Abstract
Dislocation of the graft is a well-recognized complication of Descemet's stripping automated endothelial keratoplasty (DSAEK). We describe a technique to promote adhesion of the graft during DSAEK using an anterior chamber air–fluid infusion and exchange for direct control of the pressure and medium used to tamponade the graft against the host stroma.
Descemet's stripping automated endothelial keratoplasty (DSAEK) is becoming a widely used procedure for the treatment of corneal endothelial dysfunction and disorders. The technique involves stripping Descemet's membrane off the posterior stroma of the recipient1 and replacing it with cadaveric donor tissue comprised of posterior stroma, Descemet's membrane, and endothelium, which is procured with a microkeratome and artificial anterior chamber (M.S. Gorovoy, F.W. Price Jr, “New Technique Transforms Corneal Transplantation,” Cataract Refract Surg Today, Nov/Dec 2005, pages 55–58).2 One challenge of the technique has been securing the graft to the posterior aspect of the host cornea. Dislocation of the donor is reported to occur with a rate as high as 25%2 and necessitates further surgical maneuvers to reattach the graft. We describe a technique that promotes adhesion of the graft to the host stroma.
SURGICAL TECHNIQUE
As is usually done, the donor tissue is inserted and unfolded in the anterior chamber, centered, and buoyed against the recipient stroma with air that is instilled directly beneath it. In our previous cases and as described elsewhere,2 this single infusion of air was placed in the anterior chamber from an air-filled syringe and an infusion cannula through a paracentesis site. We have modified this step to include the use of an infusion pump, which is a component of an air–fluid exchange system (Accurus Surgical System, Alcon Surgical), connected to a 30-gauge needle with flexible tubing. In a beveled fashion, the needle is inserted through the nasal peripheral cornea and positioned peripheral to the donor tissue (Figure 1). Filtered room air from the Accurus is infused into the anterior chamber at a continuously regulated pressure of approximately 30 mm Hg for 10 to 15 minutes. The pressure is set using the digital arrows on the Accurus touch screen. Although intraocular pressure (IOP) is maintained at 30 mm Hg for most of the infusion time, pressures as high as 60 mm Hg are occasionally used for 30 seconds or less and seem to visibly press the graft against the host. Afterward, air can be replaced slowly with balanced salt solution to achieve a partial air–fluid exchange in the anterior chamber under constant pressure.
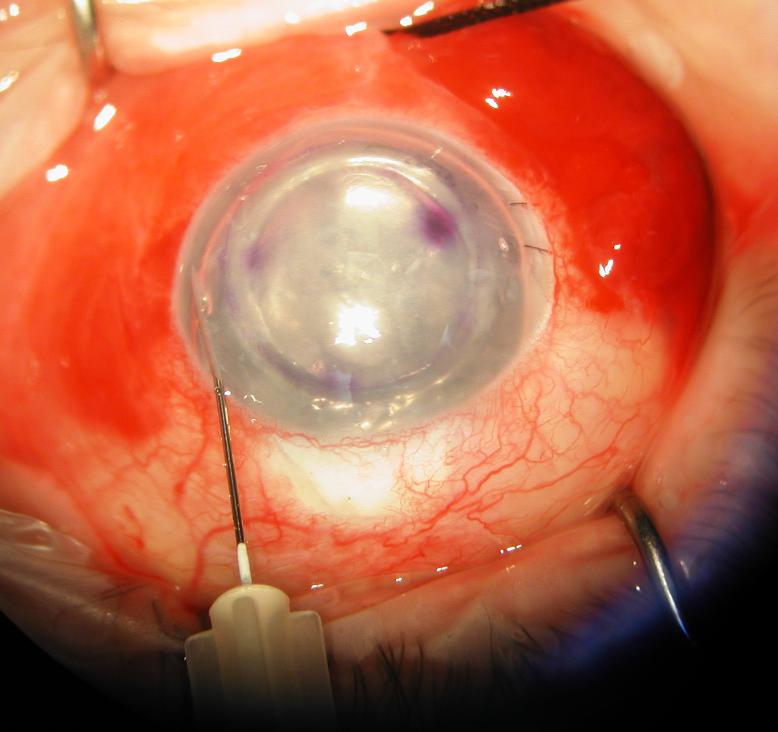
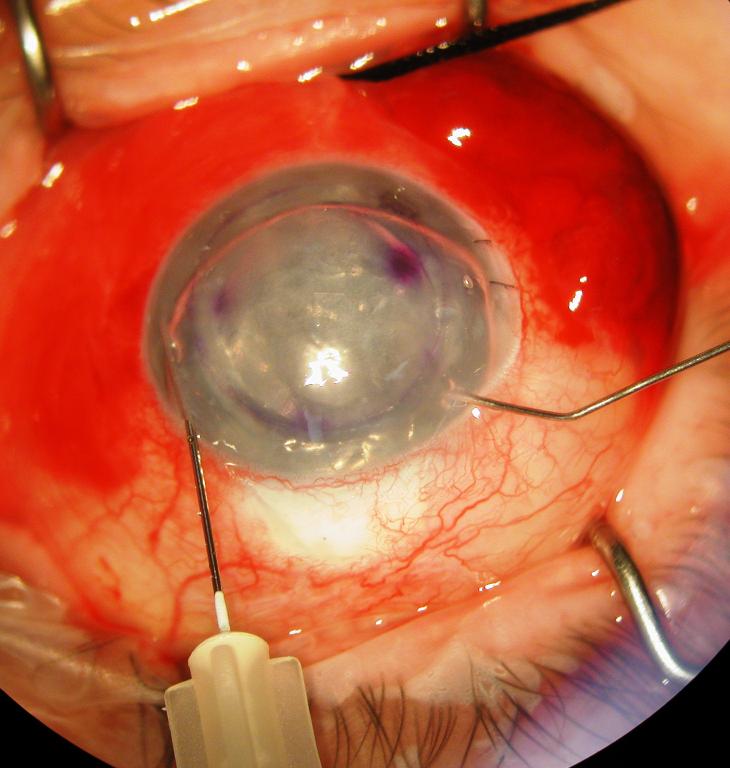
Figure 1.
Surgeon's view of the right eye depicting an infusion/air–fluid exchange technique for controlling graft tamponade pressure during DSAEK. The posterior lamellar graft is shown in its final position with a subtotal air fill maintained at 30 mm Hg by the Accurus surgical system. The system is connected via tubing to a 30-gauge needle that is inserted through a long nasal corneal tract (left). After 10 to 15 minutes, a partial air–fluid exchange is begun while the tamponade pressure is maintained (right). A manual infusion cannula is used to separate the wound margins of a paracentesis to allow controlled escape of air during the exchange.
Escape of air to lower IOP is facilitated by carefully spreading the stroma of a paracentesis site with an infusion cannula, allowing the air to be evacuated in a controlled fashion. Overall, this allows maintenance of a well-formed, pressurized anterior chamber. During this time, the graft is observed through the operating microscope to be stationed against the posterior stroma of the recipient. We attempt to exchange enough fluid for air that the temporal edge of the dilated pupil is just bared or the temporal peripheral iridotomy is exposed when the patient's head is turned temporally and the air rises to the nasal angle. With the patient's head remaining in this position and air tamponading the infusion site, the 30-gauge needle is quickly removed from the eye and pressure is placed directly over the entry site with a cotton tip applicator to avoid egress of air or fluid. More air may be burped from the anterior chamber and/or replaced with balanced salt solution as needed. We have used this technique successfully in 12 consecutive cases without graft dislocation.
DISCUSSION
Controlled pressurization of the vitreous space with infused air or fluid is an important means of retinal tamponade and subretinal fluid evacuation during vitreoretinal surgery.3 We describe a technique for applying similar principles to the problem of graft adherence in endothelial keratoplasty. In all 12 cases in which this technique was used, the graft was noted postoperatively to be well adhered to the recipient with minimal striae. We attribute this success to the sustained pressure achieved with the technique, which seems to press the graft against the host stroma, perhaps forcing fluid out of the interface and stromal tissues.
With our prior manual, noncontinuous air-injection technique, it was more difficult to maintain the pressure at the levels achievable with the current technique. However, it is still unclear what pressure is optimal for promoting graft adherence. Higher or lower pressures may be more appropriate in certain patients; specifically, very high pressures might be avoided in patients with glaucoma. With manual air instillation, IOP seemed to decrease with time after the initial infusion. This is in contrast to the stable IOP afforded by the new technique we describe. Also, evacuation of most air with simultaneous fluid infusion permits more controlled anterior chamber fluid replacement than the manual evacuation of large air bubbles from a paracentesis wound as some have advocated. This may reduce the potential for pressure fluctuations that could adversely affect graft apposition and promote striae formation and graft dislocation. The additional steps required to directly control tamponade pressure by this technique are straightforward and are worthwhile if they reduce the need for additional intraocular procedures to reposition a dislocated graft.
While this technique afforded excellent adherence in our small series of patients, some questions regarding optimization remain, such as the potentially deleterious effects of sustained air exposure4 or pressure on the endothelium. This concern is not unique to our technique, however. In fact, the direct control of tamponade time, pressure, and medium provided by this technique will enable us to quantify the minimum amount of air and pressure necessary to secure the graft to the recipient. We are also exploring the possibility that fluid pressure produced by the system without air infusion might be sufficient to achieve the same results.
Biography

Footnotes
No author has a financial or proprietary interest in any method or material mentioned.
Supported in part by a Research to Prevent Blindness, New York, New York, USA, challenge grant to the Department of Ophthalmology of the Cleveland Clinic Lerner College of Medicine and NIH 8K12 RR023264 multidisciplinary clinical research career development programs grant (W.J.D.).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Melles GRJ, Wijdh RHJ, Nieuwendaal CP. A technique to excise the Descemet's membrane from a recipient cornea (descemetorhexis) Cornea. 2004;23:286–288. doi: 10.1097/00003226-200404000-00011. [DOI] [PubMed] [Google Scholar]
- 2.Price FW, Jr, Price MO. Descemet's stripping with endothelial keratoplasty in 200 eyes; early challenges and techniques to enhance donor adherence. J Cataract Refract Surg. 2006;32:411–418. doi: 10.1016/j.jcrs.2005.12.078. [DOI] [PubMed] [Google Scholar]
- 3.Aaberg TM. Management of anterior and posterior proliferative vitreoretinopathy; the XLV Edward Jackson Memorial Lecture. Am J Ophthalmol. 1988;106:519–532. doi: 10.1016/0002-9394(88)90580-6. [DOI] [PubMed] [Google Scholar]
- 4.Van Horn DL, Edelhauser HF, Aaberg TM, Pederson HJ. In vivo effects of air and sulfur hexafluoride gas on rabbit corneal endothelium. Invest Ophthalmol. 1972;11:1028–1036. [PubMed] [Google Scholar]