Abstract
Tumor progression and metastasis are complex processes involving intricate interplay among multiple gene products. Astrocyte Elevated Gene (AEG)-1 was cloned as an HIV-1- and tumor necrosis factor α (TNF-α)-inducible transcript in primary human fetal astrocytes by a rapid subtraction hybridization approach. AEG-1 downregulates the expression of the glutamate transporter EAAT2, thus it is implicated in glutamate-induced excitotoxic damage to neurons as evident in HIV-associated neurodegeneration. Interestingly, AEG-1 expression is elevated in subsets of breast cancer, glioblastoma multiforme and melanoma cells and AEG-1 cooperates with Ha-ras to augment the transformed phenotype of normal immortal cells. Moreover, AEG-1 is overexpressed in >95% of human malignant glioma samples when compared with normal human brain. Overexpression of AEG-1 increases and siRNA inhibition of AEG-1 decreases migration and invasion of human glioma cells, respectively. AEG-1 contains a lung-homing domain facilitating breast tumor metastasis to lungs. These findings indicate that AEG-1 might play a pivotal role in the pathogenesis, progression and metastasis of diverse cancers. Our recent observations indicate that AEG-1 exerts its effects by activating the NF-κB pathway and AEG-1 is a downstream target of Ha-ras and plays an important role in Ha-ras-mediated tumorigenesis. These provocative findings are intensifying interest in AEG-1 as a crucial regulator of tumor progression and metastasis and as a potential mediator of neurodegeneration. In this review, we discuss the cloning, structure and function(s) of AEG-1 and provide recent insights into the diverse actions and intriguing properties of this molecule.
Keywords: AEG-1, Progression, Metastasis, Ha-ras oncogene, Glutamate excitotoxicity, AEG-1 promoter
1. Introduction
Cancer arises from a stepwise accumulation of genetic alterations that drive the progressive transformation of normal human cells ultimately culminating in highly malignant derivatives. This multi-step process results in alterations in cellular physiology that underlie malignant cell growth, e.g., self-sufficiency in growth signals, insensitivity to growth-inhibitory signals, evasion from apoptosis, unlimited replicative potential, and aberrant angiogenesis, as well as tissue invasion and metastasis (Fidler & Ellis, 1994; Hahn & Weinberg, 2002). The initiation and progression of solid tumors is in part dependent on a variety of signaling pathways, as are the processes that allow for invasion and metastasis. Cellular motility and extracellular matrix degradation are two major determinants of cancer cell invasion. Invasion through basement membrane from solid tumors to blood vessels and then to the secondary sites involves secretion of chemokines that help in tumor cell motility in a defined direction and proteolytic enzymes involved in extracellular matrix degradation, by the host as well as tumor cells (Liotta & Kohn, 2001).
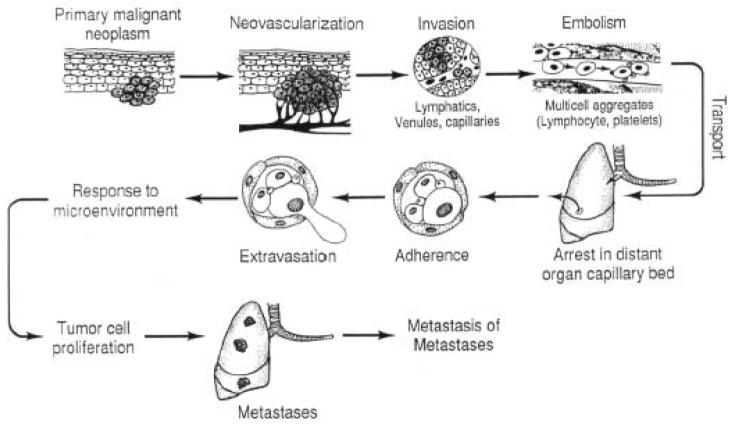
Metastases arise following the spread of cancer cells from a primary site with the formation of new tumors in a distant organ. On the molecular level, the metastatic phenotype is orchestrated by the expression of homing receptors with associated signaling molecules, their ligands, and extracellular matrix-degrading proteases (Kurschat & Mauch, 2000). Metastasis consists of a series of sequential steps that include shedding of cells from a primary tumor into the circulation, survival of the cells in the circulation, arrest in a new organ, extravasations into the surrounding tissue, initiation and maintenance of growth, and vascularization of the metastatic tumor (Fig. 1). This process involves coordination of diverse signal-transduction pathways that allow cancer cells to proliferate, remodel their surrounding environment, invade to a distant site and reestablish the tumor. The precise molecular events leading to acquisition of the metastatic phenotype remain largely ambiguous.
Fig. 1.
A schematic representation of the metastatic process. (reproduced, by permission of the publisher, from Fidler & Ellis, 1994).
We recently reported the cloning and functional characterization of an HIV-inducible gene, astrocyte elevated gene-1 (AEG-1), which is induced in primary human fetal astrocytes (PHFA) infected with HIV-1 or treated with recombinant HIV-1 envelope glycoprotein (gp120), or tumor necrosis factor-α (TNF-α) (Kang et al., 2005; Su et al., 2003a; Su et al., 2002). AEG-1 is a downstream target molecule of Ha-ras and c-myc mediating their tumor promoting effects (Lee et al., 2006). Intriguingly, AEG-1 induces increased anchorage-independent growth and invasiveness of tumor cells and increased expression of adhesion molecules by activating the NF-κB pathway, and AEG-1 can physically interact with the p65 subunit of NF-κB and modulate its function in the nucleus (Emdad et al., 2006). Moreover, AEG-1 expression is elevated in diverse neoplastic conditions; it cooperates with Ha-ras to promote transformation; and its overexpression augments invasion of transformed cells demonstrating its functional involvement in Ha-ras-mediated tumorigenesis (Kang et al., 2005, Emdad et al., 2006; Lee et al., 2006). This review explores the multiple functions of AEG-1 including effects on oncogenic signaling pathways that are linked to an invasive, metastatic phenotype as well as neurodegeneration associated with brain tumor development and progression and HIV-1 infection.
2. Cloning of AEG-1
HIV-1 infection can impact on CNS functions resulting in neurodegeneration and HIV-1 associated dementia (HAD) (Lipton & Gendelman, 1995). The pathogenic events triggered by HIV-1 in the brain ultimately result in neuronal loss and CNS dysfunction. Neurons are rarely infected with HIV-1 in vivo and most evidence suggests that macrophages and microglial cells are the primary target cells for productive HIV-1 infection in the CNS (Lipton & Gendelman, 1995). Astrocytes also can be infected with HIV-1 in vitro and in vivo, although with lower efficiency than T cells and macrophages (Brack-Werner, 1999). There is general agreement, however, that HIV-1 can persist in astrocytes for prolonged periods in a low productive, non-cytolytic state, from which it can be induced by physiologic stimuli, such as TNF-α (Shahabuddin et al., 1992; Tornatore et al., 1991).
HIV-1 infection results in downregulation of the glutamate transporter, especially the astrocyte-specific Excitatory Amino Acid Transporter-2 (EAAT2), in astrocytes (Wang et al., 2003). Lack of EAAT2 precludes removal of glutamate from synaptic clefts and the accumulation of glutamate induces excitotoxicity and damage to neurons (Choi, 1988). Treatment of astrocytes with gp120, an HIV-1 envelope protein, alters cell physiology (Benos et al., 1994a; Benos et al., 1994b), including downregulation of glutamate transport (Wang et al., 2003), which is essential for normal neuronal functions, as indicated by increased D-aspartate efflux from astrocytes. Impairment of glutamate transport is also observed after incubation of human astrocytes with TNF-α (Fine et al., 1996) or co-cultivation with T cells infected with human T cell leukemia virus type I (HTLV-I) (Szymocha et al., 2000), and similar defects are found in feline astrocytes after infection with feline immunodeficiency virus (FIV) (Yu et al., 1998). More recent studies indicate that ligation of the HIV-1 coreceptor CXCR4 on astrocytes, by either stromal cell-derived factor 1 (SDF-1) or gp120 can stimulate a novel signaling pathway that involves Ca2+-dependent release of glutamate (Sharma & Vijayaraghavan, 2001) in a process including activation of the CXCR4 receptor, an autocrine/paracrine TNF-α-dependent signaling, and prostaglandin production (Bezzi et al., 2001). These results suggest that HIV-1, gp120, and other neuropathogenic agents can alter specific signaling pathways in astrocytes in a way that may impair important physiological functions of these cells in neuronal signal transmission and response to brain injury.
Based on the profound alterations occurring in astrocyte physiology following HIV-1 infection, or treatment with gp120 and TNF-α, we assumed that modifications in gene expression would occur that correlate with and may be causative of these functional changes. Using a robust and selective rapid subtraction hybridization approach (RaSH) method developed in our laboratory (Boukerche et al., 2007; Jiang et al., 2000), we have addressed these possibilities (Su et al., 2002; Su et al., 2003b). Limited amounts of RNA and technical complexities are factors that can prevent the efficient use of classical subtraction hybridization approaches for identifying differentially expressed genes. The RaSH (Boukerche et al., 2007; Jiang et al., 2000) approach was able to ameliorate these problems in gene identification and cloning when employing subtraction hybridization. RaSH consists of library construction from double-stranded cDNAs that are enzymatically digested into small fragments, ligated to adapters and PCR amplified followed by incubation of tester and driver PCR fragments (Boukerche et al., 2007; Jiang et al., 2000). A schematic of this approach employed in early passage primary human fetal astrocytes (PHFA) and HIV-1 infection is outlined in Fig. 2. RaSH has been successfully used for identifying known and novel differentially expressed cDNAs, including genes related to terminal differentiation of cancer cells (Jiang et al., 2000; Kang et al., 2001), genes displaying elevated expression as a function of acquisition of enhanced metastatic ability (Bouckerche et al., 2004), genes displaying differential expression in HIV-1 resistant versus sensitive human T-cell clones (Simm et al., 2001), genes upregulated as a function of infection of PHFA with HIV-1 or treatment with gp120 (astrocyte elevated genes, AEGs) and genes downregulated as a function of infection of PHFA with HIV-1 or treatment with gp120 (astrocyte suppressed genes, ASGs) (Kang et al., 2005; Su et al., 2003b; Su et al., 2002).
Fig. 2.
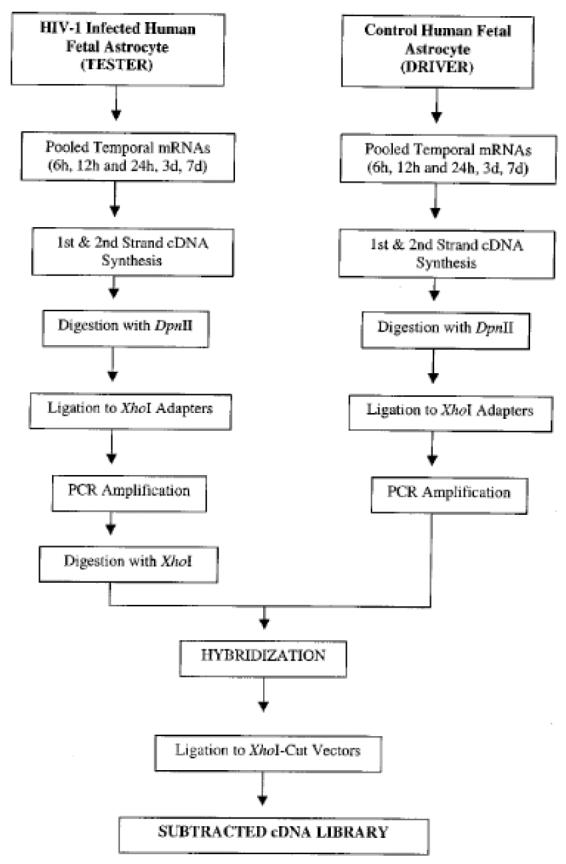
A schematic representation of the RaSH approach. For details, please refer to Su et al (2003).
To avoid cloning genes displaying normal cell cycle fluctuations in astrocytes, RNAs were isolated and pooled from 6, 12 and 24 hrs and 3 and 7 days uninfected and infected cells and were used to produce temporal cDNA libraries for RaSH. By subtracting temporal cDNAs derived from uninfected PHFA from temporal cDNAs (Su et al., 2003b; Su et al., 2002) derived from HIV-1 infected cells, a series of genes displaying elevated expression in virus infected cells, astrocyte elevated genes (AEGs), were identified. Both known and novel AEGs (total of 15), at the time of initial identification and isolation, including AEG-1, were identified that displayed early or late expression kinetics following HIV-1 infection or treatment with gp120. The comparable pattern of gene expression changes following HIV-1 infection or gp120 treatment suggested that HIV-1 exposure of astrocytes, even in the absence of productive infection, could induce profound changes in cellular gene expression and physiology.
The full-length cDNA of the novel AEG-1 gene was obtained using a novel modified rapid amplification of cDNA ends (RACE) approach called C-ORF (complete open reading frame technology) and bioinformatics (Kang & Fisher, 2007; Kang & Fisher, 2005; Kang et al., 2005). This cloning has permitted a detailed analysis of the functional properties of this intriguing gene providing new insights into the processes of cancer development, progression to metastasis and neurodegeneration.
3. Structure and localization of AEG-1
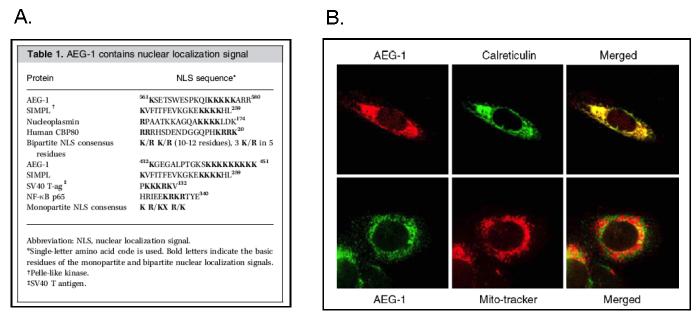
The full-length AEG-1 cDNA consists of 3611-bp, excluding the poly-A tail (Kang et al., 2005). The open reading frame (ORF) from 220- to 1968-nts encodes a putative 582 amino acid protein with a calculated molecular mass of 64-kDa with a pI of 9.33. Genomic blast search demonstrated that the AEG-1 gene consists of 12 exons/11 introns and is located at 8q22 where cytogenetic analysis of human gliomas indicated recurrent amplifications. Protein motif analysis, such as SMART, predicted a single transmembrane domain at the N-terminus of the protein (amino acids 50-70) that includes putative dileucine repeats involved in protein trafficking, which was supported by three independent transmembrane protein prediction methods (PSORT II,TMpred, and HMMTOP). However, PSORT II and TMpred predicted AEG-1 as a type Ib protein (C-terminal inside), whereas TMHMM and TopPred 2 predicted a type II protein (C-terminal outside). Analysis of the amino acid sequence of AEG-1 further revealed the presence of putative, either monopartite or bipartite, nuclear localization signals (NLS) between amino acids 79 to 91, 432 to 451 and 561 to 580 (Fig. 3A) suggesting import into the nucleus. The existence of a cleavable signal peptide was not evident based on the motif analyses.
Fig. 3.
Localization of AEG-1. A. Putative nuclear localization signals (NLS) in AEG-1. B. Subcellular localization of AEG-1 in IM-PHFA. Calreticulin and Mito-Tracker were used for specific ER and mitochondria markers, respectively. Reproduced, by permission of the publishers, from Emdad et al (2006), and Kang et al (2005), respectively.
Intracellular localization of AEG-1 was examined in various cell types, using a chicken polyclonal anti-AEG-1 antibody and immunofluorescence microscopy. Fig. 3B shows endogenous AEG-1 staining in immortalized primary human fetal astrocytes (IM-PHFA), in which AEG-1 was detected at the perinuclear region and in endoplasmic reticulum (ER)-like structures, but not at the plasma membrane, and co-localized with the ER-specific protein calreticulin, but not with the mitochondrial marker Mito-Tracker. The observed ER localization of AEG-1 favors type Ib membrane topology of the AEG-1 protein. Our recent observations, however, reveal that TNF-α treatment, as well as forced overexpression of AEG-1, which upregulates AEG-1 expression, resulted in the localization of the protein both in the cytoplasm and in the nucleus in HeLa cells (Emdad et al., 2006). This finding is analogous to the nuclear receptor co-activator SRC-3 (pCIP/ACTR/AIB-1/RAC-3/TRAM-1) that is located mainly in the cytoplasm, translocates from the cytoplasm to the nucleus on TNF-α activation (Wu et al., 2002).
A separate research group cloned mouse AEG-1 as metadherin from 4T1 mouse breast carcinoma cells and the cloning of the mouse (3D3) and rat (lyric) homologues of AEG-1 have been reported (Brown & Ruoslahti, 2004; Sutherland et al., 2004). Mouse AEG-1 protein, containing 579 amino acids, is 3 amino acids shorter than human AEG-1. There are discrepancies among the AEG-1/metadherin/3D3 reports with respect to its localization. According to Sutherland et al. (2004) Western blot analysis of HeLa cells revealed that most isoforms of 3D3/lyric were found predominantly in the nuclear pellet, although some higher molecular weight isoforms were also located in the cytoplasmic and soluble nuclear fractions. They confirmed the subcellular localization of endogenous 3D3/lyric by immunostaining various human and mouse cell lines, which showed perinuclear and cytoplasmic staining as well as some nuclear rim and general nuclear diffuse staining (Sutherland et al., 2004). Brown and Ruoslahti (2004) detected Metadherin staining throughout the cytoplasm in fixed and permeabilized 4T1 cells by using a rabbit antibody raised against the lung–homing domain of metadherin. Antibody-assisted FACS analysis suggests that metadherin has type II membrane topology (Brown & Ruoslahti, 2004). However, 3D3/lyric and AEG-1 suggest type Ib topology based on ER location, detected by immunofluorescent microscopy, and clusters of basic amino acids at the C-terminal juxtaposition of its transmembrane domain. Sequence motif analysis equivocally supports both topologies, depending on the algorithm employed. For that reason, the exact localization and membrane topology of AEG-1, 3D3/lyric and metadherin and the functional significance of this localization require further clarification.
4. Functional studies of AEG-1
4.1. AEG-1 in tumor progression and metastasis
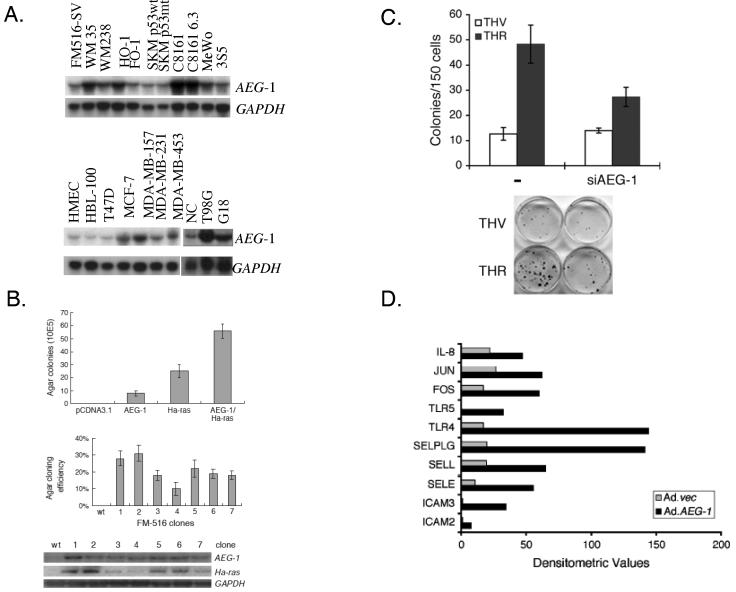
The accumulation of mutations during carcinogenesis results in six essential alterations in cell physiology that collectively dictate malignant growth: self sufficiency in cell growth, insensitivity to growth-inhibitory signals, evasion of cell death, limitless replicative proliferation, sustained angiogenesis, and tissue invasion and metastasis (Hanahan & Weinberg, 2000). Most of these alterations affect cell signaling pathways, and the majority of oncogenes are integral components of cellular signaling circuits, which are up-regulated or constitutively activated in malignant cells. Recent studies highlight a potential role of AEG-1 in promoting tumor progression and metastasis (Brown & Ruoslahti, 2004; Emdad et al., 2006; Kang et al., 2005). The current data suggests that AEG-1 is capable of promoting several aspects of tumor progression and metastasis. AEG-1 regulates several hallmarks of metastatic cancers (Fidler, 1991), including aberrant proliferation, increased adhesion and invasiveness and resistance to apoptosis under certain stress-full condition. Elevated levels of AEG-1 are detected in subsets of breast cancer, glioblastoma multiforme and melanoma cells (Fig 4A) and ectopic overexpression of AEG-1 promotes colony-forming ability of immortalized melanocytes and astrocytes in soft agar. AEG-1 also cooperates with Ha-ras to augment the transformed phenotype of normal immortal cells (Fig. 4B). Ectopic expression of AEG-1 promotes anchorage-independent growth of normal immortal human melanocytes (FM516-SV) that do not normally form colonies in soft agar. Although expression of the T24-Ha-ras oncogene in FM516-SV cells promotes a higher rate in agar growth, co-expression of AEG-1 and Ha-ras in FM516-SV cells results in a synergistic increase in colony formation in soft agar (Fig. 4B). Expression levels of Ha-ras positively correlate with the agar cloning efficiency of AEG-1/Ha-ras stable transfectants, suggesting a complementing role of AEG-1 with Ha-ras in development of the cancerous phenotype. A correlation of AEG-1 upregulation with enhanced soft agar colony forming ability is also evident in adult human astrocytes transformed by sequential expression of SV40 T antigen, hTERT and Ha-ras (THR) (Lee et al., 2006; Rich et al., 2001). Increased expression of AEG-1 in THR cells indirectly supports tumor-forming properties of AEG-1 in glial tumor cells. Of potential clinical relevance, significantly increased AEG-1 expression is detected in tissue samples by immunofluorescence studies and Western blot analyses from diverse human brain tumors including highly invasive glioblastoma multiforme, anaplastic astrocytoma and aggressive meningiomas in comparison to that from normal human brain (unpublished observations).
Fig. 4.
AEG-1 in tumor progression and metastasis. A. AEG-1 expression in various cancer cell lines. Northern blots of the indicated RNA samples were probed with AEG-1 and GAPDH probes. FM516-SV: SV40-immortalized normal human melanocyte; WM35: radial growth phase primary human melanoma; WM238, HO-1, FO-1, SKM p53wt, SKM p53mut, C8161, C8161 6.3, MeWo, 3S5: metastatic and variant human melanoma cells; HMEC: early passage human mammary epithelial cells; HBL-100: immortal normal human breast epithelial cell line; T47D, MCF-7, MDA-MB-157, MDA-MB-231, MDA-MB-453: human breast carcinoma cell lines; NC, normal cerebellum cell line; T98G, G18: human malignant glioma cell line. B. Effect on soft agar colony formation of FM516-SV cells following expression of AEG-1 and Ha-ras, alone or in combination. Upper panel, colony forming ability of FM516-SV cells transiently transfected with the indicated expression vectors. Data shown is average ± S.D. of three independent transfections. Middle panel, colony forming ability of FM516-SV cells stably transfected with AEG-1 and Ha-ras. Data shown is average ± S.D. of three independent transfections. Lower panel, expression of AEG-1 and Ha-ras mRNA in stable transfectants determined by Northern blot analysis. C. AEG-1 is required for Ha-ras-mediated colony formation. THV and THR cells were transfected with control or AEG-1 siRNA using the Lipofectamine 2000 transfection reagent according to the manufacturer's instructions. After 2 days, the cells were trypsinized and counted, and 150 cells were plated in 6-cm dishes. Colonies of ≥ 50 cells were scored after 10 days. The data are represented as mean ± SD and represent three independent experiments in triplicate. D. Ad.AEG-1 infection upregulates NF-κB downstream genes. The expression levels of NF-κB signaling pathway genes after Ad.vec and Ad.AEG-1 infection were analyzed in total RNA using the Human NF-κB signaling gene array (SuperArray Bioscience Corporation) according to the manufacturer's protocol. Graphical representation of expression levels of each gene obtained by densitometric analysis. Reproduced, by permission of the publishers, from Kang et al (2005), Lee et al (2006), and Emdad et al (2006), respectively.
Experiments have documented that AEG-1 increases anchorage-independent growth and invasiveness of HeLa cells by activating the NF-κB pathway (Emdad et al., 2006). Overexpression of AEG-1 increases and siRNA inhibition of AEG-1 decreases migration and invasion of human glioma cells, respectively. In addition, AEG-1 expression is significantly induced by oncogenic Ha-ras through the PI3K/AKT/GSK3β/c-Myc signaling pathway (Lee et al., 2006) and AEG-1 siRNA inhibits the colony formation activity of oncogenic Ha-ras (Fig. 4C). Rat lyric is an overexpressed gene in rat liver and colon tumors (Sutherland et al., 2004). Human Metadherin is overexpressed in cultured breast cancer cells and tissue sections from breast adenocarcinoma, and contains a lung-homing domain that facilitates metastasis of breast cancer cells to the lung. Antibodies reactive to the lung-homing domain of AEG-1/Metadherin and siRNA mediated knock down of AEG-1/Metadherin in breast cancer cells inhibiting experimental lung metastasis, indicating that tumor cell AEG-1/Metadherin mediates localization at the metastatic site (Brown & Ruoslahti, 2004). In preliminary studies, overexpression of AEG-1 in immortalized human melanocyte (FM516-SV) cells results in augmented adherence to sections of lung tissue (unpublished data).
An NF-κB gene array revealed that ectopic overexpression of AEG-1 results in marked upregulation NF-κB-responsive cell adhesion molecules (ICAM-2 and I-CAM-3, selectin E, selectin L, and selectin P ligand), TLR4 and TLR5, and cytokines such as IL-8 (Fig. 4D), uncovering a potential mechanism of AEG-1-mediated enhanced lung metastasis of breast cancer cells and increased adhesion of immortalized melanocytes to lung endothelial cells. Taken together, these findings indicate that in both human and rat; AEG-1 is involved in promoting tumor progression and metastasis.
Although a definitive link between HIV infection and malignant glioma is lacking, one report indicates that in a series of 8 HIV positive patients with brain malignancies, five had glioblastoma multiforme. Glioblastoma developed in these five patients >6-years after diagnosis of HIV infection suggesting that HIV-infected patients may be at risk for intracranial neoplasms other than primary central nervous system lymphoma (Blumenthal et al., 1999). In addition, an interesting connection between these two apparently independent pathological conditions is found in elevated expression of matrix metalloproteinase (MMPs). HAD associates with increased MMP-2, 7 and 9 levels which are also observed in highly metastasizing and rapidly expanding tumors and HIV-1 Tat-associated neuropathy is inhibited by MMP inhibitors (TIMP) (Conant et al., 1999; Johnson et al., 2001; Sporer et al., 1998). Accordingly, in addition to downmodulation of EAAT2 expression that results in glutamate excitotoxicity, the elevated expression of AEG-1 in HIV-infected astrocytes might contribute to HIV-1-associated neuropathy by up-regulating MMPs that are essential in tumor expansion. Indeed, our initial experiments suggest that downregulation of AEG-1 by siRNA targeting AEG-1, significantly inhibits MMP-2 and MMP-9 promoter activity in rat glioma cells (unpublished observation). Thus, the elevated expression of AEG-1 in HIV-infected astrocytes might contribute to HIV-1-associated neuropathy and malignant transformation of astrocytes.
4.2. AEG-1 and the NF-κB pathway
Cancer is a hyperproliferative disorder that results from tumor initiation and promotion, which in certain contexts can lead to acquisition of metastastic potential by an evolving tumor cell. The transcription factor nuclear factor kappa B (NF-κB) can affect oncogenesis by virtue of its capacity to regulate the expression of a plethora of genes that modulate apoptosis and cell survival as well as proliferation, inflammation, cell adhesion, tumor metastasis and angiogenesis (Baldwin, 2001a; Baldwin, 2001b; Kabrun & Enrietto, 1994). NF-κB regulates the expression of various molecules such as cell adhesion proteins, matrix metalloproteinase, cycloxygenase 2, inducible nitric oxide synthase, chemokines, and inflammatory cytokines, all of which promote tumor cell invasion and angiogenesis (Baldwin, 1996; Mettouchi et al., 1997). The ability of NF-κB to suppress apoptosis and to induce expression of proto-oncogenes such as c-myc and cyclin D1, which directly stimulate proliferation, suggests that NF-κB participates in many aspects of oncogenesis (Guttridge et al., 1999; Hinz et al., 1999). The earliest evidence for a role for NF-κB in oncogenic transformation was derived from the fact that v-Rel, a highly oncogenic retroviral homologue of c-Rel, causes carcinogenesis in avian lymphoid cells (Gilmore et al., 1996). Later studies suggested that v-Rel also has the capacity to transform mammalian cells in vivo (Carrasco et al., 1996). Transgenic mice expressing v-Rel under the control of the T-cell specific lck promoter develop T-cell lymphomas. Inhibition of NF-κB by overexpression of a degradation-resistant IκBα delays the development of T-cell lymphomas and prolongs the survival of v-Rel transgenic mice (Carrasco et al., 1996). Indeed, constitutive NF-κB activity has been observed in several different tumor cell types, including T- or B-lymphocyte leukemia, lymphoma, myeloma, melanoma, prostate, colon, breast, thyroid, pancreas, glioma, and head and neck squamous cell carcinoma cell lines, as well as in samples obtained from cancer patients, and the inhibition of NF-κB abrogates tumor cell proliferation (Bargou et al., 1997; Bours et al., 1994; Devalaraja et al., 1999; Gilmore et al., 1996; Mori et al., 1999; Mukhopadhyay et al., 2001; Visconti et al., 1997; Wang et al., 1999).
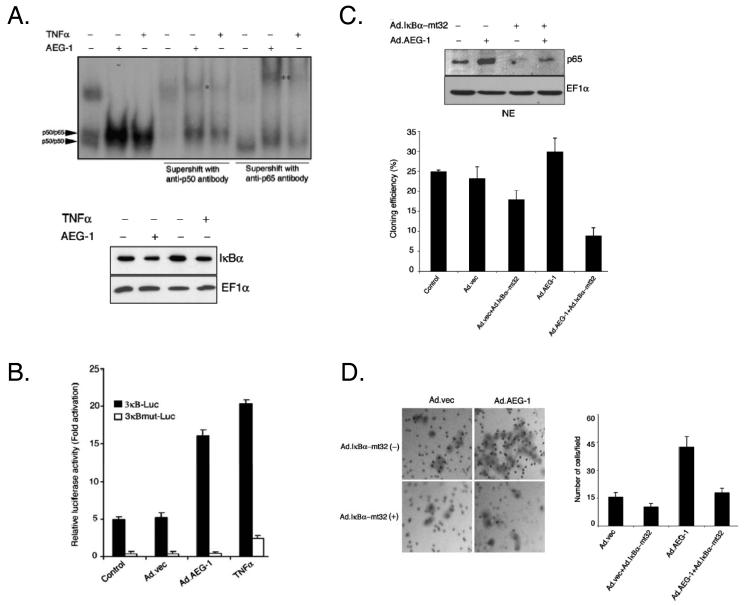
Our recent observations indicate that, AEG-1 facilitates IκBα degradation, resulting in an increase in NF-κB DNA binding activity and NF-κB promoter activity in reporter assays (Fig. 5A, and 5B). Additionally we show that, inhibition of NF-κB by IκBα-mt32 (using an adenovirus expressing the mt32IκBα superrepressor, which inhibits IκBα degradation and subsequent NF-κB nuclear translocation) significantly inhibits AEG-1-induced augmentation in soft agar colony formation and invasion of HeLa cells (Fig. 5C and 5D). AEG-1 can physically interact with the p65 component of NF-κB and modulate its function in the nucleus (Emdad et al., 2006). Taken together these data indicate that AEG-1 is decisively involved as a positive regulator of NF-κB–induced gene expression, and this activation provides a molecular basis for the ability of AEG-1 to promote anchorage-independent growth and invasion, prominent contributors to cancer progression.
Fig. 5.
AEG-1 and NF-κB pathway. A. AEG-1 activates the NF-κB pathway. Upper panel, HeLa cells were infected with Ad.AEG-1 (50 pfu/cell) or treated with TNF-[unk] (10 ng/ml) and 2 days later NF-κB binding activity was analyzed in the nuclear extracts of the cells by EMSA. Supershift analysis was performed with the indicated antibodies. *indicates a supershifted band by anti-p50 antibody; ** indicates a supershifted band by anti-p65 antibody. Lower panel, Ad.AEG-1 infection activates NF-κB by IκB[unk] degradation. HeLa cells were infected with Ad.AEG-1 (50 pfu/cell) or treated with TNF-[unk](10 ng/ml). The expressions of the indicated proteins were analyzed in cytoplasmic extracts by Western blot analysis 2 days later. B. AEG-1 activates NF-κB promoter activity. HeLa cells were infected with Ad.AEG-1 (50 pfu/cell) or treated with TNF-α (10 ng/ml) and 12 hr later were transfected with the indicated plasmids using Lipofectamine 2000. Luciferase assays were performed 48 hr after transfection using a Luciferase Reporter Gene Assay kit according to the manufacturer's protocol. The β-galactosidase activity was determined using the Galacto-Light Plus kit. Luciferase activity was normalized by β-galactosidase activity, and the data presented is the fold-activation relative to pGL3-Basic from triplicate determinations. C. Inhibition of NF-κB blocks AEG-1-induced increased anchorage independent growth. Upper panel, HeLa cells were either uninfected or infected with Ad.AEG-1 or Ad.IκBα–mt32 either alone or in combination at an m.o.i. of 50 pfu/cell for each Ad. Two days later the level of p65 was determined in the nuclear extracts by Western blot analysis. Lower panel, HeLa cells were infected as in the upper panel. Twenty-four hr later, cells (1 × 105) were replated in 0.4% agar on 0.8% base agar. Two weeks later, colonies ≥ 50 cells were counted under a dissection microscope. Data represents mean ± SD of triplicate plates in three independent experiments. D. Inhibition of NF-κB blocks AEG-1-induced enhanced matrigel invasion. Left panel, HeLa cells were infected as in Fig. 4C. Twenty-four hr later, cells (2.5 × 104) were seeded onto the upper chamber of a matrigel invasion chamber system in the absence of serum. Forty-eight hr after seeding the filters were fixed, stained and photographed. Right panel, graphical representation of the invasion assay. Data represents mean ± SD of three independent experiments. Reproduced, by permission of the publisher, from Emdad et al (2006).
4.3. AEG-1 and Ha-ras
The aberrant activation of RAS proteins has been implicated in facilitating virtually all aspects of the malignant phenotype of the cancer cell, including cellular proliferation, transformation, invasion and metastasis (Malumbres & Barbacid, 2001). Ras interacts with and regulates multiple downstream effectors that stimulate diverse cytoplasmic signaling activities (Feig & Buchsbaum, 2002; Shields et al., 2000). The most widely studied effectors of Ras signaling are the Raf serine/threonine kinases (c-Raf-1, A-Raf, B-Raf) (Chong et al., 2003). Ras promotes Raf association with the plasma membrane, where other events facilitate Raf activation. Raf then phosphorylates and activates the MEK1 and MEK2 dual specificity kinases. MEK1/2 are kinases for ERK1/2 mitogen-activated protein kinases (MAPKs). Activated MAPKs translocate to the nucleus where they regulate gene expression by modulating transcription factors, including those of the Ets family (Chambers & Tuck, 1993; Seth et al., 1992; Yordy & Muise-Helmericks, 2000).
Another commonly studied signal transduction route downstream of oncogenic Ras activation is the PI3K pathway (Rodriguez-Viciana et al., 1994; Rodriguez-Viciana et al., 1997). This kinase phosphorylates the signaling molecule phosphatidylinositol 4,5-biphosphate to form phosphatidylinositol 3,4,5-triphosphate (PIP3). PIP3 can then activate the serine/threonine kinase Akt/PKB. Ras-dependent stimulation of Akt activation leads to upregulation of the transcription factor NF-κB, and can increase cell survival, perhaps by blunting apoptotic signaling (Mayo et al., 1997).
Besides classical protein kinase cascades as Ras effector pathways in tumorigenesis, recent publications point towards an important synergistic participation of Rho GTPases, especially Rac1, RhoA and cdc42, in the cellular transformation of epithelial cells by oncogenic Ras (Sahai et al., 2001; Shields et al., 2000). Rho GTPases are mainly involved in regulating actin cytoskeleton organization and cell cycle progression and gene transcription (Etienne-Manneville & Hall, 2002; Ridley, 2001). Ras signaling also involves Rac/JNK and Rac/p38 MAPK pathways, both of which appear to be involved in cell stress responses, growth inhibition, and apoptotic signaling (Coso et al., 1995; Minden et al., 1995; Xia et al., 1995). Activation of Ras signaling pathways is essential for cells to leave a quiescent state and pass through the G1 phase of the cell cycle (Peeper et al., 1997). Under normal conditions the action of Ras and other members of the Ras pathway are stringently regulated during the cell cycle and under different growth conditions (Macaluso et al., 2002).
Amplification of ras proto-oncogenes and activating mutations that lead to the expression of constitutively active Ras proteins are observed in approximately 30% of all human cancers (Bos, 1989; Flotho et al., 1999; Stirewalt et al., 2001). In a tumor cell, the oncogenic activation of ras is a consequence of point mutations that either impair GTPase activity or enhance GTP binding affinity, resulting in a highly active proliferative signal (White et al., 1995). The effects of Ras on proliferation and tumorigenesis have been documented in immortal cell lines (Chang et al., 2003). However, antiproliferative responses of oncogenic Ras have also been observed in nontransformed cells including primary rat Schwann cells and primary fibroblast cells of human and murine origins (Bar-Sagi & Feramisco, 1985; Benito et al., 1991; Hirakawa & Ruley, 1988; Malumbres et al., 2000). Consequently, aberrant Ras signaling represents a nodal pathway regulating tumor cell growth and provides a potential target for cancer therapy.
Our recent study indicates that AEG-1 is a downstream target gene of Ha-ras and Ha-ras-mediated AEG-1 induction is regulated mainly at the transcriptional level rather than by modulating protein stability. Transfection with AEG-1 siRNA results in a significant reduction in colony formation of THR cells (Fig. 4C), indicating that AEG-1 plays an essential role as a downstream target gene in oncogenic Ha-ras-mediated proliferation and transforming activities. We further documented that Ha-ras mediates AEG-1 induction through the PI3K/GSK3β/c-Myc signaling pathway (Lee et al., 2006). Accordingly, Myc, but not USF-1, protein levels were higher in THR cells than in THV cells and LY294002, a PI3K inhibitor, reduced Myc protein levels in THR cells suggesting a correlation between GSK3β phosphorylation by Ha-ras-mediated AKT activation and Myc expression in THR cells (Lee et al., 2006). Reduction of AEG-1 expression by LY294002 was confirmed at the protein level as well as the transcription level. siRNA targeting c-myc significantly decreased AEG-1 protein expression further supporting a functional role of c-myc in Ha-ras-mediated AEG-1 expression.
PI3K-AKT signals control several growth-regulatory transcription factors. Two prominent examples are the forkhead box (FOXO) protein and NF-κB. Other transcriptional regulators whose activities are affected by PI3K-AKT signaling include MIZ-1, p53, AP-1, c-Myc, β-catenin and HIF1α (Bader et al., 2005). The exact roles of these proteins during PI3K-mediated oncogenesis are currently unknown, but they have all been linked to oncogenic transformation. AKT phosphorylates and thereby inactivates the cell-cycle inhibitor MIZ1 and also suppresses p53 activity by a mechanism that involves MDM2. By contrast, the activity of AP-1, c-Myc and β-catenin are increased by AKT. These targets are negatively controlled by GSK3β, which is inactivated by AKT-mediated phosphorylation (Bader et al., 2005). In vitro studies demonstrated that GSK3β could phosphorylate serine residues 38, 42, and 62, and threonine residue 58 within the transactivation domain of Myc, that are considered important for regulation of Myc transcriptional activity in vivo (Grimes & Jope, 2001; Henriksson et al., 1993; Pulverer et al., 1994; Saksela et al., 1992). Phosphorylation of Thr-58 and Ser-62 by GSK3β negatively regulate Myc function, resulting in decreased cellular growth and transformation. In human neuroblastoma SH-SY5Y cells, inhibition of GSK3β by lithium causes increased Myc nuclear protein levels (Grimes & Jope, 2001). Our recent study confirms that PI3K signaling in THR cells phosphorylates AKT and GSK3β, which activates c-Myc, but not USF-1, resulting in increased AEG-1 expression (Lee et al., 2006). We also observed that AEG-1 expression was mildly elevated by treatment with the MEK inhibitor PD98059 and siRNA of MEK2. Recent studies demonstrate that blockade of ERK1/2 with PD98059 cause induction of specific genes, such as IL-6, CYP1A1 and NF-κB, even though other downstream Ras signaling pathways induce their activity (de Haij et al., 2005; Janssen-Heininger et al., 1999; Shin et al., 2005).
The complex relationships among diverse extracellular conditions and cellular responses to a distinct stimulus have only recently begun to be elucidated at the mechanistic level. It will be very interesting to delineate the potential cross-talk relationship between the PI3K and MEK signaling pathways in regulating Ha-ras-mediated AEG-1 expression. Our findings elucidate a novel mechanism of Ha-ras-mediated tumorigenesis and delineate a crucial role of AEG-1 in promoting cancer development and/or maintenance (Lee et al., 2006).
4.4. AEG-1- and HIV-induced neurodegeneration
Glutamate is the main excitatory amino acid transmitter in the mammalian central nervous system and is probably involved in most aspects of normal brain function including cognition, memory and learning (Fonnum, 1984; Headley & Grillner, 1990). Glutamate acts at ionotropic and metabotropic receptors, the primary ionotropic receptor classes being NMDA and AMPA/kainite (Pitt et al., 2000). Extracellular glutamate levels are tightly regulated by transporters; there are distinct classes of transporters on neurons and on astrocytes but the bulk of glutamate uptake appears to be mediated by astrocytes (Anderson & Swanson, 2000). Recent research suggests that astrocytes play a critical role in neuronal signal transmission by enhancing synaptic activity and strength, increasing the number of synapses, and modulating neuronal activity (Beattie et al., 2002; Oliet et al., 2001; Ullian et al., 2001). Excessive glutamate exposure is toxic to neurons, probably resulting in large part from glutamate-triggered Ca2+ entry (Choi, 1988). Because glutamate is not metabolized in the extracellular environment, the maintenance of normal glutamatergic neurotransmission or the prevention of glutamate-induced neurodegenerative disorders depend on the presence of active glutamate transport systems in glial cells and neurons (Perego et al., 2000).
Five different isoforms of glutamate transporters (excitatory amino acid carriers) have now been identified and cloned: GLAST (EAAT1), GLT-1 (EAAT2), EAAC-1 (EAAT3), EAAT4, and EAAT5 (Arriza et al., 1994; Fairman et al., 1995; Kanai & Hediger, 1992; Pines et al., 1992; Storck et al., 1992). Immunolocalization studies have revealed that EAAT2 and EAAT1 are primarily expressed in astroglial cells, whereas EAAC1 and EAAT4 are present primarily in neurons (Chaudhry et al., 1995; Fairman et al., 1995; Furuta et al., 1997; Lehre & Danbolt, 1998; Pines et al., 1992). Tanaka et al have demonstrated that the astroglial EAAT2 transporter is responsible for the majority of the clearance of glutamate from neuronal synapses in the central nervous system (Tanaka et al., 1997). Impaired glutamate uptake by glial cells can result in cell death from excessive levels of glutamate and overstimulation of glutamate receptors (Choi, 1988). Indeed, downregulation of EAAT2 followed by accumulation of glutamate in the ECF and neuronal death have been documented in a wide variety of neurodegenerative disorders, including amyotrophic lateral sclerosis (ALS), Alzheimer's disease, several forms of epilepsy, ischemia/stroke, HIV-1-associated dementia (HAD), traumatic brain injury and hepatic encephalopathy (Choi, 1988; Doble, 1999; Kaul et al., 2001; Masliah et al., 1996). The importance of regulating glutamate transport is further underscored by the observation that mice lacking EAAT2 develop progressive neurodegeneration and epilepsy as a result of aberrant glutamate homeostasis (Rothstein et al., 1996; Tanaka et al., 1997).
Dementia is a major complication of HIV-1 infection (Lipton & Gendelman, 1995; Navia & Price, 1987). The incidence of HAD declined after the introduction of highly active antiretroviral therapies, but it still reaches 10% (Sacktor et al., 2001). The pathological hallmarks of HAD include neuronal loss, reactive gliosis, and white matter pallor (Budka, 1991; Everall et al., 1991; Sharer et al., 1986). HIV-1 infects infiltrating macrophages, microglial cells, and astrocytes in the brain but it is rarely found in neurons (Koenig et al., 1986; Saito et al., 1994; Wiley et al., 1986), suggesting indirect viral action in neuronal cell loss. HIV-1 infection of PHFA results in downregulation of both EAAT2 mRNA and protein and also glutamate uptake in these cells (Wang et al., 2003). In a similar manner, treatment with TNF-α, which might be secreted from HIV-infected macrophages, downregulates both EAAT2 and EAAT1 mRNA and protein and also glutamate uptake in PHFA suggesting an involvement of glutamate excitotoxicity in the pathogenesis of HAD (Su et al., 2003c; Wang et al., 2003).
Neoplastic transformation of human astrocytes causes malignant gliomas, which are often associated with seizures and neuronal necrosis (Ye et al., 1999). It has been shown that Na+-dependent glutamate uptake in glioma cell lines derived from human tumors is up to 100-fold lower than in astrocytes (Ye et al., 1999). Immunohistochemical studies document very low expression levels of EAAT2 both in glioma cell lines as well as in brain tissues from glioblastoma patients (Ye et al., 1999). This leads to excessive glutamate release from glioma cells in peritumoral brain tissue that may contribute to tumor-associated necrosis and possibly to seizures.
Since it has an enormous role in maintaining normal brain function, numerous studies have been performed to understand the regulatory mechanisms underlying the expression pattern of EAAT2 (Collier, 1998; Gegelashvili et al., 1997; Miralles et al., 2001; Mitrovic et al., 1998; Munch et al., 2000; Palmada et al., 2002; Robinson, 1998; Vandenberg et al., 1997; Zelenaia et al., 2000). Expressions of both the mRNA and protein have been shown to be downregulated by TNF-α and upregulated by TGFα, EGF and cAMP (Gegelashvili et al., 1997; Schlag et al., 1998; Zelenaia et al., 2000). However, how the gene was transcriptionally controlled had not been elucidated because of lack of information about the genomic organization of the gene. Previous attempts to identify the promoter region of the EAAT2 gene were not fruitful because of the presence of an extremely large intron (approximately 100kb) that separates exon 1 from exon 2 and incomplete sequence information for exon 1. We successfully cloned the EAAT2 promoter (EAAT2-promoter) using a sequential progressive genomic scanning (SPGS) approach (Su et al., 2003c). The EAAT2 promoter functions primarily in astrocytes (PHFA) and its activity is modulated by those agents that regulate expression of EAAT2 mRNA and protein (Su et al., 2003c).
Sitcheran et al. (2005) have shown that both TNF-α-mediated repression and EGF-mediated activation of EAAT2 expression require NF-κB, though they utilize distinct signaling pathways. TNF-α induces the classical IκBα degradation pathway to trigger NF-κB nuclear translocation and DNA binding to repress EAAT2 expression. In contrast, EGF activates EAAT2 expression independently of IκBα degradation and NF-κB nuclear translocation. HIV-1 infection downregulates EAAT2-promoter activity (Su et al., 2003c). TNF-α plays an important role in the pathogenesis of HAD. Exogenous TNF-α inhibits glutamate uptake through a reduction in Vmax for glutamate transport and activation of endogenous TNF-α by ligation of CXCR4 on astrocytes induces glutamate release (Bezzi et al., 2001). However, Wang et al. (2003) recently observed no TNF-α production following HIV-1 infection or gp120 treatment of PHFA under conditions where EAAT2 function and transcription are inhibited. TNF-α requires 24 hr to achieve maximal inhibition of glutamate uptake and it affects both EAAT1 and EAAT2 expression. HIV-1, in contrast, achieves maximal inhibition 6 hr after infection and its effects were mediated predominantly by changes in EAAT2 expression.
We recently discovered that infection of PHFA with HIV-1, treatment with gp120 or TNF-α resulted in elevated AEG-1 expression and decreased EAAT2 expression (Kang et al., 2005; Su et al., 2003a). This inverse correlation of AEG-1 and EAAT2 expression by HIV infection and AEG-1 downregulation of the EAAT2 promoter indicate a potential contribution of AEG-1 to the generation of HAD. The direct effect of AEG-1 expression on EAAT2 promoter activity was analyzed by transient co-transfection of an AEG-1 expression vector with a luciferase reporter linked to the EAAT2-promoter (Fig. 6A). Ectopic over-expression of AEG-1 specifically inhibited EAAT2 promoter activity (∼3.5 fold) in PHFA, while EAAT1 promoter activity was not affected by AEG-1. AEG-1 downregulates the EAAT2 promoter activity in IM-PHFA (h-TERT immortalized PHFA) and to a similar extent in PHFA, but not in H4 astroglioma cells (Fig. 6B). In contrast, expression of PTEN that suppresses the PI–3K signaling pathway, effectively inhibited EAAT2-promoter activity in all three-cell types (Fig. 6B). Activation of the PI–3K–Akt signaling pathway was required for upregulation of EAAT2 promoter activity by EGF and cAMP (Su et al., 2003c). In this context, high EAAT2 expression in H4 cells might result from constitutive activation of the PI–3K–Akt pathway (Knobbe et al., 2002). Taken together, AEG-1 expression induced after 3 and 7 days following HIV-1 infection or treatment with TNF-α or gp120 (Su et al., 2002) in PHFA may directly contribute to EAAT2 downregulation through a PI3K–Akt-independent pathway. Analysis of AEG-1 expression in other neurodegenerative diseases will provide a global perspective of the role of AEG-1 in the molecular pathogenesis of these disorders.
Fig. 6.
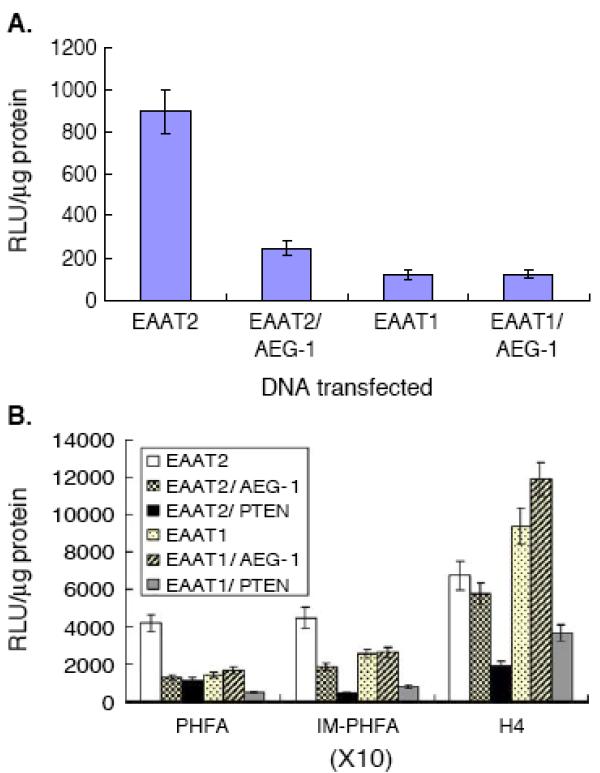
Effect of AEG-1 expression on EAAT2 promoter activity. A. A luciferase reporter vector containing the complete EAAT2 or EAAT1 promoter was co-transfected with either pcDNA3.1 or pcDNA3.1-AEG-1-HA. Two days later, cells were harvested and luciferase activity of the protein extracts was measured. Data shown is representative of multiple experiments, which varied by ≤ 15%. Results presented ± S.D. from two independent experiments. B. A luciferase reporter vector containing the complete EAAT2 or EAAT1 promoter was co-transfected with the indicated expression vectors. Samples were obtained and analyzed as above. Data shown is representative of multiple experiments, which varied by ≤ 15%. Results presented ± S.D. from two independent experiments. Reproduced, by permission of the publisher, from Kang et al (2005).
In the glioma model, promotion of tumor formation might be ascribed to AEG-1-mediated EAAT2 downmodulation. Growth of the glioma mass is restricted by limited intracranial space and neighboring tissue. Glutamate excitotoxicity to neighboring neurons resulting from active release of glutamate or by reduction of glutamate transporter activity and subsequent glutamate uptake is known to promote malignant glioma pathogenesis (Sontheimer, 2003; Ye et al., 1999). In fact, EAAT2 promoter activity in glioma cells, except H4, is lower by an order of magnitude than in PHFA and several-fold lower than in IM-PHFA (Kang et al., 2005) and our recent evidence indicates that AEG-1 expression is significantly upregulated in malignant glioma cells as well as in tumor samples (unpublished data). It is conceivable that an impairment of glutamate transporter regulation by AEG-1 may contribute to distinct biological properties of astrocytic tumors such as edema, necrosis and tumor-related seizures.
4.5. AEG-1 promoter analysis
Genomic BLAST search indicates that human AEG-1 (accession no. AF411226) consists of 12 exons and 11 introns that extend over a region of ∼82-kb and is located at chromosome 8q22.1. We isolated the 5′ upstream region of AEG-1, employing a PCR technique, using an anti-sense primer in exon 1 of the AEG-1 gene and a sense primer in the 5′ genomic sequence with genomic DNA isolated from primary human fetal astrocytes as a template. The resulting 2,759-bp fragment was cloned into the luciferase reporter plasmid pGL3-Basic (pGL3-AEG promoter). Employing primer extension analysis using an antisense oligonucleotide derived from the first exon of AEG-1, four transcription initiation sites were identified (Lee et al., 2006). Bioinformatics analysis revealed that the human AEG-1 promoter (−2710/+49) lacks consensus TATA and CAAT boxes, but contains multiple Sp1 binding motifs and high GC content. The presence of multiple transcription initiation sites, multiple Sp1 sites and high GC content has also been recognized in many other TATA-less promoters. Ha-ras overexpression results in an ∼4-fold increase in human AEG-1 promoter activity (Lee et al., 2006). Similarly, AEG-1 promoter activity is significantly higher in Ha-ras-stably transfected cells such as THR and CREF-ras cells, indicating that the AEG-1 promoter has a significant transcriptional response to the activated Ha-ras pathway (Lee et al., 2006).
To determine the intracellular signaling pathway by which Ha-ras induces AEG-1 promoter activity, a PI3K inhibitor LY294002 and a MEK inhibitor PD98059 were used. The addition of LY294002 but not PD98059, significantly attenuated Ha-ras-mediated AEG-1 promoter activation in THR cells with little change in basal AEG-1 promoter activity in THV cells (Lee et al., 2006). PTEN is a phosphatase that selectively dephosphorylates the 3 positions of both phophatidylinositol-3,4,5-triphosphate and phophatidylinositol-4,5-bisphosphate, antagonizing the diverse downstream signaling effector pathways activated by PI3K-derived phospholipids (Bader et al., 2005; Sulis & Parsons, 2003). Co-transfection of a PTEN expression plasmid with pGL3-AEG1-promoter also significantly attenuated Ha-ras-induced AEG-1 promoter activity in THR cells without affecting the basal promoter activity in THV cells (Lee et al., 2006). As observed with PD98059, siRNA for MEK1 and MEK2 showed no inhibitory effect on Ha-ras-induced AEG-1 promoter activity, indicating that the PI3K signaling pathway is involved in Ha-ras-mediated AEG-1 promoter activation.
We also determined in more detail the cis-elements in the AEG-1 promoter essential for response to Ha-ras. Serial deletions from −2710-bp to −459-bp showed an ∼3-fold increase in promoter activity in both THV and THR cell lines, indicating the presence of negative transcriptional control elements in this region (−2710/−459) (Lee et al., 2006). Deletion from −459 to −301-bp resulted in a 90% loss of basal as well as Ha-ras-induced promoter activity in THV and THR cells, suggesting that transcription factors binding to this region (−459/−301) are capable of regulating both basal as well as Ha-ras-induced AEG-1 promoter activity.
Further experiments identified the transcription factors binding to the −459/−301 region as critical elements in regulating Ha-ras-induced AEG-1 promoter activity. Employing electrophoretic mobility shift assays we demonstrated that the region −356/−302 of the AEG-1 promoter binds to Ha-ras-activated transcription factors. This region contains several putative transcription factor binding sites, including two E-box elements to which the bHLH proteins such as c-Myc and USF-1 bind. Indeed, activation of two E-box elements in the AEG-1 promoter by increased c-Myc binding was shown to be critical for the Ha-ras-mediated AEG-1 induction (Lee et al., 2006).
A number of genes involved in tumor progression and metastasis, such as osteopontin, cdc2, connexin 43 and MMP-9, show augmented expression by the cooperative action of Ras and c-Myc (Born et al., 1994; Carystinos et al., 2003; Denhardt et al., 2003; Himelstein et al., 1997; Leone et al., 1997). However, as yet no study has demonstrated that the induction of a ras-responsive gene is mediated by direct binding of c-Myc to the gene promoter. In these contexts, our studies add a new dimension to the molecular circuitry in the ras-c-myc axis. We also confirmed that the human AEG-1 promoter has both positive (459/−302) and negative regulatory regions (−738/−460). Although the positive regulatory region contains several putative transcription factor binding sites critical for basal promoter activity, such as Sp1, E-box element, CREB and Ets-2 (Ghosh et al., 2005; Kim et al., 2003; O'Leary & Kasper, 2000; Ye et al., 1993), our data indicate that two E-box elements in this region are functional and important for both basal and ras-induced promoter activity. The negative regulatory region has putative RAR-α and YY1 binding sites that have been shown to act predominantly as a repressor of transcription (Gordon et al., 2006; Schule et al., 1991; Sucharov et al., 2004). Current studies are in progress to elucidate the involvement of these transcription factors in mediating transcriptional repression of AEG-1.
5. Conclusion and future perspectives
An enhanced understanding of the regulators of common signaling pathways involved in tumor progression and metastasis will pave the way for the development of more potent and selective inhibitors of neoplasia. This should be a useful adjunct to conventional therapies, potentially permitting interference with tumor progression at several nodal points in this process. This review highlights recent studies on AEG-1, which strongly support a central role of AEG-1 in tumor progression and metastasis through the regulation of highly integrated signaling events (Fig. 7). There are many questions that remain regarding the cellular function(s) of AEG-1 and how it influences metastatic progression. Although AEG-1 interacts with the p65 component of NF-κB, it remains to be determined how this interaction affects the function of interacting partners and whether this interaction is relevant to tumor progression. Further studies are also necessary to determine if AEG-1 alters oncogenic activation of specific signaling pathways that results in differential expression of functionally relevant proteins. By identifying and characterizing the interaction of AEG-1 with its effectors, we can expand our understanding of how this molecule mechanistically engages cells to adopt a more progressive, and consequently, a more metastatic phenotype. Other important areas worth exploring include defining the role of AEG-1 in the pathogenesis of neurodegenerative diseases as well as HAD and the mechanism by which AEG-1 modulates EAAT2 promoter activity. Understanding the detailed molecular mechanism(s) of AEG-1 function and regulation will help clarify its role in the process of tumor progression and facilitate development of potential therapeutic strategies to impede not only tumor development, but also metastasis. Moreover, based on the ras-responsive nature of the AEG-1 promoter, constructs containing this region could provide useful vectors to selectively deliver tumor suppressor genes, such as p53 or mda-7/IL-24 (Fisher, 2005; Gupta et al., 2006; Lebedeva et al., 2003), for gene therapy of cancers containing activated Ras oncogenes.
Fig. 7.
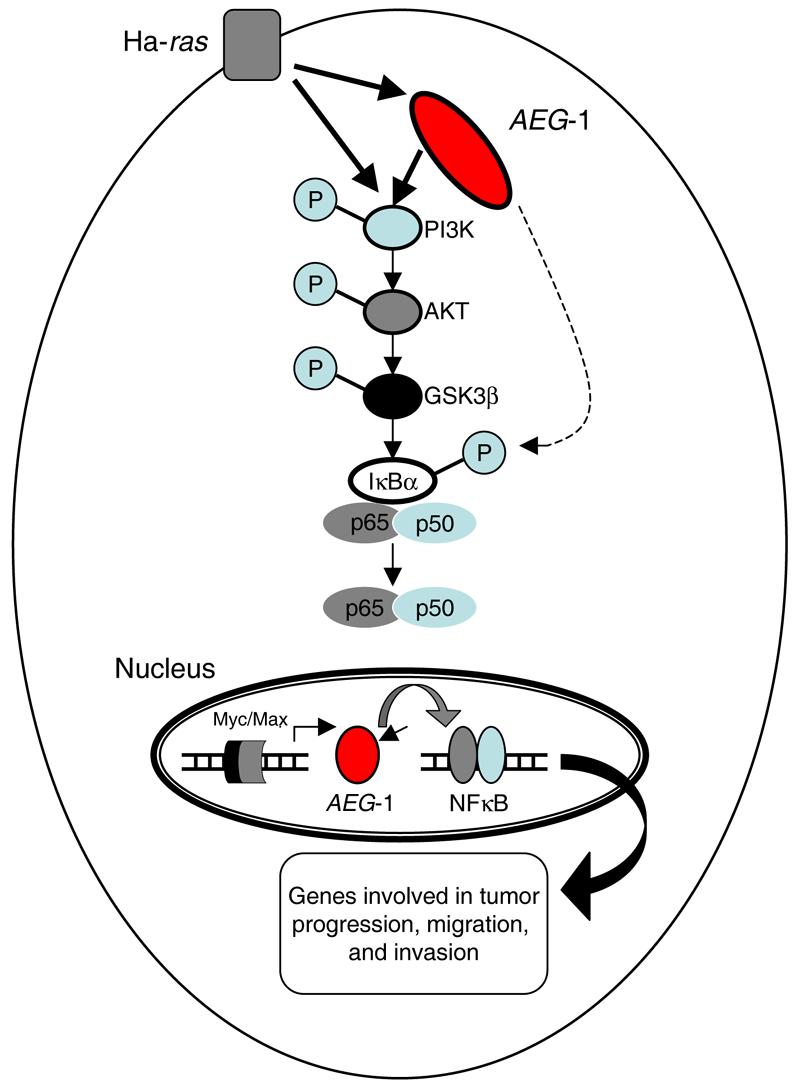
Hypothetical model of signal transduction pathways involved in Ha-ras-mediated AEG-1 induction. Ha-ras activates the PI3K signaling cascade resulting in increased binding of Myc-Max to the AEG-1 promoter and augments AEG-1 expression. AEG-1 activates the NF-κB pathway that regulates expression of genes involved in migration and invasion and thus plays a crucial role in Ha-ras-mediated tumor progression.
Acknowledgements
The present studies were supported in part by National Institutes of Health grants P01 NS31492 (DJV, PBF) and R01 CA35675 (PBF), the Samuel Waxman Cancer Research Foundation (PBF), the Chernow Endowment (PBF), a Joelle Syverson Fellowship from the American Brain Tumor Association (LE); and a grant from the Goldhirsh Foundation (DS). PBF is the Michael and Stella Chernow Urological Cancer Research Scientist and a SWCRF Investigator.
Abbreviations
- AEG-1
astrocyte elevated gene-1
- AKT
protein kinase B
- bHLH
basic-helix-loop-helix
- C-ORF
complete open reading frame
- CXCR4
chemokine (C-X-C motif) receptor 4
- EAAT2 and EAAT1
excitatory amino acid transporter 1 and 2, respectively
- EGF
epidermal growth factor
- GSK-3β
glycogen synthase kinase 3 beta
- HIF1α
hypoxia-inducible factor 1 α subunit
- HIV-1
human immunodeficiency virus-1
- HAD
HIV-associated dementia
- IM-PHFA
hTERT (human telomerase) immortalized primary human fetal astrocyte
- MIZ1
Msx-interacting-zinc finger
- MMP
matrix metalloproteinase
- ORF
open reading frame
- PHFA
primary human fetal astrocyte
- PI3K
phosphoinositide kinase-3
- PTEN
phosphatase and tensin homolog
- RAR-α
retinoic acid receptor –α
- RaSH
rapid subtraction hybridization
- THV
human adult astrocytes immortalized by SV40 T/t Ag and hTERT
- THR
stable overexpression of Ha-ras in THV cells
- TNF-α
tumor necrosis factor-α
- USF-1
upstream stimulating factor-1
- YY1
YY1 transcription factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. J Neurosci. 1994;14:5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader AG, Kang S, Zhao L, Vogt PK. Oncogenic PI3K deregulates transcription and translation. Nat Rev Cancer. 2005;5:921–929. doi: 10.1038/nrc1753. [DOI] [PubMed] [Google Scholar]
- Baldwin AS., Jr. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest. 2001a;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin AS., Jr. The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- Baldwin AS., Jr. Series introduction: the transcription factor NF-kappaB and human disease. J Clin Invest. 2001b;107:3–6. doi: 10.1172/JCI11891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Sagi D, Feramisco JR. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell. 1985;42:841–848. doi: 10.1016/0092-8674(85)90280-6. [DOI] [PubMed] [Google Scholar]
- Bargou RC, Emmerich F, Krappmann D, Bommert K, Mapara MY, Arnold W, et al. Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin's disease tumor cells. J Clin Invest. 1997;100:2961–2969. doi: 10.1172/JCI119849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, et al. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- Benito M, Porras A, Nebreda AR, Santos E. Differentiation of 3T3-L1 fibroblasts to adipocytes induced by transfection of ras oncogenes. Science. 1991;253:565–568. doi: 10.1126/science.1857988. [DOI] [PubMed] [Google Scholar]
- Benos DJ, Hahn BH, Bubien JK, Ghosh SK, Mashburn NA, Chaikin MA, et al. Envelope glycoprotein gp120 of human immunodeficiency virus type 1 alters ion transport in astrocytes: implications for AIDS dementia complex. Proc Natl Acad Sci U S A. 1994a;91:494–498. doi: 10.1073/pnas.91.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benos DJ, Hahn BH, Shaw GM, Bubien JK, Benveniste EN. gp120-mediated alterations in astrocyte ion transport. Adv Neuroimmunol. 1994b;4:175–179. doi: 10.1016/s0960-5428(06)80254-8. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Domercq M, Brambilla L, Galli R, Schols D, De Clercq E, et al. CXCR4-activated astrocyte glutamate release via TNFalpha: amplification by microglia triggers neurotoxicity. Nat Neurosci. 2001;4:702–710. doi: 10.1038/89490. [DOI] [PubMed] [Google Scholar]
- Blumenthal DT, Raizer JJ, Rosenblum MK, Bilsky MH, Hariharan S, Abrey LE. Primary intracranial neoplasms in patients with HIV. Neurology. 1999;52:1648–1651. doi: 10.1212/wnl.52.8.1648. [DOI] [PubMed] [Google Scholar]
- Born TL, Frost JA, Schonthal A, Prendergast GC, Feramisco JR. c-Myc cooperates with activated Ras to induce the cdc2 promoter. Mol Cell Biol. 1994;14:5710–5718. doi: 10.1128/mcb.14.9.5710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos JL. ras oncogenes in human cancer: a review. Cancer Res. 1989;49:4682–4689. [PubMed] [Google Scholar]
- Boukerche H, Su Z.-z., Fisher PB. Cloning differentially expressed genes using rapid subtraction hybridization (RaSH) In: Fisher PB, editor. Methods in Molecular Biology. Humana Press; New Jersey: 2007. in press. [DOI] [PubMed] [Google Scholar]
- Boukerche H, Su Z.-z., Kang D-C, Fisher PB. Identification and cloning of genes displaying elevated expression as a consequence of metastatic progression in human melanoma cells by rapid subtraction hybridization. Gene. 2004;343:191–201. doi: 10.1016/j.gene.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Bours V, Dejardin E, Goujon-Letawe F, Merville MP, Castronovo V. The NF-kappa B transcription factor and cancer: high expression of NF-kappa B- and I kappa B-related proteins in tumor cell lines. Biochem Pharmacol. 1994;47:145–149. doi: 10.1016/0006-2952(94)90448-0. [DOI] [PubMed] [Google Scholar]
- Brack-Werner R. Astrocytes: HIV cellular reservoirs and important participants in neuropathogenesis. Aids. 1999;13:1–22. doi: 10.1097/00002030-199901140-00003. [DOI] [PubMed] [Google Scholar]
- Brown DM, Ruoslahti E. Metadherin, a cell surface protein in breast tumors that mediates lung metastasis. Cancer Cell. 2004;5:365–374. doi: 10.1016/s1535-6108(04)00079-0. [DOI] [PubMed] [Google Scholar]
- Budka H. Neuropathology of human immunodeficiency virus infection. Brain Pathol. 1991;1:163–175. doi: 10.1111/j.1750-3639.1991.tb00656.x. [DOI] [PubMed] [Google Scholar]
- Carrasco D, Rizzo CA, Dorfman K, Bravo R. The v-rel oncogene promotes malignant T-cell leukemia/lymphoma in transgenic mice. Embo J. 1996;15:3640–3650. [PMC free article] [PubMed] [Google Scholar]
- Carystinos GD, Kandouz M, Alaoui-Jamali MA, Batist G. Unexpected induction of the human connexin 43 promoter by the ras signaling pathway is mediated by a novel putative promoter sequence. Mol Pharmacol. 2003;63:821–831. doi: 10.1124/mol.63.4.821. [DOI] [PubMed] [Google Scholar]
- Chambers AF, Tuck AB. Ras-responsive genes and tumor metastasis. Crit Rev Oncog. 1993;4:95–114. [PubMed] [Google Scholar]
- Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL, et al. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia. 2003;17:1263–1293. doi: 10.1038/sj.leu.2402945. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Lehre KP, van Lookeren Campagne M, Ottersen OP, Danbolt NC, Storm-Mathisen J. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15:711–720. doi: 10.1016/0896-6273(95)90158-2. [DOI] [PubMed] [Google Scholar]
- Choi DW. Glutamate neurotoxicity and diseases of the nervous system. Neuron. 1988;1:623–634. doi: 10.1016/0896-6273(88)90162-6. [DOI] [PubMed] [Google Scholar]
- Chong H, Vikis HG, Guan KL. Mechanisms of regulating the Raf kinase family. Cell Signal. 2003;15:463–469. doi: 10.1016/s0898-6568(02)00139-0. [DOI] [PubMed] [Google Scholar]
- Collier DA. Abnormal EAAT2 splicing in amyotrophic lateral sclerosis. Mol Psychiatry. 1998;3:298. [PubMed] [Google Scholar]
- Conant K, McArthur JC, Griffin DE, Sjulson L, Wahl LM, Irani DN. Cerebrospinal fluid levels of MMP-2, 7, and 9 are elevated in association with human immunodeficiency virus dementia. Ann Neurol. 1999;46:391–398. doi: 10.1002/1531-8249(199909)46:3<391::aid-ana15>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Coso OA, Chiariello M, Yu JC, Teramoto H, Crespo P, Xu N, et al. The small GTP-binding proteins Rac1 and Cdc42 regulate the activity of the JNK/SAPK signaling pathway. Cell. 1995;81:1137–1146. doi: 10.1016/s0092-8674(05)80018-2. [DOI] [PubMed] [Google Scholar]
- de Haij S, Bakker AC, van der Geest RN, Haegeman G, Vanden Berghe W, Aarbiou J, et al. NF-kappaB mediated IL-6 production by renal epithelial cells is regulated by c-jun NH2-terminal kinase. J Am Soc Nephrol. 2005;16:1603–1611. doi: 10.1681/ASN.2004090781. [DOI] [PubMed] [Google Scholar]
- Denhardt DT, Mistretta D, Chambers AF, Krishna S, Porter JF, Raghuram S, et al. Transcriptional regulation of osteopontin and the metastatic phenotype: evidence for a Ras-activated enhancer in the human OPN promoter. Clin Exp Metastasis. 2003;20:77–84. doi: 10.1023/a:1022550721404. [DOI] [PubMed] [Google Scholar]
- Devalaraja MN, Wang DZ, Ballard DW, Richmond A. Elevated constitutive IkappaB kinase activity and IkappaB-alpha phosphorylation in Hs294T melanoma cells lead to increased basal MGSA/GRO-alpha transcription. Cancer Res. 1999;59:1372–1377. [PubMed] [Google Scholar]
- Doble A. The role of excitotoxicity in neurodegenerative disease: implications for therapy. Pharmacol Ther. 1999;81:163–221. doi: 10.1016/s0163-7258(98)00042-4. [DOI] [PubMed] [Google Scholar]
- Emdad L, Sarkar D, Su Z.-z., Randolph A, Boukerche H, Valerie K, et al. Activation of the nuclear factor kappaB pathway by astrocyte elevated gene-1: implications for tumor progression and metastasis. Cancer Res. 2006;66:1509–1516. doi: 10.1158/0008-5472.CAN-05-3029. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Everall IP, Luthert PJ, Lantos PL. Neuronal loss in the frontal cortex in HIV infection. Lancet. 1991;337:1119–1121. doi: 10.1016/0140-6736(91)92786-2. [DOI] [PubMed] [Google Scholar]
- Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995;375:599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- Feig LA, Buchsbaum RJ. Cell signaling: life or death decisions of ras proteins. Curr Biol. 2002;12:R259–261. doi: 10.1016/s0960-9822(02)00787-x. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. The biology of cancer metastasis or, ‘you cannot fix it if you do not know how it works’. Bioessays. 1991;13:551–554. doi: 10.1002/bies.950131010. [DOI] [PubMed] [Google Scholar]
- Fidler IJ, Ellis LM. The implications of angiogenesis for the biology and therapy of cancer metastasis. Cell. 1994;79:185–188. doi: 10.1016/0092-8674(94)90187-2. [DOI] [PubMed] [Google Scholar]
- Fine SM, Angel RA, Perry SW, Epstein LG, Rothstein JD, Dewhurst S, et al. Tumor necrosis factor alpha inhibits glutamate uptake by primary human astrocytes. Implications for pathogenesis of HIV-1 dementia. J Biol Chem. 1996;271:15303–15306. doi: 10.1074/jbc.271.26.15303. [DOI] [PubMed] [Google Scholar]
- Fisher PB. Is mda-7/IL-24 a “magic bullet” for cancer? Cancer Res. 2005;65:10128–10138. doi: 10.1158/0008-5472.CAN-05-3127. [DOI] [PubMed] [Google Scholar]
- Flotho C, Valcamonica S, Mach-Pascual S, Schmahl G, Corral L, Ritterbach J, et al. RAS mutations and clonality analysis in children with juvenile myelomonocytic leukemia (JMML) Leukemia. 1999;13:32–37. doi: 10.1038/sj.leu.2401240. [DOI] [PubMed] [Google Scholar]
- Fonnum F. Glutamate: a neurotransmitter in mammalian brain. J Neurochem. 1984;42:1–11. doi: 10.1111/j.1471-4159.1984.tb09689.x. [DOI] [PubMed] [Google Scholar]
- Furuta A, Rothstein JD, Martin LJ. Glutamate transporter protein subtypes are expressed differentially during rat CNS development. J Neurosci. 1997;17:8363–8375. doi: 10.1523/JNEUROSCI.17-21-08363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegelashvili G, Danbolt NC, Schousboe A. Neuronal soluble factors differentially regulate the expression of the GLT1 and GLAST glutamate transporters in cultured astroglia. J Neurochem. 1997;69:2612–2615. doi: 10.1046/j.1471-4159.1997.69062612.x. [DOI] [PubMed] [Google Scholar]
- Ghosh D, Sachdev S, Hannink M, Roberts RM. Coordinate regulation of basal and cyclic 5′-adenosine monophosphate (cAMP)-activated expression of human chorionic gonadotropin-alpha by Ets-2 and cAMP-responsive element binding protein. Mol Endocrinol. 2005;19:1049–1066. doi: 10.1210/me.2004-0320. [DOI] [PubMed] [Google Scholar]
- Gilmore TD, Koedood M, Piffat KA, White DW. Rel/NF-kappaB/IkappaB proteins and cancer. Oncogene. 1996;13:1367–1378. [PubMed] [Google Scholar]
- Gordon S, Akopyan G, Garban H, Bonavida B. Transcription factor YY1: structure, function, and therapeutic implications in cancer biology. Oncogene. 2006;25:1125–1142. doi: 10.1038/sj.onc.1209080. [DOI] [PubMed] [Google Scholar]
- Grimes CA, Jope RS. The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol. 2001;65:391–426. doi: 10.1016/s0301-0082(01)00011-9. [DOI] [PubMed] [Google Scholar]
- Gupta P, Su ZZ, Lebedeva IV, Sarkar D, Sauane M, Emdad L, et al. mda-7/IL-24: Multifunctional cancer-specific apoptosis-inducing cytokine. Pharmacol Ther. 2006;111:596–628. doi: 10.1016/j.pharmthera.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS., Jr. NF-kappaB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–5799. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn WC, Weinberg RA. Modelling the molecular circuitry of cancer. Nat Rev Cancer. 2002;2:331–341. doi: 10.1038/nrc795. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Headley PM, Grillner S. Excitatory amino acids and synaptic transmission: the evidence for a physiological function. Trends Pharmacol Sci. 1990;11:205–211. doi: 10.1016/0165-6147(90)90116-p. [DOI] [PubMed] [Google Scholar]
- Henriksson M, Bakardjiev A, Klein G, Luscher B. Phosphorylation sites mapping in the N-terminal domain of c-myc modulate its transforming potential. Oncogene. 1993;8:3199–3209. [PubMed] [Google Scholar]
- Himelstein BP, Lee EJ, Sato H, Seiki M, Muschel RJ. Transcriptional activation of the matrix metalloproteinase-9 gene in an H-ras and v-myc transformed rat embryo cell line. Oncogene. 1997;14:1995–1998. doi: 10.1038/sj.onc.1201012. [DOI] [PubMed] [Google Scholar]
- Hinz M, Krappmann D, Eichten A, Heder A, Scheidereit C, Strauss M. NF-kappaB function in growth control: regulation of cyclin D1 expression and G0/G1-to-S-phase transition. Mol Cell Biol. 1999;19:2690–2698. doi: 10.1128/mcb.19.4.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa T, Ruley HE. Rescue of cells from ras oncogene-induced growth arrest by a second, complementing, oncogene. Proc Natl Acad Sci U S A. 1988;85:1519–1523. doi: 10.1073/pnas.85.5.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen-Heininger YM, Macara I, Mossman BT. Cooperativity between oxidants and tumor necrosis factor in the activation of nuclear factor (NF)-kappaB: requirement of Ras/mitogen-activated protein kinases in the activation of NF-kappaB by oxidants. Am J Respir Cell Mol Biol. 1999;20:942–952. doi: 10.1165/ajrcmb.20.5.3452. [DOI] [PubMed] [Google Scholar]
- Jiang H, Kang DC, Alexandre D, Fisher PB. RaSH, a rapid subtraction hybridization approach for identifying and cloning differentially expressed genes. Proc Natl Acad Sci U S A. 2000;97:12684–12689. doi: 10.1073/pnas.220431297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL, van Eys GJ, Angelini GD, George SJ. Injury induces dedifferentiation of smooth muscle cells and increased matrix-degrading metalloproteinase activity in human saphenous vein. Arterioscler Thromb Vasc Biol. 2001;21:1146–1151. doi: 10.1161/hq0701.092106. [DOI] [PubMed] [Google Scholar]
- Kabrun N, Enrietto PJ. The Rel family of proteins in oncogenesis and differentiation. Semin Cancer Biol. 1994;5:103–112. [PubMed] [Google Scholar]
- Kanai Y, Hediger MA. Primary structure and functional characterization of a high-affinity glutamate transporter. Nature. 1992;360:467–471. doi: 10.1038/360467a0. [DOI] [PubMed] [Google Scholar]
- Kang D-C, Jiang H, Wu Q, Pestka S, Fisher PB. Cloning and characterization of human ubiquitin-processing protease-43 from terminally differentiated human melanoma cells using a rapid subtraction hybridization protocol RaSH. Gene. 2001;267:233–242. doi: 10.1016/s0378-1119(01)00384-5. [DOI] [PubMed] [Google Scholar]
- Kang D-C, Fisher PB. Complete open reading frame (C-ORF) technique: a rapid and efficient method for obtaining complete protein coding sequences. In: Fisher PB, editor. Methods in Molecular Biology. Humana Press; New Jersey: 2007. in press. [DOI] [PubMed] [Google Scholar]
- Kang D-C, Fisher PB. Complete open reading frame (C-ORF) technology: simple and efficient technique for cloning full-length protein-coding sequences. Gene. 2005;353:1–7. doi: 10.1016/j.gene.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Kang D-C, LaFrance R, Su Z.-z., Fisher PB. Reciprocal subtraction differential RNA display: an efficient and rapid procedure for isolating differentially expressed gene sequences. Proc Natl Acad Sci U S A. 1998;95:13788–13793. doi: 10.1073/pnas.95.23.13788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D-C, Su Z.-z., Sarkar D, Emdad L, Volsky DJ, Fisher PB. Cloning and characterization of HIV-1-inducible astrocyte elevated gene-1, AEG-1. Gene. 2005;353:8–15. doi: 10.1016/j.gene.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Kaul M, Garden GA, Lipton SA. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature. 2001;410:988–994. doi: 10.1038/35073667. [DOI] [PubMed] [Google Scholar]
- Kim SY, Choi SY, Chao W, Volsky DJ. Transcriptional regulation of human excitatory amino acid transporter 1 (EAAT1): cloning of the EAAT1 promoter and characterization of its basal and inducible activity in human astrocytes. J Neurochem. 2003;87:1485–1498. doi: 10.1046/j.1471-4159.2003.02128.x. [DOI] [PubMed] [Google Scholar]
- Knobbe CB, Merlo A, Reifenberger G. Pten signaling in gliomas. Neurooncol. 2002;4:196–211. [PMC free article] [PubMed] [Google Scholar]
- Koenig S, Gendelman HE, Orenstein JM, Dal Canto MC, Pezeshkpour GH, Yungbluth M, et al. Detection of AIDS virus in macrophages in brain tissue from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- Kurschat P, Mauch C. Mechanisms of metastasis. Clin Exp Dermatol. 2000;25:482–489. doi: 10.1046/j.1365-2230.2000.00701.x. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Su ZZ, Sarkar D, Fisher PB. Restoring apoptosis as a strategy for cancer gene therapy: focus on p53 and mda-7. Semin Cancer Biol. 2003;13:169–178. doi: 10.1016/s1044-579x(02)00134-7. [DOI] [PubMed] [Google Scholar]
- Lee SG, Su ZZ, Emdad L, Sarkar D, Fisher PB. Astrocyte Elevated Gene-1 is a target gene of oncogenic Harvey-ras requiring phosphatidylinositol 3-kinase and c-Myc. Proc Natl Acad Sci U S A. 2006;103:17390–17395. doi: 10.1073/pnas.0608386103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehre KP, Danbolt NC. The number of glutamate transporter subtype molecules at glutamatergic synapses: chemical and stereological quantification in young adult rat brain. J Neurosci. 1998;18:8751–8757. doi: 10.1523/JNEUROSCI.18-21-08751.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone G, DeGregori J, Sears R, Jakoi L, Nevins JR. Myc and Ras collaborate in inducing accumulation of active cyclin E/Cdk2 and E2F. Nature. 1997;387:422–426. doi: 10.1038/387422a0. [DOI] [PubMed] [Google Scholar]
- Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- Lipton SA, Gendelman HE. Seminars in medicine of the Beth Israel Hospital, Boston. Dementia associated with the acquired immunodeficiency syndrome. N Engl J Med. 1995;332:934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- Macaluso M, Russo G, Cinti C, Bazan V, Gebbia N, Russo A. Ras family genes: an interesting link between cell cycle and cancer. J Cell Physiol. 2002;192:125–130. doi: 10.1002/jcp.10109. [DOI] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M. To cycle or not to cycle: a critical decision in cancer. Nat Rev Cancer. 2001;1:222–231. doi: 10.1038/35106065. [DOI] [PubMed] [Google Scholar]
- Malumbres M, Perez De Castro I, Hernandez MI, Jimenez M, Corral T, Pellicer A. Cellular response to oncogenic ras involves induction of the Cdk4 and Cdk6 inhibitor p15(INK4b) Mol Cell Biol. 2000;20:2915–2925. doi: 10.1128/mcb.20.8.2915-2925.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Alford M, DeTeresa R, Mallory M, Hansen L. Deficient glutamate transport is associated with neurodegeneration in Alzheimer's disease. Ann Neurol. 1996;40:759–766. doi: 10.1002/ana.410400512. [DOI] [PubMed] [Google Scholar]
- Mayo MW, Wang CY, Cogswell PC, Rogers-Graham KS, Lowe SW, Der CJ, et al. Requirement of NF-kappaB activation to suppress p53-independent apoptosis induced by oncogenic Ras. Science. 1997;278:1812–1815. doi: 10.1126/science.278.5344.1812. [DOI] [PubMed] [Google Scholar]
- Mettouchi A, Cabon F, Montreau N, Dejong V, Vernier P, Gherzi R, et al. The c-Jun-induced transformation process involves complex regulation of tenascin-C expression. Mol Cell Biol. 1997;17:3202–3209. doi: 10.1128/mcb.17.6.3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minden A, Lin A, Claret FX, Abo A, Karin M. Selective activation of the JNK signaling cascade and c-Jun transcriptional activity by the small GTPases Rac and Cdc42Hs. Cell. 1995;81:1147–1157. doi: 10.1016/s0092-8674(05)80019-4. [DOI] [PubMed] [Google Scholar]
- Miralles VJ, Martinez-Lopez I, Zaragoza R, Borras E, Garcia C, Pallardo FV, et al. Na+ dependent glutamate transporters (EAAT1, EAAT2, and EAAT3) in primary astrocyte cultures: effect of oxidative stress. Brain Res. 2001;922:21–29. doi: 10.1016/s0006-8993(01)03124-9. [DOI] [PubMed] [Google Scholar]
- Mitrovic AD, Amara SG, Johnston GA, Vandenberg RJ. Identification of functional domains of the human glutamate transporters EAAT1 and EAAT2. J Biol Chem. 1998;273:14698–14706. doi: 10.1074/jbc.273.24.14698. [DOI] [PubMed] [Google Scholar]
- Mori N, Fujii M, Ikeda S, Yamada Y, Tomonaga M, Ballard DW, et al. Constitutive activation of NF-kappaB in primary adult T-cell leukemia cells. Blood. 1999;93:2360–2368. [PubMed] [Google Scholar]
- Mukhopadhyay A, Bueso-Ramos C, Chatterjee D, Pantazis P, Aggarwal BB. Curcumin downregulates cell survival mechanisms in human prostate cancer cell lines. Oncogene. 2001;20:7597–7609. doi: 10.1038/sj.onc.1204997. [DOI] [PubMed] [Google Scholar]
- Munch C, Schwalenstocker B, Hermann C, Cirovic S, Stamm S, Ludolph A, et al. Differential RNA cleavage and polyadenylation of the glutamate transporter EAAT2 in the human brain. Brain Res Mol Brain Res. 2000;80:244–251. doi: 10.1016/s0169-328x(00)00139-x. [DOI] [PubMed] [Google Scholar]
- Navia BA, Price RW. The acquired immunodeficiency syndrome dementia complex as the presenting or sole manifestation of human immunodeficiency virus infection. Arch Neurol. 1987;44:65–69. doi: 10.1001/archneur.1987.00520130051017. [DOI] [PubMed] [Google Scholar]
- O'Leary KA, Kasper CB. Molecular basis for cell-specific regulation of the NADPH-cytochrome P450 oxidoreductase gene. Arch Biochem Biophys. 2000;379:97–108. doi: 10.1006/abbi.2000.1862. [DOI] [PubMed] [Google Scholar]
- Oliet SH, Piet R, Poulain DA. Control of glutamate clearance and synaptic efficacy by glial coverage of neurons. Science. 2001;292:923–926. doi: 10.1126/science.1059162. [DOI] [PubMed] [Google Scholar]
- Palmada M, Kinne-Saffran E, Centelles JJ, Kinne RK. Benzodiazepines differently modulate EAAT1/GLAST and EAAT2/GLT1 glutamate transporters expressed in CHO cells. Neurochem Int. 2002;40:321–326. doi: 10.1016/s0197-0186(01)00087-0. [DOI] [PubMed] [Google Scholar]
- Peeper DS, Upton TM, Ladha MH, Neuman E, Zalvide J, Bernards R, et al. Ras signalling linked to the cell-cycle machinery by the retinoblastoma protein. Nature. 1997;386:177–181. doi: 10.1038/386177a0. [DOI] [PubMed] [Google Scholar]
- Perego C, Vanoni C, Bossi M, Massari S, Basudev H, Longhi R, et al. The GLT-1 and GLAST glutamate transporters are expressed on morphologically distinct astrocytes and regulated by neuronal activity in primary hippocampal cocultures. J Neurochem. 2000;75:1076–1084. doi: 10.1046/j.1471-4159.2000.0751076.x. [DOI] [PubMed] [Google Scholar]
- Pines G, Danbolt NC, Bjoras M, Zhang Y, Bendahan A, Eide L, et al. Cloning and expression of a rat brain L-glutamate transporter. Nature. 1992;360:464–467. doi: 10.1038/360464a0. [DOI] [PubMed] [Google Scholar]
- Pitt D, Werner P, Raine CS. Glutamate excitotoxicity in a model of multiple sclerosis. Nat Med. 2000;6:67–70. doi: 10.1038/71555. [DOI] [PubMed] [Google Scholar]
- Pulverer BJ, Fisher C, Vousden K, Littlewood T, Evan G, Woodgett JR. Site-specific modulation of c-Myc cotransformation by residues phosphorylated in vivo. Oncogene. 1994;9:59–70. [PubMed] [Google Scholar]
- Rich JN, Guo C, McLendon RE, Bigner DD, Wang XF, Counter CM. A genetically tractable model of human glioma formation. Cancer Res. 2001;61:3556–3560. [PubMed] [Google Scholar]
- Ridley AJ. Rho family proteins: coordinating cell responses. Trends Cell Biol. 2001;11:471–477. doi: 10.1016/s0962-8924(01)02153-5. [DOI] [PubMed] [Google Scholar]
- Robinson MB. The family of sodium-dependent glutamate transporters: a focus on the GLT-1/EAAT2 subtype. Neurochem Int. 1998;33:479–491. doi: 10.1016/s0197-0186(98)00055-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, et al. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warne PH, Khwaja A, Marte BM, Pappin D, Das P, et al. Role of phosphoinositide 3-OH kinase in cell transformation and control of the actin cytoskeleton by Ras. Cell. 1997;89:457–467. doi: 10.1016/s0092-8674(00)80226-3. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L, Kuncl RW, et al. Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron. 1996;16:675–686. doi: 10.1016/s0896-6273(00)80086-0. [DOI] [PubMed] [Google Scholar]
- Sacktor N, Lyles RH, Skolasky R, Kleeberger C, Selnes OA, Miller EN, et al. HIV-associated neurologic disease incidence changes: Multicenter AIDS Cohort Study, 1990-1998. Neurology. 2001;56:257–260. doi: 10.1212/wnl.56.2.257. [DOI] [PubMed] [Google Scholar]
- Sahai E, Olson MF, Marshall CJ. Cross-talk between Ras and Rho signalling pathways in transformation favors proliferation and increased motility. EMBO J. 2001;20:755–766. doi: 10.1093/emboj/20.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y, Sharer LR, Epstein LG, Michaels J, Mintz M, Louder M, et al. Overexpression of nef as a marker for restricted HIV-1 infection of astrocytes in postmortem pediatric central nervous tissues. Neurology. 1994;44:474–481. doi: 10.1212/wnl.44.3_part_1.474. [DOI] [PubMed] [Google Scholar]
- Saksela K, Makela TP, Hughes K, Woodgett JR, Alitalo K. Activation of protein kinase C increases phosphorylation of the L-myc trans-activator domain at a GSK-3 target site. Oncogene. 1992;7:347–353. [PubMed] [Google Scholar]
- Schlag BD, Vondrasek JR, Munir M, Kalandadze A, Zelenaia OA, Rothstein JD, et al. Regulation of the glial Na+-dependent glutamate transporters by cyclic AMP analogs and neurons. Mol Pharmacol. 1998;53:355–369. doi: 10.1124/mol.53.3.355. [DOI] [PubMed] [Google Scholar]
- Schule R, Rangarajan P, Yang N, Kliewer S, Ransone LJ, Bolado J, et al. Retinoic acid is a negative regulator of AP-1-responsive genes. Proc Natl Acad Sci U S A. 1991;88:6092–6096. doi: 10.1073/pnas.88.14.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A, Gonzalez FA, Gupta S, Raden DL, Davis RJ. Signal transduction within the nucleus by mitogen-activated protein kinase. J Biol Chem. 1992;267:24796–24804. [PubMed] [Google Scholar]
- Shahabuddin M, Volsky B, Kim H, Sakai K, Volsky DJ. Regulated expression of human immunodeficiency virus type 1 in human glial cells: induction of dormant virus. Pathobiology. 1992;60:195–205. doi: 10.1159/000163723. [DOI] [PubMed] [Google Scholar]
- Sharer LR, Epstein LG, Cho ES, Joshi VV, Meyenhofer MF, Rankin LF, et al. Pathologic features of AIDS encephalopathy in children: evidence for LAV/HTLV-III infection of brain. Hum Pathol. 1986;17:271–284. doi: 10.1016/s0046-8177(83)80220-2. [DOI] [PubMed] [Google Scholar]
- Sharma G, Vijayaraghavan S. Nicotinic cholinergic signaling in hippocampal astrocytes involves calcium-induced calcium release from intracellular stores. Proc Natl Acad Sci U S A. 2001;98:4148–4153. doi: 10.1073/pnas.071540198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields JM, Pruitt K, McFall A, Shaub A, Der CJ. Understanding Ras: ‘it ain't over 'til it's over’. Trends Cell Biol. 2000;10:147–154. doi: 10.1016/s0962-8924(00)01740-2. [DOI] [PubMed] [Google Scholar]
- Shin SM, Cho IJ, Kim SG. CCAAT/enhancer binding protein activation by PD98059 contributes to the inhibition of AhR-mediated 3-methylcholanthrene induction of CYP1A1. Xenobiotica. 2005;35:975–987. doi: 10.1080/00498250500354584. [DOI] [PubMed] [Google Scholar]
- Simm M, Su Z.-z., Huang EY, Chen Y, Jiang H, Volsky DJ, et al. Cloning of differentially expressed genes in an HIV-1 resistant T cell clone by rapid subtraction hybridization, RaSH. Gene. 2001;269:93–101. doi: 10.1016/s0378-1119(01)00456-5. [DOI] [PubMed] [Google Scholar]
- Sitcheran R, Gupta P, Fisher PB, Baldwin AS. Positive and negative regulation of EAAT2 by NF-kappaB: a role for N-myc in TNFalpha-controlled repression. Embo J. 2005;24:510–520. doi: 10.1038/sj.emboj.7600555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sontheimer H. Malignant gliomas: perverting glutamate and ion homeostasis for selective advantage. Trends Neurosci. 2003;26:543–549. doi: 10.1016/j.tins.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Sporer B, Paul R, Koedel U, Grimm R, Wick M, Goebel FD, et al. Presence of matrix metalloproteinase-9 activity in the cerebrospinal fluid of human immunodeficiency virus-infected patients. J Infect Dis. 1998;178:854–857. doi: 10.1086/515342. [DOI] [PubMed] [Google Scholar]
- Stirewalt DL, Kopecky KJ, Meshinchi S, Appelbaum FR, Slovak ML, Willman CL, et al. FLT3, RAS, and TP53 mutations in elderly patients with acute myeloid leukemia. Blood. 2001;97:3589–3595. doi: 10.1182/blood.v97.11.3589. [DOI] [PubMed] [Google Scholar]
- Storck T, Schulte S, Hofmann K, Stoffel W. Structure, expression, and functional analysis of a Na(+)-dependent glutamate/aspartate transporter from rat brain. Proc Natl Acad Sci U S A. 1992;89:10955–10959. doi: 10.1073/pnas.89.22.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z.-z., Chen Y, Kang DC, Chao W, Simm M, Volsky DJ, et al. Customized rapid subtraction hybridization (RaSH) gene microarrays identify overlapping expression changes in human fetal astrocytes resulting from human immunodeficiency virus-1 infection or tumor necrosis factor-alpha treatment. Gene. 2003a;306:67–78. doi: 10.1016/s0378-1119(03)00404-9. [DOI] [PubMed] [Google Scholar]
- Su Z.-z., Kang D-C, Chen Y, Pekarskaya O, Chao W, Volsky DJ, et al. Identification and cloning of human astrocyte genes displaying elevated expression after infection with HIV-1 or exposure to HIV-1 envelope glycoprotein by rapid subtraction hybridization, RaSH. Oncogene. 2002;21:3592–3602. doi: 10.1038/sj.onc.1205445. [DOI] [PubMed] [Google Scholar]
- Su Z.-z., Kang D-C, Chen Y, Pekarskaya O, Chao W, Volsky DJ, et al. Identification of gene products suppressed by human immunodeficiency virus type 1 infection or gp120 exposure of primary human astrocytes by rapid subtraction hybridization. J Neurovirol. 2003b;9:372–389. doi: 10.1080/13550280390201263. [DOI] [PubMed] [Google Scholar]
- Su Z-z., Leszczyniecka M, Kang DC, Sarkar D, Chao W, Volsky DJ, et al. Insights into glutamate transport regulation in human astrocytes: cloning of the promoter for excitatory amino acid transporter 2 (EAAT2) Proc Natl Acad Sci U S A. 2003c;100:1955–1960. doi: 10.1073/pnas.0136555100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sucharov CC, Helmke SM, Langer SJ, Perryman MB, Bristow M, Leinwand L. The Ku protein complex interacts with YY1, is up-regulated in human heart failure, and represses alpha myosin heavy-chain gene expression. Mol Cell Biol. 2004;24:8705–8715. doi: 10.1128/MCB.24.19.8705-8715.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulis ML, Parsons R. PTEN: from pathology to biology. Trends Cell Biol. 2003;13:478–483. doi: 10.1016/s0962-8924(03)00175-2. [DOI] [PubMed] [Google Scholar]
- Sutherland HG, Lam YW, Briers S, Lamond AI, Bickmore WA. 3D3/lyric: a novel transmembrane protein of the endoplasmic reticulum and nuclear envelope, which is also present in the nucleolus. Exp Cell Res. 2004;294:94–105. doi: 10.1016/j.yexcr.2003.11.020. [DOI] [PubMed] [Google Scholar]
- Szymocha R, Akaoka H, Dutuit M, Malcus C, Didier-Bazes M, Belin MF, et al. Human T-cell lymphotropic virus type 1-infected T lymphocytes impair catabolism and uptake of glutamate by astrocytes via Tax-1 and tumor necrosis factor alpha. J Virol. 2000;74:6433–6441. doi: 10.1128/jvi.74.14.6433-6441.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Watase K, Manabe T, Yamada K, Watanabe M, Takahashi K, et al. Epilepsy and exacerbation of brain injury in mice lacking the glutamate transporter GLT-1. Science. 1997;276:1699–1702. doi: 10.1126/science.276.5319.1699. [DOI] [PubMed] [Google Scholar]
- Tornatore C, Nath A, Amemiya K, Major EO. Persistent human immunodeficiency virus type 1 infection in human fetal glial cells reactivated by T-cell factor(s) or by the cytokines tumor necrosis factor alpha and interleukin-1 beta. J Virol. 1991;65:6094–6100. doi: 10.1128/jvi.65.11.6094-6100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- Vandenberg RJ, Mitrovic AD, Chebib M, Balcar VJ, Johnston GA. Contrasting modes of action of methylglutamate derivatives on the excitatory amino acid transporters, EAAT1 and EAAT2. Mol Pharmacol. 1997;51:809–815. doi: 10.1124/mol.51.5.809. [DOI] [PubMed] [Google Scholar]
- Visconti R, Cerutti J, Battista S, Fedele M, Trapasso F, Zeki K, et al. Expression of the neoplastic phenotype by human thyroid carcinoma cell lines requires NFkappaB p65 protein expression. Oncogene. 1997;15:1987–1994. doi: 10.1038/sj.onc.1201373. [DOI] [PubMed] [Google Scholar]
- Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119–127. [PubMed] [Google Scholar]
- Wang Z, Pekarskaya O, Bencheikh M, Chao W, Gelbard HA, Ghorpade A, et al. Reduced expression of glutamate transporter EAAT2 and impaired glutamate transport in human primary astrocytes exposed to HIV-1 or gp120. Virology. 2003;312:60–73. doi: 10.1016/s0042-6822(03)00181-8. [DOI] [PubMed] [Google Scholar]
- White MA, Nicolette C, Minden A, Polverino A, Van Aelst L, Karin M, et al. Multiple Ras functions can contribute to mammalian cell transformation. Cell. 1995;80:533–541. doi: 10.1016/0092-8674(95)90507-3. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Schrier RD, Nelson JA, Lampert PW, Oldstone MB. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986;83:7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu RC, Qin J, Hashimoto Y, Wong J, Xu J, Tsai SY, et al. Regulation of SRC-3 (pCIP/ACTR/AIB-1/RAC-3/TRAM-1) Coactivator activity by I kappa B kinase. Mol Cell Biol. 2002;22:3549–3561. doi: 10.1128/MCB.22.10.3549-3561.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Ye K, Dinarello CA, Clark BD. Identification of the promoter region of human interleukin 1 type I receptor gene: multiple initiation sites, high G+C content, and constitutive expression. Proc Natl Acad Sci U S A. 1993;90:2295–2299. doi: 10.1073/pnas.90.6.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye ZC, Rothstein JD, Sontheimer H. Compromised glutamate transport in human glioma cells: reduction-mislocalization of sodium-dependent glutamate transporters and enhanced activity of cystine-glutamate exchange. J Neurosci. 1999;19:10767–10777. doi: 10.1523/JNEUROSCI.19-24-10767.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yordy JS, Muise-Helmericks RC. Signal transduction and the Ets family of transcription factors. Oncogene. 2000;19:6503–6513. doi: 10.1038/sj.onc.1204036. [DOI] [PubMed] [Google Scholar]
- Yu N, Billaud JN, Phillips TR. Effects of feline immunodeficiency virus on astrocyte glutamate uptake: implications for lentivirus-induced central nervous system diseases. Proc Natl Acad Sci U S A. 1998;95:2624–2629. doi: 10.1073/pnas.95.5.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelenaia O, Schlag BD, Gochenauer GE, Ganel R, Song W, Beesley JS, et al. Epidermal growth factor receptor agonists increase expression of glutamate transporter GLT-1 in astrocytes through pathways dependent on phosphatidylinositol 3-kinase and transcription factor NF-kappaB. Mol Pharmacol. 2000;57:667–678. doi: 10.1124/mol.57.4.667. [DOI] [PubMed] [Google Scholar]