Abstract
Mitogen-activated protein (MAP) kinases have been implicated in hemodynamic load induced heart failure. Both angiotensin II (Ang II) and mechanical stretch activate MAP kinases in cardiac myocytes. In this study, we used a neonatal rat ventricular myocyte (NRVM) model to determine the role of focal-adhesion kinase (FAK) in β1 integrin mediated MAP kinase activation in response to mechanical stretch in presence and absence of Ang II receptor blockade (ATB). NRVM plated on deformable membranes coated with collagen IV were exposed to 20% equiaxial static-stretch. β1 integrin signaling was blocked by adenovirus-mediated expression of a dominant-negative form of β1D integrin (tac-β1D). FAK signaling was disrupted by infecting NRVM with adenovirus expressing FAK-related non-kinase (FRNK). Western blot analysis was used to assess the phosphorylation of MAP kinases. In the presence and absence of ATB, mechanical stretch caused maximal phosphorylation of ERK, p38 and JNK at 5 min, which was significantly attenuated in NRVM expressing tac-β1D. In the presence of ATB, FRNK overexpression significantly increased basal phosphorylation of ERK (40.2 ± 8.6% p<0.05), p38 (39.5 ± 11.7%, P<0.05), JNK (86 ± 29.4%, P<0.05) and stretch-induced p38 (48.1 ± 8.7%, P<0.05) and JNK (85.0 ± 19.4%, P<0.05) phosphorylation. However, in the absence of ATB, FRNK overexpression significantly reduced basal and stretch-induced phosphorylation of only ERK. Examination of FAK activation revealed that β1 integrin was required for stretch-induced phosphorylation of FAK at Y397 and Y925, but not Y861. In summary, mechanical stretch-activated ERK1/2, p38 and JNK through FAK independent and dependent mechanisms. β1 integrin was required for FAK independent activation of all three MAP kinases, whereas cross-talk between β1 integrin and Ang II receptors mediated FAK dependent regulation of ERK1/2.
Keywords: Cardiac myocytes, focal-adhesion kinase, integrin, MAP kinase, mechanical stretch
1. Introduction
Cardiovascular diseases such as hypertension and myocardial infarction are often associated with the development of cardiac hypertrophy. The hypertrophy occurs in response to an increased mechanical load on the heart in the form of pressure or volume overload. The mechanisms that couple load to the hypertrophic growth initiation and to the transition into heart failure are poorly understood. Mechanical stretch has been postulated to trigger growth and remodeling responses in the hemodynamically overloaded myocardium [1–3]. This hypothesis is supported by in vitro studies, in which cardiac myocytes cultured on collagen or laminin-coated deformable membranes, display hypertrophic responses when exposed to mechanical stretch [4]. In this model, several key signaling molecules, including protein kinase C [5], p70/85 S6 kinase [6], as well as the MAP kinase family members ERK1/2 [4], p38 [7] and JNK [8] are activated by mechanical stretch [2,9].
A major goal in this area has been to identify the mechanisms that link biomechanical forces to the activation of signaling pathways that mediate the hypertrophic as well as maladaptive responses of cardiac myocytes to mechanical stress. However, the mechanical signaling pathways responsible for activation of the growth pathways are complex and poorly understood. Integrins, initially considered solely as molecules necessary for adhesive interactions between cells and the extracellular matrix, are now known to also act as mechanical transducing receptors, which modulate cellular growth and gene expression [10,11]. Integrins are α/β transmembrane receptors that link the force-generating actin cytoskeleton to the extracellular matrix [12]. The most abundant integrin expressed by cardiac myocytes is the β1 subunit, which exists predominantly as the β1D alternatively spliced isoform in the neonate and adult heart. The prominent location of β1D integrins at the junction of extracellular matrix to Z discs makes them candidates for acting as biomechanical sensors in cardiac myocytes. Over-expression of β1-integrin induces hypertrophic gene expression in rat cardiac myocytes [13], whereas conditional knockout of β1 integrin in ventricular myocytes results in myocardial fibrosis and chamber dilation in transgenic mice [14]. Interestingly, AT1 has been shown to mediate cardiac hypertrophy by upregulation of β1 integrin expression [15] and the AT1 receptor can act as a mechanoreceptor in cardiac tissue [16].
Results from in vivo and in vitro studies suggest that focal adhesion kinase (FAK), a downstream effector of β-integrin containing receptors [17,18], plays an important role in the regulation of cardiac growth. FAK-Y397, FAK-Y861 and FAK-Y925 have been reported to be activated by mechanical overload in rat heart [19–22]. In addition, FAK is activated by mechanical stretch in NRVM [7,23,24]. Complete knockout of FAK in the mouse results in a lethal embryonic phenotype with major defects in the cardiovascular system [25,26]. In the mouse, cardiac ventricle-restricted FAK knockout has been shown to cause eccentric cardiac hypertrophy and fibrosis [27]. This suggests that FAK has a complex role, in which it mediates cardiac development in the embryo and protects the myocardium in the adult by inhibiting growth. However, studies using isolated myocytes, indicate that FAK is a positive regulator of myocyte growth. In these studies, stretch-induced FAK and MAP kinase activation in cardiac myocytes has been linked to αvβ3, not β1 integrin signaling [21]. In addition, signaling from activation of AT1 and AT2 may have contributed to the stretch effects. Although recent studies indicate that Ang II growth effects involve β integrin [15], cross-talk between integrin and Ang II receptor signaling pathways remains to be elucidated. In this study, we used a neonatal rat ventricular myocyte (NRVM) model to determine the role of focal-adhesion kinase (FAK) in β1 integrin mediated MAP kinase activation in response to mechanical stretch in presence and absence of Ang II receptor blockade (ATB).
2. Experimental Procedures
2.1 Materials
FAK antibody (F15020, clone 77) and FITC-conjugated β1 integrin antibody (Ha2/5) were obtained from BD Transduction Laboratories (Lexington, KY); phospho-ERK-p44/42-T202/Y204 antibody (9101), ERK polyclonal antibody (9102), phospho-p38-T180/Y182 antibody (9211), p38 antibody (5F11), phospho-SAPK/JNK-T183/Y185 antibody (9251), JNK antibody (9252) phospho-FAK-Y925 (3284) and horseradish peroxidase-conjugated secondary antibodies were obtained from Cell Signaling Technology, Inc. (Danvers, MA). Phospho-FAK-Y861 (ab38458) was obtained Abcam Inc. (Cambridge, MA). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibody (MAB1501) was obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); and phospho-FAK-Y397 antibody (07–157), and antibody (7G7/B6) to the extracellular domain (residues 140–144) of the interleukin (IL)-2α receptor were obtained from Upstate Biotechnology (Lake Placid, NY). Bovine serum albumin (BSA, diagnostic grade K) was obtained from Celluliance (Kanakee, IL). Enhanced chemiluminescence (ECL) reagent (Western Lightning™) was obtained from Perkin Elmer Life Science (Boston, MA). Replication-defective adenovirus with vector containing sequences encoding green fluorescent protein (GFP) and FAK related non-kinase (FRNK) were gifts from Dr. Joan Taylor (University of North Carolina, Chapel Hill, NC), and tac-β1D and lacZ were gifts from Robert Ross (University of San Diego, San Diego, CA) [28]. Transformed 293 human embryonic kidney (HEK) cells CRL-1573 were obtained from (American Type Culture Collection, Manassas, VA) and cultured as advised by the supplier.
2.2 Isolation of neonatal rat ventricular myocytes
Primary cultures of NRVMs were prepared from 1 to 2-day-old Sprague Dawley rats as previously described [29]. Dispersed cardiac cells were separated using a discontinuous Percoll gradient, containing a density of 1.060 gm/L (nonmyocyte layer) and 1.086 gm/L (myoctye layer). The NRVM were plated on deformable membranes coated with collagen-IV [1 µg/cm²]) on Bioflex plates (Flexcell International Corp, Hillsborough, NC), at a density of 0.75 × 106 cells/well in DMEM/M199 medium and maintained at 37°C in humid air with 5% CO2. Cytosine arabinoside (100 µM) was added to prevent cell division of non-myocytes and culture media was changed to serum-free 24 h prior to initiation of experiments. The cultured myocytes were >95% pure, as revealed by microscopic observation of contractile characteristics and by flow cytometry, after staining with anti-desmin antibody (Sigma Chemical Co., St. Louis, MO). In ATB experiments, mechanical activation of AT1 and autocrine effects, due to Ang II release were blocked adding AT1 and AT2 receptor antagonists (10 µM losartan and 10 µM PD123319, respectively) to the culture media 30 min prior to stretch. NRVMs were exposed to 20% equiaxial static-stretch for various times in a Flexercell FX-3000 strain unit equipped with loading posts. This degree of stretch mimics conditions of cardiac hypertrophy [4] and did not result in cell lift-off or decreased cell viability in the present study. This study conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996).
2.3 Adenovirus infection
At 24 hr after plating, NRVM were infected with either replication-defective adenovirus encoding tac-β1D (50 multiplicity of viral infection [MOI]) or FRNK (350 MOI). Adenovirus expressing lacZ (50 MOI) and GFP (350 MOI) were used to control for nonspecific effects of adenovirus infection associated with tac-β1D and FRNK expression, respectively. Levels of expressed proteins were determined using flow cytometry (described below) and Western blot analysis (not shown). Because β1D and FAK are important for cell adhesion, NRVM were titrated with tac-β1D and FRNK expressing virus. Thus, viruses were titrated to maximize expressed protein, but prevent viral toxicity and detachment of cells from deformable membranes during stretch procedures. The shape and cell areas of NRVM expressing tac-β1D or FRNK were similar compared to control cells (non-infected and virus controls). However, the cell borders of NRVM expressing tac-β1D and FRNK had a slight “smooth” appearance. Cardiac myocytes were infected for 24 h with each adenovirus diluted in DMEM/medium 199. The medium was replaced with virus-free DMEM/medium 199, and cells were cultured for an additional 24 h prior to stretch experiments. The viral MOI was determined by dilution assay in HEK-293 cells grown in 6-well clusters.
2.4 Preparation of cell lysates
Cells were lysed in assay lysis buffer (Cell Signaling) containing 10 µg/mL aprotinin, 10 µg/mL leupeptin, 1 mM 2-(2-aminoethyl)-benzenesulfonyl fluoride, hydrochloride and 1 mM sodium orthovanadate. Insoluble material was removed by centrifugation for 15 min at 14,000 g and samples were boiled with loading buffer and protein was determined using a kit (BioRad DC Protein Assay) according to the manufacturer’s recommendation.
2.5 Western blotting
Western blot analysis was performed as previously described [30]. Briefly, equal amounts of protein (30 µg) from cell lysates were separated by SDS-PAGE and blotted onto PVDF transfer membranes. The membranes were blocked for 2 h using 5% BSA in TBST buffer (10 mM Tris, 0.1 M NaCl, 0.1% Tween 20, pH 7.4). Blots were incubated with the primary antibodies in 5% BSA in TBST buffer overnight at 4°C with light agitation. Following incubation with primary antibody, blots were washed three times for 5 min each with TBST buffer and incubated with appropriate horseradish peroxidase-labeled secondary antibodies in 1% BSA in TBST buffer for 1 h at 37°C. Following three washes of 5 min each with TBST, proteins were detected using ECL. Quantification of bands was performed using ImageQuant software. Signals from phosphoproteins were normalized to the total protein, obtained by stripping and reprobing blots with corresponding total antibody. Blots were again striped and probed with GAPDH antibody to confirm equal loading.
2.6 Flow cytometry
NRVM were harvested by incubation in 1 mM EDTA, washed with phosphate buffered saline (PBS, pH 7.4) and resuspended at 3 × 106 cells/ml in FACS buffer (PBS containing 1% FBS and 1 mM EDTA). Surface expression of β1 integrin was determined by incubating cells for 30 min at 22°C with 2.5 µg/ml of FITC-conjugated β1 antibody. To determine tac-β1D expression, cells were incubated for 15 min with 10 µg/ml IL-2α antibody (7G7/B6) and an additional 15 min with (20 µg/ml) 488-Alexa conjugated anti-mouse secondary antibody. Negative controls were cell samples incubated without primary antibody or only with fluorescent-conjugated secondary antibody. After incubation with antibody, cells were washed three times in FACS buffer and analyzed on a flow cytometer (FACSCalibur, Becton Dickinson, San Jose, CA), using Cell Quest 5.2 software. Each analysis was based on a sample of 10,000 cells.
2.7 Statistical analysis
Data are presented as the mean ± the standard error of the mean (SEM). Significant differences among groups were estimated using one-way or two-way ANOVAs followed by Tukey’s multiple comparison test (GraphPad Software Inc., San Diego, CA). A value of p<0.05 was considered to denote statistical significance.
3. Results
3.1 Mechanical stretch activates MAP kinase phosphorylation independent of AT1 and AT2
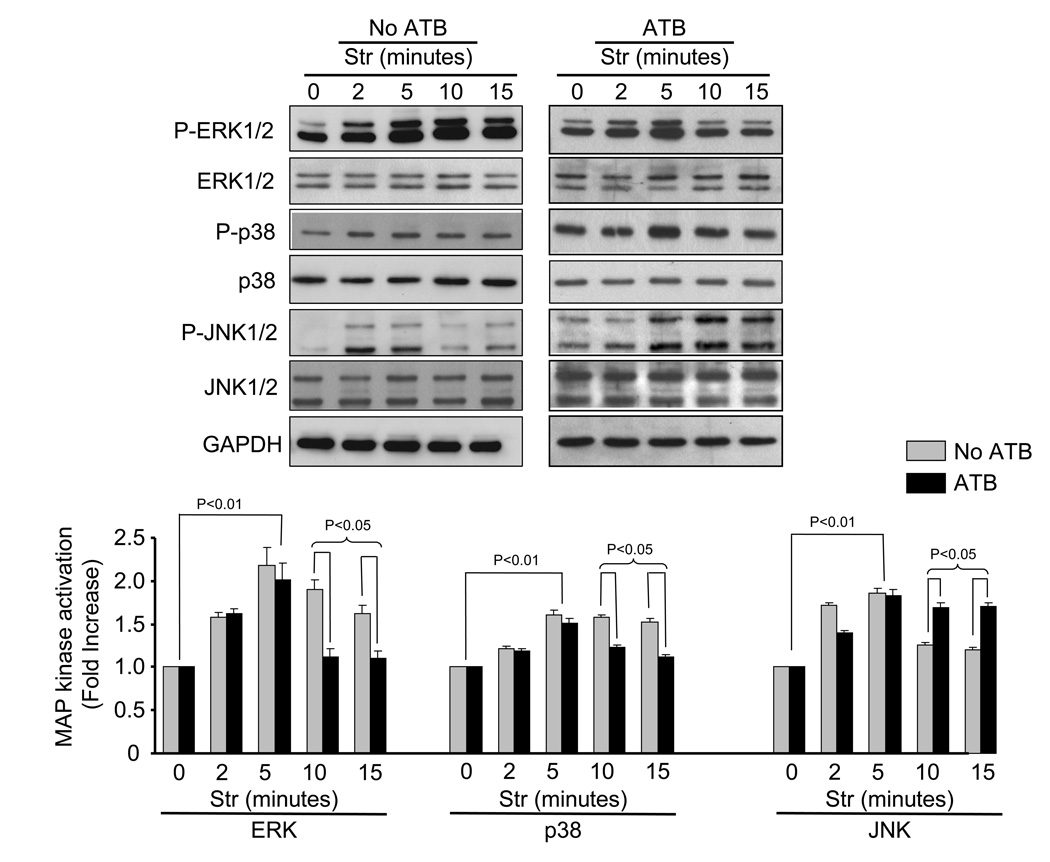
Mechanical stretch has been shown to activate MAP kinase pathways as a result of autocrine release of Ang II [31] or by direct activation of the AT1 receptor [16]. To study mechanical signaling independent of Ang II receptor activation, antagonists for AT1 (10 µM losartan) and AT2 (10 µM PD123319) were added to culture media 15 min prior to 20% mechanical stretch. Figure 1 show the time-course of stretch-induced MAP kinase activation (phosphorylation) in the presence and absence of ATB. Phosphorylation of MAP kinases was increased within 2 min, peaked at 5 min and declined. In the presence of ATB, mechanical stretch (5 min) significantly increased phosphorylation of ERK (69.3 ± 26.1%, p<0.01), p38 (51.8 ± 11%, p<0.01) and JNK (93.6 ± 38%, p<0.01) (N=5). These results indicate that mechanical stretch is sufficient to activate MAP kinases, independent of AT1 receptor activation. It was interesting to note that similar levels of MAP kinase activation were observed in the absence of ATB up to 5 min. However, at 10 and 15 min, MAP kinase phosphorylation levels were more dependent on Ang II receptor activation. In the presence of ATB, there were significant decreases in the phosphorylation levels of ERK (41 ± 7.23%, P<0.05 and 32% ± 4.7%, P<0.05 at 10 and 15 min, respectively) and p38 (22% ± 3.7%, P<0.05 and 26% ± 3.2%, P<0.05 at 10 and 15 min, respectively). In contrast, phosphorylation of JNK was increased in the presence of ATB (35% ± 3.9%, P<0.05 and 42% ± 4.1%, P<0.05 at 10 and 15 min, respectively).
Fig. 1. Time-course of stretch-induced MAP kinase phosphorylation.
Representative blots showing time-course of stretch-induced changes in ERK (p44/42-T202/Y204), p38-T180/Y182 and JNK-T183/Y185 in cell lysates harvested from NRVM in the absence and presence of AT1 and AT2 receptor blockers (ATB, 10 µM losartan and 10 µM PD123319, respectively). Bar graphs show fold changes in ERK, p38 and JNK phosphorylation after stretch (Str), respectively, when compared to non-stretch (0 min). Values are means ± SEM. N=5 experiments.
3.2 β1D integrin is required for stretch-induced phosphorylation of ERK1/2, p38, and JNK
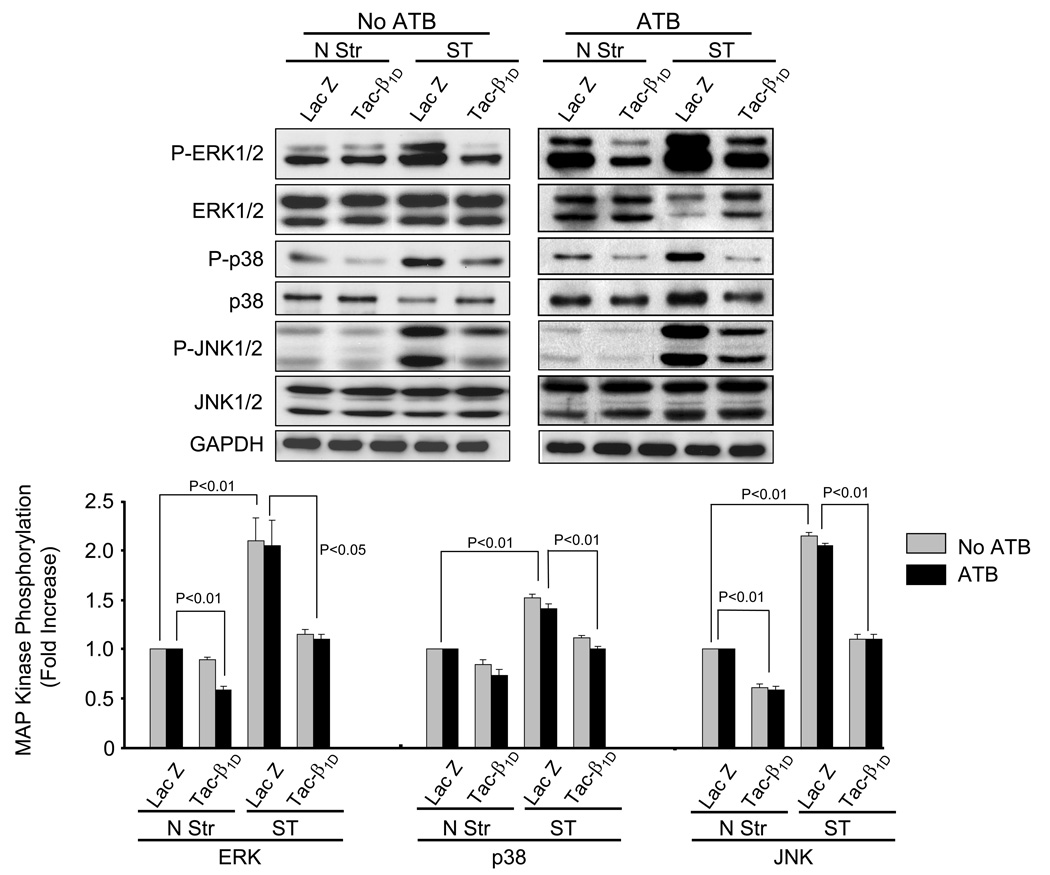
Integrins have been implicated as mechanotransducers responsible for activating growth responses in the cardiovascular system [11,13]. The predominant integrin expressed in cardiac myocytes is β1. To test whether β1 integrin couples to stretch-induced activation of MAP kinases, NRVM were infected 24 h with matched titers of recombinant adenoviruses that express either tac-β1D or control (lacZ) transgenes. The tac-β1D acts as a dominant-negative for the small amounts of expressed β1A subunit, as well as the predominantly expressed β1D subunit in myocytes. NRVM transfected with tac-β1 expressing adenovirus had high cell surface expression (mean fluorescence intensity [MFI], N = 3 experiments) of tac-β1 (498 ± 190), compared to cells transfected with lacZ (29.9 ± 15.6) (Fig. 2). The expression of β1 integrin, was modestly decreased in tac-β1 expressing cells (237 ± 79.7), compared to lacZ expressing cells (312 ± 62.7). In the presence of ATB, disruption of β1 signaling significantly inhibited stretch-dependent activation of ERK (56.5 ± 9.0%, P<0.05), p38 (31.4 ± 8.6%, P<0.01) and JNK (86.7 ± 6.2%, P<0.01) (Fig. 3). Similar changes were observed in the absence of ATB, however, under basal conditions, tac-β1D expression inhibited phosphorylation of MAP kinases. These results indicate that β1 integrin plays a significant role in stretch-induced activation of ERK, p38 and JNK.
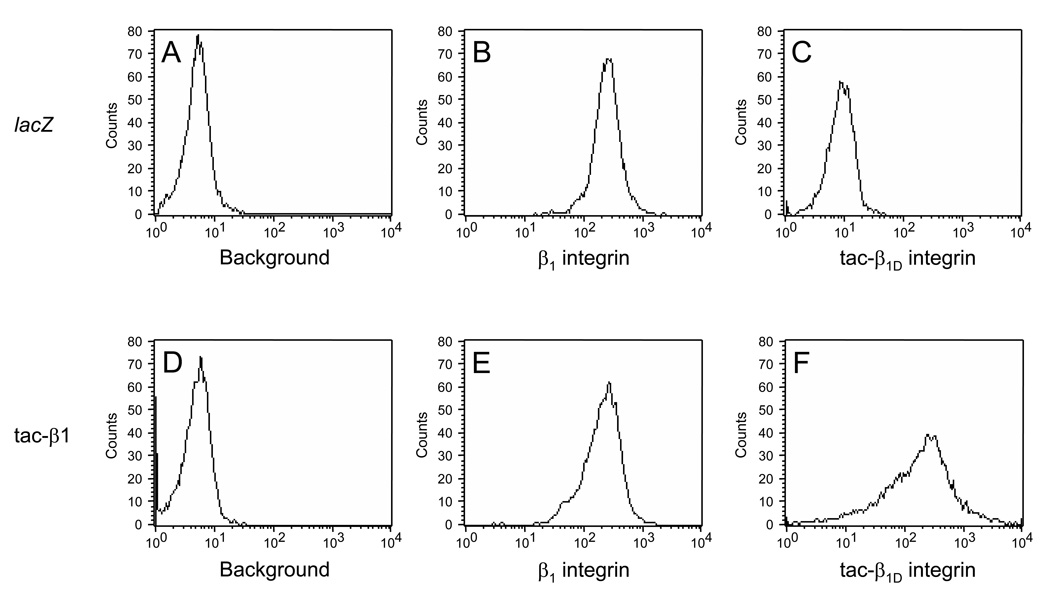
Fig. 2. Expression of β1 integrin and tac-β1 in transfected cells.
The presence of cell surface β1 integrin was verified by staining cells with FITC-conjugated β1 integrin antibody 24 h after infecting cells with adenovirus expressing lacZ (B, MFI = 264 ± 0.55) or tac-β1 (E, MFI = 240 ± 1.94). To determine expression levels tac-β1 cells were initially incubated with IL-2α receptor antibody, which detects an epitope (residues 140–144 of human IL-2 receptor α) in the extracellular domain of tac-β1 protein. Surface expression of tac-β1D was measured by detecting secondary antibody conjugated to Alexa-488, which was absent in cells infected with adenovirus expressing lacZ (C, MFI = 10.6 ± 0.07), compared to tac-β1D chimera (F, MFI = 326 ± 6.05). Background fluorescence in NRVM expressing lacZ (A, MFI = 5.17 ± 0.03) and tac-β1D (D, MFI = 5.35 ± 0.03) were determined by incubating cells in the absence of antibody. The mean fluorescent intensity and standard error (MFI ± SEM) for each distribution were obtained from a sample of 10,000 cells.
Fig. 3. β1D integrin is required for stretch-induced phosphorylation of ERK1/2, p38, and JNK.
Prior to stretching, NRVM were infected with adenovirus (24 h) which resulted in expression of tac-β1D or control protein (lacZ). After 5 min of stretch (Str) or no stretch (N Str), phosphorylation levels of ERK, p38 and JNK were determined in the presence and absence of ATB. Bar graphs show fold changes in MAP kinase phosphorylation after tac-β1D expression compared to control virus. Values are means ± SEM. N=5 experiments.
3.3 Differential activation of FAK at Y397, Y861 and Y925 in response to mechanical stretch
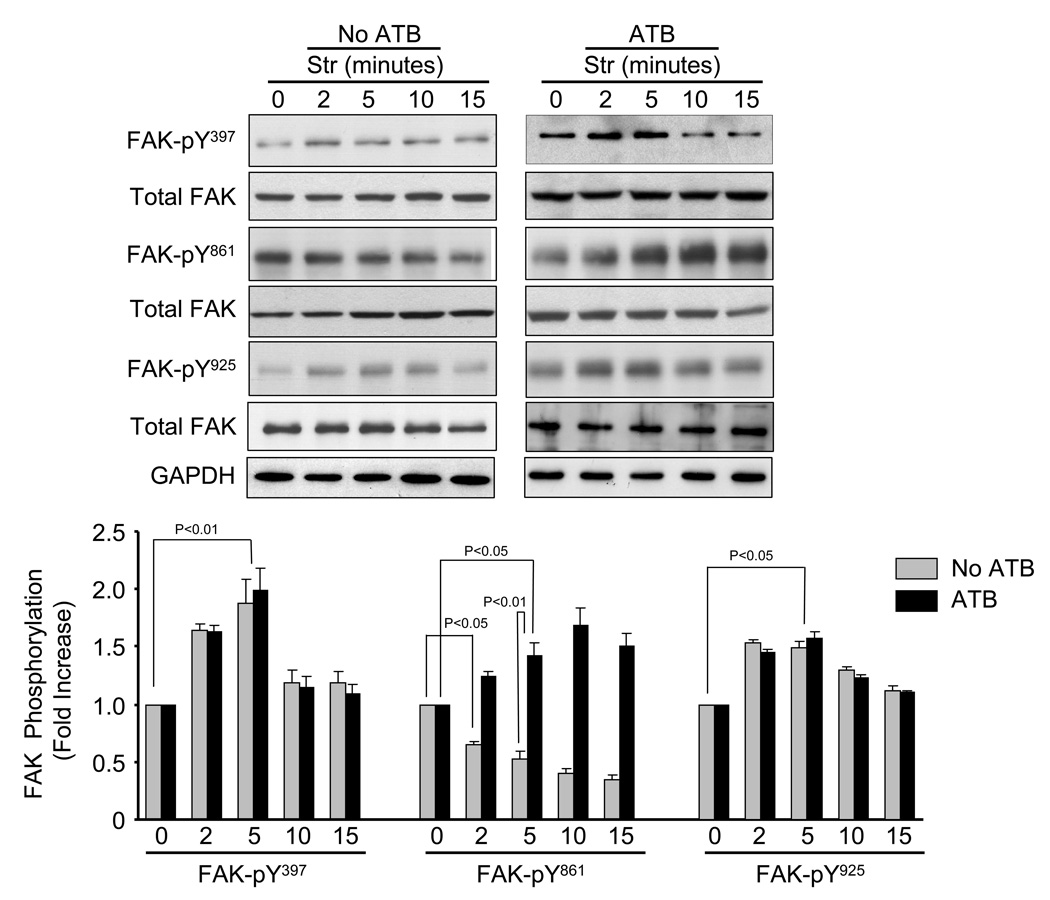
Mechanical stretch is known to activate FAK in cardiac myocytes [7,23,24]. Figure 4 shows the time-course (0 – 15 min) of stretch-induced FAK phosphorylation at Y397, Y861 and Y925 in presence and absence of ATB. In the presence of ATB, FAK phosphorylation at Y397, Y861 and Y925 were significantly increased within 2 min and maximal at 5 min (FAK-Y397, 98.5±14.1%, P<0.05; FAK-Y861, 42 ± 5.3%, P<0.05; FAK-Y925, 58 ± 6.2%, P<0.05) (Fig. 4). In the absence of ATB, stretch-induced phosphorylation of FAK-Y397 and Y925 followed the same patterns as with ATB. However, in the absence of ATB, FAK-Y861 phosphorylation was markedly decreased at all time points following mechanical stretch. These results indicate that mechanical stretch has differential effects on FAK-Y861 activation, which is dependent upon Ang II receptor activation.
Fig. 4. Time-course of stretch-induced FAK phosphorylation.
Representative blots showing time-course of stretch-induced FAK phosphorylation at Y397, Y861 and Y925. Bar graphs show fold changes in FAK phosphorylation in the absence and presence of Ang II receptor blocker (ATB) at various times of stretch (Str) compared to non-stretch (0 min). Values are means ± SEM. N=5 experiments.
3.4 Role of FAK in stretch-induced MAP kinase activation
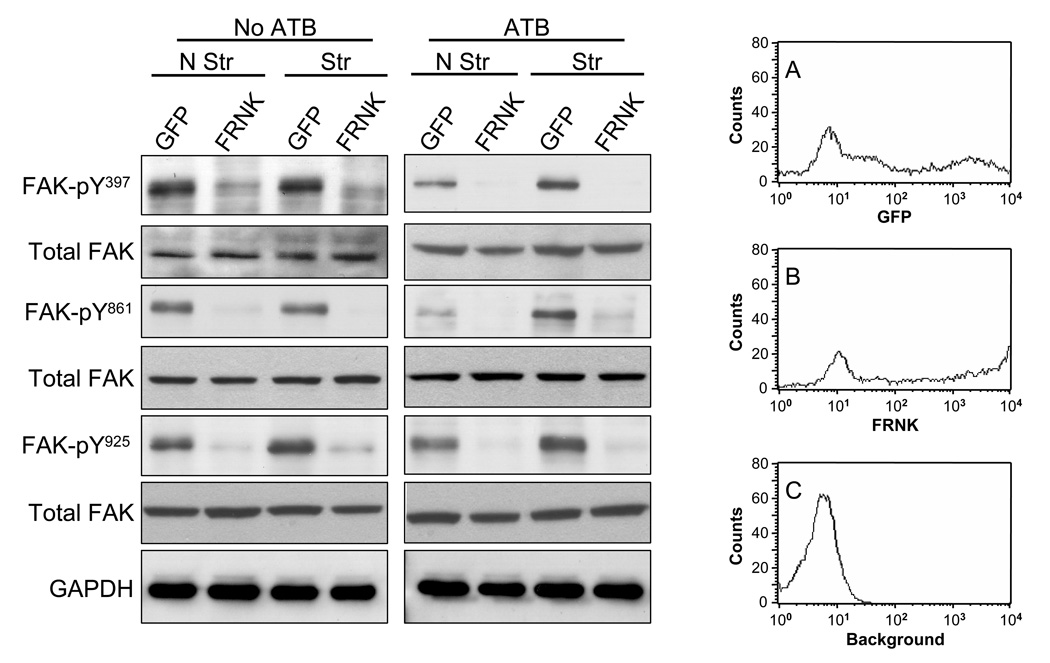
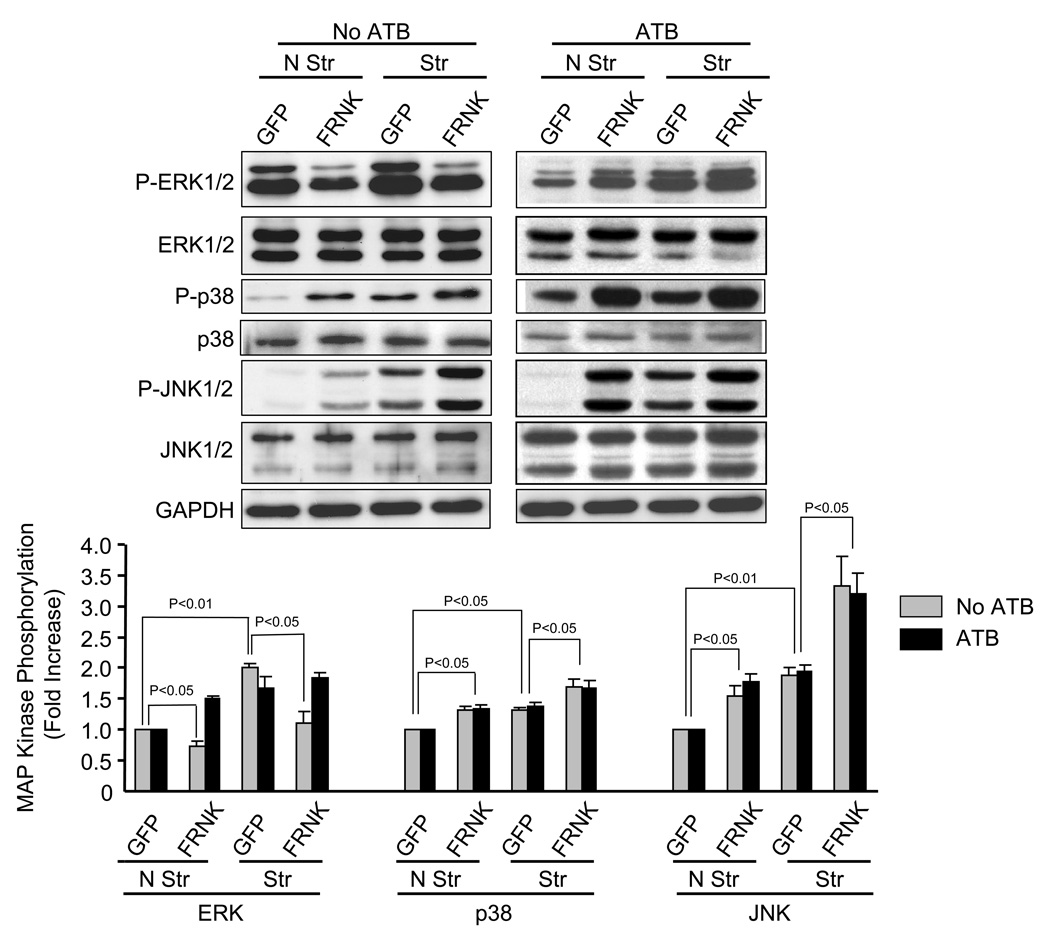
In noncardiac cells, both integrins and FAK have been shown to couple to ERK1/2 activation [32]. However, in cardiac cells MAP kinases involved in hypertrophic gene responses have been shown to be independent of FAK [33]. We therefore tested whether FAK activation is essential for stretch-induced MAP kinase activation by expressing the FRNK in NRVM. Because FRNK contains the site responsible for targeting intact FAK to focal contacts, it inhibits FAK by functioning as a dominant-negative [34–36]. Adenovirus-mediated overexpression of FRNK dramatically reduced FAK phosphorylation at Y397, Y861 and Y925 confirming that FRNK expression was sufficient to abolish basal and stretch-mediated FAK phosphorylation (Fig. 5). Expression of GFP and GFP-tagged FRNK in NRVM are shown in Fig. 5A (MFI = 3107 ± 753, n=4) and Fig. 5B (MFI = 4074 ± 391, N=4), respectively. In the absence of ATB, FRNK overexpression significantly decreased basal and stretch-induced ERK phosphorylation (Fig. 6), as previously described [37]. In contrast, FRNK overexpression increased basal and stretch-induced phosphorylation of p38 (31 ± 3.86%, P<0.05 and 30 ± 4.12%, P<0.05, respectively) and JNK (55 ± 6.27%, P<0.01 and 78 ± 11.23%, P<0.05, respectively). In the presence of ATB, overexpression of FRNK significantly increased basal phosphorylation of ERK (40.2 ± 8.6% p<0.05), p38 (39.5 ± 11.7%, P<0.05), JNK (86 ± 29.4%, P<0.05) and stretch-induced p38 (48.1 ± 8.7%, P<0.05) and JNK (85.0 ± 19.4%, P<0.05) phosphorylation.
Fig. 5. Effects of FRNK on stretch-induced FAK phosphorylation.
Representative blots showing effects of FRNK and GFP (control) expression on stretch-induced FAK phosphorylation at Y397, Y861 and Y925 in myocytes in the absence and presence of ATB. Blots are representative of 5 independent experiments. Histograms (A–C) show fluorescent levels in NRVM infected with adenovirus expressing GFP (A, MFI = 3107 ± 753, n=4), GFP-FRNK (B, MFI = 4074 ± 391, n=4), compared to no adenovirus treatment (background) (C, MFI = 5.54 ± 0.5). The mean fluorescent intensity and standard error (MFI ± SEM) for each distribution were obtained from a sample of 10,000 cells.
Fig. 6. Effect of FRNK expression on stretch-induced MAP kinase phosphorylation.
Prior to stretching, NRVM were infected with adenovirus (24 h) which resulted in expression of GFP (control) or FRNK. After 5 min of stretch (Str) or no stretch (N Str), phosphorylation levels of ERK, p38 and JNK were determined in the presence and absence of ATB. Bar graphs show fold changes in these MAP kinase phosphorylation after FRNK expression compared to control virus. Values are means ± SEM. N=5 experiments.
3.5 Regulation of stretch-mediated FAK Y397, Y861 and Y925 phosphorylation by β1- integrin
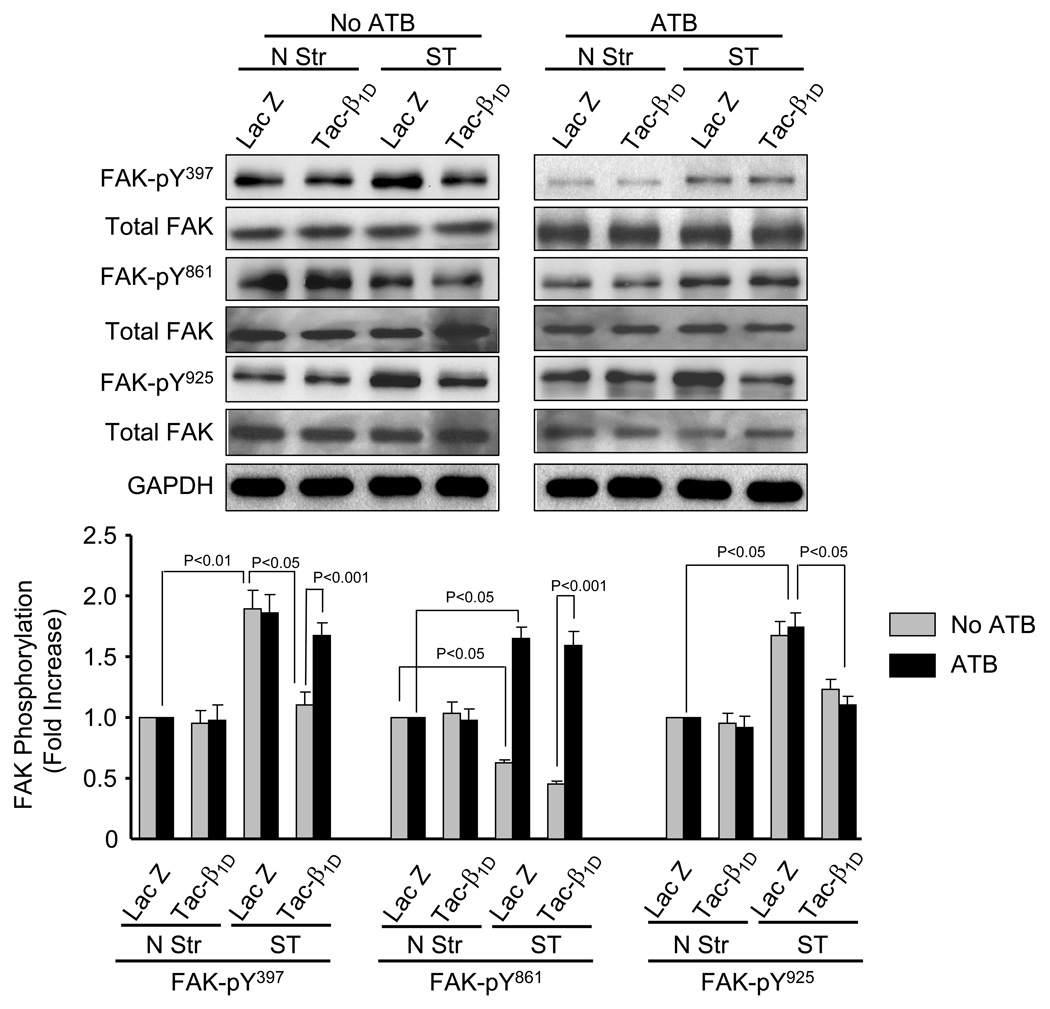
To test whether β1 integrin may couple to stretch-induced FAK activation, cell lysates from NRVM infected with lacZ and tac-β1 expressing virus were probed with phospho-specific antibody directed against FAK at Y397, Y861 and Y925. In the absence of ATB, expression of tac-β1 significantly attenuated stretch-mediated FAK phosphorylation at Y397 (41.5 ± 5.3%, P<0.05), Y861 (28.57 ± 2.9%, P<0.05) and Y925 (26.7 ± 3.6%, P<0.05). In the presence of ATB, tac-β1D reversed stretch-induced FAK phosphorylation at Y925, but not at Y397 and Y861.
4.0 Discussion
Cardiac hypertrophy is a common response to pressure overload caused by increased hemodynamic load and associated with increased mortality. This response is characterized by remodeling of the extracellular matrix and hypertrophic growth of cardiac myocytes. Mechanical stress induces activation of ERK, p38 and JNK [7], which have been associated with various aspects of hypertrophic growth in isolated cardiac myocytes and intact heart [9,38,39].Both β1 integrin and FAK are important for mechanosensing and have been implicated as key mediators of hypertrophic growth in cardiac myocytes [11,13,27,40]. The β1 integrin couples to various α-integrin subunits to form α/β receptor heterodimers which differ with regard to ligand binding and effects on cellular function. In the present study, NRVM were plated on collagen IV, which is selectively liganded by α1β1 integrin and upregulated in the pressure-overloaded myocardium [27]. Like many “embryonic genes” in the ventricle, the α1-integrin subunit is expressed in the embryonic heart, undergoes downregulation in the neonate and reinduced by mechanical loading in the adult heart through pressure-overload [41]. This suggests that both collagen IV and α1β1 integrin have roles in the mechanically overloaded myocardium.
To determine the role of β1 integrin in stretch-induced MAP kinase activation, signaling was blocked by infecting NRVM with adenovirus expressing a dominant-negative chimeric protein consisting of the cytoplasmic tail domain of β1D integrin (tac-β1D). Myocytes expressing tac-β1D showed a signficant reduction in stretch-induced activation of all three MAP kinases, indicating a central role for β1-integrin in the mechanotransduction process. One of the best known integrin-dependent pathways is centered on activation of the tyrosine kinase FAK, which recruits several structural and signaling components and leads to activation of MAP kinases. Another widely studied process, seemingly independent of FAK, is the activation of the ERK cascade through the transmembrane protein caveolin-1, the Src-family kinase Fyn and the adaptor protein Shc. To test which mechanism was operational in integrin-mediated activation of MAP kinases, the NRVM were infected with adenovirus expressing FRNK. It was intriguing to observe that in the presence of ATB, disruption of FAK signaling not only revealed that β1 integrin coupled to ERK, p38 and JNK independent of FAK, but also resulted in hyperactivation of all three MAP kinases under both stretch and non-stretch conditions in presence of ATB. A role for FAK in upstream activation of ERK has been previously demonstrated [37]. Ang II receptors appear to play an important role in coupling FAK to stretch-induced ERK activation. In the absence of ATB, overexpression of FRNK significantly reduced basal and stretch-induced ERK activation. The coupling of β1 integrin to MAP kinase activation, independent of FAK activation, is poorly understood. In keratinocytes, Shc has been shown to be necessary and sufficient for integrin-mediated ERK activation [34,42,43]. Dominant-negative inhibition of Shc activity suppressed ERK signaling, whereas overexpression of three different dominant negative forms of FAK had no effect on ERK signaling. In addition, deletion of the cytoplasmic domain of the β1 integrin prevented FAK activation without affecting ERK signaling. Caveolin appears to play an important role in FAK-independent ERK activation. A chimera containing the transmembrane domain of the α1 integrin subunit binds with caveolin-1 and causes recruitment of Shc and ERK activation without inducing FAK activation [43]. Although cardiac myocytes only express muscle-specific caveolin-3, it appears to function similar to caveolin-1 and has a major role in cardiac hypertrophy and regulation of the ERK pathway [44]. Thus, in cardiac myocytes, β1 integrin could bypass FAK by signaling to ERK through caveolin-3 and Shc. However, the potential role of Shc and caveolins in β1 integrin-mediated activation of ERK, as well as p38 and JNK, remains to be examined in cardiac myocytes. Although several studies have examined the role of FAK in mediating MAP kinase activation in non-cardiac cells, only a few have been conducted in cardiac tissue following mechanical [22, 45] or humoral stimulation [46]. In addition, these have been limited to the effects of FAK on ERK activation. To our knowledge, the present study is the first to examine the role of FAK on activation of ERK, p38 and JNK. A significant finding was the demonstration that FAK becomes a negative effector of ERK activation in the presence of Ang II receptor antagonism.
Because of the differential effects of FAK on ERK kinase activation observed in the presence of ATB, we tested whether blockade of β1 integrin and Ang II receptors affect stretch-induced FAK phosphorylation at Y397, Y861 and Y925. Our results (Fig. 7) indicate that a different mechanism is responsible for stretch-induced FAK phorphorylation for each of the sites. Stretch-induced FAK-Y925 was abolished by tac-β1D, but unaffected by ATB, suggesting that β1 integrin is the primary upstream activator. Although treatment of NRVM with tac-β1D also attenuated stretch-induced activation of FAK-Y397, complete blockade was only observed in the presence of ATB. This suggests that stretch-induced activation of FAK-Y397 requires cross-talk between β1 integrin and Ang II receptors. Stretch-induced activation of FAK-Y861 was unaffected by tac-β1D expression and enhanced by ATB. This suggests that Ang II receptor signaling negatively couples to FAK-Y861 phosphorylation and that mechanotransduction systems, other than β1D is responsible for its activation by stretch in NRVM. Although FAK-Y861 has been shown couple to JNK activation in NIH 3T3 cells [47], results in the present study suggest that JNK is activated by a FAK-Y861 independent mechanism in stretched myocytes. Stretch-induced activation of JNK was significantly attenuated by ATB (Fig. 1) and completely blocked by tac-β1D integrin expression (Fig. 3). However, it is possible that activated FAK-Y861 couples to other types of cellular function in stretched myocytes. A role for FAK-Y397 in stretch-induced ERK1/2 activation has been demonstrated in isolated perfused hearts [22] and implicated in isolated cardiac myocytes [45]. Likewise, in the absence of ATB, stretch-induced ERK activation in NRVM was blocked by overexpression of FRNK in our NRVM. This suggest that FAK or a closely related FAK family member may be involved. In osteoblasts, mechanical stretch-induced activation of ERK2-Y187 has been shown to require activation of FAK397 and praline-rich tyrosine kinase-2 (Pyk2), a tyrosine kinase highly homologous to FAK [48]. Pyk2 may regulate stretch-induced MAP kinase phosphorylation in NRVM, as basal and stretch-induced Pyk2-Y402 phosphorylation were markedly reduced in NRVM over expressing FRNK (data not shown).
Fig. 7. Stretch-induced FAK phosphorylation is differentially regulated by β1D integrin and Ang II receptor activation.
Prior to stretching, NRVM were infected with adenovirus (24 h) which resulted in expression of tac-β1D or control protein (lacZ). After 5 min of stretch (Str) or no stretch (N Str), phosphorylation levels of FAK-Y397, FAK-Y861 and FAK-Y925 were determined in the presence and absence of ATB. Bar graphs show fold changes in FAK phosphorylation compared to control virus. Values are means β1D SEM. N=4 experiments.
The upstream effectors responsible for mediating stretch-induced phosphorylation of FAK activation sites, remain to be identified. Although the AT1 receptor was blocked in experiments, mechanical stretch may have stimulated the release of another humoral factor, such as endothelin-1 [49] and VEGF [50], which are known to rapidly activate FAK in NRVM [33]. Also, mechanosensing systems, such as stretch-activated channels, cytoskeletal elements could activate FAK [51].
In summary, we have demonstrated that β1 integrin plays a critical role in mediating stretch-induced MAP kinase activation in NRVM. However, Ang II receptor signaling was found to affect phosphorylation of both MAP kinase and FAK. In the presence of AT1 and AT2 blockade, stretch-induced activation of ERK, p38 and JNK in NRVM was mediated by β1 integrin, through a FAK independent mechanism. Also, cells expressing FRNK demonstrated increased phosphorylation of ERK, p38 and JNK phosphorylation in the presence of ATB. To our knowledge, this is the first cell-based study to show that FAK or a closely related kinase (e.g. Pyk2) [35], as a negative regulator of MAP kinases. However, in the absence of ATB, expression of FRNK inhibited ERK, but increased p38 and JNK phosphorylation. Although the significance of this differential response remains to be determined, FRNK induced apoptosis in NRVM [35] could be related to activation of p38 and/or JNK. Results also revealed that stretch-induced activation of FAK at Y397, Y861 and Y925 occurs by a combination of β1 integrin and Ang II receptor dependent and independent mechanisms. Nonetheless, these results suggest that both β1 integrin and Ang II receptors play important and coordinate roles in mediating stretch-induced activation of MAP kinases in cardiac myocytes. To our knowledge, this is the first report to show that FAK or a related kinase inhibits MAP kinase activation in an isolated myocyte system. The possible role of FAK activation, with regard to MAP kinase activation, other downstream targets and myocardial growth remains to be determined under in vivo conditions. A potential role of FAK in mediating cardiac protection has demonstrated in a recent study which showed that persistent challenge of mice with myocyte-restricted FAK inactivation led to enhanced cardiac fibrosis and cardiac dysfunction, in comparison to challenged genetic controls [40]. However, future studies will be required to better understand the complex and interactive roles of β1D integrin, Ang II receptors and FAK in mediating the signaling pathways responsible for load-induced cardiac hypertrophy.
5.0 Acknowledgements
This work was supported by a grant from the National Institutes of Health (R01-HL-68838) and Scott and White Hospital.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6.0 References
- 1.Kudoh S, Komuro I, Hiroi Y, Zou Y, Harada K, Sugaya T, et al. Mechanical stretch induces hypertrophic responses in cardiac myocytes of angiotensin II type 1a receptor knockout mice. J Biol Chem. 1998;273:24037–24043. doi: 10.1074/jbc.273.37.24037. [DOI] [PubMed] [Google Scholar]
- 2.Lammerding J, Kamm RD, Lee RT. Mechanotransduction in cardiac myocytes. Ann N Y Acad Sci. 2004;1015:53–70. doi: 10.1196/annals.1302.005. [DOI] [PubMed] [Google Scholar]
- 3.Sadoshima J, Izumo S. The cellular and molecular response of cardiac myocytes to mechanical stress. Annu Rev Physiol. 1997;59:551–571. doi: 10.1146/annurev.physiol.59.1.551. [DOI] [PubMed] [Google Scholar]
- 4.Sadoshima J, Izumo S. Mechanical stretch rapidly activates multiple signal transduction pathways in cardiac myocytes: potential involvement of an autocrine/paracrine mechanism. Embo J. 1993;12:1681–1692. doi: 10.1002/j.1460-2075.1993.tb05813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yazaki Y, Komuro I. Role of protein kinase system in the signal transduction of stretch-mediated myocyte growth. Basic Res Cardiol. 1992;87 Suppl 2:11–18. doi: 10.1007/978-3-642-72477-0_2. [DOI] [PubMed] [Google Scholar]
- 6.Laser M, Kasi VS, Hamawaki M, Cooper Gt, Kerr CM, Kuppuswamy D. Differential activation of p70 and p85 S6 kinase isoforms during cardiac hypertrophy in the adult mammal. J Biol Chem. 1998;273:24610–24619. doi: 10.1074/jbc.273.38.24610. [DOI] [PubMed] [Google Scholar]
- 7.Aikawa R, Nagai T, Kudoh S, Zou Y, Tanaka M, Tamura M, et al. Integrins play a critical role in mechanical stress-induced p38-MAPK activation. Hypertension. 2002;39:233–238. doi: 10.1161/hy0202.102699. [DOI] [PubMed] [Google Scholar]
- 8.Komuro I, Kudo S, Yamazaki Y, Zou Y, Shiojima I, Yazaki Y. Mechanical stretch activates the stress-activated protein kinases in cardiac myocytes. Faseb J. 1996;10:631–636. doi: 10.1096/fasebj.10.5.8621062. [DOI] [PubMed] [Google Scholar]
- 9.Ruwhof C, van der Laarse A. Mechanical stress-induced cardiac hypertrophy: mechanisms and signal transduction pathways. Cardiovasc Res. 2000;47:23–37. doi: 10.1016/s0008-6363(00)00076-6. [DOI] [PubMed] [Google Scholar]
- 10.Knoll R, Hoshijima M, Chien K. Cardiac mechanotransduction and implications for heart disease. J Mol Med. 2003;81:750–756. doi: 10.1007/s00109-003-0488-x. [DOI] [PubMed] [Google Scholar]
- 11.Wernig F, Mayr M, Xu Q. Mechanical stretch-induced apoptosis in smooth muscle cells is mediated by beta1-integrin signaling pathways. Hypertension. 2003;41:903–911. doi: 10.1161/01.HYP.0000062882.42265.88. [DOI] [PubMed] [Google Scholar]
- 12.Brancaccio M, Hirsch E, Notte A, Selvetella G, Lembo G, Tarone G. Integrin signalling: the tug-of-war in heart hypertrophy. Cardiovasc Res. 2006;70:422–433. doi: 10.1016/j.cardiores.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 13.Ross RS, Pham C, Shai SY, Goldhaber JI, Fenczik C, Glembotski CC, et al. Beta1 integrins participate in the hypertrophic response of rat ventricular myocytes. Circ Res. 1998;82:1160–1172. doi: 10.1161/01.res.82.11.1160. [DOI] [PubMed] [Google Scholar]
- 14.Shai SY, Harpf AE, Babbitt CJ, Jordan MC, Fishbein MC, Chen J, et al. Cardiac myocyte-specific excision of the beta1 integrin gene results in myocardial fibrosis and cardiac failure. Circ Res. 2002;90:458–464. doi: 10.1161/hh0402.105790. [DOI] [PubMed] [Google Scholar]
- 15.Jai N, Okamoto H, Shimizu T, Chiba S, Matsui Y, Sugawara T, et al. A newly developed angiotensin II type 1 receptor antagonist, CS866, promotes regression of cardiac hypertrophy by reducing integrin beta1 expression. Hypertens Res. 2003;26:737–742. doi: 10.1291/hypres.26.737. [DOI] [PubMed] [Google Scholar]
- 16.Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, et al. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat Cell Biol. 2004;6:499–506. doi: 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]
- 17.Hanks SK, Calalb MB, Harper MC, Patel SK. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci USA. 1992;89:8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang HB, Dembo M, Hanks SK, Wang Y. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc Natl Acad Sci USA. 2001;98:11295–11300. doi: 10.1073/pnas.201201198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fonseca PM, Inoue RY, Kobarg CB, Crosara-Alberto DP, Kobarg J, Franchini KG. Targeting to C-terminal myosin heavy chain may explain mechanotransduction involving focal adhesion kinase in cardiac myocytes. Circ Res. 2005;96:73–81. doi: 10.1161/01.RES.0000152390.99806.A5. [DOI] [PubMed] [Google Scholar]
- 20.Franchini KG, Torsoni AS, Soares PH, Saad MJ. Early activation of the multicomponent signaling complex associated with focal adhesion kinase induced by pressure overload in the rat heart. Circ Res. 2000;87:558–565. doi: 10.1161/01.res.87.7.558. [DOI] [PubMed] [Google Scholar]
- 21.Laser M, Willey CD, Jiang W, Cooper Gt, Menick DR, Zile MR, et al. Integrin activation and focal complex formation in cardiac hypertrophy. J Biol Chem. 2000;275:35624–35630. doi: 10.1074/jbc.M006124200. [DOI] [PubMed] [Google Scholar]
- 22.Domingos PP, Fonseca PM, Nadruz W, Jr., Franchini KG. Load-induced focal adhesion kinase activation in the myocardium: role of stretch and contractile activity. Am J Physiol Heart Circ Physiol. 2002;282:H556–H564. doi: 10.1152/ajpheart.00534.2001. [DOI] [PubMed] [Google Scholar]
- 23.Seko Y, Takahashi N, Tobe K, Kadowaki T, Yazaki Y. Pulsatile stretch activates mitogen-activated protein kinase (MAPK) family members and focal adhesion kinase (p125(FAK)) in cultured rat cardiac myocytes. Biochem Biophys Res Commun. 1999;259:8–14. doi: 10.1006/bbrc.1999.0720. [DOI] [PubMed] [Google Scholar]
- 24.Torsoni AS, Constancio SS, Nadruz W, Jr., Hanks SK, Franchini KG. Focal adhesion kinase is activated and mediates the early hypertrophic response to stretch in cardiac myocytes. Circ Res. 2003;93:140–147. doi: 10.1161/01.RES.0000081595.25297.1B. [DOI] [PubMed] [Google Scholar]
- 25.Furuta Y, Ilic D, Kanazawa S, Takeda N, Yamamoto T, Aizawa S. Mesodermal defect in late phase of gastrulation by a targeted mutation of focal adhesion kinase, FAK. Oncogene. 1995;11:1989–1995. [PubMed] [Google Scholar]
- 26.Ilic D, Furuta Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, et al. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 27.Peng X, Kraus MS, Wei H, Shen TL, Pariaut R, Alcaraz A, et al. Inactivation of focal adhesion kinase in cardiomyocytes promotes eccentric cardiac hypertrophy and fibrosis in mice. J Clin Invest. 2006;116:217–227. doi: 10.1172/JCI24497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pham CG, Harpf AE, Keller RS, Vu HT, Shai SY, Loftus JC, Ross RS. Striated muscle-specific beta(1D)-integrin and FAK are involved in cardiac myocyte hypertrophic response pathway. Am J Physiol Heart Circ Physiol. 2000;279:H2916–H2926. doi: 10.1152/ajpheart.2000.279.6.H2916. [DOI] [PubMed] [Google Scholar]
- 29.Fukuzawa J, Booz GW, Hunt RA, Shimizu N, Karoor V, Baker KM, Dostal DE. Cardiotrophin-1 increases angiotensinogen mRNA in rat cardiac myocytes through STAT3: an autocrine loop for hypertrophy. Hypertension. 2000;35:1191–1196. doi: 10.1161/01.hyp.35.6.1191. [DOI] [PubMed] [Google Scholar]
- 30.Dostal DE, Booz GW, Baker KM. Regulation of angiotensinogen gene expression and protein in neonatal rat cardiac fibroblasts by glucocorticoid and beta-adrenergic stimulation. Basic Res Cardiol. 2000;95:485–490. doi: 10.1007/s003950070025. [DOI] [PubMed] [Google Scholar]
- 31.Yano M, Kim S, Izumi Y, Yamanaka S, Iwao H. Differential activation of cardiac c-jun amino-terminal kinase and extracellular signal-regulated kinase in angiotensin II-mediated hypertension. Circ Res. 1998;83:752–760. doi: 10.1161/01.res.83.7.752. [DOI] [PubMed] [Google Scholar]
- 32.Schlaepfer DD, Broome MA, Hunter T. Fibronectin-stimulated signaling from a focal adhesion kinase-c-Src complex: involvement of the Grb2, p130cas, and Nck adaptor proteins. Mol Cell Biol. 1997;17:1702–1713. doi: 10.1128/mcb.17.3.1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eble DM, Strait JB, Govindarajan G, Lou J, Byron KL, Samarel AM. Endothelin-induced cardiac myocyte hypertrophy: role for focal adhesion kinase. Am J Physiol Heart Circ Physiol. 2000;278:H1695–H1707. doi: 10.1152/ajpheart.2000.278.5.H1695. [DOI] [PubMed] [Google Scholar]
- 34.Lin TH, Aplin AE, Shen Y, Chen Q, Schaller M, Romer L, et al. Integrin-mediated activation of MAP kinase is independent of FAK: evidence for dual integrin signaling pathways in fibroblasts. J Cell Biol. 1997;136:1385–1395. doi: 10.1083/jcb.136.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heidkamp MC, Bayer AL, Kalina JA, Eble DM, Samarel AM. GFP-FRNK disrupts focal adhesions and induces anoikis in neonatal rat ventricular myocytes. Circ Res. 2002;90:1282–1289. doi: 10.1161/01.res.0000023201.41774.ea. [DOI] [PubMed] [Google Scholar]
- 36.Sundberg LJ, Galante LM, Bill HM, Mack CP, Taylor JM. An endogenous inhibitor of focal adhesion kinase blocks Rac1/JNK but not Ras/ERK-dependent signaling in vascular smooth muscle cells. J Biol Chem. 2003;278:29783–29791. doi: 10.1074/jbc.M303771200. [DOI] [PubMed] [Google Scholar]
- 37.Sawhney RS, Cookson MM, Omar Y, Hauser J, Brattain MG. Integrin alpha2-mediated ERK and calpain activation play a critical role in cell adhesion and motility via focal adhesion kinase signaling: identification of a novel signaling pathway. J Biol Chem. 2006;281:8497–8510. doi: 10.1074/jbc.M600787200. [DOI] [PubMed] [Google Scholar]
- 38.Ueyama T, Kawashima S, Sakoda T, Rikitake Y, Ishida T, Kawai M, et al. Requirement of activation of the extracellular signal-regulated kinase cascade in myocardial cell hypertrophy. J Mol Cell Cardiol. 2000;32:947–960. doi: 10.1006/jmcc.2000.1135. [DOI] [PubMed] [Google Scholar]
- 39.Clerk A, Sugden PH. Activation of protein kinase cascades in the heart by hypertrophic G protein-coupled receptor agonists. Am J Cardiol. 1993;83:64H–69H. doi: 10.1016/s0002-9149(99)00261-1. [DOI] [PubMed] [Google Scholar]
- 40.DiMichele LA, Doherty JT, Rojas M, Beggs HE, Reichardt LF, Mack CP, Taylor JM. Myocyte-restricted focal adhesion kinase deletion attenuates pressure overload-induced hypertrophy. Circ Res. 2006;99:636–645. doi: 10.1161/01.RES.0000240498.44752.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ross RS. The extracellular connections: the role of integrins in myocardial remodeling. J Card Fail. 2002;8:S326–S331. doi: 10.1054/jcaf.2002.129263. [DOI] [PubMed] [Google Scholar]
- 42.Wary KK, Mainiero F, Isakoff SJ, Marcantonio EE, Giancotti FG. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–743. doi: 10.1016/s0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- 43.Wary KK, Mariotti A, Zurzolo C, Giancotti FG. A requirement for caveolin-1 and associated kinase Fyn in integrin signaling and anchorage-dependent cell growth. Cell. 1998;94:625–634. doi: 10.1016/s0092-8674(00)81604-9. [DOI] [PubMed] [Google Scholar]
- 44.Woodman SE, Park DS, Cohen AW, Cheung MW, Chandra M, Shirani J, et al. Caveolin-3 knock-out mice develop a progressive cardiomyopathy and show hyperactivation of the p42/44 MAPK cascade. J Biol Chem. 2002;277:38988–38997. doi: 10.1074/jbc.M205511200. [DOI] [PubMed] [Google Scholar]
- 45.Torsoni AS, Marin TM, Velloso LA, Franchini KG. RhoA/ROCK signaling is critical to FAK activation by cyclic stretch in cardiac myocytes. Am J Physiol Heart Circ Physiol. 2005;289(4):H1488–H1496. doi: 10.1152/ajpheart.00692.2004. [DOI] [PubMed] [Google Scholar]
- 46.Qin J, Liu ZX. FAK-related nonkinase attenuates hypertrophy induced by angiotensin-II in cultured neonatal rat cardiac myocytes. Acta Pharmacol Sin. 2006;27(9):1159–1164. doi: 10.1111/j.1745-7254.2006.00370.x. [DOI] [PubMed] [Google Scholar]
- 47.Lim Y, Han I, Jeon J, Park H, Bahk YY, Oh ES. Phosphorylation of focal adhesion kinase at tyrosine 861 is crucial for Ras transformation of fibroblasts. J Biol Chem. 2004;279(28):29060–29065. doi: 10.1074/jbc.M401183200. [DOI] [PubMed] [Google Scholar]
- 48.Boutahar N, Guignandon A, Vico L, Lafage-Proust MH. Mechanical strain on osteoblasts activates autophosphorylation of focal adhesion kinase and proline-rich tyrosine kinase 2 tyrosine sites involved in ERK activation. J Biol Chem. 2004;279(29):30588–30599. doi: 10.1074/jbc.M313244200. [DOI] [PubMed] [Google Scholar]
- 49.Yamazaki T, Komuro I, Kudoh S, Zou Y, Shiojima I, Hiroi Y, Mizuno T, et al. Endothelin-1 is involved in mechanical stress-induced cardiomyocyte hypertrophy. J Biol Chem. 1996;271:3221–3228. doi: 10.1074/jbc.271.6.3221. [DOI] [PubMed] [Google Scholar]
- 50.Yamada K, Green KG, Samarel AM, Saffitz JE. distinct pathways regulate expression of cardiac electrical and mechanical junction proteins in response to stretch. Circ Res. 2005;97(4):346–353. doi: 10.1161/01.RES.0000178788.76568.8a. [DOI] [PubMed] [Google Scholar]
- 51.Lal H, Guleria RS, Foster DM, Lu G, Watson LE, Sanghi S, et al. Intergins: novel therapeutic targets for cardiovascular diseases. Cardiovasc Hematol Agents Med Chem. 2007;5:109–132. doi: 10.2174/187152507780363223. [DOI] [PubMed] [Google Scholar]