Abstract
The role of arginine vasopressin (Avp) as an adrenocorticotropin (ACTH) secretagogue is mediated by the Avp 1b receptor (Avpr1b) found on anterior pituitary corticotropes. Avp also potentiates the actions of corticotropin-releasing hormone (Crh) and appears to be an important mediator of the hypothalamic-pituitary-adrenal (HPA) axis response to chronic stress. To investigate the role of Avp in the HPA axis response to stress, we measured plasma ACTH and corticosterone (CORT) levels in Avpr1b knockout (KO) mice and wild-type controls in response to two acute (restraint and insulin administration) and one form of chronic (daily restraint for 14 days) stress. No significant difference was found in the basal plasma levels of ACTH and CORT between the two genotypes. Acute restraint (30 min) increased plasma ACTH and CORT to a similar level in both the Avpr1b mutant and wild-type mice. In contrast, plasma ACTH and CORT levels induced by hypoglycemia were significantly decreased in the Avpr1b KO mice when compared to wild-type littermates. There was no difference in the ACTH response to acute and chronic restraint in wild-type mice. In the Avpr1b KO group subjected to 14 sessions of daily restraint, plasma ACTH was decreased when compared to wild-type mice. On the other hand, the CORT elevations induced by restraint did not adapt in the Avpr1b KO or wild-type mice. The data suggests that the Avpr1b is required for the normal pituitary and adrenal response to some acute stressful stimuli, and is necessary only for a normal ACTH response during chronic stress.
Keywords: Avp 1b receptor, adrenocorticotropic hormone, corticosterone, hypoglycemia, restraint stress
Introduction
The neurohypophysial hormone vasopressin (Avp) is the hormonal regulator of water homeostasis and has major effects on behaviour and vascular tone (e.g. see 1-5). In addition to this, it is also a key regulator of the hypothalamic-pituitary-adrenal (HPA) axis (6,7). These actions are mediated through a family of G protein-coupled receptors; the Avp 1a receptor (Avpr1a) which regulates vascular tone and has many putative roles in the central nervous system, the Avp 2 receptor (Avpr2) which controls renal collecting duct water permeability, and the Avp 1b receptor (Avpr1b or V3R) which is predominantly found in the corticotropes of the anterior pituitary (8), where it is involved in the regulation of adrenocorticotropin (ACTH) release.
Diverse homeostatic challenges including cognitive (e.g. restraint) and noncognitive (e.g. infection) stressors activate the HPA axis and sympathoadrenal systems. The key CNS site integrating the neuroendocrine adjustments to stress is the hypothalamic paraventricular nucleus (PVN). This nucleus is comprised of two major neurosecretory components: the magnocellular (mPVN) and parvocellular (pPVN) subdivisions (6,9). The mPVN together with the supraoptic (SON) nuclei of the hypothalamus constitute the neurohypophysial system that is the primary source of Avp and the related peptide oxytocin (Oxt) released into the systemic circulation from neurons terminating in the posterior pituitary. Neurons in the more medially situated pPVN are the principal CNS source of corticotropin-releasing hormone (Crh), which is a major physiological regulator of pituitary ACTH secretion. Under basal conditions about 50% of these neurons also express Avp (10). Numerous neural inputs and blood- and CSF-borne factors dynamically influence the activity of pPVN Crh and Avp neurons (11,12). Moreover, there is evidence that the neurohypophysial and HPA axes may functionally overlap but the extent of this interaction is not fully understood (6,7,13,14). Crh neurons originating from the pPVN project to the external zone of the median eminence from where Crh is released into the portal vessels bathing the anterior pituitary. Crh and Avp stimulate ACTH secretion by interacting with the Crh-type 1 receptor (Crhr1) and Avpr1b, respectively, on the pituitary corticotrope. ACTH in turn potently induces the secretion of glucocorticoids (corticosterone (CORT) in rodents) from the adrenal cortex, which exert a negative feedback action on the pituitary, PVN and other brain regions such as the hippocampus to restrict the dramatic initial release of ACTH and CORT (6).
Regulation of CORT (via ACTH) secretion is critical for life and is necessary for the mammalian response to stress. Although Crh seems to be the dominant ACTH secretagogue in rodents in response to most acute stressors (e.g. restraint), Avp synergizes with Crh in activating the release of ACTH (6), and in both Crh- and Crhr1-deficient mice, Avp seems to be sufficient to maintain adequate HPA activity for survival (15-17; although for the Crh mutants, exogenous CORT is needed for lung maturation at birth (15)). In fact, there is a compensatory increase in the basal hypothalamic vasopressinergic system in Crhr1 KO animals (18). However, the precise role of Avp release in the response to stress remains controversial because the vast majority of studies looking at Avp have used indirect or correlative measurements of Avp- pPVN neuron activation after a stress challenge (19). Some of the best evidence for Avp involvement in the HPA axis response to stress derives from studies demonstrating that Avp immunoneutralization inhibits the rise in plasma ACTH produced by diverse stressors such as restraint, insulin-induced hypoglycemia and lipopolysaccharide (20-22). Additional studies (in particular those in which Avp levels in pituitary portal blood have been sampled) indicate that Avp may be released, sometimes preferentially over Crh, in response to some acute stressors such as insulin-induced hypoglycemia (23-32).
There is evidence that Avp may become the dominant ACTH secretagogue in some chronic stress situations (33,34). If an acute stress is repeated over a number of days, adaptation or desensitization of the HPA axis can occur resulting in diminished responsiveness to this (homotypic) stressor (33,34). Avp and the pituitary Avpr1b appear to play major roles in this adaptive response. Repeated restraint stress in rats results in increased expression of Avp in pPVN Crh-containing neurons (35). Both pituitary Crhr1 and Avpr1bs are activated and undergo regulatory variations during stress, but only the changes in Avpr1b levels parallel the changes in pituitary ACTH responsiveness (34,36). In addition, exogenous Avp but not Crh alters ACTH levels in chronically restrained rats even though these animals are able to respond to an acute heterotypic stress (37). Furthermore, recent studies have shown that an acute restraint episode following repeated restraint results in a rapid increase in Avp- but not Crh-hnRNA in the pPVN (38).
Avpr1b-deficient mice generated by us exhibit markedly reduced aggression and modestly impaired social recognition (5). The Avpr1b knockout (KO) mice exhibited a normal CORT response to an acute physical-psychological stress (resident-intruder)(5). In a subsequent study, the ACTH and CORT responses to forced swim were substantially reduced in another line of Avp1rb KO mice (39). The present experiments were designed to test the hypothesis that a functional Avpr1b is required for a normal HPA axis response (as measured by plasma ACTH and/or CORT levels) to acute and repeated restraint and insulin-induced hypoglycemia in mice.
Materials and Methods
Animals
Adult (8-12 weeks) littermates (a mix of the C57BL/6J and 129X1/SvJ strains) of crosses using mice heterozygous for the Avpr1b mutation (5) were group housed (three to four per cage) under controlled light and temperature (21 ± 2 C) with food and water available ad libitum and maintained on a 14 h light, 10 h dark cycle (lights on at 0500h). Male mice were used for all studies with the exception of the diurnal CORT measurements, where plasma from both males and females was collected. Studies were performed between 0900-1200h. All procedures were conducted in accordance with the Animal Scientific Procedures Act (1986) United Kingdom and the appropriate University of Bristol Ethical Review Process.
Basal measurements
For basal histology, in situ hybridization histochemistry and plasma CORT (am) levels, animals were sacrificed 1.5 h after lights on. Trunk blood was collected by decapitation (within 5 sec after removal from the home cage). Brains and pituitaries were dissected, frozen on dry-ice and stored at −80 C until sectioning. Adrenals from male mice were post-fixed in Bouin's solution (Sigma-Aldrich, UK) for 4 h at RT and destained in 70% ethanol. The tissue was embedded in paraffin and 6μm sections cut for staining by haemotoxylineosin (Veternary Diagnostic Histopathology Service, Dept. Pathology & Microbiology, University of Bristol, UK). For pm plasma CORT levels, animals were sacrificed 1.5 h before lights off.
Pituitary bioassay
KO and wild-type mice were sacrificed by cervical dislocation. Pituitaries were removed and placed into ice-cold Krebs-Ringer buffer (Sigma #K4002) containing 15mM sodium bicarbonate, 2.6mM calcium chloride, 0.1% bovine serum albumin and 100μg/ml ascorbic acid (incubation buffer). The neural/intermediate lobes were carefully dissected away from the anterior lobe under a dissecting microscope (magnification, x10) to yield hemissected pituitaries - these were further divided into two to yield four pieces of approximately equal size. Two pituitary quarters (randomly selected from either pooled KO or wild-type pituitary tissue) were equilibrated in 1.5 ml incubation buffer in the wells of a 12-well plate (Netwells 15mm, 74μm pore size mesh grid, Corning) for 1 h. The equilibration buffer was removed and the tissue incubated with 1.5 ml fresh incubation buffer with or without 10nM Avp (Bachem, UK), 1nM Crh (Bachem, UK) or 10nM Avp + 1nM Crh for 2 h. At the end of the experiment, buffer was removed, microfuged for 2 min at 13,000×g (4 C) and supernatant promptly frozen at −20 C. Total ACTH/well was measured (see below) in unextracted incubation buffer. All incubations were at 37 C in a 95% O2/5% CO2atmosphere.
Restraint stress
Restraint stress was performed using 50 ml (Falcon) plastic tubes in which packing with paper tissue at the rear was used to achieve a comparable degree of restraint for each animal. Age-matched Avpr1b KO and wild-type mice were single-housed and divided into four groups (4-6 mice/group). One control group of mice was handled daily and sacrificed on the morning of day 14. A second group of mice were handled daily for 13 days and sacrificed immediately after 30 min restraint on day 14 (acute restraint). The two other groups were restrained daily for 30 min for 13 days and sacrificed either without further restraint on day 14 or immediately after a final restraint episode on day 14 (repeated restraint). Based on previous studies in restrained rats (e.g., see ref.35) and acute stress in mice (40), and the number of mice at our disposal, we chose to investigate a single time point (30 min restraint) to optimize detection of both restraint-induced plasma ACTH and CORT. This precluded any time-course study on the dynamics of ACTH and CORT secretion in the Avpr1b KO and wild-type mice.
Insulin-induced hypoglycemia
Single-housed mice were fasted overnight (12 h) with water freely available. KO and wild-type animals (4-5/group) were injected i.p. with 175-200 μl vehicle (0.9% saline; controls) or insulin (0.75 or 3.0 IU/kg diluted in vehicle; Actrapid (human insulin; 100IU/ml from Novo Nordisk, Denmark)) and sacrificed 1 h later.
Hormone analysis
All experiments were performed at least twice and samples measured in duplicate or triplicate (CORT RIA). Plasma obtained from trunk blood collected into heparinized tubes was used for all hormonal measurements. Total plasma CORT was measured (10 μl plasma diluted in 500 μl assay buffer) using antiserum kindly supplied by Dr G. Makara (Institute of Experimental Medicine, Budapest, Hungary) as described previously (41). Each experiment was processed in the same assay with intra-assay variation of less than 10%. The tracer was [125I]-corticosterone (ICN Biomedicals, Irvine, CA, USA) with a specific activity of 2-3 mCi/μg. The sensitivity of the assay was 10 ng/ml. Plasma ACTH was measured as described previously (42) using a rabbit anti-rat ACTH primary antibody (donated by G. Makara) and [125I]ACTH (Amersham Biosciences, Little Chalfont, UK). Glucose was measured by an automated enzymatic assay (hexokinase method; courtesy Dept. Clinical Biochemistry, Bristol Royal Infirmary, Bristol, U.K.).
In situ hybridization histochemistry (ISHH)
Coronal PVN and axial pituitary sections (12μM) were thaw-mounted onto polylysine-coated slides and stored at −80 C until hybridization. The Avp, Otx and Crh oligonucleotide probes used in this study were B50 (5'-GTAGACCCGGGGCTTGGCAGAATCCACG GACTCCCGTGTCCCAGCCAG-3'; bp 3928-3345 corresponding to the carboxy peptide after neurophysinII in the Avp precursor; GenBank Acc#M88354)), B51 (5'-CAAGCAGG CAGCAAGCGAGACTGGGGCAGGCCATGGCATTGGTGCTCA-3'; bp1072-1119 just prior to the start of the signal peptide and teminating before mature Oxt in the Oxt precursor; GenBank Acc#M88355)) and Crh (5'-CAGTTTCCTGTTGCTGTGAGCTTG CTGAGCTAACTGCTCTGCCCGGGC-3'; bp 1685-1732 of GeneBank Acc#AY128673), respectively. The specificity of the probes has been described previously (43,44). Proopiomelanocortin (Pomc) mRNA was detected with a riboprobe, generated by PCR essentially as described previously (45) using reverse-transcribed 129Sv mouse pituitary mRNA as template, 2.5 units of Amplitaq polymerase (Applied Biosystems, CA, USA), and two primers encompassing bp 147-622 (B56, upstream: 5'-GCGAATTCGG CCCCAGGAACAGCAGC-3'; B57, downstream: 5'-GCAAGCTTGGAATGAGACCC CTG-3') of exon 3 of the mouse Pomc gene (46; GenBank Acc#V01529). The primers contain the recognition sequences for the restriction endonucleases EcoRI (B56) and HindIII (B57) to facilitate subcloning of the PCR product into the RNA-generating vector pGEM4Z (Promega, WI, USA). The integrity of the probe was verified by DNA sequencing. The oligonucleotides were end-labelled with 35S-dATP as described (47) and sense and antisense Pomc riboprobes were generated using T7 and SP6 polymerases with 35S-UTP and the MAXIscript in vitro transcription kit (Ambion, TX, USA). Biotin-labelling of the Pomc riboprobe was carried out using biotin-16-UTP (Roche Applied Science, Germany) in place of the radiolabelled nucleotide. All in situ hybridization experiments were performed as described in detail (http://intramural.nimh.nih.gov/lcmr/snge/protocols/ISHH/ISHH.html). 35S-labelled sections were exposed to Kodak XAR film together with 14C-labelled standards (Amersham Biosciences, UK) for 2 (Avp and Oxt) or 14 (Crh) days. Biotin-labelled pituitary sections were processed using the TSA amplification kit (Perkin Elmer Life Sciences, MA, USA) according to the manufacturer's instructions. After the final wash, slides were coverslipped using Vectashield (Vector Laboratories Inc., CA, USA) and viewed under a fluorescence microscope. No specific hybridization was detected with the sense probe.
Data analysis
For ISHH data, analysis of the autoradiographic images of probe bound and visualized on film was measured using NIH image analysis software (W.Rasband, version 1.62; rsb.info.nih.gov/nih-image/) and values were calibrated with respect to the autoradiographic images of the C-14 standards. For each animal (total of four KO and four wild-type mice), four adjacent sections of PVN or pituitary/slide for each probe were measured. Pomc mRNA was measured only in the anterior lobe of the pituitary - the intermediate (and posterior) lobe was excluded from analysis. The relative number of Pomc mRNA-expressing cells in anterior pituitaries from KO (n = 3) or wild-type (n = 3) mice was determined by capturing images from three axial sections under x100 magnification. The total number of Pomc-positive cells was counted for the entire field-of-view of each area.
ISHH data was statistically compared using one-way ANOVA followed by Fisher's PLSD post-hoc test. The statistical differences between hormone measurement groups were determined by two-way ANOVA followed by Bonferroni's post-hoc test using GraphPad Prism (version 4.0b) software. P < 0.05 was considered statistically significant.
Results
Basal HPA axis parameters
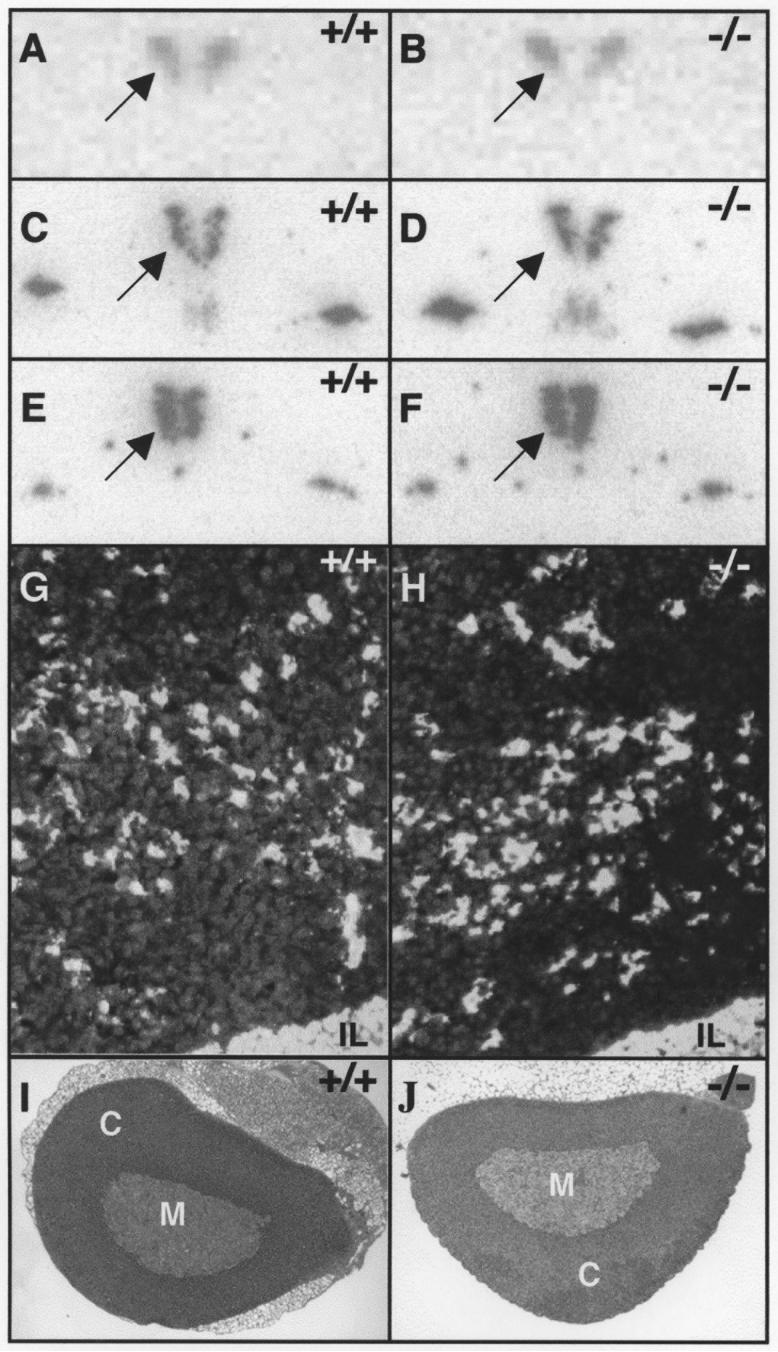
Loss of Avpr1b-dependent pathways in the Avpr1b KO mice may result in compensatory changes in the distribution or expression levels of components of the HPA axis. ISHH analyses showed no differences in the level or spatial distribution of Crh, Avp or Oxt mRNA expression in the PVN of naïve, wild-type and KO mice (Fig.1A-F; Table 1). There were no changes in the level of Pomc mRNA expression, or in the relative number of Pomc-mRNA-expressing cells in the anterior pituitary of mutant compared to wild-type animals (Fig.1G,H; Table 1). Histological analysis of adrenal glands from male mice revealed no gross morphological changes in the medulla, or zona glomerulosa, zona reticularis or zona fasciculata (the major site of CORT production) regions of the cortex (Fig.1I,J).
Figure 1.
Basal expression of neuropeptide mRNAs in PVN and pituitary and histology of the adrenal in Avpr1b KO mice.
There was no difference in the expression (dark labelling on light background) of Crh (A,B), Avp (C,D) or Oxt (E,F) mRNAs in the PVN (arrowed) of wild-type (left panels) or Avpr1b KO (right panels) mice. A-F are images scanned directly from X-ray film and have been graphically adjusted in Adobe Photoshop CS to optimise brightness and contrast. The level of Pomc mRNA expression and relative numbers of Pomc mRNA-expressing cells (white labelling on dark background) were similar in the anterior pituitaries from wild-type (G) or Avpr1b KO (H) mice. There was no gross histological differences in the appearances of the adrenal glands between the two genotypes (I, wild-type; J, KO). IL, intermediate lobe of the pituitary; M, adrenal medulla; C, adrenal cortex.
Table 1.
Mean PVN Avp, Oxt, and Crh, and anterior pituitary pomc mRNA levels as a percentage of the wild-type, control values (n = 4 mice/group)
| Group | Avp mRNA | Oxt mRNA | Crh mRNA | pomc mRNA |
|---|---|---|---|---|
| wild-type | 100 (2) | 100 (4) | 100 (13) | 100 (4) |
| KO | 105 (3) | 94 (4) | 109 (12) | 95 (7) |
There are no significant differences (P > 0.05) for relative Avp, Oxt, Crh or pomc mRNA levels between un-stressed, adult (8-12 weeks), male Avpr1b KOs and wild-type mice. Results are expressed as % mean (± SEM).
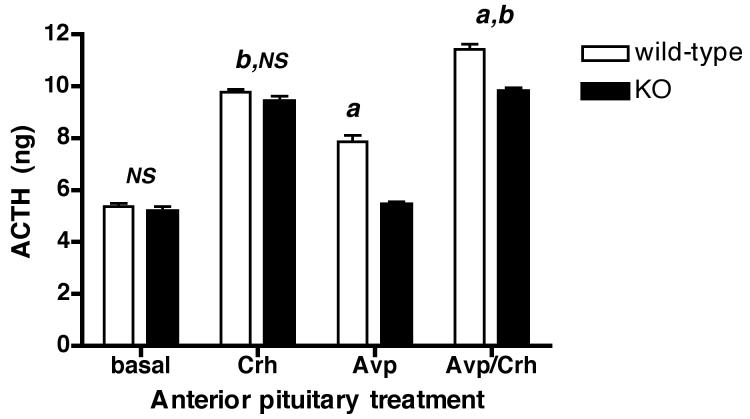
To prove that the Avpr1b in the Avpr1b mutants was specifically impaired, cultured pituitary segments were stimulated with Avp, Crh or a combination of both peptides and ACTH release was measured. Fig.2 shows that there is no difference in basal or Crh (1nM)-stimulated ACTH release from anterior pituitary tissue from Avpr1b KO or wild-type mice. Avp (10nM) stimulated ACTH release (although to a significantly lower level than the smaller Crh (1nM) dose) from wild-type but not Avpr1b KO pituitaries. Basal and Avp-stimulated ACTH release from pituitaries of Avpr1b KO mice was of a similar magnitude. Fig. 2 also shows that Avp had a small but statistically significant potentiating effect on Crh-stimulated ACTH release in pituitaries from wild-type animals: in contrast, there was no difference between Crh/Avp- or Crh-stimulated ACTH release from Avpr1b KO pituitaries. Thus Avp does not stimulate ACTH release or potentiate Crh-induced ACTH release from Avpr1b-deficient pituitaries.
Figure 2.
Vasopressin does not stimulate ACTH secretion from anterior pituitary segments obtained from Avpr1b KO mice.
Isolated anterior pituitary segments from wild-type or Avpr1b KO mice were incubated in vitro with buffer alone (basal), Crh (1nM), Avp (10nM), or a combination of both ACTH secretagogues (Avp/Crh). Values are mean total ACTH (ng)/well ± SEM, n = 4 separate pituitary preparations. a, P <0.001 Avp KO vs. Avp wild-type; Avp/Crh KO vs. Avp/Crh wild-type; Avp wild-type vs. basal wild-type; Avp/Crh wild-type vs. Crh wild-type. b, P <0.0001 Avp/Crh KO vs. Avp KO; Avp/Crh KO vs. basal KO; Crh KO vs. basal KO; Crh wild-type vs. Crh basal; Avp/Crh wild-type vs. Avp wild-type; Avp/Crh wild-type vs. basal wild-type. NS, not significant (P > 0.05) KO vs. wild-type basal; Crh wild-type vs. Crh KO; Crh KO vs. Avp/Crh KO; basal KO vs. Avp KO.
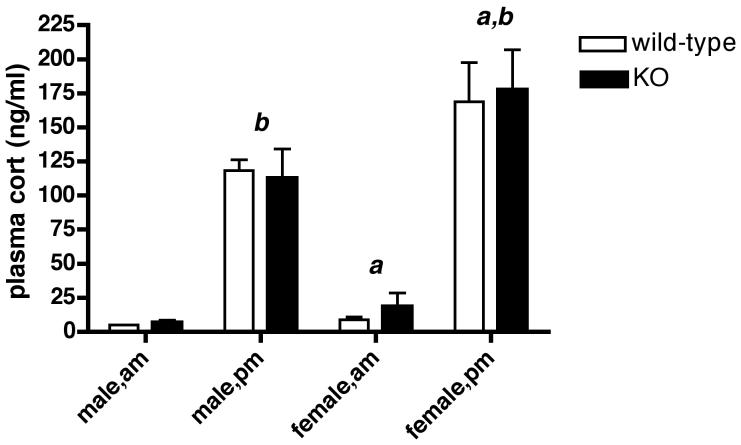
Fig. 3 shows that the characteristic diurnal rise in circulating CORT that occurs in the afternoon is intact in naïve, male and female Avpr1b KOs. Plasma CORT levels in the morning or afternoon were indistinguishable between the genotypes. Basal morning and afternoon plasma CORT levels were significantly increased (P < 0.05) in female, compared to male mice, irrespective of the genotype. Sexual dimorphism is a well-known feature of the HPA axis, with higher plasma CORT levels consistently reported for female compared with male rodents (e.g. see ref.48).
Figure 3.
Diurnal variation in plasma CORT secretion in wild-type and Avpr1b KO mice.
Values are mean ± SEM, n = 3-5 samples for each time-point. a, P < 0.05 female am vs. male am; female pm vs. male pm. b, P < 0.0001 male am vs. male pm; female am vs. female pm. There were no significant differences (P > 0.05) between am or pm values for male or female mice of both genotypes.
Hormone levels following stress
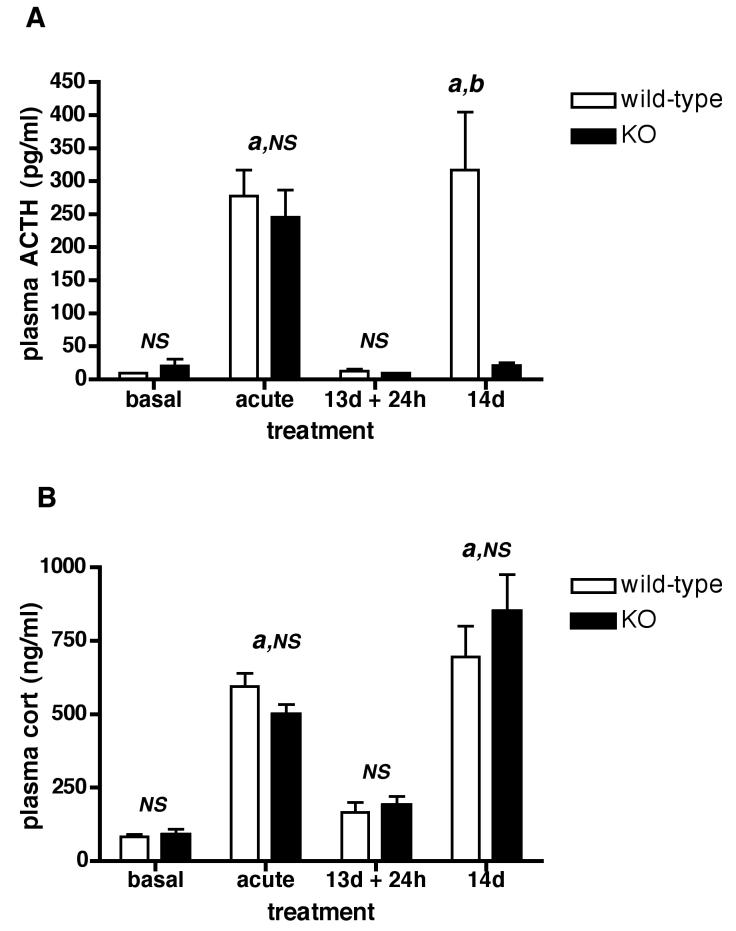
The effect of acute and repeated restraint on the plasma levels of ACTH and CORT in Avpr1b KO and wild-type mice is shown in Fig. 4 (A,B). In naïve wild-type mice, 30 min restraint increased plasma ACTH and CORT - there was no significant difference (P > 0.05) between these values and those obtained for Avpr1b KO animals (see Fig.4A,B - acute). Irrespective of genotype, there was no statistical difference between basal ACTH level after 13 d of repeated restraint and the level found in the control, handled groups of mice. After restraint on day 14 plasma ACTH levels were dramatically reduced (but not absent) in Avpr1b KO but not wild-type mice (Fig.4A - 14d). In contrast, the plasma CORT levels in repeatedly restrained mice were similar in each genotype (Fig.4B - 14d).
Figure 4.
Effect of acute and repeated restraint stress on plasma ACTH (A) and CORT (B) levels in Avpr1b mutant and control mice.
basal = control, naïve mice briefly handled 30 min prior to sacrifice; acute = mice subjected to 30 min acute restraint; 13d + 24h = 24 h after 13 daily 30 min restraint sessions; 14d = daily restraint fro 14 d, sacrifice immediately following the last 30 min restraint session. Values are mean ± SEM, n = 4-5 mice/group. Four out of five 14d restraint Avpr1b KO samples had detectable ACTH; in contrast, the vast majority (14/16) of samples in the Avpr1b KO and wild-type basal and 13d + 24h groups had no measurable ACTH (the assay limit of 10 pg/ml was recorded for these samples). a, P < 0.001 13d restraint + 24h wild-type vs. 14d restraint KO; b, P < 0.0001 acute restraint vs. basal; 14d restraint wild-type vs. 14d restraint KO (ACTH only). NS, not significant (P > 0.05) basal wild-type vs. basal KO; acute wild-type vs. acute KO; 13d + 24h wild-type vs. 13d + 24h KO; 14d wild-type vs. 14d KO (CORT only).
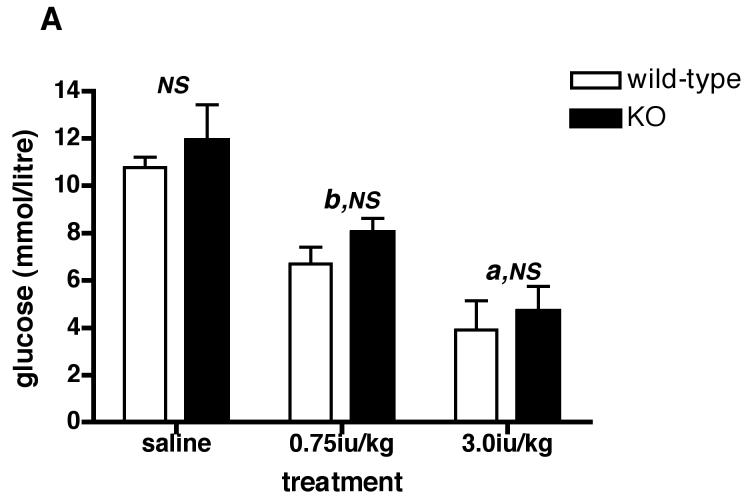
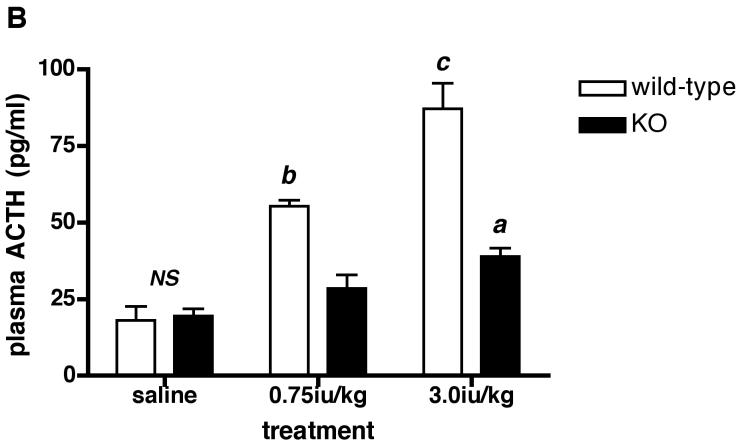
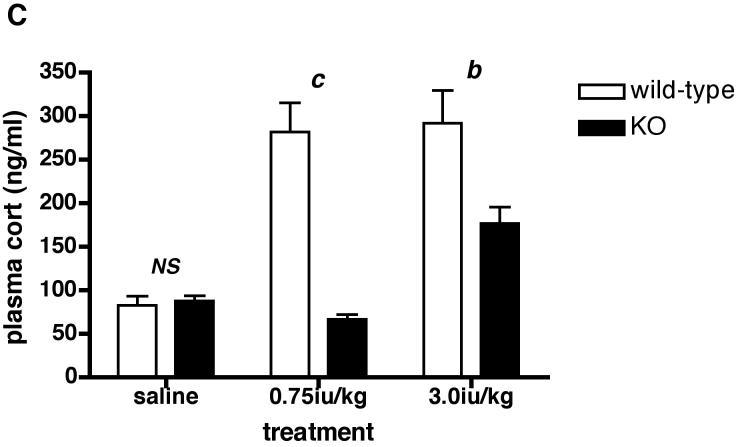
Since there was no difference in plasma ACTH or CORT between acutely restrained Avpr1b KO and wild-type mice, we tested the effects of another acute stressor (hypoglycemia) on HPA axis activation in the animals. Fig. 5A shows that 0.75iu/kg insulin reduces (P < 0.01) plasma glucose by approx. 33% and 38% in Avpr1b KO and wild-type mice, respectively. A higher dose of insulin (3.0iu/kg) causes a further fall in plasma glucose such that levels are now approx. 60% lower than saline-injected control animals for both genotypes. As described in many studies (see refs.19, 22-31), the hypoglycemia associated with peripheral insulin administration activated the HPA axis, as shown by the increase in plasma ACTH (approx. 3-5-fold; see Fig.5B) and CORT (approx. 4-5-fold; see Fig.5C) levels in wild-type mice (Fig.5B). In contrast, the 0.75iu/kg insulin dose did not increase plasma ACTH or CORT levels in the Avpr1b KO animals - the CORT levels in Avpr1b KO plasma following the higher insulin dose (3.0iu/kg) were approx. 40-50% lower (P < 0.001 for ACTH levels; P < 0.01 for CORT levels) than those found in the plasma of wild-type mice. The results show that an intact Avpr1b is required for a normal HPA axis response to acute insulin-induced hypoglycemia but not acute restraint.
Figure 5.
Levels of plasma glucose (A), ACTH (B) and CORT (C) following peripheral (ip) administration of insulin (Actrapid) to Avpr1b-deficient mice.
Mice were sacrificed 1 h after treatment with saline vehicle, 0.75iu/kg or 3.0iu/kg insulin. Values are mean ± SEM, n = 3-4 mice/treatment. Plasma glucose = A: a, P < 0.05 0.75iu/kg insulin vs. 3.0iu/kg insulin. b, P < 0.01 saline vs. insulin-injected. b, P < 0.01 saline vs. insulin-injected. NS, no significant difference (P > 0.05) saline wild-type vs. saline KO; 0.75iu/kg insulin wild-type vs. 0.75iu/kg insulin KO; 3.0iu/kg insulin wild-type vs. 3.0iu/kg insulin KO. Plasma ACTH = B: a, P < 0.05 3.0iu/kg insulin KO vs. saline KO. b, P < 0.01 0.75iu/kg insulin wild-type vs. 0.75iu/kg insulin KO; 0.75iu/kg insulin wild-type vs. saline wild-type; 3.0iu/kg insulin wild-type vs. 0.75iu/kg insulin wild-type. c, P < 0.001 3.0iu/kg insulin wild-type vs. 3.0iu/kg insulin KO; 3.0iu/kg insulin wild-type vs. saline wild-type. NS, no significant difference (P > 0.05) saline wild-type vs. saline KO. Plasma CORT = C: b, P < 0.01 3.0iu/kg insulin wild-type vs. 3.0iu/kg insulin KO; 0.75iu/kg insulin KO vs. 3.0iu/kg insulin KO. c, P < 0.001 0.75iu/kg insulin wild-type vs. 0.75iu/kg insulin KO. NS, no significant difference (P >0.05) saline wild-type vs. saline KO.
Discussion
In previous studies mice with a targeted disruption of the Avpr1b have been shown to exhibit markedly reduced male–male territorial aggression (in the resident-intruder paradigm)(5) and impaired social motivation (49). In the present study we have verified that the pituitary Avpr1b is not functional in the Avpr1b mutants - Avp does not stimulate ACTH release or potentiate Crh-induced ACTH release from Avpr1b-deficient pituitaries - and have used the KO animals to further investigate the role of Avp in the HPA axis response to stress. Basal levels of Avp, Oxt and Crh gene expression in the hypothalamic PVN, and Pomc (the precursor for ACTH and β-endorphin) gene expression in the anterior pituitary are normal in the Avpr1b KOs. In the absence of any gross anatomical deficit in the adrenal, male and female Avpr1b KOs have normal levels of circulating CORT and an intact diurnal CORT rhythm. The main finding(s) of this study are that an intact Avpr1b is required for an HPA axis response to acute insulin-induced hypoglycemia but not acute restraint in male mice. In addition, our results show a lack of adaptation in plasma CORT levels to repeated restraint in wild-type and Avpr1b mutant mice, and suggest that Avp (and its cognate Avpr1b) only seems necessary for a full ACTH response to the final episode of restraint in a 14 d ‘chronic’ (repeated) restraint paradigm.
There is a wealth of data suggesting that Avp participates in the HPA axis response to acute stressors, including restraint or immobilization stress (e.g., see refs. 35,38,66,71 and Introduction). Many of these studies indicate that the co-operation between pPVN Crh and Avp (often indirectly assessed by measuring peptide hnRNA/mRNA levels by ISHH following a stress) is required for a normal ACTH/CORT response to some, but not all acute stressors. We have previously shown that Avpr1b KO mice exhibit a normal CORT response to a brief physical-psychological stress (exposure to an intruder mouse)(5). However, there is evidence that in acute stress, ACTH responses do not always parallel CORT responses (e.g., ref.50), and that ACTH-independent activation of adrenal CORT secretion through neural or humoral factors can occur (51,52). Such factors may also act in concert to modulate adrenocortical sensitivity to ACTH and be stressor-specific, and include immune- or adrenal-produced cytokines such as interleukin-6 (53,54), sympathetic (e.g., splanchnic nerve) activity (55) and locally produced peptides and other factors that may have a paracrine function (51). Results in another Avpr1b KO line demonstrated that while the HPA response to forced swim is reduced in Avpr1b KOs, stress-stimulated ACTH release appears more closely related to Avp stimulation than CORT release (39). In the present study, the HPA axis response to acute restraint stress was not compromised in male Avpr1b KO mice and suggests that Avp is not required for the acute restraint-induced elevation in ACTH and CORT secretion. It is possible that the restraint procedure employed in this study was sufficiently stressful to override any contribution from Avp - e.g., ACTH secretion may be entirely (over)driven by the major ACTH secretagogue Crh. This concept is supported by a study (38) that found that there was mainly a pPVN Crh (and not Avp) hnRNA and mRNA response to acute restraint in naïve rats. In addition, there are many other ‘minor’ agents with potential ACTH releasing activity (such as vasoactive intestinal peptide, angiotensin, ghrelin, orexins, isatin (56-59)) that may compensate in part for the lack of Avp action at the Avpr1b. The normal ACTH response to acute restraint in our Avpr1b KOs cannot be accounted for by substitutive or compensatory effects of Oxt stimulation of corticotrope function because Oxt's action on corticotrope ACTH release are mediated by the Avpr1b (60,61). It should be noted that an Avpr1b anatagonist SSR149415 blocks acute restraint-induced ACTH secretion in rats (62), although the specificity of this compound has been questioned by others (63).
Our results on restraint stress are compatible with those of a previous study in the Avp-deficient Brattleboro rat by Zelena and coworkers (64). In their study, normal ACTH and CORT responses to acute restraint were maintained in Avp-deficient animals. These authors also found that the fast ACTH response to the 11th and the 15th restraint (but not 5th or 8th) was significantly lower in male, but not female Brattleboro rats compared to that obtained for acute restraint or to the 11th restraint in control animals. In our repeated restraint study only one time point (14 d) was investigated - it is conceivable that, as in the Brattleboro rat, a deficit in the ACTH response may be observed in the last session of a shorter period of repeated restraint. In both the Brattleboro rat and Avpr1b KO mice it appears that there is sufficient ACTH remaining after (and secreted during) the final restraint session to elicit a full CORT response. It is important to note that the glucocorticoid response to ACTH saturates at low circulating ACTH levels (65). In addition, our results may reflect increased adrenocortical sensitivity to ACTH with or without the concomitant participation of ACTH-independent neural or humoral factors as already outlined above. The extrapituitary regulation of adrenocortical function is known to be very pertinent to chronic forms of stress (52). Future studies may determine whether there are changes in adrenocortical function (e.g., is there evidence of increased expression of cytokines and/or cytokine receptors or an increased sympatho-adrenomedullary drive?) in the Avpr1b KO subjected to chronic stress. Notwithstanding the above considerations, it should be emphasized that there are alternative explanations for the discordant ACTH and CORT responses to repeated restraint stress in the Avpr1b KO mice, e.g., ACTH levels (driving a normal CORT response) may have peaked earlier than the one time point (30 min) assayed in this study.
Assuming that the dynamics of restraint-induced release of ACTH secretagogues is similar in Avpr1b KO and wild-type mice, our findings also suggest that Crh (and/or other stimulators of ACTH secretion) does not compensate for the reduced pituitary ACTH responsiveness to the final acute restraint session in the repeated restraint experiments in the Avpr1b KOs. This conclusion is supported by the studies of Ma and coworkers (38) which showed that a final episode of acute restraint following 13 d of repeated daily restraint in rats increased the expression of pPVN Avp hn- and m-RNAs but not pPVN Crh hn/mRNAs. In addition, a ‘related’ stress such as repeated immobilization increases Avp expression in Crh-containing fibres in the external zone of the median eminence without altering Crh levels (66). A critical difference between our results and those of others using repeated restraint procedures (as outlined in Ma and coworkers (38)) is that the ACTH and CORT responses in wild-type mice, and CORT responses in the Avpr1b KOs did not adapt to the final restraint stress. In the Avpr1b KOs the ACTH response adapted after 14 d of daily, repeated restraint stress. When rodents are repeatedly subjected to the same stressor on a daily basis, a reduction of the ACTH or CORT response with days is frequently, but not always observed (67). Adaptation appears to be clearer and more consistent with low to middle intensity stressors that do not possess a physical component (67), and its extent can be strain-dependent (68).
In contrast to our results on acute restraint, the HPA response (ACTH and CORT - see Fig.5) to hypoglycemia evoked by acute peripheral insulin administration is dramatically reduced in the Avpr1b KOs. Insulin-induced hypoglycemia is a potent physiological stimulus of the HPA axis and both Crh and/or Avp appear to act as ACTH secretagogues in studies on man (25-27), sheep (28,29,32), horse (31) and rat (21,23,24). The relative roles of Crh and Avp in mediating the ACTH and CORT response to insulin-induced hypoglycemia are uncertain, and although one study suggested that there was a preferential release of Avp over Crh and Oxt into hypophysial portal blood in rats (23), most studies interpret their findings as suggesting a synergistic effect of Crh and Avp in simulating ACTH release. The origin of Avp was not addressed in this study – insulin increases plasma levels of Avp (69), presumably derived from the magnocellular vasopressinergic neurons in the PVN or SON, which may act on the corticotrope Avpr1b (6,13,14). On the other hand, others have shown that insulin increases Avp (and Crh) turnover in the external zone of the median eminence (30), suggesting that the peptide originates from the pPVN. It is clear that low and high doses of insulin may have different effects on Crh/Avp release (32) depending on the pathway(s) that are activated. Insulin-induced hypoglycemia may induce HPA axis responses via two major pathways - neuronal components that impinge on the Crh neuron and humoral activation of the HPA axis via a pituitary-dependent pathway (11). It is possible that there is less inhibition of CORT release with the high (3.0iu/kg) compared with low (0.75iu/kg) insulin doses in the Avpr1b KOs because a more Avp-independent pathway(s) is being activated. Additional studies using different stressors with varying levels of intensity (e.g., more moderate forms of restraint) and that activate different hierarchal pathways will be required to shed more understanding on the role of the Avpr1b in acute stress.
In conclusion, the data suggests that the Avpr1b is required for the normal pituitary and adrenal response to some acute stressful stimuli, and is necessary only for a normal ACTH response during chronic restraint stress, and that other hypothalamic peptides such as Crh do not compensate for the loss of the Avpr1b in maintaining the normal response to these stressors. The Avpr1b KO should provide a useful model to investigate the role of Avp in other aspects of HPA axis function, for example facilitation and sensitization, where Avp has been implicated (70,71).
Acknowledgements
This manuscript is dedicated to the memory of Dr. Michael S. Harbuz. The work was supported by Wellcome Trust (U.K.) grants to SJL and A-MO'C. WSY is supported by the NIMH Intramural Research Program (Z01-MH-002498-16). The authors thank Mr. Alistair Don and Ms. Lisa Chang for technical support. We also thank Mr. Wayne Tainton for glucose assays. The results of this work were presented in part at the 35th International Society for Psychoneuroendocrinology (ISPNE) Congress, Glasgow, Scotland, July, 2004.
Footnotes
Disclosure statement: The authors have nothing to declare
Publisher's Disclaimer: NIH statement: This is an un-copyedited author manuscript copyrighted by The Endocrine Society. This may not be duplicated or reproduced, other than for personal use or within the rule of “Fair Use of Copyrighted Materials” (section 107, Title 17, U.S. Code) without permission of the copyright owner, The Endocrine Society. From the time of acceptance following peer review, the full text of this manuscript is made freely available by The Endocrine Society at http://www.endojournals.org/. The final copy edited article can be found at http://www.endojournals.org/. The Endocrine Society disclaims any responsibility or liability for errors or omissions in this version of the manuscript or in any version derived from it by the National Institutes of Health or other parties. The citation of this article must include the following information: author(s), article title, journal title, year of publication and DOI.
References
- 1.Knepper MA. Molecular physiology of urinary concentrating mechanism: regulation of aquaporin water channels by vasopressin. Am J Physiol Renal Physiol. 1997;272:F3–F12. doi: 10.1152/ajprenal.1997.272.1.F3. [DOI] [PubMed] [Google Scholar]
- 2.Young LJ, Nilsen R, Waymire KG, MacGregor GR, Insel TR. Increased affiliative response to vasopressin in mice expressing the V1a receptor from a monogamous vole. Nature. 1999;400:766–768. doi: 10.1038/23475. [DOI] [PubMed] [Google Scholar]
- 3.Storm EE, Tecott LH. Social circuits: Peptidergic regulation of mammalian social behaviour. Neuron. 2005;47:483–486. doi: 10.1016/j.neuron.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Koshimizu T, Nasa Y, Tanoue A, Oikawa R, Kawahara Y, Kiyono Y, Adachi T, Tanaka T, Kuwaki T, Mori T, Takeo S, Okamura H, Tsujimoto G. V1a vasopressin receptors maintain normal blood pressure by regulating circulating blood volume and baroreflex sensitivity. Proc Natl Acad Sci USA. 2006;103:7807–7812. doi: 10.1073/pnas.0600875103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wersinger SR, Ginns EI, O'Carroll A-M, Lolait SJ, Young WS., III Vasopressin V1b receptor knockout reduces aggressive behaviour in male mice. Molecular Psychiatry. 2002;7:975–984. doi: 10.1038/sj.mp.4001195. [DOI] [PubMed] [Google Scholar]
- 6.Antoni F. Vasopressinergic control of pituitary adrenocorticotropin secretion comes of age. Front Neuroendocrinol. 1993;14:76–122. doi: 10.1006/frne.1993.1004. [DOI] [PubMed] [Google Scholar]
- 7.Volpi S, Rabadan-Diehl C, Aguilera G. Vasopressinergic regulation of the hypothalamic pituitary adrenal axis and stress adaptation. Stress. 2004;7:75–83. doi: 10.1080/10253890410001733535. [DOI] [PubMed] [Google Scholar]
- 8.Lolait SJ, O'Carroll A-M, Mahan LC, Felder CC, Button DC, Young WS, III, Mezey E, Brownstein MJ. Extrapituitary expression of the rat V1b vasopressin receptor gene. Proc Natl Acad Sci USA. 1995;92:6783–6787. doi: 10.1073/pnas.92.15.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunningham ET, Jr, Sawchenko PE. Reflex control of magnocellular vasopressin and oxytocin secretion. Trends Neurosci. 1991;14:406–411. doi: 10.1016/0166-2236(91)90032-p. [DOI] [PubMed] [Google Scholar]
- 10.Whitnall MH. Regulation of the hypothalamic corticotropin-releasing hormone neurosecretory system. Prog Neurobiol. 1993;40:573–629. doi: 10.1016/0301-0082(93)90035-q. [DOI] [PubMed] [Google Scholar]
- 11.Pacak K, Palkovits M. Stressor specificity of central neuroendocrine responses: Implications for stress-related disorders. Endocr Rev. 2001;22:502–548. doi: 10.1210/edrv.22.4.0436. [DOI] [PubMed] [Google Scholar]
- 12.Herman JP, Figueiredo H, Mueller NK, Ulrich-Lai Y, Ostrander MM, Choi DC, Cullinan WE. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo-pituitary-adrenocortical responsiveness. Front Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Wotjak C, Naruo T, Muraoka S, Simchen R, Landgraf R, Engelmann M. Forced swimming stimulates the expression of vasopressin and oxytocin in magnocellular neurons of the rat hypothalamic paraventricular nucleus. Eur J Neurosci. 2001;13:2273–2281. doi: 10.1046/j.0953-816x.2001.01613.x. [DOI] [PubMed] [Google Scholar]
- 14.Wotjak T, Ludwig M, Ebner K, Russell JA, Singewald S, Landgraf R, Engelmann M. Vasopressin from hypothalamic magnocellular neurons has opposite effects at the adenohypophysis and in the supraoptic nucleus on ACTH secretion. Eur J Neurosci. 2002;16:477–485. doi: 10.1046/j.1460-9568.2002.02101.x. [DOI] [PubMed] [Google Scholar]
- 15.Muglia L, Jacobson L, Dikkes P, Majzoub JA. Corticotropin-releasing hormone deficiency reveals major fetal but not adult glucocorticoid need. Nature. 1995;373:427–432. doi: 10.1038/373427a0. [DOI] [PubMed] [Google Scholar]
- 16.Timpl P, Spanagel R, Sillaber T, Kresse A, Reul JMHM, Stalla GK, Blanquet V, Steckler T, Holsboer F, Wurst W. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nature Genetics. 1998;19:162–166. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- 17.Smith GW, Aubry J-M, Dellu F, Contarino A, Bilezikjian M, Gold LH, Chen R, Marchuk Y, Hauser C, Bentley CA, Sawchenko PE, Koob GF, Vale W, Lee K-F. Corticotropin releasing factor receptor 1-deficient mice display decreased anxiety, impaired stress response, and aberrant neuroendocrine development. Neuron. 1998;20:1093–1102. doi: 10.1016/s0896-6273(00)80491-2. [DOI] [PubMed] [Google Scholar]
- 18.Muller MB, Landgraf R, Preil J, Sillaber I, Kresse AE, Keck ME, Zimmerman S, Holsboer F, Wurst W. Selective activation of the hypothalamic vasopressinergic system in mice deficient for the corticotropin-releasing hormone receptor 1 is dependent on glucocorticoids. Endocrinology. 2000;141:4262–4269. doi: 10.1210/endo.141.11.7767. [DOI] [PubMed] [Google Scholar]
- 19.Makara GB, Mergl Z, Zelena D. The role of vasopressin in hypothalamo-pituitary-adrenal axis activation during stress: an assessment of the evidence. Ann NY Acad Sci. 2004;1018:151–161. doi: 10.1196/annals.1296.018. [DOI] [PubMed] [Google Scholar]
- 20.Kjaer A, Knigge U, Bach FW, Warberg J. Histamine- and stress-induced secretion of ACTH and beta-endorphin: involvement of corticotropin-releasing hormone and vasopressin. Neuroendocrinology. 1992;56:419–28. doi: 10.1159/000126258. [DOI] [PubMed] [Google Scholar]
- 21.Suda T, Nakano Y, Tozawa F, Sumitomo T, Sata Y, Yamada M, Demura H. The role of corticotropin-releasing factor and vasopressin in hypoglycemia-induced proopiomelanocortin gene expression in the rat anterior pituitary gland. Brain Res. 1992;579:303–308. doi: 10.1016/0006-8993(92)90065-h. [DOI] [PubMed] [Google Scholar]
- 22.Turnbull AV, Lee S, Rivier C. Mechanisms of hypothalamic-pituitary-adrenal axis stimulation by immune signals in the adult rat. Ann NY Acad Sci. 1998;840:434–443. doi: 10.1111/j.1749-6632.1998.tb09582.x. [DOI] [PubMed] [Google Scholar]
- 23.Plotsky PM, Bruhn TO, Vale W. Hypophysiotropic regulation of adrenocorticotropin secretion in response to insulin-induced hypoglycemia. Endocrinology. 1985;117:323–329. doi: 10.1210/endo-117-1-323. [DOI] [PubMed] [Google Scholar]
- 24.Paulmyer-Lacroix O, Anglade G, Grino M. Insulin-induced hypoglycaemia increases colocalization of corticotrophin-releasing factor and arginine vasopressin mRNAs in the rat hypothalamic paraventricular nucleus. J Mol Endocrinol. 1994;13:313–320. doi: 10.1677/jme.0.0130313. [DOI] [PubMed] [Google Scholar]
- 25.Baylis PH, Heath DA. Plasma-arginine-vasopressin response to insulin-induced hypoglycaemia. Lancet. 1977;2:428–430. doi: 10.1016/s0140-6736(77)90610-9. [DOI] [PubMed] [Google Scholar]
- 26.Fisher BM, Baylis PH, Frier BM. Plasma oxytocin, arginine vasopressin and atrial natriuretic peptide responses to insulin-induced hypoglycaemia in man. Clin Endocrinol (Oxf) 1987;26:179–185. doi: 10.1111/j.1365-2265.1987.tb00775.x. [DOI] [PubMed] [Google Scholar]
- 27.Ellis MJ, Schmidli RS, Donald RA, Livesey JH, Espiner EA. Plasma corticotrophin-releasing factor and vasopressin responses to hypoglycaemia in normal man. Clin Endocrinol (Oxf) 1990;32:93–100. doi: 10.1111/j.1365-2265.1990.tb03754.x. [DOI] [PubMed] [Google Scholar]
- 28.Engler D, Pham T, Fullerton MJ, Ooi G, Funder JW, Clarke IJ. Studies of the secretion of corticotropin-releasing factor and arginine vasopressin into the hypophysial-portal circulation of the conscious sheep. I. Effect of an audiovisual stimulus and insulin-induced hypoglycemia. Neuroendocrinology. 1989;49:367–381. doi: 10.1159/000125141. [DOI] [PubMed] [Google Scholar]
- 29.Guillaume V, Conte-Devolx B, Magnan E, Boudouresque F, Grino M, Cataldi M, Muret L, Priou A, Figarili JC, Oliver C. Effect of chronic active immuniztion with antiarginine vasopressin on pituitary-adrenal function in sheep. Endocrinology. 1992;130:3007–3014. doi: 10.1210/endo.130.5.1315264. [DOI] [PubMed] [Google Scholar]
- 30.Berkenbosch F, De Goeij DCE, Tilders FJH. Hypoglycemia enhances turnover of corticotropin-releasing factor and of vasopressin in the zona externa of the rat median eminence. Endocrinology. 1989;125:28–34. doi: 10.1210/endo-125-1-28. [DOI] [PubMed] [Google Scholar]
- 31.Alexander SL, Roud HK, Irvine CHG. Effect of insulin-induced hypoglycaemia on secretion patterns and rates of corticotropin-rleasing hormone, arginine vasopressin and adrenocorticotrophin in horses. J Endocrinol. 1997;153:401–409. doi: 10.1677/joe.0.1530401. [DOI] [PubMed] [Google Scholar]
- 32.Caraty A, Grino M, Locatelli A, Guillaume V, Boudouresque F, Conte-Devoix B, Oliver C. Insulin-induced hypoglycenia stimulates corticotropin-releasing factor and arginine vasopressin secretion into hypophysial portal blood of conscious, unrestrained rams. J Clin Invest. 1990;85:1716–1721. doi: 10.1172/JCI114626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harbuz MS, Lightman SL. Stress and the hypothalamo-pituitary-adrenal axis: acute, chronic and immunological activation. J. Endocrinol. 1992;134:327–339. doi: 10.1677/joe.0.1340327. [DOI] [PubMed] [Google Scholar]
- 34.Aguilera G. Regulation of pituitary ACTH secretion during chronic stress. Front Neuroendocrinol. 1994;15:321–350. doi: 10.1006/frne.1994.1013. [DOI] [PubMed] [Google Scholar]
- 35.De Goeij DCE, Jezova D, Tilders FJ. Repeated stress enhances vasopressin synthesis in corticotropin releasing factor neurons in the paraventricular nucleus. Brain Res. 1992;577:165–168. doi: 10.1016/0006-8993(92)90552-k. [DOI] [PubMed] [Google Scholar]
- 36.Rabadan-Diehl C, Lolait SJ, Aguilera G. Regulation of pituitary vasopressin V1b receptor mRNA during stress in the rat. J Neuroendocrinol. 1995;7:903–910. doi: 10.1111/j.1365-2826.1995.tb00734.x. [DOI] [PubMed] [Google Scholar]
- 37.Hashimoto K, Suemaru S, Takao T, Sugarwara M, Makino S, Ota S. Corticotropin-releasing hormone and pituitary-adrenocortical responses to chronically stressed rats. Regul Pept. 1988;23:117–126. doi: 10.1016/0167-0115(88)90019-5. [DOI] [PubMed] [Google Scholar]
- 38.Ma X-M, Levy A, Lightman SL. Emergence of an isolated arginine vasopressin (AVP) response to stress after repeated restraint: A study of both AVP and corticotropin-releasing hormone messenger ribonucleic acid (RNA) and heteronuclear RNA. Endocrinology. 1997;138:4351–4357. doi: 10.1210/endo.138.10.5446. [DOI] [PubMed] [Google Scholar]
- 39.Tanoue A, Ito S, Honda K, Oshikawa S, Kitagawa Y, Koshimizu T, Mori T, Tsujimoto G. The vasopressin V1b receptor critically regulates hypothalamic-pituitary-adrenal axis activity under both stress and resting conditions. J Clin Invest. 2004;113:302–309. doi: 10.1172/JCI19656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Keeney A, Jessop DS, Harbuz MS, Marsden CA, Hogg S, Blackburn-Munro RE. Differential effects of acute and chronic social defeat stress on HPA axis function and hippocampal serotonin release in mice. J Neuroendocrinology. 2006;18:330–338. doi: 10.1111/j.1365-2826.2006.01422.x. [DOI] [PubMed] [Google Scholar]
- 41.Harbuz MS, Rees RG, Eckland DJA, Jessop DS, Brewerton D, Lightman SL. Paradoxical responses of hypothalamic CRF mRNA and CRF-41 peptide and adenohypophyseal POMC mRNA during chronic inflammatory stress. Endocrinology. 1992;130:1394–1400. doi: 10.1210/endo.130.3.1537299. [DOI] [PubMed] [Google Scholar]
- 42.Jessop DS, Eckland DJA, Todd K, Lightman SL. Osmotic regulation of hypothalamo-neurointermediate lobe corticotrophin-releasing factor-41 in the rat. J Endocrinol. 1989;120:119–124. doi: 10.1677/joe.0.1200119. [DOI] [PubMed] [Google Scholar]
- 43.Young WS, III, Mezey E, Siegel RE. Vasopressin and oxytocin mRNAs in adrenalectomized and Brattleboro rats: analysis by quantitative in situ hybridisation histochemistry. Brain Res. 1986;387:231–241. doi: 10.1016/0169-328x(86)90029-x. [DOI] [PubMed] [Google Scholar]
- 44.Young WS, III, Mezey E, Siegel RE. Quantitative in situ hybridisation histochemistry reveals increased levels of corticotropin-releasing factor mRNA after adrenalectomy in rats. Neurosci Lett. 1986;70:198–203. doi: 10.1016/0304-3940(86)90463-5. [DOI] [PubMed] [Google Scholar]
- 45.O'Carroll A-M, Raynor K, Lolait SJ, Reisine T. Characterization of cloned human somatostatin receptor SSTR5. Mol Pharmacol. 1994;46:291–298. [PubMed] [Google Scholar]
- 46.Slominski A, Ermak G, Hwang J, Mazurkiewicz J, Corliss D, Eastman A. The expression of proopiomelanocortin (POMC) and of corticotropin releasing hormone receptor (CRH-R) genes in mouse skin. Biochim Biophys Acta. 1996;1289:247–251. doi: 10.1016/0304-4165(95)00159-x. [DOI] [PubMed] [Google Scholar]
- 47.Young WS, III, Bonner TJ, Brann MR. Mesencephalic dopamine neurons regulate the expression of neuropeptide mRNAs in the rat forebrain. Proc Natl Acad Sci USA. 1986;83:9827–9831. doi: 10.1073/pnas.83.24.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kitay JI. Sex differences in adrenal cortical secretion in the rat. Endocrinology. 1961;68:818–824. doi: 10.1210/endo-68-5-818. [DOI] [PubMed] [Google Scholar]
- 49.Wersinger SR, Kelliher KR, Zufall F, Lolait SJ, O'Carroll A-M, Young SW., 3rd Social motivation is reduced in vasopressin 1b receptor null mice despite normal performance in an olfactory discrimination task. Horm Behav. 2004;46:638–645. doi: 10.1016/j.yhbeh.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 50.Bhatnagar S, Sun LM, Raber J, Manes S, Julius DMF, Dallman MF. Changes in anxiety-related behaviour and hypothalamic pituitary adrenal activity in mice lacking the 5-HT-3A receptor. Physiol Behav. 2004;81:545–555. doi: 10.1016/j.physbeh.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 51.Ehrhart-Bornstein M, Hinson JP, Bornstein SF, Scherbaum WA, Vinson GP. Intraadrenal interactions in the regulation of adrenocortical steroidogenesis. Endocr Rev. 1998;19:101–143. doi: 10.1210/edrv.19.2.0326. [DOI] [PubMed] [Google Scholar]
- 52.Bornstein SR, Chrousos GP. Adrenocorticotropin (ACTH)- and non-ACTH-mediated regulation of the adrenal cortex: neural and immune inputs. J Clin Endocrinol Metab. 1999;84:1729–1736. doi: 10.1210/jcem.84.5.5631. [DOI] [PubMed] [Google Scholar]
- 53.Zhou D, Kusnecov AW, Shurin MR, DePaoli M, Rabin BS. Exposure to physical and psychological stressors elevates plasma interleukin 6: relationship to the activation of hypothalamic-pituitary-adrenal axis. Endocrinology. 1993;133:2523–2530. doi: 10.1210/endo.133.6.8243274. [DOI] [PubMed] [Google Scholar]
- 54.Bethin KE, Vogt SK, Muglia LJ. Interleukin-6 is an essential, corticotropin-releasing hormone-independent stimulator of the adrenal axis during immune system activation. Proc Natl Acad Sci USA. 2000;97:9317–9322. doi: 10.1073/pnas.97.16.9317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jasper MS, Engeland WC. Spanchnicotomy increases adrenal sensitivity to ACTH in nonstressed rats. Am J Physiol. 1997;273:E363–E368. doi: 10.1152/ajpendo.1997.273.2.E363. [DOI] [PubMed] [Google Scholar]
- 56.Carrasco GA, Van de Kar LD. Neuroendocrine pharmacology of stress. Eur J Pharmacol. 2003;463:235–272. doi: 10.1016/s0014-2999(03)01285-8. [DOI] [PubMed] [Google Scholar]
- 57.Giordano R, Picu A, Broglio F, Bonelli L, Baldi M, Berardelli R, Ghigo E, Arvat E. Ghrelin, hypothalamus-pituitary-adrenal (HPA) axis and Cushing's syndrome. Pituitary. 2004;7:243–248. doi: 10.1007/s11102-005-1173-6. [DOI] [PubMed] [Google Scholar]
- 58.Spinazzi R, Andreis PG, Rossi GP, Nussdorfer GG. Orexins in the regulation of the hypothalamic-pituitary-adrenal axis. Pharmacol Rev. 2006;58:46–57. doi: 10.1124/pr.58.1.4. [DOI] [PubMed] [Google Scholar]
- 59.Medvedev A, Igosheva N, Crumeyrolle-Arias M, Glover V. Isatin: Role in stress and anxiety. Stress. 2005;8:175–183. doi: 10.1080/10253890500342321. [DOI] [PubMed] [Google Scholar]
- 60.Link H, Dayanithi G, Fohr KJ, Gratzl M. Oxytocin at physiological concentrations evokes adrenocorticotropin (ACTH) release from corticotrophs by increasing intracellular free calcium mobilized mainly from intracellular stores. Oxytocin displays synergistic or additive effects on ACTH-releasing factor or arginine vasopressin-induced ACTH secretion, respectively. Endocrinology. 1992;130:2183–2191. doi: 10.1210/endo.130.4.1312449. [DOI] [PubMed] [Google Scholar]
- 61.Schlosser SF, Almeida OFX, Patchev VK, Yassouridis A, Elands J. Oxytocin-stimulated release of adrenocorticotropin from the rat pituitary is mediated by arginine vasopressin receptors of the V1b type. Endocrinology. 1994;135:2058–2063. doi: 10.1210/endo.135.5.7956927. [DOI] [PubMed] [Google Scholar]
- 62.Serradeil-Le Gal C, Wagnon J, Simiand J, Griebel G, Lacour C, Guillon G, Barberis C, Brossard G, Soubrie P, Nisato D, Pascal M, Pruss R, Scatton B, Maffrand J-P, Le Fur G. Characterization of (2S,4R)-1-[5-Chloro-1[(2,4-dimethoxyphenyl)sulfonyl]-3-(2-methoxy-phenyl)-2-oxo-2.3-dihydro-1H-indol-3-yl]-4-hydroxy-N,N-dimethyl-2-pyrrolidine carboxamide (SSR149415), a selective and orally active vasopressin V1b receptor antagonist. J Pharmacol Exp Ther. 2002;300:1122–1130. doi: 10.1124/jpet.300.3.1122. [DOI] [PubMed] [Google Scholar]
- 63.Griffante C, Green A, Curcuruto O, Haslam CP, Dickinson BA, Arban R. Selectivity of d[Cha4]AVP and SSR149415 at human vasopressin and oxytocin receptors: evidence that SSR149415 is a mixed V1b/oxytocin receptor antagonist. Brit J Pharmacol. 2005;146:744–751. doi: 10.1038/sj.bjp.0706383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zelena D, Foldes A, Mergl Z, Barna I, Kovacs KJ, Makara GB. Effects of repeated restraint stress on hypothalamo-pituitary-adrenocortical function in vasopressin-deficient Brattleboro rats. Brain Res Bull. 2004;15:521–530. doi: 10.1016/j.brainresbull.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 65.Dallman MF, Viau VG, Bhatnagar S, Gomez F, Laugero K, Bell ME. Corticotropin-releasing factor, corticosteroids, stress, and sugar: Energy balance, the brain, and behaviour. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behaviour. Vol. 1. USA: Academic Press; 2002. pp. 571–631. [Google Scholar]
- 66.deGoeij DEC, Kvetnansky R, Whitnall MH, Jesova D, Berkenbosch F, Tilders FJH. Repeated stress-induced activation of corticotropin-releasing factor neurons enhances vasopressin stores and colocalization with corticotropin releasing factor neurons in the median eminence of rats. Neuroendocrinology. 1991;53:150–159. doi: 10.1159/000125712. [DOI] [PubMed] [Google Scholar]
- 67.Marti O, Armario A. Anterior pituitary response to stress: time-related changes and adaptation. Int J Dev Neurosci. 1998;16:241–260. doi: 10.1016/s0736-5748(98)00030-6. [DOI] [PubMed] [Google Scholar]
- 68.Dhabhar FS, McEwen BS, Spencer RL. Adaptation to prolonged or repeated stress - comparison between rat strains showing intrinsic differences in reactivity to acute stress. Neuroendocrinology. 1997;65:360–368. doi: 10.1159/000127196. [DOI] [PubMed] [Google Scholar]
- 69.Baylis PH, Robertson GL. Rat vasopressin response to insulin-induced hypoglycemia. Endocrinology. 1980;107:1975–1979. doi: 10.1210/endo-107-6-1975. [DOI] [PubMed] [Google Scholar]
- 70.Schmidt ED, Binnekade R, Jansze AAJW, Tilders FJH. Short stressor induced long-lasting increases of vasopressin stores in hypothalamic corticotropin-releasing hormone (CRH) neurons in adult rats. J Neuroendocrinol. 1996;8:703–712. [PubMed] [Google Scholar]
- 71.Ma X-M, Lightman SL, Aguilera G. Vasopressin and corticotropin-releasing hormone gene responses to novel stress in rats adapted to repeated restraint. Endocrinology. 1999;140:3623–3632. doi: 10.1210/endo.140.8.6943. [DOI] [PubMed] [Google Scholar]