1992;106:254–261. doi: 10.1037/0735-7036.106.3.254. [DOI] [PubMed] [Google Scholar]
- 56.Kehoe P, Blass EM. Opioid-mediation of separation distress in 10-day-old rats: reversal of stress with maternal stimuli. Dev Psychobiol. 1986;19:385–398. doi: 10.1002/dev.420190410. [DOI] [PubMed] [Google Scholar]
- 57.Kehoe P, Boylan CB. Behavioral effects of kappa-opioid-receptor stimulation on neonatal rats. Behav Neurosci. 1994;108:418–423. doi: 10.1037//0735-7044.108.2.418. [DOI] [PubMed] [Google Scholar]
- 58.Kehoe P, Boylan CB. Cocaine-induced effects on isolation stress in neonatal rats. Behav Neurosci. 1992;106:374–379. doi: 10.1037//0735-7044.106.2.374. [DOI] [PubMed] [Google Scholar]
- 59.Kehoe P, Clash K, Skipsey K, Shoemaker WJ. Brain dopamine response in isolated 10-day-old rats: assessment using D2 binding and dopamine turnover. Pharmacol Biochem Behav. 1996;53:41–49. doi: 10.1016/0091-3057(95)00196-4. [DOI] [PubMed] [Google Scholar]
- 60.Kirby RF, Blumberg MS. Maintenance of arterial pressure in infant rats during moderate and extreme thermal challenge. Dev Psychobiol. 1998;32:169–176. [PubMed] [Google Scholar]
- 61.Kraebel KS, Brasser SM, Campbell JO, Spear LP, Spear NE. Developmental differences in temporal patterns and potentiation of isolation-induced ultrasonic vocalizations: Influence of temperature variables. Dev Psychobiol. 2002;40:147–159. doi: 10.1002/dev.10022. [DOI] [PubMed] [Google Scholar]
- 62.Landers MS, Sullivan RM. Norepinephrine and associative conditioning in the neonatal rat somatosensory system. Brain Res Dev Brain Res. 1999;114:261–264. doi: 10.1016/s0165-3806(99)00026-7. [DOI] [PubMed] [Google Scholar]
- 63.Landers MS, Sullivan RM. Vibrissae-evoked behavior and conditioning before functional ontogeny of the somatosensory vibrissae cortex. J Neurosci. 1999;19:5131–5137. doi: 10.1523/JNEUROSCI.19-12-05131.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Levine S, Mody T. The long-term psychobiological consequences of intermittent postnatal separation in the squirrel monkey. Neurosci Biobehav Rev. 2003;27:83–89. doi: 10.1016/s0149-7634(03)00011-3. [DOI] [PubMed] [Google Scholar]
- 65.Marlier L, Schaal B. Human newborns prefer human milk: conspecific milk odor is attractive without postnatal exposure. Child Dev. 2005;76:155–168. doi: 10.1111/j.1467-8624.2005.00836.x. [DOI] [PubMed] [Google Scholar]
- 66.Mayer AD, Rosenblatt JS. Hormonal interaction with stimulus and situational factors in the initiation of maternal behavior in nonpregnant rats. J Comp Physiol Psychol. 1980;94:1040–1059. doi: 10.1037/h0077744. [DOI] [PubMed] [Google Scholar]
- 67.Mennella JA, Moltz H. Pheromonal emission by pregnant rats protects against infanticide by nulliparous conspecifics. Physiol Behav. 1989;46:591–595. doi: 10.1016/0031-9384(89)90337-5. [DOI] [PubMed] [Google Scholar]
- 68.Miczek KA, Weerts EM, Vivian JA, Barros HM. Aggression, anxiety and vocalizations in animals: GABAA and 5-HT anxiolytics. Psychopharmacology (Berl) 1995;121:38–56. doi: 10.1007/BF02245590. [DOI] [PubMed] [Google Scholar]
- 69.Moles A, Kieffer BL, D’Amato FR. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science. 2004;304:1983–1986. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- 70.Monk CS, Pine DS, Charney DS. A developmental and neurobiological approach to early trauma research. Semin Clin Neuropsychiatry. 2002;7:137–146. doi: 10.1053/scnp.2002.31793. [DOI] [PubMed] [Google Scholar]
- 71.Moriceau S, Sullivan RM. Unique neural circuitry for neonatal olfactory learning. J Neurosci. 2004;24:1182–1189. doi: 10.1523/JNEUROSCI.4578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Muller JM, Brunelli SA, Moore H, Myers MM, Shair HN. Maternally modulated infant separation responses are regulated by D2-family dopamine receptors. Behav Neurosci. 2005;119:1384–1388. doi: 10.1037/0735-7044.119.5.1384. [DOI] [PubMed] [Google Scholar]
- 73.Muller JM, Myers MM, Moore H, Shair HN. Nucleus accumbens dopamine D2 receptor activity disrupts rat pups’ response to loss of maternal contact. J Neurosci. doi: 10.1037/0735-7044.122.1.119. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Myers MM, Ali N, Weller A, Brunelli SA, Tu AY, Hofer MA, Shair HN. Brief maternal interaction increases number, amplitude, and bout size of isolation-induced ultrasonic vocalizations in infant rats (Rattus norvegicus) J Comp Psychol. 2004;118:95–102. doi: 10.1037/0735-7036.118.1.95. [DOI] [PubMed] [Google Scholar]
- 75.Naito H, Inoue M, Makino J. Ultrasonic isolation calls in genetically high- and low-emotional rat pups. Experimental Animals/Japanese Association for Laboratory Animal Science. 2000;49:289–294. doi: 10.1538/expanim.49.289. [DOI] [PubMed] [Google Scholar]
- 76.Naito H, Tonoue T. Sex difference in ultrasound distress call by rat pups. Behav Brain Res. 1987;25:13–21. doi: 10.1016/0166-4328(87)90041-6. [DOI] [PubMed] [Google Scholar]
- 77.Nelson EE, Panksepp J. Brain substrates of infant-mother attachment: contributions of opioids, oxytocin, and norepinephrine. Neurosci Biobehav Rev. 1998;22:437–452. doi: 10.1016/s0149-7634(97)00052-3. [DOI] [PubMed] [Google Scholar]
- 78.Nitschke W, Bell RW, Zachman T. Distress vocalizations of young in three inbred strains of mice. Dev Psychobiol. 1972;5:363–370. doi: 10.1002/dev.420050409. [DOI] [PubMed] [Google Scholar]
- 79.Noirot E. Ultrasounds in young rodents. II. Changes with age in albino rats. Anim Behav. 1968:16. doi: 10.1016/0003-3472(68)90123-1. [DOI] [PubMed] [Google Scholar]
- 80.Okon EE. The temperature relations of vocalizations in infant Golden Hamsters and Wistar rats. Journal of Zoology, London. 1971;164:227–237. [Google Scholar]
- 81.Olivier B, Molewijk E, van Oorschot R, van der Heyden J, Ronken E, Mos J. Rat pup ultrasonic vocalization: effects of benzodiazepine receptor ligands. Eur J Pharmacol. 1998;358:117–128. doi: 10.1016/s0014-2999(98)00603-7. [DOI] [PubMed] [Google Scholar]
- 82.Oswalt GL, Meier GW. Olfactory, thermal, and tactual influences on infantile ultrasonic vocalization in rats. Dev Psychobiol. 1975;8:129–135. doi: 10.1002/dev.420080205. [DOI] [PubMed] [Google Scholar]
- 83.Podhorna J, Brown RE. Flibanserin has anxiolytic effects without locomotor side effects in the infant rat ultrasonic vocalization model of anxiety. Br J Pharmacol. 2000;130:739–746. doi: 10.1038/sj.bjp.0703364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Porter RH, Winberg J. Unique salience of maternal breast odors for newborn infants. Neurosci Biobehav Rev. 1999;23:439–449. doi: 10.1016/s0149-7634(98)00044-x. [DOI] [PubMed] [Google Scholar]
- 85.Rifkin A, Glickman SE. Separation as a natural cue to danger in the spotted hyena (Crocuta crocuta) Dev Psychobiol. 2004;44:199–207. doi: 10.1002/dev.20003. [DOI] [PubMed] [Google Scholar]
- 86.Rivera S, Sanfeliu C, Garcia M, Comellas F, Rodriguez-Farre E. Increase in rat pup ultrasonic isolation calls induced by lindane. Neurotoxicology. 1992;13:235–239. [PubMed] [Google Scholar]
- 87.Roitman MF, van Dijk G, Thiele TE, Bernstein IL. Dopamine mediation of the feeding response to violations of spatial and temporal expectancies. Behav Brain Res. 2001;122:193–199. doi: 10.1016/s0166-4328(01)00189-9. [DOI] [PubMed] [Google Scholar]
- 88.Roth TL, Sullivan RM. Consolidation and expression of a shock-induced odor preference in rat pups is facilitated by opioids. Physiol Behav. 2003;78:135–142. doi: 10.1016/s0031-9384(02)00961-7. [DOI] [PubMed] [Google Scholar]
- 89.Roth TL, Sullivan RM. Endogenous opioids and their role in odor preference acquisition and consolidation following odor-shock conditioning in infant rats. Dev Psychobiol. 2001;39:188–198. doi: 10.1002/dev.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 91.Shair HN, Brunelli SA, Hofer MA. Lack of evidence for mu-opioid regulation of a socially mediated separation response. Physiol Behav. 2005;83:767–777. doi: 10.1016/j.physbeh.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 92.Shair HN, Brunelli SA, Hofer MA. Prior experience with adult male odor reduces fear responses and can induce potentiation of USV in isolated rat pups exposed to an adult male. Dev Psychobiol. 2003;41:107. [Google Scholar]
- 93.Shair HN, Brunelli SA, Masmela JR, Boone E, Hofer MA. Social, thermal, and temporal influences on isolation-induced and maternally potentiated ultrasonic vocalizations of rat pups. Dev Psychobiol. 2003;42:206–222. doi: 10.1002/dev.10087. [DOI] [PubMed] [Google Scholar]
- 94.Shair HN, Jasper A. Decreased venous return is neither sufficient nor necessary to elicit ultrasonic vocalizations of infant rat pups. Behav Neurosci. 2003;117:840–853. doi: 10.1037/0735-7044.117.4.840. [DOI] [PubMed] [Google Scholar]
- 95.Shair HN, Masmela JR, Brunelli SA, Hofer MA. Potentiation and inhibition of ultrasonic vocalization of rat pups: regulation by social cues. Dev Psychobiol. 1997;30:195–200. doi: 10.1002/(sici)1098-2302(199704)30:3<195::aid-dev2>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 96.Shair HN, Masmela JR, Hofer MA. The influence of olfaction on potentiation and inhibition of ultrasonic vocalization of rat pups. Physiol Behav. 1999;65:769–772. doi: 10.1016/s0031-9384(98)00218-2. [DOI] [PubMed] [Google Scholar]
- 97.Shoemaker WJ, Kehoe P. Effect of isolation conditions on brain regional enkephalin and beta- endorphin levels and vocalizations in 10-day-old rat pups. Behav Neurosci. 1995;109:117–122. doi: 10.1037//0735-7044.109.1.117. [DOI] [PubMed] [Google Scholar]
- 98.Smotherman WP, Bell RW, Starzec J, Elias J, Zachman TA. Maternal responses to infant vocalizations and olfactory cues in rats and mice. Behav Biol. 1974;12:55–66. doi: 10.1016/s0091-6773(74)91026-8. [DOI] [PubMed] [Google Scholar]
- 99.Sullivan RM, Hall WG. Reinforcers in infancy: classical conditioning using stroking or intra- oral infusions of milk as UCS. Dev Psychobiol. 1988;21:215–223. doi: 10.1002/dev.420210303. [DOI] [PubMed] [Google Scholar]
- 100.Sullivan RM, Hofer MA, Brake SC. Olfactory-guided orientation in neonatal rats is enhanced by a conditioned change in behavioral state. Dev Psychobiol. 1986;19:615–623. doi: 10.1002/dev.420190612. [DOI] [PubMed] [Google Scholar]
- 101.Sullivan RM, Stackenwalt G, Nasr F, Lemon C, Wilson DA. Association of an odor with activation of olfactory bulb noradrenergic beta-receptors or locus coeruleus stimulation is sufficient to produce learned approach responses to that odor in neonatal rats. Behav Neurosci. 2000;114:957–962. doi: 10.1037/0735-7044.114.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sullivan RM, Wilson DA. The locus coeruleus, norepinephrine, and memory in newborns. Brain Res Bull. 1994;35:467–472. doi: 10.1016/0361-9230(94)90160-0. [DOI] [PubMed] [Google Scholar]
- 103.Sullivan RM, Wilson DA. Role of the amygdala complex in early olfactory associative learning. Behav Neurosci. 1993;107:254–263. doi: 10.1037//0735-7044.107.2.254. [DOI] [PubMed] [Google Scholar]
- 104.Sullivan RM, Zyzak DR, Skierkowski P, Wilson DA. The role of olfactory bulb norepinephrine in early olfactory learning. Brain Res Dev Brain Res. 1992;70:279–282. doi: 10.1016/0165-3806(92)90207-d. [DOI] [PubMed] [Google Scholar]
- 105.Takahashi LK. Ontogeny of behavioral inhibition induced by unfamiliar adult male conspecifics in preweanling rats. Physiol Behav. 1992;52:493–498. doi: 10.1016/0031-9384(92)90336-z. [DOI] [PubMed] [Google Scholar]
- 106.Takahashi LK. Organizing action of corticosterone on the development of behavioral inhibition in the preweanling rat. Brain Res Dev Brain Res. 1994;81:121–127. doi: 10.1016/0165-3806(94)90074-4. [DOI] [PubMed] [Google Scholar]
- 107.Weber M, Paxinos G, Richardson R. Conditioned changes in ultrasonic vocalizations to an aversive olfactory stimulus are lateralized in 6-day-old rats. Dev Psychobiol. 2000;37:121–128. doi: 10.1002/1098-2302(200011)37:3<121::aid-dev1>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 108.Weller A, Feldman R. Emotion regulation and touch in infants: the role of cholecystokinin and opioids. Peptides. 2003;24:779–788. doi: 10.1016/s0196-9781(03)00118-9. [DOI] [PubMed] [Google Scholar]
- 109.Wiedenmayer CP, Barr GA. Developmental changes in responsivity to threat are stimulus-specific in rats. Dev Psychobiol. 2001;39:1–7. doi: 10.1002/dev.1022. [DOI] [PubMed] [Google Scholar]
- 110.Wiedenmayer CP, Barr GA. Ontogeny of defensive behavior and analgesia in rat pups exposed to an adult male rat. Physiol Behav. 1998;63:261–269. doi: 10.1016/s0031-9384(97)00439-3. [DOI] [PubMed] [Google Scholar]
- 111.Wiedenmayer CP, Lyo D, Barr GA. Rat pups reduce ultrasonic vocalization after exposure to an adult male rat. Dev Psychobiol. 2003;42:386–391. doi: 10.1002/dev.10112. [DOI] [PubMed] [Google Scholar]
- 112.Wilson DA, Sullivan RM. Neurobiology of associative learning in the neonate: early olfactory learning. Behav Neural Biol. 1994;61:1–18. doi: 10.1016/s0163-1047(05)80039-1. [DOI] [PubMed] [Google Scholar]
- 113.Winberg J, Porter RH. Olfaction and human neonatal behaviour: clinical implications. Acta Paediatr. 1998;87:6–10. doi: 10.1080/08035259850157787. [DOI] [PubMed] [Google Scholar]
- 114.Winslow JT, Insel TR. Effects of central vasopressin administration to infant rats. Eur J Pharmacol. 1993;233:101–107. doi: 10.1016/0014-2999(93)90354-k. [DOI] [PubMed] [Google Scholar]
- 115.Winslow JT, Insel TR. Endogenous opioids: do they modulate the rat pup’s response to social isolation? Behav Neurosci. 1991;105:253–263. doi: 10.1037//0735-7044.105.2.253. [DOI] [PubMed] [Google Scholar]
- 116.Winslow JT, Insel TR. Infant rat separation is a sensitive test for novel anxiolytics. Prog Neuropsychopharmacol Biol Psychiatry. 1991;15:745–757. doi: 10.1016/0278-5846(91)90003-j. [DOI] [PubMed] [Google Scholar]
- 117.Winslow JT, Insel TR. Serotonergic modulation of the rat pup ultrasonic isolation call: studies with 5HT1 and 5HT2 subtype-selective agonists and antagonists. Psychopharmacology. 1991;105:513–520. doi: 10.1007/BF02244372. [DOI] [PubMed] [Google Scholar]
- 118.Young LJ, Wang Z, Insel TR. Neuroendocrine bases of monogamy. Trends Neurosci. 1998;21:71–75. doi: 10.1016/s0166-2236(97)01167-3. [DOI] [PubMed] [Google Scholar]
- 119.Zimmerberg B, Brunelli SA, Hofer MA. Reduction of rat pup ultrasonic vocalizations by the neuroactive steroid allopregnanolone. Pharmacol Biochem Behav. 1994;47:735–738. doi: 10.1016/0091-3057(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 120.Zimmerberg B, Drucker PC, Weider JM. Differential behavioral effects of the neuroactive steroid allopregnanolone on neonatal rats prenatally exposed to alcohol. Pharmacol Biochem Behav. 1995;51:463–468. doi: 10.1016/0091-3057(95)00008-k. [DOI] [PubMed] [Google Scholar]