Abstract
Background:
We reviewed previous studies comparing schizophrenia patients and healthy subjects for performance on the Iowa Gambling Task (IGT) (a laboratory task designed to measure emotion-based decision-making), and found mixed results. We hypothesize that deficits in IGT performance in schizophrenia may be more specifically related to concurrent substance use disorders. To test this hypothesis, we compared schizophrenia patients with (SCZ(+)) or without (SCZ(−)) cannabis use disorders, to healthy subjects, on measures of cognition and IGT performance.
Methods:
A comprehensive battery of cognitive tests and the IGT were administered to three groups of subjects: (1) 13 subjects with DSM-IV diagnosis of schizophrenia and no concurrent substance use disorders (mean age: 28 ± 12 (SD); 54% males); (2) 14 subjects with schizophrenia and concurrent cannabis use disorders (mean age: 29 ± 9 (SD); 71% males); and (3) 20 healthy subjects (mean age 33 ± 10 (SD); 60% males).
Results:
Compared to the healthy group, both schizophrenia groups were cognitively more impaired, and did worse on IGT performance. There were no differences between SCZ(+) and SCZ(−) patients on most of the cognitive tests, and IGT performance.
Conclusions:
Schizophrenia patients show widespread impairments in several cognitive domains and emotion-based decision-making. These results are consistent with the evidence that schizophrenia reflects a dorsolateral and orbitofrontal/ventromedial prefrontal cortex dysfunction. More intriguing, it appears that the concurrent abuse of cannabis has no compounding effects on cognition, as well as emotion/affect-based decision-making.
Keywords: Schizophrenia, Cannabis, Decision-making, Iowa Gambling Task, Cognition
1. Introduction
Schizophrenia is a chronic illness with deficits in emotional processing (for a review, see e.g., Tremeau, 2006). It has been proposed that emotions play a key role in decision-making (Bechara et al., 1997; Bechara, 2005). Several studies have looked at emotion-based decision-making in schizophrenia using the Iowa Gambling Task (IGT), a laboratory task specifically developed to measure decision-making in patients with lesions of the orbito-/ventromedial prefrontal cortex and with compromised emotions (Bechara et al., 1994).
Table 1 summarizes the studies comparing IGT performance between patients with schizophrenia and healthy subjects.
Table 1.
Studies comparing patients with schizophrenia or schizoaffective disorders and normal controls for the performance at the Iowa Gambling Task (IGT)
| Authors | Subjects | Assessments | Results | Comments |
|---|---|---|---|---|
| Studies showing differences in IGT performance between groups | ||||
| Beninger et al. (2003) | 36 patients (half on typical (67% male; age: 46 (mean)± 1 (SD)) and half (67% male; age 42±12) on atypical anti-psychotics) and 18 controls (67% male; age: 45±3). Exclusion of patients with substance abuse in the past month. |
IGT (real card version), MMSE, BPRS, WCST, declarative memory questionnaire |
IGT: patients on atypicals had fewer choices from advantageous decks compared to patients on typicals or controls a. Patient groups differ from controls for declarative memory and MMSE. Patients on typicals perform worse at the WCST than patients on atypicals and controls |
No information about substance use disorders in controls. No information about past history of substance use disorders in patients |
| Ritter et al. (2004) | 20 chronic patients (100% male, age: 48±6) and 15 controls (100% male, age: 47±10). Exclusion of subjects with substance use disorders for the past 3 months |
IGT (computerized version) North American Adult Reading Test, WCST, BPRS, SANS |
IGT: Patients had a larger differential between disadvantageous and advantageous cards and lost more money than controls. There were no differences between groups across blocks a. A history of alcohol use disorder as a covariate did not change results. Both groups perform poorly at the WCST. |
No correlations between IGT variables and WCST or symptoms. |
| Shurman et al. (2005) | 39 patients (72% male, age: 33±10) and 10 controls (50% male, age: 32±4). Exclusion of subjects with a history of substance use disorders |
IGT (computerized version) WCST, DMST, PANSS, SANS, SAPS |
IGT: Patients had smaller total net scores and earned less money than controls. Group differences for IGT performance across blocks a was significant at p=0.08. Patients had a preference for decks with low frequency and high punishments. Patients had worse WCST and DMST performances than controls. Total money amount earned at IGT was negatively correlated with SANS score. |
|
| Kester et al. (2006) | 15 adolescents with schizophrenia (60% male, age: 16±3) and 25 controls (56% male, age: 17±2). Exclusion of subjects with substance use disorders |
IGT (computerized version), WRAT-3, BPRS, SANS, PAS, GAS, and WCST |
Patients are doing worse than controls for IGTa and WCST |
|
| Studies showing no differences in IGT performance between groups | ||||
| Wilder et al. (1998) | 12 patients (91% male, age: 33±6) and 30 controls (41% male; age: 30±10). Exclusion of subjects with substance use disorders for the past 6 months b |
IGT (real card version) b, CVLT, LNSP, WCST WAIS-R, and WRAT-R reading test |
No differences between groups for number of choices made from each deck and overall money lost at the IGT. Patients had a preference for decks with low frequency and high magnitude punishments. No correlations between IGT and cognitive variables |
No analysis of substance use disorder data |
| Cavallaro et al. (2003) | 110 patients with chronic schizophrenia (60% male; age: 33±9), 67 patients with OCDc (49% male; age: 30±9) and 56 controls (40% male; age: 31±6). Exclusion of patients with “multiple diagnoses” |
IGT (real card version), WCST (real card version), Tower of Hanoi |
Number of choices for disadvantageous decks did not differ between schizophrenia and control. Schizophrenia performed worse at the WCST and tower of Hanoi compared of controls |
Unclear if subjects with substance use disorders were excluded from the study |
| Evans et al. (2005) | 19 patients (gender not reported; age: 38±10) and 19 controls case-matched for age and level of education |
IGT (real card version) WCST, COWAT, SANS, SAPS |
No differences between groups for IGT performance and subjective experience across blocks a. Correlation between behavioral performance and subjective ratings No correlations between IGT outcomes and severity of symptoms |
No information on substance use disorders. No comparison of groups for WCST and COWAT performances |
| Rodríguez-Sánchez et al. (2005) | 80 first-episode (FE) patients (69% male, age: 26±7) and 22 controls 55% male, age: 26±6). Exclusion of patients with substance dependence |
IGT (computerized version) WAIS-III backward digits, FAS, TMT, SANS, SAPS |
No differences between groups for IGT (net scores, total net score, choices per Deck). Patients had worse cognition than controls |
No information on history of substance abuse in patients |
| Thurnbull et al. (2006) d | 21 chronic patients (61% male, age: 13±1) and 21 controls matched for age and education. No exclusion of substance use disorders |
IGT (real card version), COWAT, WCST, tests of set-shifting abilities, SANS, SAPS |
No differences between groups for IGTa. High levels of negative symptoms were associated with difficulties of reversal contingencies |
No information on substance use disorders. Not specified if same sample as Evans et al. (2005) |
CVLT = California Verbal Learning Test; WCST = Wisconsin Card Sorting Test; LNSP = Letter Number Span; WAIS-R = Wechsler Adult Intelligence Scale—Revised; WRAT-3 = Wide Range Achievement Test—Third Edition; BPRS = Brief Psychiatric Rating Scale; SANS = Scale for the Assessment of Negative Symptoms; DMST = Delayed match to Sample Task; PANSS = Positive and Negative Syndrome Scale; SAPS = Scale for the Assessment of Positive Symptoms; FAS = verbal fluency test; TMT = Trail Making Test; COWAT = Controlled Oral Word Association; PAS = Premorbid Adjustment Scale; GAS = Global Assessment Scale.
Two-way analysis of variance (ANOVA; groups by net scores interaction effects).
Personal communication from Dr. T. Goldberg.
Results for OCD patients are not reviewed.
Includes same patients as Evans et al. (2005).
In several studies, patients with schizophrenia showed poor IGT performance compared to healthy subjects. Beninger et al. (2003) compared 36 patients with schizophrenia and 18 healthy controls and found that patients on atypical, but not typical, antipsychotics performed worse on the IGT compared to healthy controls. Ritter et al. (2004) compared 20 patients with schizophrenia or schizoaffective disorder with 15 healthy subjects, and found that patients chose more cards from the disadvantageous decks than the advantageous deck compared to healthy subjects. Shurman et al. (2005) compared 39 patients with schizophrenia and 10 healthy controls and found worse performance on the IGT in schizophrenia patients. In a recent study of our group, Kester et al. (2006) found worse IGT performance in 15 adolescents with schizophrenia compared to 25 normal controls. In contrast, several other studies did not find poor IGT performance in schizophrenia patients compared to healthy subjects. Wilder et al. (1998) compared 11 patients with schizophrenia and 20 healthy controls and found no significant differences between groups for IGT performance. Cavallaro et al. (2003) found no differences in IGT performance between 110 patients and 56 normal controls. Evans et al. (2005) did not find differences in IGT performance between 19 patients and 19 age- and level of education-matched normal controls. Rodríguez-Sánchez et al. (2005) compared 80 first-episode patients with schizophrenia spectrum disorders (schizophrenia, schizoaffective disorder and schizophreniform disorder) with 22 healthy subjects. There were no differences between groups in IGT performance although healthy subjects had a preference for “low frequency–high punishment” decks compared to patients. Thus, IGT performance is impaired in some (Beninger et al., 2003; Ritter et al., 2004, Shurman et al., 2005; Kester et al., 2006), but not all (Wilder et al., 1998; Rodríguez-Sánchez et al., 2005; Evans et al., 2005) studies.
Deficits in IGT performance in schizophrenia may be related to deficits of other areas of the prefrontal cortex. In contrast to the orbitofrontal region, which has been associated with the emotional aspects of behavior and inhibition of inappropriate behavior, the dorsolateral region is involved in working memory, language production, and executive functioning. Previous studies in schizophrenia have found cognitive deficits associated with the dorsolateral region including attention, working and declarative memory, verbal fluency, and executive function (for a review, see Williamson, 2006). The IGT may involve several dorsolateral cognitive functions such as working memory, attention, and executive function that may explain the observed deficits in schizophrenia. Some of the reviewed studies examined correlations between the IGT and tests that index parcellated aspects of dorsolateral prefrontal function. Compared to patients on typical antipsychotics, Beninger et al. (2003) found that patients on atypical antipsychotics had worse performance at the IGT but better performance on the Wisconsin Card Sorting Test (WCST — a test involving attention, working memory, and executive function). Ritter et al. (2004) found decreased performances on the IGT and WCST in schizophrenia but did not find a correlation between the two tests. Similarly, Shurman et al. (2005) reported worse performance on the IGT, WCST, and Delayed Match to Sample Task (a test assessing spatial working memory) in schizophrenia patients compared to healthy subjects but did not report a correlation between the two tests. Kester et al. (2006) reported deficits on the IGT and WCST in adolescents with schizophrenia compared to healthy adolescents, but did not find a correlation between the two tests. Thus, these studies suggest widespread deficits of prefrontal cortical functions in schizophrenia but no direct relationship between deficits of the orbitofrontal and dorsolateral prefrontal cortex.
Deficits in IGT performance in schizophrenia may be more specifically related to the co-occurrence of substance use disorders. Rates of concurrent substance use disorders are high in schizophrenia, and several studies have associated poor IGT performance with alcohol (Mazas et al., 2000; Bechara et al., 2001), psychostimulants (Bechara et al., 2001; Bolla et al., 2003), opiates (Petry et al., 1998; Mintzer and Stitzer, 2002), and marijuana (Whitlow et al., 2004; Bolla et al., 2005) abuse, as well as polysubstance abuse (Grant et al., 2000). Most drugs of abuse increase dopaminergic activity, and converging evidence from animal and human studies suggests that addiction is associated with dopaminergic dysfunction (Kalivas and Volkow, 2005). It has been suggested that individuals with addictive behaviors have reduced dopamine (D2) receptor density and dopamine release resulting in a decreased sensitivity of reward circuits to stimulation by natural rewards (Volkow et al., 2004). Further, there is evidence that emotion-based decision-making is sensitive to changes in dopaminergic activity. Czernecki et al. (2002) reported that patients with Parkinson's disease have deficits on both a reversal task and the IGT, neither of which was sensitive to l-dopa. Recently, we found that the acute administration of a branched-chain amino acid mixture (BCAA) valine, leucine, and isoleucine in healthy subjects increases prolactin levels and impairs IGT performance (Sevy et al., 2006). The acute administration of a BCAA mixture has been demonstrated to lower the plasma ratio of tyrosine + phenylalanine to BCAA and to increase prolactin levels secondary to a decrease in dopaminergic activity (Harmer et al., 2001; Gijsman et al., 2002).
As described in Table 1, several studies excluded subjects with recent use of substances (Wilder et al., 1998; Beninger et al., 2003; Ritter et al., 2004), but it was not clear whether subjects with a lifetime history of substance use disorders were excluded. Some studies did not provide information regarding the inclusion of subjects with substance use disorders (Cavallaro et al., 2003; Evans et al., 2005; Thurnbull et al., 2006). One study included patients with cocaine and alcohol use disorders, but the “healthy” subject group also included individuals using cocaine and alcohol (Ritter et al., 2004). However, the authors reported that alcohol abuse/dependence as a covariate did not significantly change the results. To date, there have been no studies that directly compared IGT performance between schizophrenia patients with and without substance use disorders. In contrast, several studies have compared schizophrenia patients with and without substance use disorders with regard to cognitive functions dependent on the dorsolateral prefrontocortical region. Studies have reported some cognitive deficits in cocaine (Sevy et al., 1990; Serper et al., 2000) and alcohol (Allen et al., 1999; Bowie et al., 2005) abusers compared to non-substance users, but other studies did not find cognitive differences between substance abusers and non-substance users (Cleghorn et al., 1991; Nixon et al., 1996; Addington and Addington, 1997; Pencer and Addington, 2003). Discrepancies between studies in schizophrenia patients abusing alcohol may be age related, with cognitive deficits becoming more apparent in older patients abusing alcohol (Allen et al., 1999; Bowie et al., 2005).
Thus, we postulated that (1) schizophrenia patients have widespread cognitive deficits associated with the orbitofrontal prefrontal cortex (OFPFC) and dorsolateral prontal cortex (DLPFC) compared to healthy subjects; (2) deficits of the OFPFC are more specifically associated with a history of cannabis use disorders; and (3) deficits of the OFPFC are not associated with deficits of the DLPFC or other areas of the cortex. The latter hypothesis is also based on previous findings in individuals with substance use disorders showing no association between IGT deficits and cognitive deficits related to other areas of brain function (Grant et al., 2000).
To test the first hypothesis, we compared schizophrenia patients and healthy subjects on a battery of cognitive tests, which tap into DLPFC function, and with the IGT, which assays OFPFC function. We applied a computational model, the Expectancy-Valence model (Busemeyer and Stout, 2002; Yechiam et al., 2005) to identify the relative contributions of distinct components (attention to past outcomes, relative weight of wins and losses, choice strategies) to decisions made during performance of the IGT.
To examine the second hypothesis, we compared schizophrenia patients with and without cannabis use disorders on the same measures. We focused on cannabis because it is the most commonly used illicit drug in schizophrenia (Murray et al., 2003).
Finally, we tested the third hypothesis by examining correlations between performance on the IGT and performance on cognitive tests indexing parcellated aspects of the DLPFC.
2. Materials and methods
2.1. Subjects
2.1.1. Patients with schizophrenia
Twenty-seven patients were recruited from various inpatient and outpatient programs at the North Shore-Long Island Jewish Health System through referrals from clinicians and fliers that were posted in these programs. Inclusion criteria were: (1) current diagnosis of schizophrenia or schizoaffective disorder confirmed by a structured clinical interview for DSM-IV Axis I disorders (SCID-IV/Patient edition; First et al., 1998); (2) no DSM-IV criteria for a current substance-induced psychotic disorder or a psychotic disorder due to a general medical condition.
Thirteen patients with schizophrenia and no substance use disorders (SCZ(−)) had no past or current DSM-IV diagnosis for substance or alcohol use disorders.
Fourteen patient with schizophrenia and cannabis use disorders (SCZ(+)) had the additional following inclusion criteria: (1) current DSM-IV diagnosis for cannabis abuse or dependence; (2) having cannabis as a main drug of choice; (3) being in a supervised program (inpatient, residential, or intensive day program) in order for the staff to monitor substance use and accurately determine the duration of sobriety; and (3) being sober for at least 1 week.
2.1.2. Healthy subjects
Twenty normal controls were recruited at the Zucker Hillside Hospital for other studies on cognition in schizophrenia. Consenting subjects were assessed with the SCID-IV/non Patient edition (First et al., 2001) and were excluded if they had any history of psychiatric and/or substance use disorders. Six healthy subjects (30%) had a history of occasional use of marijuana and smoked marijuana 10 times or less during their lifetime. All the healthy subjects were sober for more than 3 months prior to testing.
The study was conducted according to the guidelines of the Institutional Review Board of the North Shore — Long Island Jewish Health System (Lake Success, NY). All subjects were paid for their participation in the study.
2.2. Experimental design and procedures
2.2.1. Assessments of cognition
Premorbid intelligence was estimated with the Wide Range Achievement Test—Third Edition, Reading subtest (WRAT-3R) (Spreen and Strauss, 1998).
Visual attention and working memory was assessed with the CPT Identical Pairs test (CPT-IP) (Cornblatt et al., 1988, 1989).
Auditory attention and verbal working memory was measured with the Wechsler Adult Intelligence Scale—Revised, Digit Span Subtest (WAIS-R Digit Span).
Verbal learning and memory was assessed with the California Verbal Learning Test (CVLT) (Delis et al., 1987).
Verbal fluency was measured with the Controlled Oral Word Association Test (COWAT) (Spreen and Strauss, 1998).
Processing speed and executive function was assessed with the Trail Making Test Parts A and B is drawn from the Halstead-Reitan Neuropsychological Battery (Reitan, 1979).
Cognitive tests are described in the Supplemental materials.
2.2.2. Assessment of emotion-based decision-making
Emotion-based decision-making was assessed with the computerized version of the IGT (Bechara, 2005). The IGT is described in the Supplemental materials section.
2.3. Data analysis
Groups were compared using either a Chi-square test or Fisher's exact test, as appropriate, for categorical variables, and analysis of variance (ANOVA) for Continuous variables. Correlations were analyzed using Spearman rank correlation coefficients. A result was considered statistically significant at the 0.05 level of significance. Repeated measures analysis of covariance (RM ANCOVA) was used to analyze net scores, where the model contained one repeated (within) factor (net scores), two covariates of “years of education” and “WRAT-3 Reading Subtest”, and a between factor (grouping) of patient group: schizophrenia patients vs. healthy subjects or SCZ(−) vs. SCZ(+). Power calculation is described in Supplemental materials.
3. Results
3.1. Schizophrenia patients vs. healthy subjects
3.1.1. Demographics (Table 2)
Table 2.
Demographics, cognition, and emotion-based decision-making in patients with schizophrenia and healthy subjects
| Patients with schizophrenia (n=27) | Healthy subjects (n=20) | pa | Confidence intervals | |
|---|---|---|---|---|
| Demographics | ||||
| Age, in years | 30±9 (18–46) b | 33±10 (19–50) | ns | (−8.61, 2.61) |
| Gender (male/female) | 17 m/10 f | 12 m/8 f | nsc | |
| Years of education | 12±2 (9–15) | 15±2 (12–18) | <0.0011 | (−4.19, −1.81) |
| Cognitive testingd: | ||||
| WRAT-3 reading subtest | 89±15 (60–111) | 101±11 (76–117) | <0.0029 | (−20.00, −4.00) |
| CPT-IP, fndprime | 1±0.7 (−0.3–2.6) | 2±0.9 (0.7–4.0) | <0.0002 | (−1.47, −0.53) |
| Digit Span Forward | 6±1 (4–9) | 7±2 (4–11) | <0.0145 | (−1.90, −0.11) |
| Digit Span Backward | 4±0.9 (2–6) | 6 ±2 (2–11) | <0.0017 | (−2.87, −1.13) |
| CVLT | 35±12 (11–60) | 54±11 (34–76) | <0.0001 | (−25.89, −12.11) |
| COWAT, Phonemic Fluency | 32±11 (14–62) | 40±13 (18–65) | <0.0435 | (−15.06, −0.94) |
| COWAT, Semantic Fluency | 14±5 (6–23) | 24±4 (19–31) | <0.0001 | (−12.74, −7.26) |
| Trail Making A | 41±24 (14–132) | 29±10 (17–50) | <0.0443 | (0.49, 23.51) |
| Trail Making B | 130±65 (39–300) | 67±32 (36–125) | <0.0007 | (31.15, 94.85) |
| Iowa Gambling Task: | ||||
| Total amount of money won/lost ($) | −1296±862 (−3015–500) | −981±945 (−2640–830) | ns | (−848.55, 218.55) |
| Total number of cards chosen from: | ||||
| Deck A′ | 20±7 (2–36) | 18±5 (12–27) | ns | (−1.70, 5.70) |
| Deck B′ | 32±11 (16–56) | 31±8 (19–56) | ns | (−4.85, 6.85) |
| Deck C′ | 23±6 (13–41) | 23±6 ( 8–33) | ns | (−3.57, 3.57) |
| Deck D′ | 24±8 (11–43) | 27±6 (12–38) | ns | (−7.29, 1.29) |
| Net scorese: | ||||
| Trials 1–20 | −2.6±4 (−16–4) | −1.0±5 (−8–14) | ns | (−4.24, 1.04) |
| Trials 21–40 | 1.0±6 (−16–14) | 0.2±4 (−10–8) | ns | (−2.32, 3.92) |
| Trials 41–60 | −0.3±7 (−14–18) | −1.9±7 (−14–14) | ns | (−2.56, 5.76) |
| Trials 61–80 | −0.8±6 (−16–10) | 2.4±8 (−12–20) | ns | (−7.31, 0.91) |
| Trials 81–100 | −2.6±8 (−18–12) | 1.4±9 (−20–20) | ns | (−9.01, 1.01) |
| Total net score (sum of Net scores) | −5.0±18 (−46–22) | 1.2±17 (−40–32) | ns | (−16.65, 4.25) |
| Categorical scores | ||||
| Trials 1–60 (0/1)f | 14/13 | 13/7 | nsc | |
| Trials 61–100 (0/1) | 15/12 | 4/16 | <0.02c | |
| Expectancy-Valence Model | ||||
| Recency parameter | 0.24±0.32 (0–1) | 0.37±0.42 (0–1) | ns | (−0.35, 0.09) |
| Attention to Wins/Losses parameter | 0.42±0.43 (0–1) | 0.40±0.37 (0–1) | ns | (−0.22, 0.26) |
| Choice consistency parameter | 0.48±2.9 (−5–5) | −1.04±3.17 (−5–5) | ns | (−0.27, 3.31) |
T-test except when indicated.
Mean±standard deviation (range).
Chi-square test.
On all tests except Trails A and Trails B, higher scores represent better performance. Trails A and B are time to completion, thus higher scores reflect worse performance.
Net score: number of cards chosen from advantageous decks (C′ or D′) minus number of cards chosen from disadvantageous decks (A′ or B′) during 20 trials.
Categorical scores = 1 if ∑ net scores for trials 1–60 or trials 61–100 ≥ 0, and =0 if ∑ net scores for trials 1–60 or trials 61–100 <0.
Groups did not differ for age or sex. Schizophrenia patients had less years of education than healthy subjects (F=24.5; df=2,45; p<0.0011). There was a higher proportion of tobacco users in the schizophrenia group compared to the healthy subject group (48% vs. 5%, p<0.01).
3.1.2. Cognition and emotion-based decision-making (Table 2)
There was a significant difference between the two groups for scores at the WRAT-3R (F=10.05; df=1,41; p<0.0029), CPT-IP fndprime (F=16.85; df=1,37; p<0.0002), Digit Span Forward (F=6.53; df=1,40; p<0.0145), Digit Span Backward (F=11.28; df=1,40; p<0.0017), CVLT (F=26.89; df=1,40; p<0.0001), COWAT Phonemic (F=4.43; df=1,31; p<0.0435) and Semantic (F=26.91; df=1,33; p<0.0001) Fluency, Trail Making A (F=4.31; df=1,40; p<0.0443) and B (F=13.53; df=1,40; p<0.0007). There were no differences between these two groups for any of the IGT variables (Fig. 1).
Fig. 1.
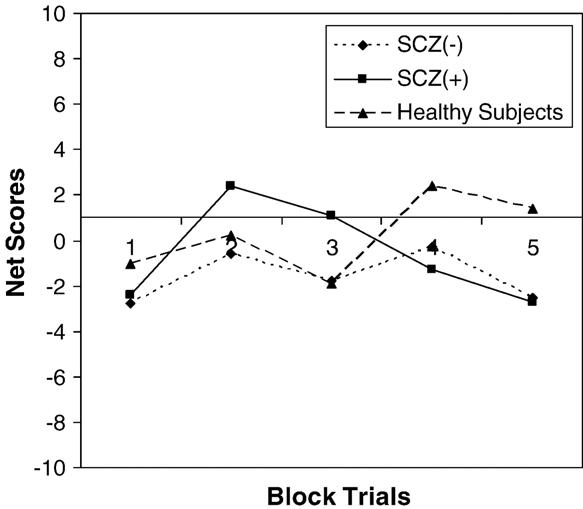
Iowa Gambling Task in SCZ(+), SCZ(−) and healthy subjects.
Mean net scores for trials 61–100 suggest that healthy subjects had a learning curve in the positive direction, whereas schizophrenia patients had a negative learning curve. Lack of findings may have been due to the large variance of net scores. Hence, net scores were categorized into two groups: if the sum of the net scores was greater than or equal to 0 vs. the sum of the net scores being less than 0 for both trials 1–60 and trials 61–100. There was no difference between these two groups for the categorized net scores for trials 1–60. However, for trials 61–100 there was a lower proportion of schizophrenia patients whose sum of the net scores was greater than or equal to 0 compared to the healthy controls (Chi-square=6.031; df=1; p<0.02).
3.2. Schizophrenia patients without substance use disorders (SCZ(−) and with cannabis use disorders (SCZ(+))
3.2.1. Clinical characteristics (Table 3)
Table 3.
Demographics, cognition and clinical characteristics of schizophrenia patients with (SCZ(+)) and without (SCZ(−)) cannabis use disorders and healthy subjects
| Patients with schizophrenia |
p | Confidence intervals |
||
|---|---|---|---|---|
| SCZ(−) (n = 13) | SCZ(+) (n = 14) | |||
| Age, in years | 31±9 (18–46)a | 29±9 (18–45) | ns | (−5.14, 9.14) |
| Gender (male/female) | 7/6 | 10/4 | nsb | |
| Years of education | 13±1 (11–15) | 11±1 (9–14) | <0.0005 | (1.21, 2.79) |
| Age at onset of psychosis, in years | 21±5 (12–29) | 20±4 (15–32) | ns | (−2.58, 4.58) |
| Age at onset of cannabis use, in years | 16±1 (14–18) | |||
| Duration of psychotic illness, in years | 10±9 (0–28) | 9±9 (1–27) | ns | (−6.14, 8.14) |
| Symptoms: | ||||
| Brief Psychiatric Rating Scale (BPRS) Total Score | 28±8 (19–47) | 29±7 (19–46) | ns | (−6.95, 4.95) |
| Scale for the Assessment of Negative Symptoms (SANS) Total Score | 5.8±4.7 (0–13) | 8.0±3.9 (2–16) | ns | (−5.61, 1.21) |
| Subscales: | ||||
| Flat affect | 1.8±1.5 (0–4) | 2.0±1.3 (0–4) | ns | (−1.31, 0.91) |
| Alogia | 1.0±1.2 (0–3) | 1.1±1.2 (0–3) | ns | (−1.05, 0.85) |
| Avolition | 1.3±1.4 (0–3) | 2.5±1.3 (0–5) | <0.0317 | (−2.27,−0.13) |
| Anhedonia | 1.7±1.4 (0–4) | 2.3±1.4(0–4) | ns | (−1.71, 0.51) |
| Cognitive testingc: | ||||
| WRAT-3 reading subtest | 85±15 (61–107) | 92±14 (60–111) | ns | (−18.50, 4.50) |
| CPT-IP, fndprime | 0.9±0.7 (−0.3–2.1) | 1±0.8 (0.0–2.6) | ns | (−0.70, 0.50) |
| Digit Span Forward | 6±1 (4–7) | 7±1 (5–9) | <0.0485 | (−1.79, −0.21) |
| Digit Span Backward | 4±1 (2–5) | 4±1 (3–6) | ns | (−0.79, 0.79) |
| CVLT | 33±10 (11–48) | 36±14 (18–60) | ns | (−12.71, 6.72) |
| COWAT, Phonemic Fluency | 32±7 (19–42) | 31±14 (14–62) | ns | (−7.89, 9.89) |
| COWAT, Semantic Fluency | 14±6 (6–23) | 14±5 (8–23) | ns | (−4.37, 4.37) |
| Trail Making A | 49±28 (29–132) | 34±18 (14–87) | ns | (−3.52, 33.52) |
| Trail Making B | 151±72 (61–300) | 110±53 (39–213) | ns | (−8.86, 90.86) |
| Iowa Gambling Task: | ||||
| Total amount of money won/lost ($) | −1157±807 | −1424±921 | ns | (−421.82, 955.82) |
| (−2400–460) | (−3015–500) | |||
| Total number of cards chosen from: | ||||
| Deck A′ | 21±8 (2–36) | 20±6 (10–29) | ns | (−4.58, 6.58) |
| Deck B′ | 33±11 (21–56) | 32±11 (16–51) | ns | (−7.73, 9.73) |
| Deck C′ | 24±7 (13–41) | 22±5 (14–34) | ns | (−2.80, 6.80) |
| Deck D′ | 22±6 (11–33) | 26±8 (14–43) | ns | (−9.64, 1.64) |
| Net scoresd | ||||
| Trials 1–20 | −2.8±5 (−16–4) | −2.4±4 (−10–2) | ns | (−3.98, 3.18) |
| Trials 21–40 | −0.6±7 (−16–14) | 2.4±6 (− 6–14) | ns | (−8.16, 2.16) |
| Trials 41–60 | −1.8±5 (−10–6) | 1.1±9 (−14–18) | ns | (−8.74, 2.94) |
| Trials 61–80 | −0.3±4 (−8–8) | −1.3±7 (−16–10) | ns | (−3.57, 5.57) |
| Trials 81–100 | −2.5±8 (−18–12) | −2.7±8 (−18–8) | ns | (−6.15, 6.55) |
| Total Net score (sum of Net scores) | −8.0±17 (−46–14) | −2.9±18 (−30–22) | ns | (−19.00, 8.81) |
| Categorical scores | ||||
| Trials 1–60 (0/1)e | 9/4 | 5/9 | nsb | |
| Trials 61–100 (0/1) | 7/6 | 8/6 | nsb | |
| Expectancy-Valence Model | ||||
| Recency parameter | 0.16±0.17 (0–0.50) | 0.31±0.41 (0–1) | ns | (−0.40, 0.10) |
| Attention to Wins/Losses parameter | 0.41±0.42 (0–1) | 0.44±0.44 (0–1) | ns | (−0.37, 0.31) |
| Choice consistency parameter | 0.11±3.2 (−5–5) | 0.81±2.79 (−5–5) | ns | (−3.08, 1.68) |
Mean ± standard deviation (range).
Chi-square or Fisher's Exact (when n < 5 in one of the cells) test.
On all tests except Trails A and Trails B, higher scores represent better performance. Trails A and B are time to completion, thus higher scores reflect worse performance.
Net score: number of cards chosen from advantageous decks (C′ or D′) minus number of cards chosen from disadvantageous decks (A′ or B′) during 20 trials.
Categorical scores = 1 if ∑ net scores for trials 1–60 or trials 61–100 ≥ 0, and =0 if ∑ net scores for trials 1–60 or trials 61–100 < 0.
14 schizophrenia patients out of 27 had a concurrent cannabis use disorder (abuse or dependence) and had a history of smoking one “joint” (about 0.5 g of cannabis) or more per day, 4 days or more per week (Table 3).
Seven of them had also concurrent alcohol use disorders, and among those patients with alcohol use disorders, three had cocaine use disorders and one had opioid use disorders. Ten subjects were abstinent for more than 1 year, 3 subjects were abstinent for 2 months or more, and one subject was abstinent for 2 weeks. Hence, the period of sobriety was long enough in our sample to rule out an acute effect of cannabis on cognition. Eleven patients were on atypical antipsychotics (including four patients on clozapine), one patient on typical antipsychotics, and two patients on a combination of atypical and typical antipsychotics. Two patients were on anticholinergic medications. The onset of cannabis use preceded the onset of psychosis in 11 patients (79%), occurred at the same time as the onset of psychosis in 2 patients (14%), and started 1 year after the onset of psychosis in one patient (7%). Among SCZ(−) patients, 11 patients were on atypical medications (including two patients on clozapine), and two patients were on a combinations of typical and atypical antipsychotic medications. One patient was taking anticholinergic medication.
There were no significant differences between these two groups for age, sex, and marital status. SCZ(−) patients had on average 2 more years of education compared to SCZ(+) patients (p<0.0243). Age at onset of psychosis, duration of psychotic illness, BPRS and SANS total scores, and SANS subscores for flat affects, alogia, and anhedonia did not differ between groups. However, SCZ(+) patients had higher mean SANS subscore for avolition than SCZ(−) patients (F=5.18; df=1,25; p<0.0317). There was a higher proportion of tobacco users in the SCZ(+) group compared to the SCZ(−) group (79% vs. 15%, p<0.01).
3.2.2. Cognition and emotion-based decision-making (Table 3)
There were no differences between patients with and without cannabis use disorders for scores at the WRAT-3R, CPT-IP fndprime, CVLT, Phonemic and Semantic Fluency, Trail Making A and B, or for any of the IGT variables. SCZ(+) patients had higher digit span forward scores (t=2; df=1,23; p<0.05) compared to SCZ(−) patients. There were no differences between SCZ(+) patients with (n=7) and without (n=7) alcohol use disorders. However, sample size may be too small to detect differences between patients with (n=7) and without (n=7) alcohol use disorders.
3.3. Associations between IGT and cognitive test performances
In the schizophrenia group (n=27) there were no statistically significant correlations between total net scores and any of the cognitive scores.
4. Discussion
Similar to previous studies (for a review, see Nuechterlein et al., 2004) and in support of our first hypothesis, we found that patients with schizophrenia had cognitive deficits for attention, working memory and executive functioning compared to normal controls. In agreement with some but not all of the reviewed studies, we found differences between schizophrenia patients and normal controls for IGT performance. These findings suggest that patients with schizophrenia have impaired cognitive functioning, thought to be more linked to the dorsolateral prefrontal cortex, and impaired emotion-based decision-making more linked to the ventromedial/orbital prefrontal cortex.
There are multiple possible reasons for discrepancies between studies looking at IGT performance in schizophrenia. First, most of the studies (including ours) included small sample size and negative findings may be due to the large variance of net scores. Second, as suggested by Rodríguez-Sánchez et al. (2005), there is a wide disparity in performance by healthy subjects across studies. Third, IQ and education may be correlated with IGT performance (Rodríguez-Sánchez et al., 2005), which may explain IGT performance differences in studies that did not control for educational or IQ level (Shurman et al., 2005). Finally, discrepancies between studies may be related to the heterogeneity of diagnostic groups. Many of the negative studies included schizophrenia and schizoaffective disorder (Wilder et al., 1998; Ritter et al., 2004; Rodríguez-Sánchez et al., 2005). Positive studies have generally included only patients with schizophrenia (Beninger et al., 2003; Shurman et al., 2005; Kester et al., 2006), although some studies that included only patients with schizophrenia failed to find differences between groups (Cavallaro et al., 2003; Evans et al., 2005; Thurnbull et al., 2006).
We did not find significant differences between SCZ(−) and SCZ(+) patients for many of the cognitive tests administered, with the exception of Digit Span Forward. Our results are in agreement with a previous study showing no differences between schizophrenia patients with (n=38) and without (n=25) a history of cannabis abuse for performance at tests assessing intelligence, memory, learning, fluency, and problem solving (Cleghorn et al., 1991). However, in a study comparing schizophrenia patients with (n=26) and without (n=37) cannabis use 10–12 years after the admission for a first-episode of psychosis, Stirling et al. (2005) reported that cannabis users had better cognitive functioning than patients without cannabis use in several domains including design memory, verbal fluency, object assembly, block design, picture completion, picture arrangement, and face recognition memory. Contrary to our expectations, there were no differences between SCZ(−) and SCZ(+) patients for IGT performance. Other factors such as better premorbid functioning and socialization (Arndt et al., 1992; Sevy et al., 2001) may be more critical than impaired emotion-based decision-making for increasing the risk of substance use disorders in patients with schizophrenia.
In support of our third hypothesis, we did not find any statistically significant correlations between IGT total scores and cognitive scores. Our results suggest that deficits of the DLPFC and OPFC are not directly related to one another.
Our study has some limitations. There was a higher proportion of tobacco users in the schizophrenia group compared to the healthy subject group (48% vs. 5%) and in the SCZ(+) group compared to the SCZ(−) group (79% vs. 15%). It has been suggested that nicotine may help schizophrenia patients to be more vigilant, focused, and improve their working memory (Lee et al., 1997; George et al., 2002). Although all subjects were asked not to smoke before testing, nicotine levels were not assessed in this study. Hence, there is a possibility that some patients had improved cognitive performance following nicotine use. Since most of the schizophrenia smokers were cannabis abusers, nicotine may have improved cognition of SCZ(+) patients to a level similar to SCZ(−) patients, which may have resulted in differences between groups being too small to detect. Our negative results may also be due to small sample sizes. The reported confidence intervals for the difference between the means raise the possibility that the statistical power was too low to detect small differences between SCZ(+) and SCZ(−) groups for cognition and emotion-based decision-making. Future studies will have to include larger sample sizes in order to confirm our preliminary findings.
In summary, findings on IGT performance in schizophrenia have been mixed. Similar to several previous studies, we found deficits in cognitive functioning and emotion-based decision-making in schizophrenia. However, concurrent abuse of cannabis had no compounding effects on cognition, as well as emotion-/affect-based decision-making. Future studies will be needed to determine if the neurobiological mechanisms underlying addiction differ between individuals with and without schizophrenia.
Supplementary Material
Acknowledgements
We thank Dr. Terry Goldberg for his helpful comments and Ms. Isabella Riojas who assisted with the preparation and proof-reading of the manuscript.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.schres.2007.01.005.
References
- Addington J, Addington D. Substance abuse and cognitive functioning in schizophrenia. J. Psychiatry Neurosci. 1997;22:99–104. [PMC free article] [PubMed] [Google Scholar]
- Allen DN, Goldstein G, Aldarondo F. Neurocognitive dysfunction in patients diagnosed with schizophrenia and alcoholism. Neuropsychology. 1999;13:62–68. doi: 10.1037//0894-4105.13.1.62. [DOI] [PubMed] [Google Scholar]
- Arndt SA, Tyrrell G, Flaum M, Andreasen NC. Comorbidity of substance abuse and schizophrenia. The role of pre-morbid adjustment. Psychol. Med. 1992;22:379–388. doi: 10.1017/s0033291700030324. [DOI] [PubMed] [Google Scholar]
- Bechara A. Decision-making, impulse control, and loss of willpower to resist drugs: a neurocognitive perspective. Nat. Neurosci. 2005;8:1458–1463. doi: 10.1038/nn1584. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Beninger RJ, Wasserman J, Zanibbi K, Charbonneau D, Mangels J, Beninger BV. Typical and atypical antipsychotic medications differentially affect two nondeclarative memory tasks in schizophrenic patients: a double dissociation. Schizophr. Res. 2003;61:281–292. doi: 10.1016/s0920-9964(02)00315-8. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk FR, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. NeuroImage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. NeuroImage. 2005;26:480–492. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Bowie CR, Serper MR, Riggio S, Harvey PD. Neurocognition, symptomatology, and functional skills in older alcohol-abusing schizophrenia patients. Schizophr. Bull. 2005;31:175–182. doi: 10.1093/jschbul/sbi001. [DOI] [PubMed] [Google Scholar]
- Busemeyer JR, Stout JC. A contribution of cognitive decision models to clinical assessment. Decomposing performance on the Bechara gambling task. Psychol. Assess. 2002;14:253–262. doi: 10.1037//1040-3590.14.3.253. [DOI] [PubMed] [Google Scholar]
- Cavallaro R, Cavedini P, Mistretta P, Bassi T, Angelone SM, Ubbiali A, Bellodi L. Basal-corticofrontal circuits in schizophrenia and obsessive-compulsive disorder: a controlled, double dissociation study. Biol. Psychiatry. 2003;54:437–443. doi: 10.1016/s0006-3223(02)01814-0. [DOI] [PubMed] [Google Scholar]
- Cleghorn JM, Kaplan MD, Szechtman B, Szechtman H, Brown GM, Franco S. Substance abuse and schizophrenia: effect on symptoms but not on neurocognitive function. J. Clin. Psychiatry. 1991;52:26–30. [PubMed] [Google Scholar]
- Cornblatt BA, Risch NJ, Faris G, Friedman D, Erlenmeyer-Kimling L. The Continuous Performance Test, identical pairs version (CPT-IP): I. New findings about sustained attention in normal families. Psychiatry Res. 1988;26:223–238. doi: 10.1016/0165-1781(88)90076-5. [DOI] [PubMed] [Google Scholar]
- Cornblatt BA, Lenzenweger MF, Erlenmeyer-Kimling L. The continuous performance test, identical pairs version: II. Contrasting attentional profiles in schizophrenic and depressed patients. Psychiatry Res. 1989;29:65–85. doi: 10.1016/0165-1781(89)90188-1. [DOI] [PubMed] [Google Scholar]
- Czernecki V, Pillon B, Houeto JL, Pochon JB, Levy R, Dubois B. Motivation, reward, and Parkinson's disease: influence of dopatherapy. Neuropsychologia. 2002;40:2257–2267. doi: 10.1016/s0028-3932(02)00108-2. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test: Adult Version. The Psychological Corporation; San AntonioTX: 1987. [Google Scholar]
- Evans CEY, Bowman CH, Turnbull OH. Subjective awareness on the Iowa Gambling Task. The key role of emotional experience in schizophrenia. J. Clin. Exp. Neuropsychol. 2005;27:656–664. doi: 10.1081/13803390490918354. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders-Patient Edition. Biometrics Research Department, New York State Psychiatric Institute; New York: 1998. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, et al. Structured Clinical Interview for DSM-IV-TR Axis I Disorders-Non-patient Edition. Biometrics Research Department, New York State Psychiatric Institute; New York: 2001. [Google Scholar]
- George TP, Vessicchio JC, Termine A, Sahady DM, Head CA, Pepper WT, Kosten TR, Wexler BE. Effects of smoking abstinence on visuospatial working memory function in schizophrenia. Neuropsychopharmacology. 2002;26:75–85. doi: 10.1016/S0893-133X(01)00296-2. [DOI] [PubMed] [Google Scholar]
- Gijsman HJ, Scarnà A, Harmer CJ, McTavish SFB, Odontiadis J, Cowen PJ, Goodwin GM. A dose-finding study on the effects of branch Chain amino acids on surrogate markers of brain dopamine function. Psychopharmacology. 2002;160:192–197. doi: 10.1007/s00213-001-0970-5. [DOI] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision making. Neuropsychologia. 2000;38:1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- Harmer CJ, McTavish SFB, Clark L, Goodwin GM, Cowen PJ. Tyrosine depletion attenuates dopamine function in healthy volunteers. Psychopharmacology. 2001;154:105–111. doi: 10.1007/s002130000613. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: a pathology of motivation and choice. Am. J. Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Kester HM, Sevy S, Yechiam E, Burdick K, Cervellione KL, Kumra S. Decision-making impairments in adolescents with early-onset schizophrenia. Schizophr. Res. 2006;85:113–123. doi: 10.1016/j.schres.2006.02.028. [DOI] [PubMed] [Google Scholar]
- Lee C, Frangou S, Russel MA, Gray JA. Effect of haloperidol on nicotine-induced enhancement of vigilance in human subjects. J. Psychopharmacol. 1997;11:253–257. doi: 10.1177/026988119701100309. [DOI] [PubMed] [Google Scholar]
- Mazas CA, Finn PR, Steinmetz JE. Decision-making biases, antisocial personality, and early-onset alcoholism. Neurobiol., Psychosoc., Dev. Correl. Drink. 2000;24:1036–1040. [PubMed] [Google Scholar]
- Mintzer MZ, Stitzer ML. Cognitive impairment in methadone maintenance patients. Drug Alcohol Depend. 2002;67:41–51. doi: 10.1016/s0376-8716(02)00013-3. [DOI] [PubMed] [Google Scholar]
- Murray RM, Grech A, Phillips P, Johnson S. In: What is the Relationship Between Substance Abuse and Schizophrenia. Murray RM, Jones PB, Susser E, van Os J, Cannon M, editors. Cambridge University Press; UK: 2003. pp. 317–342. [Google Scholar]
- Nixon SJ, Hallford HG, Tivis RD. Neurocognitive function in alcoholic, schizophrenic, and dually diagnosed patients. Psychiatry Res. 1996;64:35–45. doi: 10.1016/0165-1781(96)02911-3. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Barch DM, Gold JM, Goldberg TE, Green MF, Heaton RK. Identification of separable cognitive factors in schizophrenia. Schizophr. Res. 2004;72:29–39. doi: 10.1016/j.schres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Pencer A, Addington J. Substance use and cognition in early psychosis. J. Psychiatry Neurosci. 2003;28:48–54. [PMC free article] [PubMed] [Google Scholar]
- Petry NM, Bickel WK, Arnett M. Shortened time horizons and insensitivity to future consequences in heroin addicts. Addiction. 1998;93:729–738. doi: 10.1046/j.1360-0443.1998.9357298.x. [DOI] [PubMed] [Google Scholar]
- Reitan RM. Manual for Administration of Neuropsychological Test Batteries for Adults and Children. Reitan Neuropsychological Laboratory; Tuscon, AZ: 1979. [Google Scholar]
- Ritter LM, Meador-Woodruff JH, Dalack GW. Neurocognitive measures of prefrontal cortical dysfunction in schizophrenia. Schizophr. Res. 2004;68:65–73. doi: 10.1016/S0920-9964(03)00086-0. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Sánchez JM, Crespo-Facorro B, Iglesias RP, Bosch CGB, Álvarez M, Llorca J, Vázquez-Barquero JL. Prefrontal cognitive functions in stabilized first-episode patients with schizophrenia spectrum disorders: a dissociation between dorsolateral and orbitofrontal functioning. Schizophr. Res. 2005;77:279–288. doi: 10.1016/j.schres.2005.04.023. [DOI] [PubMed] [Google Scholar]
- Serper MR, Bergman A, Copersino ML, Chou J-CY. Learning and memory impairment in cocaine dependent and comorbid schizophrenic patients. Psychiatry Res. 2000;93:387–394. doi: 10.1016/s0165-1781(99)00122-5. [DOI] [PubMed] [Google Scholar]
- Sevy S, Kay SR, Opler L, van Praag HM. Significance of cocaine history in schizophrenia. J. Nerv. Ment. Dis. 1990;178:642–648. doi: 10.1097/00005053-199010000-00005. [DOI] [PubMed] [Google Scholar]
- Sevy S, Robinson DR, Holloway S, Alvir JM, Woerner MG, Bilder R, Goldman R, Lieberman J, Kane J. Correlates of substance abuse in patients with first-episode schizophrenia and schizoaffective disorder. Acta Psychiatr. Scand. 2001;104:367–374. doi: 10.1034/j.1600-0447.2001.00452.x. [DOI] [PubMed] [Google Scholar]
- Sevy S, Hassoun Y, Bechara A, Yechiam E, Napolitano B, Burdick K, Delman H, Malhotra AK. Emotion-based decision-making in healthy subjects: short-term effects of reducing dopamine levels. Psychopharmacology. 2006;188:228–235. doi: 10.1007/s00213-006-0450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shurman B, Horan WP, Nuechterlein KH. Schizophrenia patients demonstrate a distinctive pattern of decision-making impairment on the Iowa Gambling Task. Schizophr. Res. 2005;72:215–224. doi: 10.1016/j.schres.2004.03.020. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neurological Tests: Administration, Norms, and Commentary. 2nd ed. Oxford University Press; New York: 1998. [Google Scholar]
- Stirling J, Lewis S, Hopkins R, White C. Cannabis use prior to first onset psychosis predicts spared neurocognition at 10-year follow-up. Schizophr. Res. 2005;75:135–137. doi: 10.1016/j.schres.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Thurnbull OH, Evans CEY, Kemish K, Park S, Bowman CH. A novel set-shifting modification of the Iowa Gambling Task: flexible emotion-based learning in schizophrenia. Neuropsychology. 2006;3:290–298. doi: 10.1037/0894-4105.20.3.290. [DOI] [PubMed] [Google Scholar]
- Tremeau F. A review of emotion deficits in schizophrenia. Dialogues Clin. Neurosci. 2006;8:59–70. doi: 10.31887/DCNS.2006.8.1/ftremeau. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Swanson JM. Dopamine in drug abuse and addiction: results from imaging studies and treatment implications. Mol. Psychiatry. 2004;9:557–569. doi: 10.1038/sj.mp.4001507. [DOI] [PubMed] [Google Scholar]
- Whitlow CT, Liguori A, Livengood LB, Hart SL, Mussat-Whitlow BJ, Lamborn CM, Laurienti PJ, Porrino LJ. Long-term heavy marijuana users make costly decisions on a gambling task. Drug Alcohol Depend. 2004;76:107–111. doi: 10.1016/j.drugalcdep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Wilder KE, Weinberger DR, Goldberg TE. Operant conditioning and the orbitofrontal cortex in schizophrenic patients: unexpected evidence for intact functioning. Schizophr. Res. 1998;30:169–174. doi: 10.1016/s0920-9964(97)00135-7. [DOI] [PubMed] [Google Scholar]
- Williamson P. Mind, Brain, and Schizophrenia. Oxford University Press; New York: 2006. pp. 65–74. [Google Scholar]
- Yechiam E, Busemeyer JR, Stout JC, Bechara A. Using cognitive models to map relations between neuropsychological disorders and human decision-making deficits. Psychol. Sci. 2005;16:973–978. doi: 10.1111/j.1467-9280.2005.01646.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


