Summary
Neurexins have been proposed to function as major mediators of the coordinated pre- and postsynaptic apposition. However, key evidence for this role in vivo has been lacking, particularly due to gene redundancy. Here, we have obtained null mutations in the single Drosophila neurexin gene (dnrx). dnrx loss of function prevents the normal proliferation of synaptic boutons at glutamatergic neuromuscular junctions, while dnrx gain of function in neurons has the opposite effect. DNRX mostly localizes to the active zone of presynaptic terminals. Conspicuously, dnrx null mutants display striking defects in synaptic ultrastructure with the presence of detachments between pre- and postsynaptic membranes, abnormally long active zones, and increased number of T-bars. These abnormalities result in corresponding alterations in synaptic transmission, which reduced quantal content. Together, our results provide compelling evidence for an in vivo role of neurexins in the modulation of synaptic architecture and adhesive interactions between pre- and postsynaptic compartments.
Keywords: Synapse, neurotransmission, active zone, trans-synaptic adhesion, postsynaptic density, neuromuscular junction
Introduction
Synapse development and function form the basis of many neuronal processes, including formation and function of neural circuits, the ability to learn, and to store and recall memories. Thus, elucidating the mechanisms by which synapses develop and are modified is a central question in neurobiology. Over the past few decades a number of factors have been identified that play major roles in synapse morphogenesis and synaptic plasticity. Among these, trans-synaptic cell adhesion and signaling molecules that mediate the interactions between pre- and postsynaptic membranes stand out. They are thought to mediate target recognition, induce pre- and postsynaptic specializations and their alignment during synaptogenesis, maintain the integrity of synapses, and regulate synaptic assembly and disassembly during synaptic development and remodeling (Gerrow and El-Husseini, 2006; Scheiffele, 2003; Yamagata et al., 2003). In particular, neurexins and their postsynaptic binding partners the neuroligins are emerging as key synapse organizing molecules.
Neurexins were first identified as primary receptors for α-latrotoxin, a neurotoxin that triggers massive neurotransmitter release (Ushkaryov et al., 1992). There are three neurexin genes in mammals, each of which has two promoters generating α- and β-neurexins. Neurexins are subject to extensive alternative splicing, generating a large number of variants, which may mediate target recognition and synaptic specificity (Missler and Sudhof, 1998; Rowen et al., 2002; Tabuchi and Sudhof, 2002). Recent studies on the functional significance of a small subset of neurexin splicing variants support this idea (Comoletti et al., 2006; Chih et al., 2006; Graf et al., 2006). The extracellular region of neurexins binds to neuroligins (Ichtchenko et al., 1995; Boucard et al., 2005) and dystroglycan (Sugita et al., 2001). Neuroligins are localized to postsynaptic densities (PSDs) (Song et al., 1999) and associated with neurotransmitter receptors by interaction with scaffolding proteins (Irie et al., 1997; Meyer et al., 2004). Intracellularly, neurexins interact with the synaptic vesicle protein synaptotagmin (Hata et al., 1993), and PDZ domain proteins CASK (Hata et al., 1996) and Mints (Biederer and Sudhof, 2000), which are linked to the synaptic vesicle exocytosis machinery (Atasoy et al., 2007; Ho et al., 2003). Thus, the trans-synaptic interaction between neurexin and neuroligin may bridge the synaptic cleft aligning the presynaptic neurotransmitter release machinery with postsynaptic densities. Important findings from cell culture studies indicate that neurexins and neuroligins could act bidirectionally to induce pre- and postsynaptic assembly, thus controlling synapse formation (Scheiffele et al., 2000; Graf et al., 2004; Dean et al., 2003; Nam and Chen, 2005; Chih et al., 2005a; Prange et al., 2004; Levinson et al., 2005; Fu et al., 2003). Interestingly, phenotypic analyses of α-neurexins triple knockout mice suggest that α-neurexins are required for neurotransmitter release but dispensable for synapse formation (Missler et al., 2003; Zhang et al., 2005). Thus, despite the expanding evidence indicating that neurexins may act as synaptic recognition and organizer molecules in synapse development and function, the complexity and redundancy of neurexin genes in mammals pose a tremendous difficulty in understanding their function in vivo.
A potential strategy to resolve these issues is to use simpler model systems, such as the fruit fly, to investigate the in vivo function of neurexins. Unfortunately, the first neurexin-related gene isolated in Drosophila, neurexin IV (nrx IV), is primarily expressed in epithelial and glial cells, where it is required for the organization and function of septate junctions (Baumgartner et al., 1996; Banerjee et al., 2006; Faivre-Sarrailh et al., 2004). Nrx IV has an identical domain structure to Contactin-associated protein 1 (Caspr1), a member of the Caspr family which is distantly related to the neurexin family and mediates neuronglia interactions (Bellen et al., 1998; Bhat et al., 2001; Peles et al., 1997; Poliak et al., 1999). A major breakthrough, however, came about upon the near completion of the Drosophila genome project (Adams et al., 2000), which led to the identification of a single gene with striking conservation with mammalian neurexins, resurrecting the initial idea of using the Drosophila system to understand neurexin function at synapses.
Here we report the isolation of Drosophila neurexin (dnrx) null mutants and the characterization of its function during synapse development. Our results demonstrate that dnrx plays a critical role in the cytoarchitecture of synapses, and during synapse development and function. These studies provide a better understanding of neurexin function in an intact organism, and offer a strong basis for the interpretation of observations at mammalian central synapses.
Results
DNRX is the Single Drosophila Homolog of Vertebrate Neurexins
To identify a neurexin homolog in Drosophila, the rat neurexin 1α cDNA sequence (Ushkaryov et al., 1992) was blasted against the Drosophila genomic and EST databases. We identified an EST (LP03809) with significant homology to the C-terminal sequences of vertebrate neurexins. This EST was subsequently used to screen a 0–20 hr old embryonic cDNA library to obtain a full-length dnrx cDNA (Gen Bank # EF460788). In contrast to the presence of 3 neurexin genes in mammals, our genome-wide search revealed only a single neurexin gene (CG7050) in Drosophila (also reported by Tabuchi and Sudhof, 2002; Zeng et al., 2007), which we named Drosophila neurexin (dnrx). The DNRX protein has an identical domain structural organization to mammalian α-neurexins (Fig. 1A). The large extracellular region consists of an N-terminal signal peptide and three LamG-EGF-LamG repeats. Although the cytoplasmic region of DNRX is longer than the mammalian counterparts, the PDZ binding motif at the C-terminus is highly conserved (Fig. 1C). Overall, DNRX is 36–37% identical to human α-neurexins, and shares high amino acid sequence identity with mammalian neurexins within each individual protein domain (Fig. 1A).
Figure 1. Molecular Analysis of DNRX.
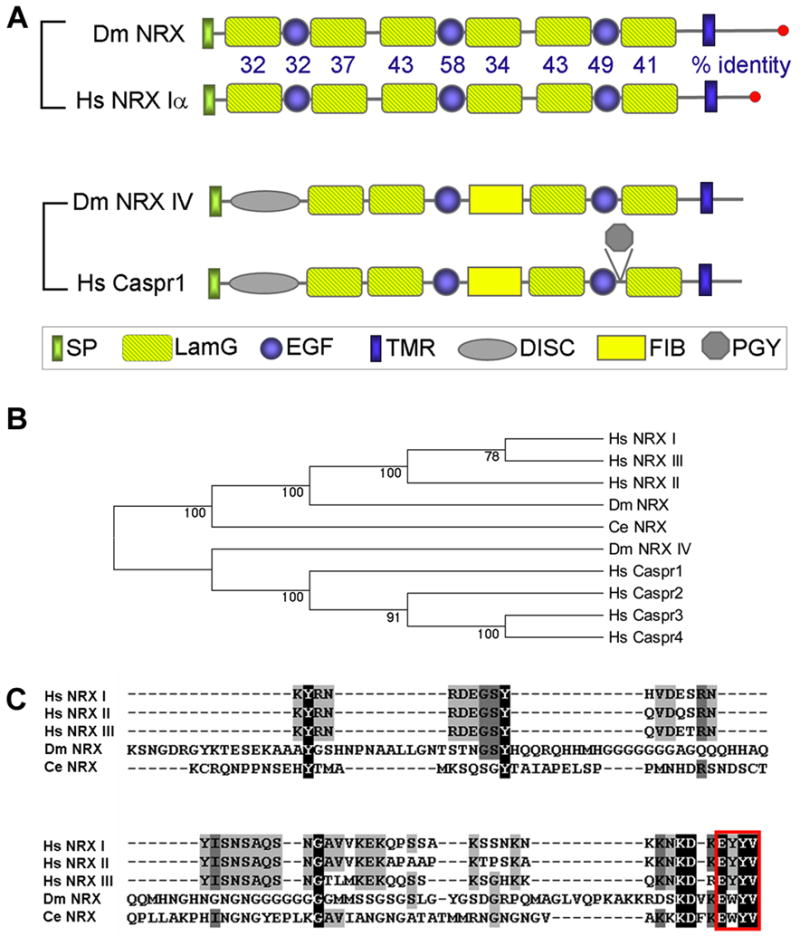
(A) Domain structure of DNRX, neurexin Iα, neurexin IV and Caspr1. The percent amino acid identity between DNRX and human neurexin Iα in specific domains is indicated. SP, signal peptide; LamG, laminin G domain; EGF, EGF repeat; DISC, discoidin-like domain; FIB, a region similar to fibrinogen; PGY, PGY repeat; TMR, transmembrane region. Dm, Drosophila melanogaster; Hs, Homo sapiens; Ce, C. elegans.
(B) Phylogenetic analysis of human, Drosophila, and C. elegans neurexins, Drosophilas neurexin IV and human Caspr proteins using the neighbor-joining method (Mega 3.1; Kumar et al., 2004). DNRX and NRXIV belong to neurexin and Caspr subfamily, respectively. Numbers along each branch are the bootstrap confidence value.
(C) Multiple sequence alignment (ClustalW) of cytoplasmic sequences of Drosophila, C. elegans and human neurexins. The PDZ binding motif at the C-termini of neurexins is boxed.
Previously, the Drosophila gene neurexin IV (nrx IV) was identified and proposed to belong to the neurexin family (Baumgartner et al., 1996). However, the domain arrangement of NRX IV differs from that of classical neurexins (Fig. 1A). Furthermore, our phylogenetic analysis shows that DNRX is the closest homolog of vertebrate neurexins, whereas NRX IV is only distantly related to neurexins (Fig. 1B).
DNRX Is Expressed in Central Neurons and Concentrated at Active Zones of Glutamatergic Neuromuscular Junctions (NMJs)
To determine where DNRX function might be required during development, we performed in situ hybridization to examine dnrx expression during embryonic stages. In situ hybridization using two independent RNA probes revealed that dnrx expression was enriched in central neurons (Fig. 2A, B), but undetectable in muscle cells (Fig. 2C). dnrx mRNA first appeared in subsets of central neurons at late stage 14, when axon pathfinding is nearly complete and the differentiation of presynaptic terminals is about to begin. This expression reached its highest levels at stages 16 and 17, when a larger number of neurons in the brain and ventral nerve cord expressed dnrx at elevated levels (Fig. 2A, B). Low levels of dnrx expression could also be detected in small subsets of peripheral nervous system neurons.
Figure 2. DNRX Is Expressed in Central Neurons and Concentrated at Active Zones of Glutamatergic NMJs.
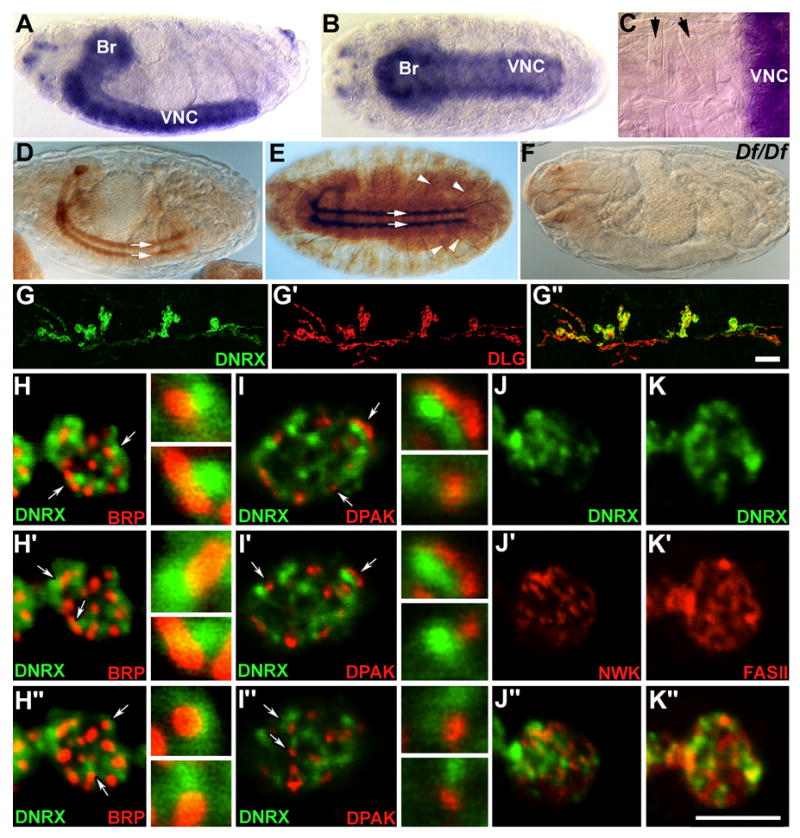
(A–C) In situ hybridization analysis of dnrx expression in wild-type embryos. (A) Lateral and (B) ventral view of dnrx mRNA distribution in the brain (Br) and ventral nerve cord (VNC) of a stage-17 whole-mount embryo. (C) Higher magnification of a dissected embryo showing that dnrx mRNA is enriched in central neurons, but undetectable in muscle cells (arrows).
(D and E) Lateral and ventral view of wild-type embryos stained with anti-DNRX antibody showing that DNRX is localized in the CNS neuropil and axonal tracts (arrows), and motor axons (E, arrowheads).
(F) A Df(3R)5C1 homozygous embryo stained with anti-DNRX showing the specificity of antibody.
(G–G″) Double-staining of wide-type 3rd instar larval NMJ 6/7 with anti-DNRX (green) and anti-DLG (red), which labels glutamatergic type I boutons. The merged DNRX and DLG images show that DNRX is located at type I boutons.
(H–K″) Confocal images of synaptic boutons double-labeled with anti-DNRX (green) and synaptic markers (red) showing DNRX is concentrated at active zones, but also extends into periactive zones.
(H–I″) Three consecutive single confocal slices from NMJs labeled with (H–H″) anti-DNRX and anti-BRP showing that bright DNRX spots are juxtaposed and slightly colocalized with BRP spots (Given that DNRX spots were associated to one side of BRP spots, such juxtaposition could not be seen in every slice, but could be verified in a sequence of single slices), and (I–I″) anti-DNRX and anti-DPAK showing that DPAK and DNRX largely do not colocalize. Columns to the right side of H–I″ are high magnification views of BRP-DNRX or DPAK-DNRX spots indicated in the low magnification panels by arrows.
(J–J″) Single confocal scan of synaptic boutons double-stained for DNRX and NWK, which localizes to the periactive zone of presynaptic terminals, shows that DNRX and NWK localize to distinct areas and do not display any significant overlap.
(K–K″) Single confocal scan of synaptic boutons double-stained for DNRX and FAS II, a cell-adhesion molecule defines the periactive zone of pre- and postsynaptic membrane, shows that most of DNRX staining regions do not overlap with that of FAS II. Scale bars: G″, 15 μm; K″, 5 μm.
The subcellular localization of DNRX was determined by immunocytochemical analysis using an affinity-purified polyclonal antibody generated against the cytoplasmic region of DNRX. In embryos DNRX was concentrated in neuropil regions of the brain and ventral nerve cord, axon tracts of the ventral nerve cord, and motor axons (Fig. 2D, E). The immunoreactivity was absent in homozygous embryos of Df(3R)5C1 which uncovers the dnrx gene, demonstrating the specificity of the DNRX antibody (Fig. 2F). A similar DNRX distribution was observed in 3rd instar larvae (data not shown). In addition, DNRX was present at the glutamatergic type I boutons of the larval body wall muscles. This was confirmed by double staining with an antibody against the scaffolding protein Discs-large (DLG) (Fig. 2G–G″), which labels type I boutons, but not other bouton types (Fig. 2G′; Lahey et al., 1994).
Confocal microscopy analysis revealed that within synaptic boutons DNRX immunoreactivity did not have a uniform distribution, but displayed discontinuous patches (Fig. 2G–K). To define the precise subcellular localization of DNRX in these patches, we used a number of synaptic markers. A presynaptic marker, Bruchpilot (BRP; Wagh et al., 2006; Kittel et al., 2006), is thought to label at least one component of the active zone, the T-bar (see below). Bright DNRX patches were juxtaposed and slightly overlapped with almost every BRP spot, as revealed by examining the labels in thin consecutive confocal slices (Fig. 2H–H″, arrows and insets). In addition, DNRX appeared to surround BRP immunoreactivity. Notably, the postsynaptic density (PSD) marker Drosophila p21-activated kinase (DPAK, Sone et al., 2000), also appeared juxtaposed to DNRX patches, but in contrast to BRP, minimal overlap between the labels was observed (Fig. 2I–I″, arrows and insets).
We next double labeled NMJ preparation with DNRX and the so called periactive zone markers, such as the SH3 adaptor protein Nervous Wreck (NWK) (Coyle et al., 2004) and the cell adhesion molecule Fasciclin II (FASII) (Sone et al., 2000). In general, DNRX showed no overlap with NWK, although occasionally colocalization between less intense DNRX regions and NWK immunoreactive regions was observed (Fig. 2J). In the case of FasII, there are significant regions of non overlap with some regions displaying partial overlap (Fig. 2K). Thus, DNRX appears to be concentrated at active zones, but also extends into periactive zones.
Genetic Analysis of dnrx
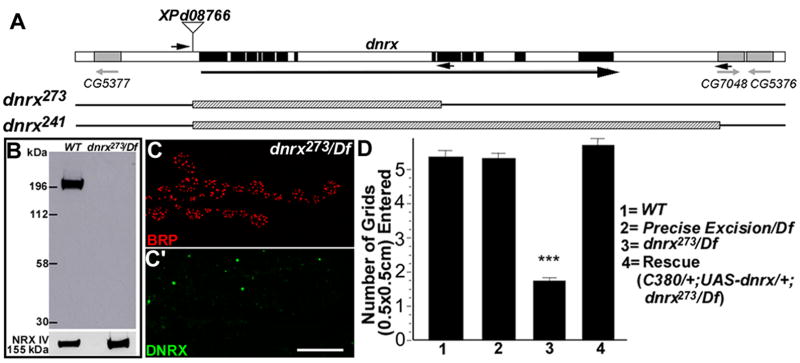
To determine the role of DNRX in synaptic development and function, we generated dnrx null mutants. The dnrx gene is predicted to comprise 13 exons and 12 introns, spanning ~13.4 kb. To disrupt the dnrx locus, we carried out an excision screen with a P-element (XPd08766) located ~200 base pairs upstream of dnrx, which led to the isolation of two dnrx mutant alleles, dnrx273 and dnrx241 (Fig. 3A). The break points of the deletions were molecularly determined by PCR and sequence analysis. The dnrx273 allele had an ~8-kb deletion within the dnrx locus, which removed most of the coding sequence for the extracellular region of DNRX, from the start codon to the 4th LamG domain. dnrx241 uncovered the entire dnrx gene and the upstream region of an adjacent transcription unit (Fig. 3A). Western blot analysis with the DNRX C-terminus specific antibody showed that ~200 kDa DNRX band in wild-type was absent in dnrx273/Df(3R)5C1, and there was no detectable truncated protein in dnrx mutants (Fig. 3B). Immunostaining with this antibody also showed no detectable protein in homozygous mutant embryos (data not shown) and NMJs (Fig. 3C–C′) from both dnrx alleles, confirming that dnrx273 and dnrx241 are null alleles. In this study, the following allelic combinations were used for phenotypic analysis: dnrx273/Df(3R)5C1, dnrx273/dnrx273, and/or dnrx273/dnrx241. A precise excision of the P-element and a wild type strain were used as controls.
Figure 3. Generation and Characterization of dnrx Mutants.
(A) Genomic structure of dnrx with the intron-exon organization. Exons are indicated by black boxes and introns by open boxes. The adjacent genes and directions of their transcription are denoted by gray boxes and arrows, respectively. The P element XPd08766 is inserted ~200 bp upstream of dnrx. dnrx alleles (dnrx273 and dnrx241) with their deleted regions indicated by hatched boxes were generated by imprecise excisions of XPd08766. The primers used for PCR and sequencing to define the break points of deletions are indicated by black arrows.
(B) Western blot of adult head membrane extracts of wild-type and dnrx273/Df flies probed with anti-DNRX (upper panel) and re-probed with anti-NRX IV (lower panel). The ~200 kDa DNRX band is absent in dnrx273/Df mutants. NRX IV is the loading control.
(C–C′) Double-staining of dnrx273/Df mutant NMJ with anti-BRP (red) and anti-DNRX (green) showing that DNRX immunoreactivity is absent from synaptic boutons (C′) where only BRP immunoreactivity is detected (C). Scale bar: 15 μm.
(D) Quantification of locomotor activity of wall-wandering 3rd instar larvae. The precise excision/Df performed as well as wild-type, whereas dnrx273/Df displayed significantly reduced locomotor activity (***P<0.0001). This phenotype was completely rescued with neuronal expression of a dnrx full-length cDNA (C380/+; UAS-dnrx/+; dnrx273/Df). For all genotypes, n=20. Data are mean±SEM.
dnrx Mutants Display Decreased Locomotor Activity and Abnormal NMJs
α-neurexin triple knockout mice and most double-knockout mutants die prematurely due to respiratory problems (Missler et al., 2003; Zhang et al., 2005). In contrast, 10% of the dnrx273/Df(3R)5C1 progeny died at pupal stages while the remaining progeny survived to adulthood and were fertile. This partial lethality was rescued by expressing a dnrx full-length cDNA in neurons using the C380-Gal4 driver (Budnik et al., 1996). dnrx mutants exhibited severely impaired behavior in larval stages, being uncoordinated and sluggish. Locomotor activity was reduced in all dnrx mutants, including the two dnrx alleles over deficiency and in allelic combinations. For the dnrx273/Df(3R)5C1 line, this phenotype was quantified in a larval locomotor assay. In this assay, the number of grids on a horizontal agar surface entered by individual 3rd-instar wandering larvae within a 30 sec time window over a test period of 180 sec was counted. While control animals passed about 5 grids on average, dnrx273/Df(3R)5C1 mutants entered less than 2 grids (Fig. 3D). This phenotype was completely rescued by expressing DNRX in neurons using the C380-Gal4 driver (Fig. 3D). Thus, decreased locomotor activity is due to dnrx loss-of-function in neurons.
Given that DNRX is localized at larval NMJs, we reasoned that abnormal development and function of NMJs might be responsible for the behavioral deficits. Compared to controls (Fig. 4A and 4E), dnrx mutants (Fig. 4B, 4C, 4F and 4G) had shortened axon branches with fewer boutons. In addition, mutant NMJ branches often contained long intervening axon stretches devoid of synaptic boutons. Further quantification revealed that dnrx mutants had a 40–60% decrease in bouton number (Fig. 4I and 4J). As with the behavioral abnormalities, the reduced bouton number was fully rescued by expression of the UAS-dnrx transgene in neurons (Fig. 4D, 4H, 4I and 4J). Taken together, these results suggest that DNRX is required for proper proliferation of synaptic boutons during larval development, which is necessary for coordinated matching of pre-and postsynaptic compartments during this period of intense growth (Griffith and Budnik, 2006).
Figure 4. DNRX Loss-of-function Leads to Reduced Synaptic Bouton Number at Larval NMJs.
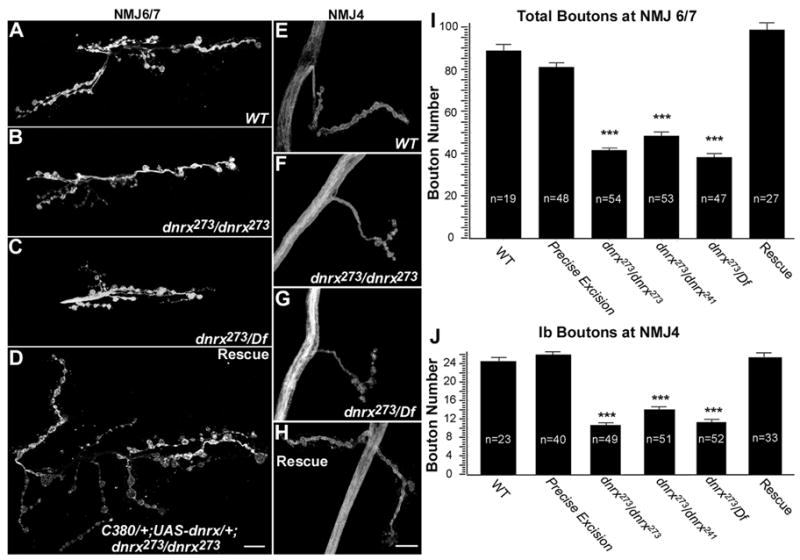
(A–H) NMJ morphology at muscle 6/7(A–D) and muscle 4 (E–H) of larval abdominal segment 3 labeled with anti-HRP. Compared to wild-type (A and E), dnrx null mutants dnrx273/dnrx273 (B and F) and dnrx273/Df (C and G) have less NMJ expansion, shorter axonal branches, and fewer boutons. A rescue line with neuronal expression of UAS-dnrx cDNA in dnrx273/dnrx273 background appears to restore NMJ morphology (D and H). Scale bars: 15 μm.
(I and J) Quantification of total bouton number at NMJ 6/7 (I) and type Ib bouton number at NMJ4 (J). Both quantifications show that dnrx mutants dnrx273/dnrx273, dnrx273/dnrx241 and dnrx273/Df3R(5C1) have a significant decrease in average bouton number when compared to wild-type and precise excision homozygotes. The reduced bouton number is completely rescued by restoring DNRX expression in neurons (rescue: C380/+; UAS-dnrx/+; dnrx273/dnrx273). ***P<0.001, data are mean±SEM.
In addition to synaptic regions, DNRX was localized to axons as well. Therefore, we also investigated whether axon guidance and pathfinding were altered in dnrx mutants. The specificity of axon pathfinding was examined by immunostaining with BP102 and FASII antibodies which stain all CNS axons and both a subset of CNS axonal fascicles and peripheral motor axons, respectively. No apparent defects were observed in both CNS axonal pathways and muscle innervation patterns in dnrx mutants (data not shown).
Neuronal Expression of DNRX Promotes Proliferation of Synaptic Boutons
Studies in cell culture have indicated that neurexins and their postsynaptic binding partners, the neuroligins, induce synapse formation (Graf et al., 2004; Chih et al., 2005a; Scheiffele et al., 2000; Dean et al., 2003). Therefore, we asked whether dnrx could similarly induce the formation of new synaptic boutons. Expression of a full-length dnrx transgene in wild-type background, using two different neuron-specific Gal4 drivers, the pan-neural driver elav (Lin and Goodman, 1994) and neural driver C380, significantly enhanced the formation of synaptic boutons (Fig. 5C, compare with 5A and 5B). Increasing dnrx gene dosage in all neurons by one copy resulted in over a 30% increase in the number of synaptic boutons, while increasing it by two copies resulted in more than 40% enhancement (Fig. 5D). A similar trend was observed by using C380-Gal4 (Fig. 5D).
Figure 5. Neuronal But Not Muscular Overexpression of DNRX Promotes Proliferation of Synaptic Boutons.
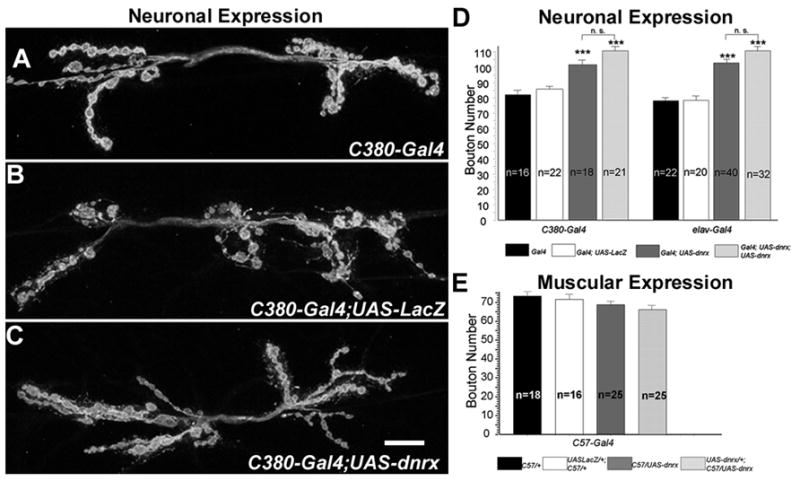
(A–C) NMJ morphology at muscle 6/7 in animals with DNRX overexpression in neurons. Compared with control animals (A) that lack dnrx transgene (C380-Gal4) or (B) that overexpress an unrelated gene (C380-Gal4; UAS-lacZ) in neurons, (C) animals with DNRX overexpression in neurons (C380-Gal4; UAS-dnrx) have more branching and boutons. Scale bar: 15 μm.
(D) Quantification of bouton number shows that animals overexpressing either one(Gal4; UAS-dnrx) or two-copy (Gal4; UAS-dnrx; UAS-dnrx) dnrx transgene driven by either neuron (C-380) or panneuron (elav) Gal4 divers have a significant increase in average bouton number, when compared to control animals that lack a dnrx transgene (Gal4) or that express an unrelated gene (Gal4; UAS-LacZ). ***P<0.001, Data are mean±SEM.
(E) Quantification of bouton number shows that there is no significant change in average bouton number between animals that express dnrx transgene in muscles driven by C57 Gal4 and control animals. Data are mean±SEM.
Recent studies on murine central synapses suggest that postsynaptic neurexins affect glutamate receptor function, and may also inhibit neuroligin function via cis-interaction with neuroligins on the postsynaptic membrane (Kattenstroth et al., 2004; Taniguchi et al., 2007). To explore the potential functional relevance of DNRX in the postsynaptic compartment at NMJs, we examined whether muscle expression of DNRX had any effect on synaptic growth. Compared to genetic background matched controls, expression of either one or two copies of a dnrx transgene in muscle cells, using the C57 Gal4 driver (Budnik et al., 1996), did not result in any significant change in bouton number (Fig. 5E). Taken together, the DNRX loss- and gain-of-function analyses demonstrate that DNRX is necessary to promote the proliferation of synaptic boutons, and further suggest that for this function DNRX is required in the pre- but not in the postsynaptic compartment.
DNRX Is Required for Pre- and Postsynaptic Differentiation
We also explored whether the expression and localization of synaptic proteins were affected in dnrx mutants. The abundance and localization of periactive zone proteins, such as the cell adhesion molecule FASII, the cytoskeleton adaptor protein NWK, and the endocytotic protein Dap160 (Roos and Kelly, 1999; Koh et al., 2004; Marie et al., 2004), appeared unchanged in dnrx mutants (Suppl. Fig. S1). Several other synaptic proteins, including the scaffolding PDZ-protein DLG and the microtubule-binding protein Futsch (Roos et al., 2000; Hummel et al., 2000), also appeared unaffected in dnrx mutants (data not shown). However, profound abnormalities in the distribution of synaptic vesicle and active zone proteins, as well as glutamate receptor (GluR) clusters were observed in dnrx mutant NMJs.
In wild-type, synaptotagmin (Syt), a synaptic vesicle protein, is efficiently transported to presynaptic terminals, therefore it is seldom observed within motor axons (Fig. 6A–A′ and 6D; Littleton et al., 1993;). In dnrx mutants, however, intense punctate Syt staining was often observed in motor axons (Fig. 6B–B′, D). Similarly, the active zone protein, BRP, which is rarely seen in wild-type axons was also mislocalized to mutant axons (Fig. 6C–C″). Notably, BRP immunoreactivity colocalized with Syt at these accumulations along the motor axons, suggesting that DNRX is involved in the proper recruitment, localization, or transport of key synaptic components during presynaptic differentiation.
Figure 6. Distribution of Pre- and Postsynaptic Proteins Is Altered in dnrx Mutants.
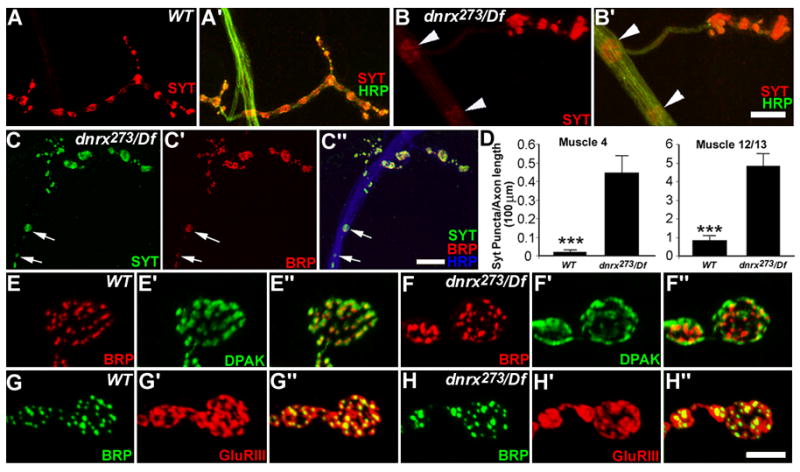
(A–B′) NMJ 4 stained with anti-Syt (red) and anti-HRP (green) in wild type (A, A′) and dnrx mutants (B, B′). dnrx mutants display accumulation of Syt staining puncta in motor axons (B and B′, arrowheads).
(C–C″) NMJ 4 of dnrx mutant stained with anti-Syt (green), anti-BRP(red) and anti-HRP (blue) showing co-localization of Syt- and BRP-staining (arrows) in accumulated puncta along the axon.
(D) Quantification of Syt-puncta observed in axons innervating muscle 4 and muscle 12/13 shows that dnrx mutants have significantly increased Syt accumulation per unit axon length. n>76; ***P<0.001. Data are mean±SEM.
(E–F″) Synaptic boutons of wild-type (E–E″) and dnrx mutant (F–F″) double-stained for BRP (red) and DPAK (green), which labels the pre- and postsynaptic active zone, respectively. The pre- and postsynaptic alignment appears grossly unaffected in dnrx mutants. However, the distribution of DPAK within synaptic boutons is increased in dnrx mutant.
(G–H″) Synaptic boutons of wild-type (G–G″) and dnrx mutants (H–H″) double-stained for BRP (green) and GluRIII (red). The apposition of GluRIII and BRP staining appears normal in dnrx mutants. However, the distribution of GluRIII cluster is enlarged in dnrx mutant. Scale bar: B′, C″, 15μm; H, 5 μm.
At mammalian synapses it has been hypothesized that neurexins are required for the alignment of pre- and postsynaptic compartments (Graf et al., 2004; Yamagata et al., 2003). Therefore, we next examined whether pre- and postsynaptic apposition was affected in dnrx mutants by double-labeling synaptic boutons with the presynaptic active zone marker BRP and the PSD markers DPAK and GluRIII (Marrus et al., 2004). No gross defects in pre- and postsynaptic alignment was observed (Fig. 6E″, 6F″, 6G″ and 6H″). DPAK and GluRIII clusters were exactly juxtaposed to active zones in dnrx mutants. However, the size of DPAK and GluRIII clusters was markedly enlarged in mutant boutons (Fig. 6F′ and 6H′ [compare with 6E′ and 6G′]). Enlargement of GluRIIA clusters was also observed, while the GluRIIA intensity in boutons was not statistically different between mutant and wild-type (Suppl. Fig. 2). Taken together, these results suggest that although the juxtaposition of pre- and postsynaptic components is not altered at least at light microscopy, the distribution of PSD proteins is affected in dnrx mutants.
Active Zones and PSDs Are Altered in dnrx Mutants
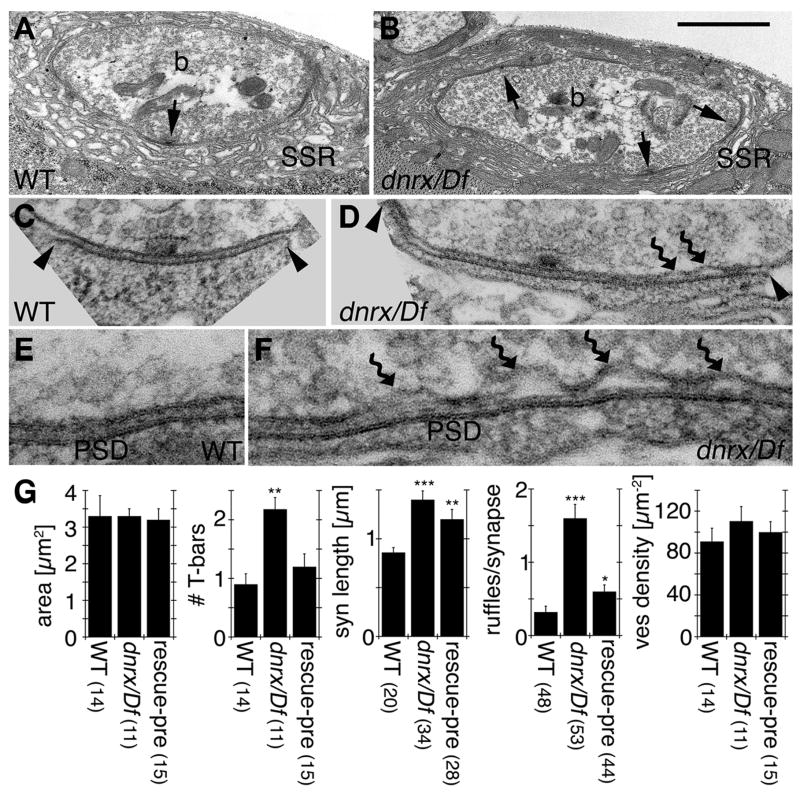
To determine the significance of the light microscopic phenotypes described above, we carried out ultrastructural and functional analyses of NMJs in dnrx mutants. For the ultrastructural studies, dnrx mutant and control NMJs were serially sectioned and subjected to a morphometric analysis. dnrx mutants had striking structural abnormalities in active zones and PSDs of Type Ib boutons. Wild type boutons are characterized by the presynaptic compartment containing synaptic vesicles, mitochondria and endosomes (b in Fig. 7A). At the presynaptic membrane, active zones are composed of regulatory electron dense structures, the T-bars (arrow in Fig. 7A, Fig. 7C) containing BRP/Cast (Kittel et al., 2006; Wagh et al., 2006), and the pre-synaptic densities (PRDs), the likely sites for synaptic vesicle fusion (Fig. 7C). The PRDs are exactly juxtaposed to PSDs (between arrowheads in Fig. 7C, Fig. 7E), which contain GluRs in high-density clusters (Prokop and Meinertzhagen, 2006). Separating both membranes is the synaptic cleft which has a uniform size, and is filled with material that differs in electron density and structure from the rest of the bouton extracellular space (Fig. 7A, C, E).
Figure 7. Ultrastructural Analysis of Type I Synaptic Bouton in dnrx Mutants.
(A–F) TEM micrographs of (A, C, E) wild type, and (B, D, F) dnrx273/Df mutants. (A, B) are low magnification view of synaptic bouton midlines (b), showing the presynaptic active zones (arrows) and post synaptic SSR. Note that in dnrx273/Df mutants the number of active zones is increased. (C, D) Higher magnification view of the PSD (between arrowheads) and its juxtaposed PRD containing a T-bar. In dnrx273/Df mutants both PSDs and PRDs are much longer than wild type. In addition, the presynaptic membrane shows signs of detachment from the PSD (curved arrows).
(E, F) High magnification view of a region of the PRD and apposed PSD. While in wild type the synaptic cleft between the PSD and PRD has a constant size, in dnrx273/Df mutants this size is variable, due to the presence of detachments of the presynaptic membrane (curved arrows). Also note that at sites of detachments the PRD no longer displays its typical electron density.
(G) Morphometric analysis of synaptic boutons, showing bouton midline area, number of T-bars, synapse length, number of ruffles/synapse, and vesicle density. Numbers in parenthesis below genotypes correspond to the number of samples (see Experimental Procedures). ***P<0.001; **P<0.01; and *P<0.05. Data are mean±SEM. Scale bar: A, B, 1 μm; C, D, 0.2 μm; E, F, 0.1 μm.
Several features of the active zones and PSDs were altered in dnrx/Df mutants. PRDs and apposed PSDs were over 60% longer in the mutants compared to controls (Fig. 7D, G). This is in agreement with the light microscopic studies showing that DPAK and GluR clusters were increased in size in dnrx mutants. In addition, the number of T-bars per bouton was increased by more than 2-fold (Fig. 7B, G). Most strikingly, the PRD showed signs of detachment from the PSD, a phenotype that is rarely seen in wild type (Fig. 7D, F, G). In these dnrx mutant synapses, PRDs showed bleb-like invaginations at several points, and at these sites the typical electron density of PRDs was lost (Fig. 7F). The material at the synaptic cleft also appeared altered, but no defects were observed at the corresponding sites of the PSDs (Fig. 7F). Thus, in dnrx mutants, synapses within synaptic boutons are dramatically altered, showing sites of presynaptic membrane detachment, abnormally long active zones, and increased number of T-bars. The increase in the number of T-bars and the aberrant detachment of PRD were completely or almost completely rescued by expressing a dnrx transgene in the mutant background, respectively (Fig. 7G). However, the increased length of the active zones was only partially rescued by the transgene, suggesting that this phenotype might be highly sensitive to DNRX dosage. Our ultrastructural analysis of dnrx mutant synaptic boutons provides the first direct in vivo evidence supporting the model that neurexins are involved in adhesion between the pre- and the postsynaptic cells.
dnrx Mutants Have Defects in Synaptic Transmission which Correlate with the Alterations in Synapse Ultrastructure
To assess the functional consequences of the reduced bouton number, the enlarged GluR clusters, the increase in the size of active zones and PSDs, and the abnormal detachments of PRDs, we carried out an electrophysiological analysis of NMJs (Fig. 8). For these experiments, the amplitude of spontaneous miniature excitatory potentials (mEJPs) was measured by intracellular recordings of muscles at low Ca++ concentrations. In addition, the amplitude and kinetics of evoked excitatory potentials (EJPs) were measured by stimulating the segmental nerve containing the motor axons. Several defects were observed in dnrx mutants. Evoked synaptic transmission was reduced, as manifested by a small, but significant decrease in the amplitude of EJPs. In addition, the frequency and amplitude of mEJPs was dramatically increased, suggesting both a pre- and a postsynaptic defect. Overall, quantal content was reduced, indicating defective synaptic transmission. The defect in EJP amplitude was completely rescued by expressing a dnrx transgene in neurons using C380-Gal4 or ubiquitously using T80-Gal4. However, no rescue of the mEJP amplitude or frequency was observed, consistent with the ultrastructural studies, in which the size of the active zones and PSDs was only partially rescued by the dnrx transgene. This partial rescue is not surprising given that neuronal expression of a dnrx transgene in wild-type background also have deleterious effects on quantal content. It is notable that the functional abnormalities were observed in homozygous dnrx273 mutants, dnrx273/dnrx241 combinations, as well as dnrx over a deficiency chromosome. Thus, they are unlikely to result from any genetic background effects.
Figure 8. Electrophysiological Analysis of NMJs.
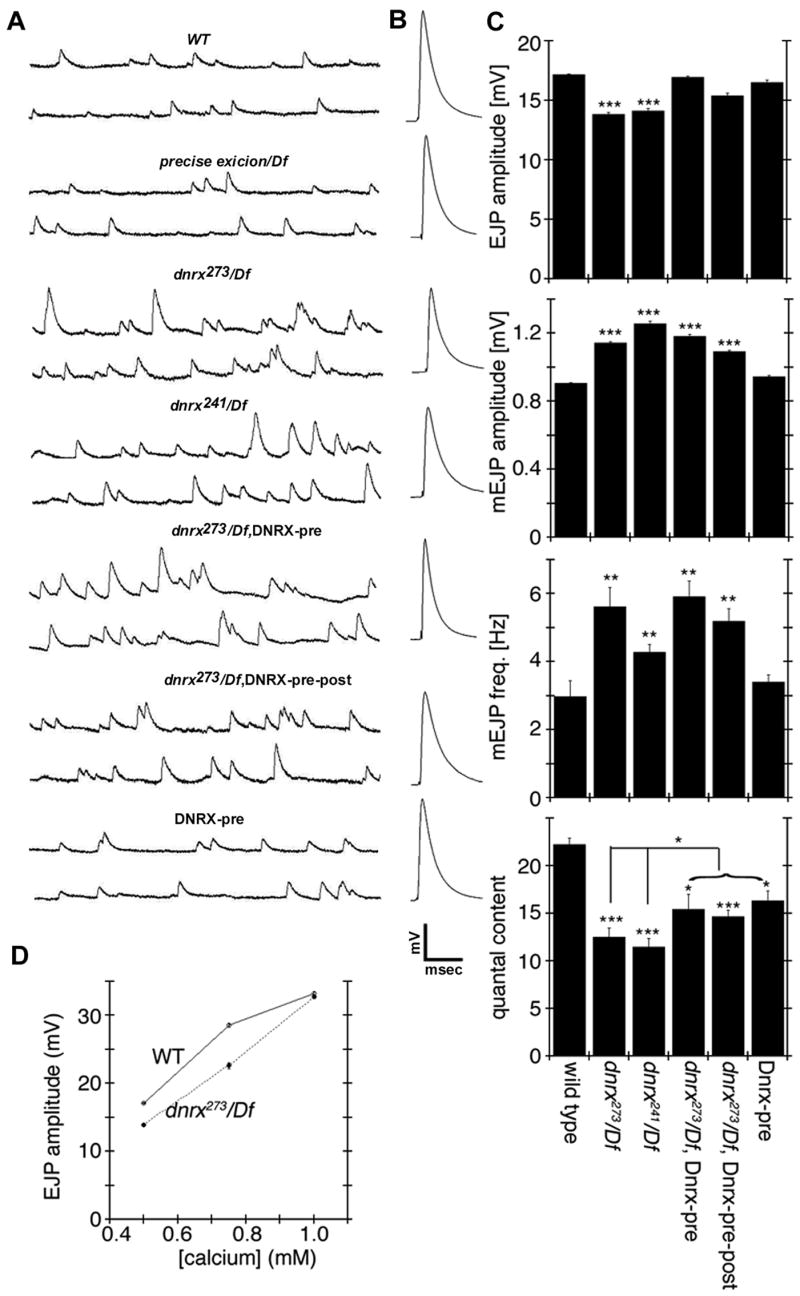
(A) Representative traces of mEJPs in the genotypes indicated. Note that both the frequency and amplitude of mEJPs is enhanced in dnrx/Df mutants.
(B) Representative traces of evoked responses, showing a decrease in peak amplitude in dnrx/Df mutants. Calibration scale: A, 3 mV and 230 msec; B, 6 mV and 10 msec.
(C) Quantification of EJP and mEJP amplitude, mEJP frequency, and quantal content (EJP/mEJP amplitude). ***P<0.001; **P<0.01; and *P<0.05. Data are mean±SEM.
(D) EJP amplitude as a function of external Ca++ concentration in wild type and dnrx mutants.
Mutations in dnrx Have Abnormal Calcium Sensitivity without Altering the Distribution of Presynaptic Calcium Channels
Studies in mammals have suggested that neurexins are involved in coupling Ca++ channels to synaptic vesicle release apparatus (Missler et al., 2003). Therefore, we next examined whether the Ca++ sensitivity of neurotransmitter release could be altered in dnrx mutants. For these experiments, the amplitude of evoked responses was measured at three Ca++ concentrations, 0.4, 0.75, and 1 mM. As previously described, there was a significant decrease in EJP amplitude in dnrx mutants at 0.4 mM Ca++. Surprisingly, however, this defect was completely restored when recordings were performed at 1 mM Ca++.
The change in the slope of Ca++ dependency of release could be due to either changes in Ca++ coupling to exocytosis, or an abnormality in the distribution of presynaptic Ca++ channels. To address this issue we examined the distribution of Cacophony (Cac), the presynaptic N-type Ca++ channel at these synapses (Smith et al., 1996; Littleton and Ganetzky, 2000). Ca++ channels are extremely sensitive to fixation. Therefore, we used a GFP-tagged Cac transgene (Cac-GFP) to visualize Ca++ channel in vivo. Previous studies have demonstrated that expression of this transgene faithfully replicates endogenous Cac distribution and function (Kawasaki et al., 2004). Cac-GFP transgene was expressed in neurons using elav-Gal4, and samples were imaged live in a spinning disk confocal microscope. There were no statistically significant differences either in the size or intensity of Cac-GFP clusters between controls and dnrx mutants (Suppl. Fig. S3), indicating that the defect in Ca++ sensitivity is most likely the result of changes in coupling rather than Ca++ channel distribution.
Discussion
Although cell adhesion molecules have long been postulated and in several cases, shown to be major participants in synapse development and plasticity, the impact of their function and the molecular mechanisms that they activate remain a puzzle. Particularly intriguing is the function of neurexins, which may provide clues to our understanding of synapse organization. We have isolated null mutants in the single Drosophila dnrx gene. We show that dnrx mutants have striking abnormalities in synapse development and function. A recent study reported that Drosophila neurexin is required for synapse formation in the adult CNS (Zeng et al., 2007). In the current study, we not only demonstrate a primary role of DNRX in regulating the formation of synapses, but also reveal the crucial role of DNRX in the proper development of active zones and regulating synaptic function in an intact organism, thus providing novel insights into understanding the function of neurexins in vivo.
Function of DNRX during Synapse Development
Our studies provide compelling evidence that DNRX plays a prime role during the expansion of the NMJ and in particular, in defining the cytoarchitecture of the active zones within synaptic boutons. First, in dnrx mutants synaptic bouton proliferation is severely disrupted, and therefore NMJ expansion is significantly stunted. Second, DNRX gain of function promotes the formation of new boutons in a gene dosage-dependent manner. Third, the ultrastructural analyses show that PRDs are not apposed normally to PSDs displaying signs of abnormal adhesion to the PSD, although every PRD is exactly juxtaposed to the PSD. Fourth, in dnrx mutants critical components of the presynaptic compartment, such as synaptic vesicle proteins and active zone components are ectopically localized within axons. Fifth, the distribution of GluRs at the PSD is abnormally large, although this phenotype may arise as a consequence of the presynaptic defects observed in dnrx mutants (discussed below).
The great majority of abnormal phenotypes in dnrx mutants could be completely rescued by expressing a wild type dnrx transgene in neurons; although in some instances the rescue was partial. However, even in the later case expressing dnrx in both muscles and neurons did not further improve the residual abnormalities, suggesting that dnrx functions primarily if not exclusively in the presynaptic compartment.
The partial rescue of some of the phenotypes, such as the defects in mEJPs and the morphology of active zones, might be due to the high sensitivity of these processes to the right levels and correct temporal expression of dnrx, which is not completely mimicked by the UAS/Gal4 system. This view is supported by the observation that overexpression of dnrx in a wild type background also decreased quantal content, suggesting that increased dnrx dosage may have detrimental effects on synapse structure and/or function. However, the data strongly support that the abnormal phenotypes arise from the lack of dnrx. First, all our experiments were carried out in mutants over a deficiency chromosome in an independent genetic background. Second, a precise excision of the P-element did not show any of the mutant phenotypes. Together these data establish a specific role for DNRX in proper synaptic development.
Role of DNRX in Active Zone Morphogenesis
One of our important findings is that dnrx mutants displayed defective active zones with larger PRD, and especially containing regions of detachment from the PSD. These detachment sites implicate DNRX as a mediator of cell adhesion between the pre- and the postsynaptic cell, in accordance with previous suggestions in mammalian neurons (Scheiffele et al., 2000; Graf et al., 2004; Dean et al., 2003). While a complete detachment of active zones is not observed, dnrx mutants have a significant decrease in the number of boutons. This raises the possibility that the phenotypes we observe is from those boutons that are maintained, and that a more drastic consequence is a failure to form synaptic boutons. Nevertheless, the lack of complete detachment of active zones in dnrx null mutants suggests that DNRX, although an important synapse organization molecule, is not sufficient for trans-synaptic cell adhesion.
Another notable phenotype in dnrx mutants was the presence of enlarged PRDs and increased number of T-bars. A major feature of Drosophila larval NMJ is its ability to compensate for decreased postsynaptic responses by upregulating neurotransmitter release. For instance, a decrease in the number of postsynaptic GluRs results in an increase in neurotransmitter release, thus maintaining the amplitude of evoked responses (Petersen et al., 1997). It is plausible that the enlarged PRDs and increase in number of T-bars in dnrx mutants are a compensatory mechanism to adjust for defective presynaptic cell adhesion and/or reduced neurotransmitter release (Murthy et al., 2001; Stewart et al., 1996). In support of this notion, in dnrx mutants there was a 50% decrease in synaptic bouton number, but this was accompanied by a 2-fold increase in the number of T-bars, such that the total number of T-bars/NMJ remained constant, despite the changes in bouton number. Similarly, defective presynaptic cell adhesion and/or reduced neurotransmitter release could lead to an increase in GluR accumulation (O’Brien et al., 1998). In our studies we found that the length of the PSD was enlarged in dnrx mutants as well as the distribution of GluR clusters.
Functional Consequences of Altering DNRX Function
The above structural abnormalities were accompanied by corresponding functional deficits. In dnrx mutants the frequency of mEJPs was strikingly increased. Further, although the T-bars were rescued by expression of a dnrx transgene, the length of the PRDs was not, and a similar lack of rescue was observed for mEJP frequency. Thus, there appears to be a notable correlation between the size of the PRD and mEJP frequency perhaps due to increased probability of synaptic vesicle release with increased synapse size. In addition, we also observed a substantial increase in mEJP amplitude. Two factors may contribute to this change. First, the distribution of GluR clusters was enlarged while the GluR intensity remained unchanged, suggesting that more GluRs were accumulated at mutant synapses. An additional contributing factor is that mEJP frequency was increased, and we observed instances of summation.
Overall, despite the increase in PRD size and the maintenance of overall T-bar number, evoked events had a decrease in amplitude, and quantal content. Recent studies have suggested that a major constituent of the T-bars is BRP/CAST (Kittel et al., 2006; Wagh et al., 2006). In brp mutants T-bars fail to form, but PRDs appear unaltered. Further, although EJP amplitude is decreased, mEJP amplitude and frequency are normal. This has led to the model that T-bars per se are not required for synaptic transmission, but that they regulate the efficiency of transmission. In dnrx mutants, PRDs are disproportionately large, which could result in asynchronous release, leading to an EJP with decreased amplitude. It is also possible that the presynaptic membrane detachments observed in dnrx mutants could contribute to the functional impairment of neurotransmitter release.
A recent study demonstrated that in dnrx mutant larvae associative learning is impaired in an olfactory choice paradigm (Zeng et al., 2007). However, in this study larval locomotion was not assessed. Our study showing that locomotor behavior is impaired in dnrx mutants raises the possibility that the poor performance of mutant larvae in the conditioning assay might also result from the locomotor abnormalities. Zeng et al also reported that the number of T-bars in the calyx of the mushroom bodies, the learning centers of the fly, was reduced in adult flies. In contrast, we found a significant increase in the number of T-bar/bouton, and since dnrx mutants have fewer boutons, this translated in the maintenance of T-bar number per NMJ. The differing results might be due to different mechanisms regulating T-bar formation in the two tissues.
DNRX Function in Relation to Mammals
The presence of a neurexin in Drosophila strengthened the view that neurexins are highly conserved across species (Tabuchi and Sudhof, 2002). The synaptic DNRX expression pattern and its function show remarkable parallels with mammalian neurexins. Moreover, the proteins that have been shown to interact with mammalian neurexins also have homologs in Drosophila, which further supports the idea that the function of neurexins and underlying signaling mechanism are evolutionarily conserved. Among these, Drosophila neuroligin and/or dystroglycan (Dg) (Deng et al., 2003) might be potential DNRX ligands. Drosophila neuroligin transcription exhibits almost an identical temporal and spatial expression pattern as dnrx during embryonic stages (Unpublished Data). dg is highly expressed in the somatic musculature of embryos (BDGP gene expression report). dg mutants are embryonic lethal, and perturbation of Dg function by RNAi as well as genetic interaction studies suggest an involvement of Dg in muscle maintenance and axonal pathfinding in adult flies (Shcherbata et al., 2007). Future studies on the identification and characterization of DNRX binding partners in Drosophila should provide additional insights into the mechanisms by which neurexins function in synapse development and function.
Extensive cell culture studies of neurexins and neuroligins and functional studies using α-neurexin knockout mice have established a central role for neurexins as synaptic adhesive and organizing molecules. Our studies on DNRX provide novel evidence in an intact organism that neurexin is required for important aspects of synapse development and function. Our DNRX gain-of-function analysis reveals that overexpression of DNRX is sufficient to promote the formation of synaptic boutons in vivo, in agreement with the findings from cell culture studies suggesting that neurexin-neuroligin trans-synaptic complexes can induce pre- and post-synaptic differentiation and synapse formation (Graf et al., 2004; Scheiffele et al., 2000; Dean et al., 2003; Chih et al., 2005b). Moreover, the accumulations of synaptic vesicle and active zone proteins along axons of dnrx null mutants further support the notion that neurexins may recruit or organize synaptic proteins or organelles during presynaptic differentiation. Phenotypic analyses of α-neurexin knockout mice demonstrated that α-neurexin is essential for synaptic transmission in a process that depends on presynaptic voltage-dependent Ca++ channels (Missler et al., 2003; Zhang et al., 2005). However, triple knockout mice have normal surface expression of Ca++ channels. These findings have led to the hypothesis that neurexins regulate the coupling between Ca++ channels and the neurotransmitter release machinery. Similarly, in dnrx null mutants we found that the Ca++ sensitivity of evoked responses was abnormal, but the distribution or levels of presynaptic Ca++ channel Cac was unchanged, consistent with the above hypothesis. Notably, Syt I, a synaptic vesicle protein that binds Ca++ and has been proposed to function as a Ca++ sensor (Geppert et al., 1994; Yoshihara and Littleton, 2002) during synaptic vesicle exocytosis, was partly mislocalized to axons in dnrx mutants. Furthermore, the structure of active zones was impaired in these mutants. Therefore, the organization of active zone proteins including the assembly of neurotransmitter release machinery might be affected in dnrx mutants.
In conclusion, our studies in Drosophila demonstrate that DNRX is required for both synapse development and function, and in particular for proper formation of active zones. Our studies provide compelling evidence for an in vivo role of neurexins in the modulation of synaptic architecture and adhesive interactions between pre- and postsynaptic compartments.
Experimental Procedures
Detailed procedures are provided in the Supplementary Material.
Supplementary Material
Acknowledgments
We thank Drs. Hugo Bellen, Steve Crews and Alan Fanning for comments and discussions. We also thank Dr. Michael Chua for assistance on quantification analysis of GluR, Norberto Gherbesi for assistance in ultrastructural analysis, Raehum Paik and Anilkumar Pillai for technical assistance, and the staff of the UMassmed Electron Microscopy Facility. Finally, we thank Drs. Hugo Bellen, Jeff Sekelsky, Aaron DiAntonio, Nicholas Harden, Barry Ganetzky, Wu-Min Deng and the Bloomington stock center (supported by Grant No. 0342468) for many valuable reagents. This work was supported by grants from the National Institute of Neurological Disorders and Stroke, NS050356 (M.A.B.) and National Institute of Mental Health, MH070000 (V.B.), and funds from the State of North Carolina (M.A.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams MD, Celniker SE, Holt RA, Evans CA, Gocayne JD, Amanatides PG, Scherer SE, Li PW, Hoskins RA, Galle RF, et al. The genome sequence of Drosophila melanogaster. Science. 2000;287:2185–95. doi: 10.1126/science.287.5461.2185. [DOI] [PubMed] [Google Scholar]
- Adams MD, McVey M, Sekelsky JJ. Drosophila BLM in double-strand break repair by synthesis-dependent strand annealing. Science. 2003;299:265–7. doi: 10.1126/science.1077198. [DOI] [PubMed] [Google Scholar]
- Ashley J, Packard M, Ataman B, Budnik V. Fasciclin II signals new synapse formation through amyloid precursor protein and the scaffolding protein dX11/Mint. J Neurosci. 2005;25:5943–5955. doi: 10.1523/JNEUROSCI.1144-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasoy D, Schoch S, Ho A, Nadasy KA, Liu X, Zhang W, Mukherjee K, Nosyreva ED, Fernandez-Chacon R, Missler M, Kavalali ET, Sudhof TC. Deletion of CASK in mice is lethal and impairs synaptic function. Proc Natl Acad Sci U S A. 2007;104:2525–30. doi: 10.1073/pnas.0611003104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee S, Pillai AM, Paik R, Li J, Bhat MA. Axonal ensheathment and septate junction formation in the peripheral nervous system of Drosophila. J Neurosci. 2006;26:3319–29. doi: 10.1523/JNEUROSCI.5383-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner S, Littleton JT, Broadie K, Bhat MA, Harbecke R, Lengyel JA, Chiquet-Ehrismann R, Prokop A, Bellen HJ. A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell. 1996;87:1059–68. doi: 10.1016/s0092-8674(00)81800-0. [DOI] [PubMed] [Google Scholar]
- Bellen HJ, Budnik V. The neuromuscular junction. In: Ashburner M, Hawley S, Sullivan B, editors. Drosophila, a laboratory manual. Cold Spring Harbor Laboratory; Cold Spring Harbor, NY: 2000. [Google Scholar]
- Bellen HJ, Lu Y, Beckstead R, Bhat MA. Neurexin IV, caspr and paranodin--novel members of the neurexin family: encounters of axons and glia. Trends Neurosci. 1998;21:444–9. doi: 10.1016/s0166-2236(98)01267-3. [DOI] [PubMed] [Google Scholar]
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, Salzer JL, Bellen HJ. Axonglia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 2001;30:369–83. doi: 10.1016/s0896-6273(01)00294-x. [DOI] [PubMed] [Google Scholar]
- Biederer T, Sudhof TC. Mints as adaptors. Direct binding to neurexins and recruitment of munc18. J Biol Chem. 2000;275:39803–6. doi: 10.1074/jbc.C000656200. [DOI] [PubMed] [Google Scholar]
- Boucard AA, Chubykin AA, Comoletti D, Taylor P, Sudhof TC. A splice code for trans-synaptic cell adhesion mediated by binding of neuroligin 1 to alpha- and beta-neurexins. Neuron. 2005;48:229–36. doi: 10.1016/j.neuron.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Budnik V, Koh YH, Guan B, Hartmann B, Hough C, Woods D, Gorczyca M. Regulation of synapse structure and function by the Drosophila tumor suppressor gene dlg. Neuron. 1996;17:627–40. doi: 10.1016/s0896-6273(00)80196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chih B, Engelman H, Scheiffele P. Control of excitatory and inhibitory synapse formation by neuroligins. Science. 2005;307:1324–8. doi: 10.1126/science.1107470. [DOI] [PubMed] [Google Scholar]
- Chih B, Gollan L, Scheiffele P. Alternative splicing controls selective trans-synaptic interactions of the neuroligin-neurexin complex. Neuron. 2006;51:171–8. doi: 10.1016/j.neuron.2006.06.005. [DOI] [PubMed] [Google Scholar]
- Comoletti D, Flynn RE, Boucard AA, Demeler B, Schirf V, Shi J, Jennings LL, Newlin HR, Sudhof TC, Taylor P. Gene selection, alternative splicing, and post-translational processing regulate neuroligin selectivity for beta-neurexins. Biochemistry. 2006;45:12816–27. doi: 10.1021/bi0614131. [DOI] [PubMed] [Google Scholar]
- Connolly JB, Tully T. Behaviour, learning, and memory. In: Roberts DB, editor. Drosophila, a practical approach. Oxford University Press; New York: 1998. [Google Scholar]
- Coyle IP, Koh YH, Lee WC, Slind J, Fergestad T, Littleton JT, Ganetzky B. Nervous wreck, an SH3 adaptor protein that interacts with Wsp, regulates synaptic growth in Drosophila. Neuron. 2004;41:521–34. doi: 10.1016/s0896-6273(04)00016-9. [DOI] [PubMed] [Google Scholar]
- Dean C, Scholl FG, Choih J, DeMaria S, Berger J, Isacoff E, Scheiffele P. Neurexin mediates the assembly of presynaptic terminals. Nat Neurosci. 2003;6:708–16. doi: 10.1038/nn1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng WM, Schneider M, Frock R, Castillejo-Lopez C, Gaman EA, Baumgartner S, Ruohola-Baker H. Dystroglycan is required for polarizing the epithelial cells and the oocyte in Drosophila. Development. 2003;130:173–84. doi: 10.1242/dev.00199. [DOI] [PubMed] [Google Scholar]
- Faivre-Sarrailh C, Banerjee S, Li J, Hortsch M, Laval M, Bhat MA. Drosophila contactin, a homolog of vertebrate contactin, is required for septate junction organization and paracellular barrier function. Development. 2004;131:4931–42. doi: 10.1242/dev.01372. [DOI] [PubMed] [Google Scholar]
- Fu Z, Washbourne P, Ortinski P, Vicini S. Functional excitatory synapses in HEK293 cells expressing neuroligin and glutamate receptors. J Neurophysiol. 2003;90:3950–7. doi: 10.1152/jn.00647.2003. [DOI] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Sudhof TC. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–27. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- Gerrow K, El-Husseini A. Cell adhesion molecules at the synapse. Front Biosci. 2006;11:2400–19. doi: 10.2741/1978. [DOI] [PubMed] [Google Scholar]
- Graf ER, Kang Y, Hauner AM, Craig AM. Structure function and splice site analysis of the synaptogenic activity of the neurexin-1 beta LNS domain. J Neurosci. 2006;26:4256–65. doi: 10.1523/JNEUROSCI.1253-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf ER, Zhang X, Jin SX, Linhoff MW, Craig AM. Neurexins induce differentiation of GABA and glutamate postsynaptic specializations via neuroligins. Cell. 2004;119:1013–26. doi: 10.1016/j.cell.2004.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith LC, Budnik V. Plasticity and second messengers during synapse development. Int Rev Neurobiol. 2006;75:237–65. doi: 10.1016/S0074-7742(06)75011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harden N, Lee J, Loh HY, Ong YM, Tan I, Leung T, Manser E, Lim L. A Drosophila homolog of the Rac- and Cdc42-activated serine/threonine kinase PAK is a potential focal adhesion and focal complex protein that colocalizes with dynamic actin structures. Mol Cell Biol. 1996;16:1896–908. doi: 10.1128/mcb.16.5.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata Y, Butz S, Sudhof TC. CASK: a novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J Neurosci. 1996;16:2488–94. doi: 10.1523/JNEUROSCI.16-08-02488.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata Y, Davletov B, Petrenko AG, Jahn R, Sudhof TC. Interaction of synaptotagmin with the cytoplasmic domains of neurexins. Neuron. 1993;10:307–15. doi: 10.1016/0896-6273(93)90320-q. [DOI] [PubMed] [Google Scholar]
- Ho A, Morishita W, Hammer RE, Malenka RC, Sudhof TC. A role for Mints in transmitter release: Mint 1 knockout mice exhibit impaired GABAergic synaptic transmission. Proc Natl Acad Sci U S A. 2003;100:1409–14. doi: 10.1073/pnas.252774899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel T, Krukkert K, Roos J, Davis G, Klambt C. Drosophila Futsch/22C10 is a MAP1B-like protein required for dendritic and axonal development. Neuron. 2000;26:357–70. doi: 10.1016/s0896-6273(00)81169-1. [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C, Sudhof TC. Neuroligin 1: a splice site-specific ligand for beta-neurexins. Cell. 1995;81:435–43. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- Irie M, Hata Y, Takeuchi M, Ichtchenko K, Toyoda A, Hirao K, Takai Y, Rosahl TW, Sudhof TC. Binding of neuroligins to PSD-95. Science. 1997;277:1511–5. doi: 10.1126/science.277.5331.1511. [DOI] [PubMed] [Google Scholar]
- Kattenstroth G, Tantalaki E, Sudhof TC, Gottmann K, Missler M. Postsynaptic N-methyl-D-aspartate receptor function requires alpha-neurexins. Proc Natl Acad Sci U S A. 2004;101:2607–12. doi: 10.1073/pnas.0308626100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki F, Zou B, Xu X, Ordway RW. Active zone localization of presynaptic calcium channels encoded by the cacophony locus of Drosophila. J Neurosci. 2004;24:282–5. doi: 10.1523/JNEUROSCI.3553-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittel RJ, Wichmann C, Rasse TM, Fouquet W, Schmidt M, Schmid A, Wagh DA, Pawlu C, Kellner RR, Willig KI, Hell SW, Buchner E, Heckmann M, Sigrist SJ. Bruchpilot promotes active zone assembly, Ca2+ channel clustering, and vesicle release. Science. 2006;312:1051–4. doi: 10.1126/science.1126308. [DOI] [PubMed] [Google Scholar]
- Koh TW, Verstreken P, Bellen HJ. Dap160/intersectin acts as a stabilizing scaffold required for synaptic development and vesicle endocytosis. Neuron. 2004;43:193–205. doi: 10.1016/j.neuron.2004.06.029. [DOI] [PubMed] [Google Scholar]
- Lahey T, Gorczyca M, Jia XX, Budnik V. The Drosophila tumor suppressor gene dlg is required for normal synaptic bouton structure. Neuron. 1994;13:823–35. doi: 10.1016/0896-6273(94)90249-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson JN, Chery N, Huang K, Wong TP, Gerrow K, Kang R, Prange O, Wang YT, El-Husseini A. Neuroligins mediate excitatory and inhibitory synapse formation: involvement of PSD-95 and neurexin-1beta in neuroligin-induced synaptic specificity. J Biol Chem. 2005;280:17312–9. doi: 10.1074/jbc.M413812200. [DOI] [PubMed] [Google Scholar]
- Lin DM, Goodman CS. Ectopic and increased expression of Fasciclin II alters motoneuron growth cone guidance. Neuron. 1994;13:507–23. doi: 10.1016/0896-6273(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Littleton JT, Bellen HJ, Perin MS. Expression of synaptotagmin in Drosophila reveals transport and localization of synaptic vesicles to the synapse. Development. 1993;118:1077–88. doi: 10.1242/dev.118.4.1077. [DOI] [PubMed] [Google Scholar]
- Littleton JT, Ganetzky B. Ion channels and synaptic organization: analysis of the Drosophila genome. Neuron. 2000;26:35–43. doi: 10.1016/s0896-6273(00)81135-6. [DOI] [PubMed] [Google Scholar]
- Marie B, Sweeney ST, Poskanzer KE, Roos J, Kelly RB, Davis GW. Dap160/intersectin scaffolds the periactive zone to achieve high-fidelity endocytosis and normal synaptic growth. Neuron. 2004;43:207–19. doi: 10.1016/j.neuron.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Marrus SB, Portman SL, Allen MJ, Moffat KG, DiAntonio A. Differential localization of glutamate receptor subunits at the Drosophila neuromuscular junction. J Neurosci. 2004;24:1406–15. doi: 10.1523/JNEUROSCI.1575-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McVey M, Larocque JR, Adams MD, Sekelsky JJ. Formation of deletions during double-strand break repair in Drosophila DmBlm mutants occurs after strand invasion. Proc Natl Acad Sci U S A. 2004;101:15694–9. doi: 10.1073/pnas.0406157101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer G, Varoqueaux F, Neeb A, Oschlies M, Brose N. The complexity of PDZ domain-mediated interactions at glutamatergic synapses: a case study on neuroligin. Neuropharmacology. 2004;47:724–33. doi: 10.1016/j.neuropharm.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Missler M, Sudhof TC. Neurexins: three genes and 1001 products. Trends Genet. 1998;14:20–6. doi: 10.1016/S0168-9525(97)01324-3. [DOI] [PubMed] [Google Scholar]
- Missler M, Zhang W, Rohlmann A, Kattenstroth G, Hammer RE, Gottmann K, Sudhof TC. Alpha-neurexins couple Ca2+ channels to synaptic vesicle exocytosis. Nature. 2003;423:939–48. doi: 10.1038/nature01755. [DOI] [PubMed] [Google Scholar]
- Murthy VN, Schikorski T, Stevens CF, Zhu Y. Inactivity produces increases in neurotransmitter release and synapse size. Neuron. 2001;32:673–82. doi: 10.1016/s0896-6273(01)00500-1. [DOI] [PubMed] [Google Scholar]
- Nam CI, Chen L. Postsynaptic assembly induced by neurexin-neuroligin interaction and neurotransmitter. Proc Natl Acad Sci U S A. 2005;102:6137–42. doi: 10.1073/pnas.0502038102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien RJ, Kamboj S, Ehlers MD, Rosen KR, Fischbach GD, Huganir RL. Activity-dependent modulation of synaptic AMPA receptor accumulation. Neuron. 1998;21:1067–78. doi: 10.1016/s0896-6273(00)80624-8. [DOI] [PubMed] [Google Scholar]
- Peles E, Joho K, Plowman GD, Schlessinger J. Close similarity between Drosophila neurexin IV and mammalian Caspr protein suggests a conserved mechanism for cellular interactions. Cell. 1997;88:745–6. doi: 10.1016/s0092-8674(00)81920-0. [DOI] [PubMed] [Google Scholar]
- Petersen SA, Fetter RD, Noordermeer JN, Goodman CS, DiAntonio A. Genetic analysis of glutamate receptors in Drosophila reveals a retrograde signal regulating presynaptic transmitter release. Neuron. 1997;19:1237–48. doi: 10.1016/s0896-6273(00)80415-8. [DOI] [PubMed] [Google Scholar]
- Poliak S, Gollan L, Martinez R, Custer A, Einheber S, Salzer JL, Trimmer JS, Shrager P, Peles E. Caspr2, a new member of the neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron. 1999;24:1037–47. doi: 10.1016/s0896-6273(00)81049-1. [DOI] [PubMed] [Google Scholar]
- Prange O, Wong TP, Gerrow K, Wang YT, El-Husseini A. A balance between excitatory and inhibitory synapses is controlled by PSD-95 and neuroligin. Proc Natl Acad Sci U S A. 2004;101:13915–20. doi: 10.1073/pnas.0405939101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop A, Meinertzhagen IA. Development and structure of synaptic contacts in Drosophila. Semin Cell Dev Biol. 2006;17:20–30. doi: 10.1016/j.semcdb.2005.11.010. [DOI] [PubMed] [Google Scholar]
- Roos J, Hummel T, Ng N, Klambt C, Davis GW. Drosophila Futsch regulates synaptic microtubule organization and is necessary for synaptic growth. Neuron. 2000;26:371–82. doi: 10.1016/s0896-6273(00)81170-8. [DOI] [PubMed] [Google Scholar]
- Roos J, Kelly RB. The endocytic machinery in nerve terminals surrounds sites of exocytosis. Curr Biol. 1999;9:1411–4. doi: 10.1016/s0960-9822(00)80087-1. [DOI] [PubMed] [Google Scholar]
- Rowen L, Young J, Birditt B, Kaur A, Madan A, Philipps DL, Qin S, Minx P, Wilson RK, Hood L, Graveley BR. Analysis of the human neurexin genes: alternative splicing and the generation of protein diversity. Genomics. 2002;79:587–97. doi: 10.1006/geno.2002.6734. [DOI] [PubMed] [Google Scholar]
- Scheiffele P. Cell-cell signaling during synapse formation in the CNS. Annu Rev Neurosci. 2003;26:485–508. doi: 10.1146/annurev.neuro.26.043002.094940. [DOI] [PubMed] [Google Scholar]
- Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–69. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- Shcherbata HR, Yatsenko AS, Patterson L, Sood VD, Nudel U, Yaffe D, Baker D, Ruohola-Baker H. Dissecting muscle and neuronal disorders in a Drosophila model of muscular dystrophy. EMBO J. 2007;26:481–493. doi: 10.1038/sj.emboj.7601503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LA, Wang X, Peixoto AA, Neumann EK, Hall LM, Hall JC. A Drosophila calcium channel alpha1 subunit gene maps to a genetic locus associated with behavioral and visual defects. J Neurosci. 1996;16:7868–79. doi: 10.1523/JNEUROSCI.16-24-07868.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone M, Suzuki E, Hoshino M, Hou D, Kuromi H, Fukata M, Kuroda S, Kaibuchi K, Nabeshima Y, Hama C. Synaptic development is controlled in the periactive zones of Drosophila synapses. Development. 2000;127:4157–68. doi: 10.1242/dev.127.19.4157. [DOI] [PubMed] [Google Scholar]
- Song JY, Ichtchenko K, Sudhof TC, Brose N. Neuroligin 1 is a postsynaptic cell-adhesion molecule of excitatory synapses. Proc Natl Acad Sci U S A. 1999;96:1100–5. doi: 10.1073/pnas.96.3.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart BA, Schuster CM, Goodman CS, Atwood HL. Homeostasis of synaptic transmission in Drosophila with genetically altered nerve terminal morphology. J Neurosci. 1996;16:3877–86. doi: 10.1523/JNEUROSCI.16-12-03877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugita S, Saito F, Tang J, Satz J, Campbell K, Sudhof TC. A stoichiometric complex of neurexins and dystroglycan in brain. J Cell Biol. 2001;154:435–45. doi: 10.1083/jcb.200105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuchi K, Sudhof TC. Structure and evolution of neurexin genes: insight into the mechanism of alternative splicing. Genomics. 2002;79:849–59. doi: 10.1006/geno.2002.6780. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, Gollan L, Scholl FG, Mahadomrongkul V, Dobler E, Limthong N, Peck M, Aoki C, Scheiffele P. Silencing of neuroligin function by postsynaptic neurexins. J Neurosci. 2007;27:2815–24. doi: 10.1523/JNEUROSCI.0032-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault ST, Singer MA, Miyazaki WY, Milash B, Dompe NA, Singh CM, Buchholz R, Demsky M, Fawcett R, Francis-Lang HL, et al. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat Genet. 2004;36:283–7. doi: 10.1038/ng1314. [DOI] [PubMed] [Google Scholar]
- Torroja L, Packard M, Gorczyca M, White K, Budnik V. The Drosophila beta-amyloid precursor protein homolog promotes synapse differentiation at the neuromuscular junction. J Neurosci. 1999;19:7793–7803. doi: 10.1523/JNEUROSCI.19-18-07793.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushkaryov YA, Petrenko AG, Geppert M, Sudhof TC. Neurexins: synaptic cell surface proteins related to the alpha-latrotoxin receptor and laminin. Science. 1992;257:50–6. doi: 10.1126/science.1621094. [DOI] [PubMed] [Google Scholar]
- Wagh DA, Rasse TM, Asan E, Hofbauer A, Schwenkert I, Durrbeck H, Buchner S, Dabauvalle MC, Schmidt M, Qin G, Wichmann C, Kittel R, Sigrist SJ, Buchner E. Bruchpilot, a protein with homology to ELKS/CAST, is required for structural integrity and function of synaptic active zones in Drosophila. Neuron. 2006;49:833–44. doi: 10.1016/j.neuron.2006.02.008. [DOI] [PubMed] [Google Scholar]
- Yamagata M, Sanes JR, Weiner JA. Synaptic adhesion molecules. Curr Opin Cell Biol. 2003;15:621–32. doi: 10.1016/s0955-0674(03)00107-8. [DOI] [PubMed] [Google Scholar]
- Yoshihara M, Littleton JT. Synaptotagmin I functions as a calcium sensor to synchronize neurotransmitter release. Neuron. 2002;36:897–908. doi: 10.1016/s0896-6273(02)01065-6. [DOI] [PubMed] [Google Scholar]
- Zeng X, Sun M, Liu L, Chen F, Wei L, Xie W. Neurexin-1 is required for synapse formation and larvae associative learning in Drosophila. FEBS Lett. 2007;581:2509–16. doi: 10.1016/j.febslet.2007.04.068. [DOI] [PubMed] [Google Scholar]
- Zhang W, Rohlmann A, Sargsyan V, Aramuni G, Hammer RE, Sudhof TC, Missler M. Extracellular domains of alpha-neurexins participate in regulating synaptic transmission by selectively affecting N- and P/Q-type Ca2+ channels. J Neurosci. 2005;25:4330–42. doi: 10.1523/JNEUROSCI.0497-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.