Abstract
The pre-clinical global ischemia model transient bilateral common carotid artery occlusion addresses the unique cascade of events leading to delayed neuronal cell death. However, the inconsistent occurrence of posterior communicating arteries (PcomA) in mice might cause high outcome variability. To determine a means for reducing variability, CD1 mice were subjected to bilateral common carotid artery occlusion for 12 to 40 min. Occlusion duration ≥18 min was applied to mice with bilateral regional cerebral blood flow (rCBF) ≥10% of baseline at 2.5 min of ischemia. However, only groups with ischemic duration ≤18 min were used for statistical analysis because of the high mortality in the other groups. After 7 days, patency of PcomA and hippocampal neuronal loss in the CA1 subfield were evaluated. Outcome variability was reduced when hemispheres containing PcomA were excluded from analysis; ischemic outcome was not affected by the presence of a contralateral PcomA. Extending ischemic duration based on rCBF did not reduce outcome variability because the initial rCBF could not reliably predict PcomA. Therefore, after an optimal ischemic duration, evaluating hippocampal injury in each hemisphere independently according to the existence of PcomA is an effective and reliable method to obtain consistent results in this pre-clinical mouse model.
Keywords: Cerebral blood flow, Global ischemia, Hippocampus, Posterior communicating artery
1. Introduction
Clinical and experimental evidence suggest a causal relationship between hippocampal damage and the development of cognitive deficits and dementia (Volpe and Petito, 1985). Transient global ischemia causes selective vulnerability and delayed neuronal cell death in several regions of the brain. Among them, the CA1 subfield of the hippocampus has attracted interest for the investigation of delayed neuronal death because it was reported to be the region most vulnerable to ischemic insult (Kirino, 1982; Pulsinelli et al., 1982; Smith et al., 1984). Numerous factors consequently participate in the process of delayed cell death (Kreisman et al., 2000), but the exact mechanism of this selected vulnerability is still unclear. Therefore, optimized, reproducible, animal models are vital for being able to study genes and signaling pathways that may be involved in this cascade as well as for testing potential pharmaceutical interventions (Goto et al., 2003; Shah et al., 2006).
Several animal models have been developed to study the mechanism of delayed hippocampal neuronal injury. Techniques such as cardiocirculatory arrest (Bottiger et al., 1997), 3-vessel occlusion (Kameyama et al., 1985), 4-vessel occlusion (Pulsinelli and Brierley, 1979), and 2-vessel occlusion with systemic hypotension (Eklof and Siesjo, 1972; Nordstrom and Rehncrona, 1977) have been tried in mice after being established in larger animals (Bottiger et al., 1999; Sheng et al., 1999; Yonekura et al., 2004). Mice are preferable to use because they are more economical and relatively more homogeneous within strains than larger animals. In addition, genetically modified mice are reliable tools for studying particular gene functions. However, the relative complexity of the surgical process in mice made these techniques challenging and unpopular. Consequently, the simpler method of bilateral common carotid artery occlusion is now more frequently used as a mouse model of global cerebral ischemia.
A drawback of the bilateral common carotid artery occlusion model is the high variability in ischemic outcome when used in mice. One possible reason for this variability is the application of a universal occlusion time in strains of mice with different genetic backgrounds. It is now apparent that the sensitivities to ischemic insult between different mouse strains is a problematic issue (Sheldon et al., 1998; Wellons et al., 2000) that necessitates optimizing ischemic duration for each mouse strain. In addition, we and others have noted variability stemming from individual differences in collateral flow through the circle of Willis, even within one strain (Fujii et al., 1997; Kitagawa et al., 1998; Sheng et al., 1999; Beckmann, 2000; Wellons et al., 2000). Therefore, knowing the patency of PcomA of an individual mouse before conducting surgery would be ideal for optimizing this model.
Some investigators have suggested that residual cerebral blood flow (rCBF) after the initiation of ischemia could be used as a predictor of the presence of PcomA (Kitagawa et al., 1998; Yonekura et al., 2004). It was also reported that mice undergoing transient global ischemia by 3-vessel occlusion in which the effect of collateral blood flow had been eliminated by successful basilar artery occlusion would have a rCBF <10% of baseline at 2.5 min after ischemia was initiated (Yonekura et al., 2004). To test these hypotheses, here we measured rCBF 2.5 min after bilateral common carotid arterial occlusion and then checked the intracranial vascular structure after the mice were sacrificed to evaluate whether a correlation existed. We then investigated the feasibility of adjusting the occlusion duration based on the changes in rCBF.
The main goals of this study were to: 1) determine the optimal ischemic duration in CD1 mice lacking PcomA in either brain hemisphere; 2) in mice with unilateral PcomA, determine whether collateral flow in the hemisphere containing the PcomA affects the ischemic outcome of the contralateral hemisphere, and test the feasibility of minimizing variability by evaluating only the hemispheres lacking PcomA; 3) determine whether a stable outcome in mice with bilateral PcomA can be accomplished by extending ischemic duration in mice with a rCBF ≥10% of baseline at 2.5 min after ischemia. During the optimization process, we integrated the monitoring of mean blood pressure, CBF, body temperature, and blood gas analysis. Each hemisphere was studied independently to compare the differences in rCBF and neuronal loss and determine potential effects of PcomA.
2. Materials and Methods
2.1. Animals
All animal protocols were approved by the Animal Care and Use Committee of Johns Hopkins University, in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals. Adult male CD1 mice (5-6 weeks old; Charles River Laboratories, Wilmington, MA) were maintained in a 12-h light/dark cycle, with food and water ad libitum. One week before surgery, each mouse was anesthetized by 2% isoflurane, and a temperature transponder IPTT-300 was implanted into its abdomen with a needle unit (Bio Medic Data Systems, Seaford, DE).
2.2. Global ischemia
Weights and core temperatures of the mice were recorded before the surgical procedure, and then anesthesia was induced with isoflurane (5.0% induction, 2% maintenance). After the trachea was intubated with a 20-G i.v. catheter, the lungs were mechanically ventilated. A temporal subcutaneous needle thermistor was placed adjacent to the skull and another thermistor was placed in the rectum. Pericranial and core temperatures were strictly controlled between 36.5 and 37.5°C by a heating pad and an overhead incandescent lamp. The right femoral artery was cannulated via surgical incision with PE 10 polyethylene tubing (Becton Dickinson Primary Care Diagnostics, Sparks, MD). Mean blood pressure was monitored by a transducer (Argon Medical Devices, Athens, TX) connected to the femoral artery catheter and coupled with an acquisition system (Gould Instruments, Valley View, OH) for computerized recording. Blood was drawn for gas analysis 10 min before and 15 min after the ischemic period. Cerebral cortical microperfusion was measured continuously from 10 min before to 15 min after ischemia by placing the probes of LDPM UNIT-PF 5010, (Perimed AB, Stockholm, Sweden) on the skull 3 mm lateral to the bregma.
Via ventral incision of the neck, both common carotid arteries were carefully dissected away from the vagus and encircled with 6-0 suture to enable later occlusion with aneurysm clips. After recording the baseline rCBF for 10 min, global ischemia was induced by occluding the common carotid arteries with aneurysm clips for 12, 15, 18, 20, 30, or 40 min. The number of mice in each surgical group was 9, 27, 13, 5, 4, and 4, respectively. Only those mice with bilateral rCBF ≥10% of baseline during the first 2.5 min of ischemia underwent ischemia for ≥18 min. After ischemia, the clips were removed to initiate reperfusion, and plasticity of arteries was confirmed by visual inspection. A group of 8 sham-operated mice underwent all of the procedures, except that their arteries were not occluded. After 15 min reperfusion, the incisions were sutured, and isoflurane was discontinued. The trachea was extubated upon recovery of the righting reflex. Before being returned to their cages at room temperature, the mice were placed in a warm chamber (33°C) for 48 h to maintain the body temperature at approximately 37°C and eliminate the protective effect of hypothermia (Jia et al., 2006; Ohtaki et al., 2006). Core temperatures were recorded after 30 min and then every day until the mice were sacrificed 7 days later. This post-ischemic recovery period was chosen to ensure maturation of delayed neuronal death because it was previously shown in gerbils that 7 days was required for protein synthesis to return to normal levels (Sorimachi et al., 1999; Sorimachi et al., 2002).
2.3. Assessment of vascular structure and injury of hippocampus
After a 7-day recovery period, the mice were deeply anesthetized by intraperitoneal injection with phentobarbiturate solution (30 mg/kg) and perfused transcardially with 0.9% saline. Then, mice in the sham-operated group, and the 12- and 20-min ischemic groups were perfused with 4% paraformaldehyde, whereas mice in the 15- and 18-min ischemic groups were injected with 0.8-mL latex in the ascending aorta. The brains were then removed and stored in 4% paraformaldehyde at 4°C. After 24 h, the intracranial vasculature of the latex-infused brains was evaluated under a dissecting microscope and recorded by digital camera. For statistical analysis, we classified the mice into two groups: mice having no anastomosis or capillary anastomosis were classified as PcomA(-), and mice having a truncal PcomA were classified as PcomA(+) (Murakami et al., 1998). Finally, all of the brains were paraffin-embedded, sliced coronally into 10-μm sections, and stained with Hematoxylin and Eosin. Latex perfusion appeared to have no effect on later Hematoxylin and Eosin staining. Injury to the CA1 subfield in the representative section of the dorsal hippocampus (1.68±0.10 mm posterior of the bregma) was evaluated under a light microscope at 100× magnification by manually counting the viable neurons. Viable neurons were defined as those with a blue hue, an intact plasma membrane, and a visible nucleus. The neuron density was expressed as neuron number/observed field (100× oil objective lens).
2.4. Exclusion Criteria, Mortality, and Statistical analysis
Based on the existence of the PcomA, there were 0 to 2 hemispheres per mouse that could be included in statistical analysis. Accordingly, the n value of each group refers to the number of hemispheres and is identified in the Results section. The mortality of the sham, 12-min ischemic, 15-min ischemic, 18-min ischemic, 20-min ischemic, and ≥30 min ischemic groups was 0, 11, 25, 38, 80, and 100%, respectively. Because the mortality of the mice that underwent ischemia for ≥20 min was very high, these groups were not included in statistical analysis. Data are expressed as mean±S.E.M. Statistical analysis was performed by one-way ANOVA followed by Bonferroni post hoc test. Significance was set at p<0.05.
3. Results
3.1. Optimal ischemic duration in CD1 mice
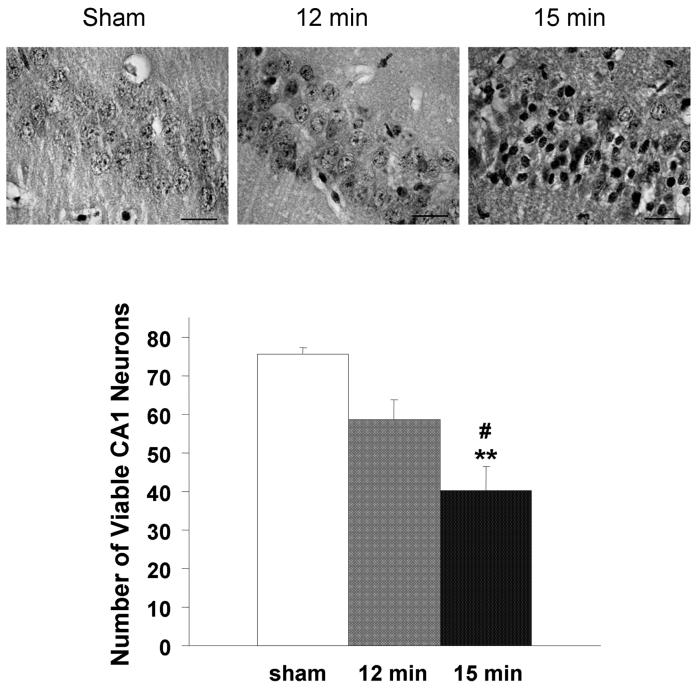
Hippocampal injury of the 12-min ischemic group (n=16) was not significantly different than that of the sham-operated group (n=16), whereas the number of viable neurons in the CA1 subfield of the15-min ischemic group (n=40) was significantly less than that of the sham-operated and 12-min ischemic groups (Fig. 1).
Fig. 1.
Neuronal loss after different ischemic durations. The number of viable CA1 neuronal cells was counted under a light microscope. Pyknotic eosinophilic neurons indicated ischemic damage. Neuronal damage was expressed as the mean number of surviving neurons in the CA1 subfield of each observed field (100 × oil lens). The number of viable neurons in hemispheres exposed to 15-min ischemia followed by 7-day reperfusion (n = 40) was significantly less than that of hemispheres from sham-operated mice (n = 16) and mice that underwent 12-min ischemia (n = 16). There were no significant differences between the 12-min ischemia group and the sham-operated group. **p<0.01 vs sham, #p<0.05 vs 12-min.
3.2. Ischemic outcome based on rCBF
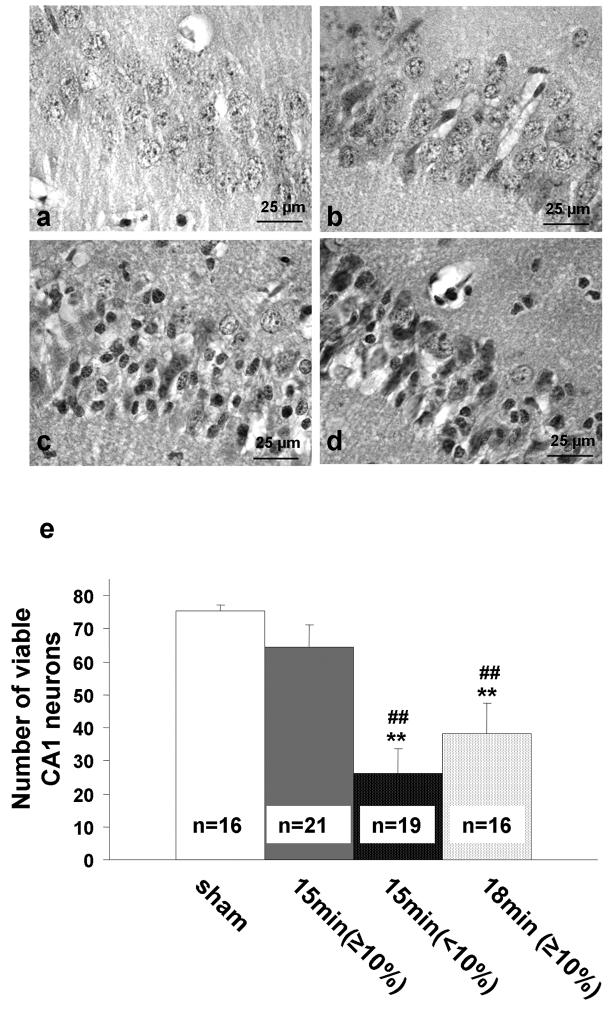
When the mean rCBF at 2.5 min of ischemia was <10% of baseline (n=17), hippocampal neuronal loss was significantly greater than when it was ≥10% of baseline (n=23). Extending ischemic duration to 18 min (n=16) when the bilateral rCBF during the initial 2.5 min of ischemia was ≥10% of baseline caused the average level of hippocampal injury to increase to the same level as that of the 15-min ischemic group with initial rCBF of <10% of baseline (p>0.05; Fig. 2). However, in some mice that underwent 18 min of ischemia, the hippocampal neuronal loss was extremely severe in one hemisphere, to the point that even some regions of the neocortex and subcortical region exhibited morphological changes, but mild in the contralateral hemisphere.
Fig. 2.
Relation between regional cerebral blood flow (rCBF) and the number of viable neurons. (a) sham-operated group; (b) hemispheres with an rCBF ≥10% of baseline at 2.5 min of occlusion and subjected to 15 min ischemia; (c) hemispheres with an rCBF <10% of baseline at 2.5 min of occlusion and subjected to 15 min ischemia; (d) hemispheres with an rCBF ≥10% of baseline at 2.5 min of occlusion and subjected to 18 min ischemia. (e) quantification of neuronal viability. **p<0.01 compared with sham-operated group; ##p <0.01 compared with 15-min ischemia group and group with rCBF ≥10% baseline.
3.3. Relationship between ischemic outcomes and contralateral PcomA
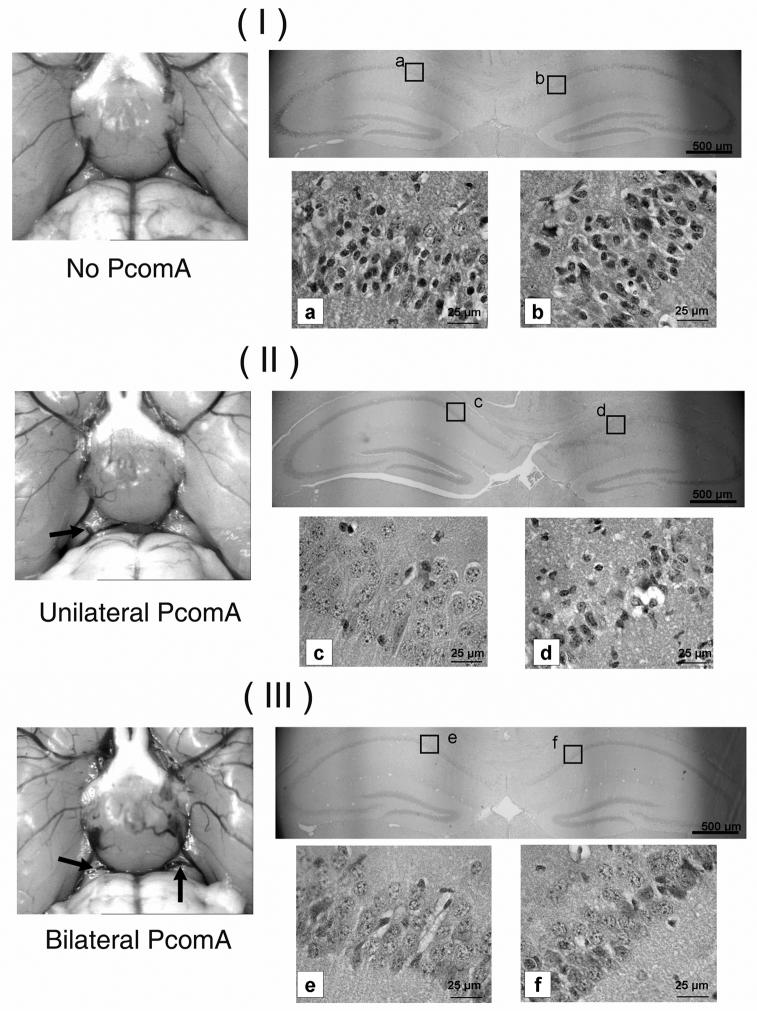
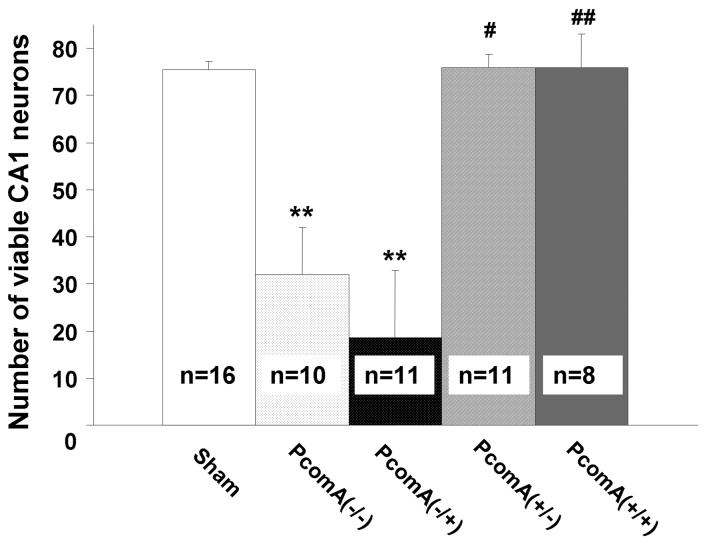
A strong correlation was observed between the patency of PcomA and the resulting hippocampal injury (Fig. 3). In the 15-min ischemic group, 9 of 20 (45%) mice were observed to have asymmetric hippocampal damage. In such cases, viable neurons were nearly absent in the hippocampal CA1 subfields of one hemisphere, while the other hemisphere had almost no damage. A clear difference in injury was observed in the hippocampal CA1 subfields between the PcomA(+) hemisphere and the PcomA(-) hemisphere even in the same animal. The ischemic outcome was not affected by the patency of the contralateral PcomA (Fig. 4).
Fig. 3.
Representative photographs of mouse PcomA (indicated by arrows) and the effect of PcomA on the outcomes of 15-min ischemic injury. (I) No PcomA; apparent ischemic injury was observed in CA1 subfields of hippocampus of both hemispheres (a, b); (II) Unilateral PcomA; apparent ischemic injury was observed in the CA1 subfield of the hippocampus of the hemisphere without PcomA (c); no injury was apparent in the CA1 subfield of the contralateral hemisphere (d). (III) Bilateral PcomA; no apparent ischemic injury was observed in the hippocampal CA1 subfields in either hemisphere (e, f).
Fig. 4.
Effect of PcomA on the number of viable neurons after 15 min of global ischemia. In the hippocampal CA1 subfield, neuronal loss in the hemispheres without PcomA was significantly greater than that in hemispheres with PcomA and in sham-operated mice. The ischemic outcome was not affected by the patency of the contralateral PcomA. PcomA(-/-): PcomA absent in both hemispheres; PcomA(-/+):PcomA absent in the ipsilateral hemisphere and present in the contralateral hemisphere; PcomA(+/-): PcomA present in the ipsilateral hemisphere and absent in the contralateral hemisphere; PcomA(+/+): PcomA present in both hemispheres. **p <0.01 vs sham; #p<0.05, ##p <0.01 vs PcomA(-/-).
3.4. Relationship between PcomA and rCBF
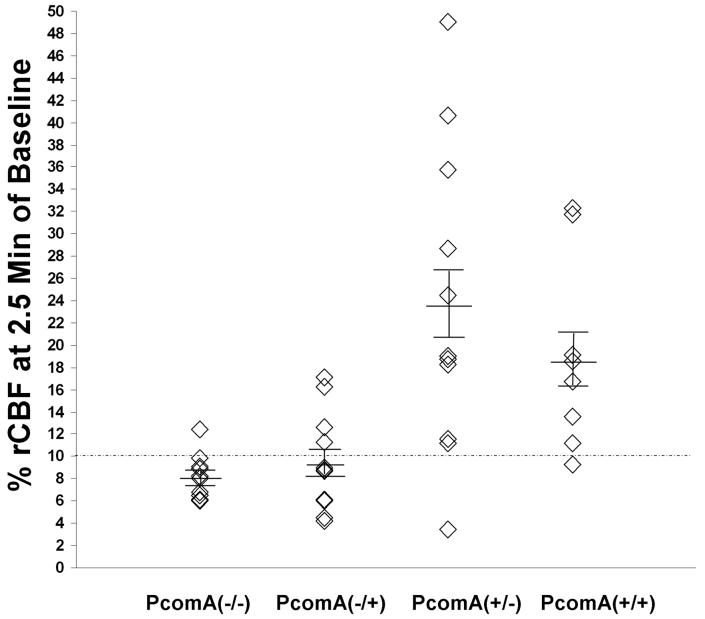
The relationship between the PcomA and the rCBF during ischemia also was investigated (Fig. 5). In the 15-min ischemic group, during the initial 2.5 min of ischemia, the rCBF of the PcomA(-) hemispheres ranged from 4.2 to 17.1%, whereas the rCBF of the PcomA(+) hemispheres ranged from 3.5 to 49.0%. There was large rCBF variation among the PcomA(+) hemispheres when the contralateral hemisphere was PcomA(-) (Fig. 5). In addition, although all the mice in the 18-min ischemia group had bilateral rCBF ≥10% of baseline at 2.5 min of occlusion, 3 of 8 (37.5%) had unilateral PcomA, indicating that rCBF was not good enough as a predictor of PcomA patency.
Fig. 5.
The relationship between rCBF at 2.5 min of ischemia and PcomA. Little variability in rCBF was observed in the hemispheres without PcomA, particularly if the contralateral hemisphere lacked PcomA as well. The greatest variability in rCBF was observed among hemispheres with PcomA when the contralateral hemisphere lacked PcomA. PcomA(-/-): PcomA absent in both hemispheres, n = 10; PcomA(-/+): PcomA absent in the ipsilateral hemisphere and present in the contralateral hemisphere, n = 11; PcomA(+/-): PcomA present in the ipsilateral hemisphere and absent in the contralateral hemisphere, n = 11; PcomA(+/+): PcomA present in both hemispheres, n = 8.
3.5. Physiological parameters
No significant differences in the arterial blood gases were observed among the groups of mice before the induction of ischemia, and no differences were apparent between the pre- and post-ischemic levels. Mean blood pressure increased by more than 20 mm Hg during ischemia, but there was no significant difference among the three groups during ischemia. Housing the mice in a warm chamber for 48 h after surgery controlled body temperatures to between 36.5 and 37.5°C; there was no significant difference among groups.
4. Discussion
In this study, we found that the presence of clearly defined PcomA significantly affects the degree of neuronal loss in the hippocampal CA1 subfield following global ischemia in mice. As depicted in figure 5, there was no significant difference in hippocampal neuronal loss between the PcomA(+) hemispheres and the hemispheres of the sham-operated mice. Moreover, there was significant neuronal loss in the CA1 subfield of hippocampus in the PcomA(-) hemispheres even when the contralateral hemisphere was PcomA(+). Taken together, these findings suggest that ischemic outcome in the PcomA(-) hemisphere was not affected by the patency of the contralateral PcomA. Therefore, all PcomA(-) hemispheres could be analyzed independently with high reproducibility. This model could be efficiently used only if the hemispheres that substantially underwent effective ischemic insult were included in further analysis; consequently, we propose establishing a routine system to exclude hemispheres with PcomA. The rCBF could be used as an initial estimate of the existence of PcomA; after the mice are sacrificed, the intracranial arterial structure could then be evaluated to ensure that only the hemispheres without PcomA are included in further analysis. In our study, 82% of the mice had at least one hemisphere in which PcomA was absent. Therefore, we estimate that at least one hemisphere could be used for analysis in most mice.
Our findings show that in CD1 mice, 15 min is the optimal ischemia time for creating a reproducible hippocampal neuronal cell loss in the CA1 subfield of hemispheres without PcomA. Moreover, we documented that the degree of neuronal loss can vary greatly between the two hemispheres of a mouse, and that ischemic outcome of one hemisphere did not affect that of the other when the patency of the PcomA differed. Thus, correlating the analysis of delayed neuronal cell death 7 days after 15-min ischemia with exclusion of the PcomA(+) hemispheres greatly reduced variations in our model.
To our knowledge, there is no optimum method for predicting the patency of PcomA before surgery. In our study, rCBF was not sufficient for predicting the patency of PcomA because the presence of PcomA was associated with a large variance in rCBF (3.5% to 49.0%) and some of the hemispheres without PcomA had a rCBF ≥10%. Despite the hypoplasia of the circle of Willis in mice, the patency of contralateral PcomA might still contribute to this rCBF overlap. Other factors, such as vessel constriction, might also affect rCBF. In such cases, the rCBF may be very low even when the PcomA is present.
We initially hypothesized that if the bilateral rCBF was ≥10% of baseline at 2.5 min, increasing ischemia time to 18 min would equalize hippocampal damage with that seen at 15 min when initial rCBF was <10% of baseline. However, since in some of those cases, PcomA was absent in one hemisphere, the result was severe hippocampal neuronal loss on the PcomA(-) side but only mild loss on the PcomA(+) side. These results may suggest that 18-min ischemia is not sufficient to cause consistent hippocampal injury in a hemisphere with PcomA. However, as previously noted, longer ischemia caused high mortality. Thus, this technique was not a viable option.
Four-vessel occlusion and 3-vessel occlusion are global ischemia models developed for controlling outcome variation caused by collateral blood flow. In these models, both common carotid arteries as well as the basilar or vertebral artery are occluded. Both models are useful for inducing cerebral ischemia without damaging organs (heart, kidney) other than brain. Collateral injury of other organs frequently occurs in systemic hypoxia and hypotension models and may alter physiological mechanisms of post-ischemic survival (Pulsinelli and Brierley, 1979). However, despite the complexity of the surgical process, the 4-vessel and 3-vessel occlusion models also have their drawbacks, including high mortality, collateral injury of brain stem, and post-ischemic seizures (Ginsberg and Busto, 1989). Moreover, the difficulty in confirming complete electrocauterization leads to considerable variation in the degree of ischemia (Kameyama et al., 1985). Another model for induction of global ischemia is the cardiac arrest model. This model has been used successfully in rats, but results in mice have been conflicting (Bottiger et al., 1999; Kawahara et al., 2002). The inconsistent results might stem from the technical difficulty in rescuing mice when the cardiac arrest is long enough to cause reproducible hippocampal neuronal injury. Therefore, by excluding the hemispheres with PcomA when using the bilateral carotid artery occlusion model, cerebral ischemia can be induced reproducibly without the disadvantages associated with the other models.
In summary, bilateral common carotid artery occlusion is an efficient global ischemia model that produces reproducible hippocampal injuries in CD1 mice. The optimal occlusion time in the absence of hemispheric PcomA was found to be 15 min. Prolonging ischemia time to 18 min in those mice with bilateral rCBF ≥10% of baseline at 2.5 min of ischemia did not solve the problem of the dichotomous outcome because the initial rCBF was not sufficient for predicting the patency of the posterior communicating artery. The existence of PcomA must be confirmed by intracranial vessel assessment. Right and left hemispheres of an individual mouse can be reproducibly studied independently according to the presence of PcomA, and only those hemispheres without PcomA should be involved in the analysis. With these modifications to the protocol, this global ischemia model can function better for testing novel hypotheses and potential new drug treatments in mice.
Acknowledgements
This research was supported by NIH grants AG022971, and NS046400 (SD). The authors gratefully thank Claire Levine for her assistance in the preparation of the manuscript, Ellen Gordes and Kathleen Kibler for technical assistance, and all Doré lab team members for insightful input.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beckmann N. High resolution magnetic resonance angiography non-invasively reveals mouse strain differences in the cerebrovascular anatomy in vivo. Magn Reson Med. 2000;44:252–8. doi: 10.1002/1522-2594(200008)44:2<252::aid-mrm12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Bottiger BW, Krumnikl JJ, Gass P, Schmitz B, Motsch J, Martin E. The cerebral ‘no-reflow’ phenomenon after cardiac arrest in rats--influence of low-flow reperfusion. Resuscitation. 1997;34:79–87. doi: 10.1016/s0300-9572(96)01029-5. [DOI] [PubMed] [Google Scholar]
- Bottiger BW, Teschendorf P, Krumnikl JJ, Vogel P, Galmbacher R, Schmitz B, et al. Global cerebral ischemia due to cardiocirculatory arrest in mice causes neuronal degeneration and early induction of transcription factor genes in the hippocampus. Brain Res Mol Brain Res. 1999;65:135–42. doi: 10.1016/s0169-328x(98)00298-8. [DOI] [PubMed] [Google Scholar]
- Eklof B, Siesjo BK. The effect of bilateral carotid artery ligation upon the blood flow and the energy state of the rat brain. Acta Physiol Scand. 1972;86:155–65. doi: 10.1111/j.1748-1716.1972.tb05322.x. [DOI] [PubMed] [Google Scholar]
- Fujii M, Hara H, Meng W, Vonsattel JP, Huang Z, Moskowitz MA.Strain-related differences in susceptibility to transient forebrain ischemia in SV-129 and C57black/6 mice Stroke 1997281805–10. discussion 11 [DOI] [PubMed] [Google Scholar]
- Ginsberg MD, Busto R. Rodent models of cerebral ischemia. Stroke. 1989;20:1627–42. doi: 10.1161/01.str.20.12.1627. [DOI] [PubMed] [Google Scholar]
- Goto S, Sampei K, Alkayed NJ, Doré S, Koehler RC. Characterization of a new double-filament model of focal cerebral ischemia in heme oxygenase-2-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2003;285:R222–30. doi: 10.1152/ajpregu.00067.2003. [DOI] [PubMed] [Google Scholar]
- Jia X, Koenig MA, Shin HC, Zhen G, Yamashita S, Thakor NV, et al. Quantitative EEG and neurological recovery with therapeutic hypothermia after asphyxial cardiac arrest in rats. Brain Res. 2006;1111:166–75. doi: 10.1016/j.brainres.2006.04.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kameyama M, Suzuki J, Shirane R, Ogawa A. A new model of bilateral hemispheric ischemia in the rat--three vessel occlusion model. Stroke. 1985;16:489–93. doi: 10.1161/01.str.16.3.489. [DOI] [PubMed] [Google Scholar]
- Kawahara N, Kawai K, Toyoda T, Nakatomi H, Furuya K, Kirino T. Cardiac arrest cerebral ischemia model in mice failed to cause delayed neuronal death in the hippocampus. Neurosci Lett. 2002;322:91–4. doi: 10.1016/s0304-3940(02)00101-5. [DOI] [PubMed] [Google Scholar]
- Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239:57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Yang G, Mabuchi T, Yagita Y, Hori M, et al. Cerebral ischemia after bilateral carotid artery occlusion and intraluminal suture occlusion in mice: evaluation of the patency of the posterior communicating artery. J Cereb Blood Flow Metab. 1998;18:570–9. doi: 10.1097/00004647-199805000-00012. [DOI] [PubMed] [Google Scholar]
- Kreisman NR, Soliman S, Gozal D. Regional differences in hypoxic depolarization and swelling in hippocampal slices. J Neurophysiol. 2000;83:1031–8. doi: 10.1152/jn.2000.83.2.1031. [DOI] [PubMed] [Google Scholar]
- Murakami K, Kondo T, Kawase M, Chan PH. The development of a new mouse model of global ischemia: focus on the relationships between ischemia duration, anesthesia, cerebral vasculature, and neuronal injury following global ischemia in mice. Brain Res. 1998;780:304–10. doi: 10.1016/s0006-8993(97)01217-1. [DOI] [PubMed] [Google Scholar]
- Nordstrom CH, Rehncrona S. Postischemic cerebral blood flow and oxygen utilization rate in rats anesthetized with nitrous oxide or phenobarbital. Acta Physiol Scand. 1977;101:230–40. doi: 10.1111/j.1748-1716.1977.tb06002.x. [DOI] [PubMed] [Google Scholar]
- Ohtaki H, Nakamachi T, Dohi K, Yofu S, Hodoyama K, Matsunaga M, et al. Controlled normothermia during ischemia is important for the induction of neuronal cell death after global ischemia in mouse. Acta Neurochir Suppl. 2006;96:249–53. doi: 10.1007/3-211-30714-1_53. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Brierley JB. A new model of bilateral hemispheric ischemia in the unanesthetized rat. Stroke. 1979;10:267–72. doi: 10.1161/01.str.10.3.267. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11:491–8. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- Shah ZA, Namiranian K, Klaus J, Kibler K, Doré S. Use of an optimized transient occlusion of the middle cerebral artery protocol for the mouse stroke model. J Stroke Cerebrovasc Dis. 2006;15:133–8. doi: 10.1016/j.jstrokecerebrovasdis.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Sheldon RA, Sedik C, Ferriero DM. Strain-related brain injury in neonatal mice subjected to hypoxia-ischemia. Brain Res. 1998;810:114–22. doi: 10.1016/s0006-8993(98)00892-0. [DOI] [PubMed] [Google Scholar]
- Sheng H, Laskowitz DT, Pearlstein RD, Warner DS. Characterization of a recovery global cerebral ischemia model in the mouse. J Neurosci Methods. 1999;88:103–9. doi: 10.1016/s0165-0270(99)00018-7. [DOI] [PubMed] [Google Scholar]
- Smith ML, Auer RN, Siesjo BK. The density and distribution of ischemic brain injury in the rat following 2-10 min of forebrain ischemia. Acta Neuropathol (Berl) 1984;64:319–32. doi: 10.1007/BF00690397. [DOI] [PubMed] [Google Scholar]
- Sorimachi T, Abe H, Takeuchi S, Tanaka R. Neuronal damage in gerbils caused by intermittent forebrain ischemia. J Neurosurg. 1999;91:835–42. doi: 10.3171/jns.1999.91.5.0835. [DOI] [PubMed] [Google Scholar]
- Sorimachi T, Abe H, Takeuchi S, Tanaka R. Ischemic depolarization monitoring: evaluation of protein synthesis in the hippocampal CA1 after brief unilateral ischemia in a gerbil model. J Neurosurg. 2002;97:104–11. doi: 10.3171/jns.2002.97.1.0104. [DOI] [PubMed] [Google Scholar]
- Volpe BT, Petito CK. Dementia with bilateral medial temporal lobe ischemia. Neurology. 1985;35:1793–7. doi: 10.1212/wnl.35.12.1793. [DOI] [PubMed] [Google Scholar]
- Wellons JC, 3rd, Sheng H, Laskowitz DT, Burkhard Mackensen G, Pearlstein RD, Warner DS. A comparison of strain-related susceptibility in two murine recovery models of global cerebral ischemia. Brain Res. 2000;868:14–21. doi: 10.1016/s0006-8993(00)02216-2. [DOI] [PubMed] [Google Scholar]
- Yonekura I, Kawahara N, Nakatomi H, Furuya K, Kirino T. A model of global cerebral ischemia in C57 BL/6 mice. J Cereb Blood Flow Metab. 2004;24:151–8. doi: 10.1097/01.WCB.0000096063.84070.C1. [DOI] [PubMed] [Google Scholar]