Abstract
The more individuals hypothesis (MIH) postulates that productivity increases species richness by increasing mean equilibrium population size, thereby reducing the probability of local extinction. We tested the MIH for invertebrates colonizing microcosms that simulated tree holes by manipulating productivity through additions of leaf or animal detritus and subsequently determining the relationships among richness, total abundance, abundance per species, and measures of productivity. We quantified productivity as the rate of microorganism protein synthesis, microorganism metabolic rate, nutrient ion concentration, and type and amount of detritus. Microcosms with animal detritus attracted more species, more individuals per species, and more total individuals than did microcosms with similar amounts of leaf detritus. Relationships between richness or abundance and productivity varied with date. Richness in June increased as a linear function of productivity, whereas the power function predicted by the MIH fit best in July. Abundance in June and July was best described by a power function of productivity, but the linear function predicted by the MIH fit best in September. Abundance per species was best described by a power function of productivity in June and July. Path analysis showed that the indirect effect of productivity through abundance on richness that is predicted by MIH was important in all months, and that direct links between productivity and richness were unnecessary. Our results support many of the predictions of the MIH, but they also suggest that the effects of abundance on richness may be more complex than expected.
Keywords: Detritus, Microorganism productivity, Mosquitoes, Species richness, Tree hole microcosm
Introduction
Quantifying the relationship between species richness and productivity and understanding its mechanistic basis are critical challenges to contemporary ecology. Productivity has been defined as the rate at which energy flows in an ecosystem (Rosenzweig 1995). The forms of richness–productivity relationships (for reviews see Waide et al. 1999; Mittelbach et al. 2001) vary considerably among habitats (Waide et al. 1999; Mittelbach et al. 2001) and with the scale of observation (Weiher 1999; Chase and Leibold 2002) and the directness of the productivity estimate (Groner and Novoplansky 2003). Although richness often shows strong relationships to productivity (Abrams 1995; Rosenzweig 1971, 1995; Waide et al. 1999), empirical evidence for or against different mechanisms causing a particular relationship is limited (Srivastava and Lawton 1998; Hurlbert 2004).
Few empirical studies have tested the mechanistic basis of productivity–richness relationships (Abrams 1995; Rosenzweig 1995; Waide et al. 1999). One hypothesis that has received attention is the more individuals hypothesis (hereafter MIH) (Srivastava and Lawton 1998; Yanoviak 2001; Hurlbert 2004, 2006). This mechanism is based on species-energy theory (Wright 1983; Rosenzweig and Abramsky 1993; Abrams 1995) and is a refinement of the ideas of Preston (1962). The MIH postulates that greater productivity supports greater population densities, which lower extinction rates of rare species. This increase in persistence of rare species increases diversity. An alternative mechanism by which productivity may affect richness via abundance is the sampling effect (Kaspari et al. 2003; Evans et al. 2005). This mechanism assumes that more productive sites attract more individuals drawn at random from a species pool, resulting in more productive sites containing more species. Srivastava and Lawton (1998) tested the MIH for invertebrate communities in tree holes in England. They found no support for this hypothesis when female invertebrates were allowed to oviposit in containers in the field, because even though richness increased with a surrogate of productivity (i.e., initial leaf litter quantity), abundance was invariant. These authors did find that abundance and productivity were positively related, but only when productivity was artificially reduced for established communities (Srivastava and Lawton 1998). These mixed results led Srivastava and Lawton (1998) to conclude that for tree holes, increases in richness with productivity was not simply a result of more individuals. In a similar aquatic container system – pitcher plants – increases in resources (dead ants) led to an increase in protozoan richness, which may have resulted from an increase in the abundance of rare taxa in higher resource treatments (Kneitel and Miller 2002), a result consistent with the MIH.
Current ideas about the relationship between diversity and productivity often focus on plant communities, where net primary production is the obvious measure of productivity (Waide et al. 1999; Mittelbach et al. 2001). Less attention has been directed at understanding relationships between productivity at one trophic level and richness at the next higher trophic level, and also to how richness and productivity are related in detritus-based systems. Detritus represents the dominant energy input to many ecosystems (O’Neill and Reichle 1980), and it plays an important role in aquatic animal communities (Anderson and Macfadyen 1976), where it can affect trophic dynamics, species interactions, and ecosystem functioning (Moore et al. 2004). Inputs of terrestrial plant detritus to aquatic systems have been well studied (Cloe and Garman 1996). Plant-derived detritus usually must pass through decomposer trophic levels before its energy is available to higher trophic levels, while detritus from animal sources (e.g., terrestrial invertebrates) is likely to be more available for direct ingestion by consumers (Mason and MacDonald 1982; Garman 1991). A more efficient transfer of animal- versus plant-derived energy has been suggested for top consumers in streams (e.g., fish; see Garman 1991; Cloe and Garman 1996; Nakano et al. 1999) and for the most common consumer in water-filled tree holes (Yee and Juliano 2006).
Because primary production in tree holes is essentially absent (Carpenter 1983), allochthonous inputs of detritus serve as the energy source for tree hole food webs. Detritus inputs into tree holes are dominated by senescent leaves (Kitching 2001), nutrient-bearing stem flow (water flowing along tree trunks after precipitation, Carpenter 1983), and terrestrial invertebrate carcasses (Yee 2006). Tree holes are colonized by a diverse community of aquatic macroinvertebrates, many of which are specialists on this habitat (Kitching 2000). There have been numerous tests of the effects of leaf litter inputs (e.g., Léonard and Juliano 1995; Walker et al. 1997; Srivastava and Lawton 1998) and stem flow (e.g., Kitching 1971; Carpenter 1983; Walker et al. 1991) on populations and communities in tree holes, but only one study has examined the role of dead invertebrates as an energy source for aquatic inhabitants in this system (Yee and Juliano 2006). No study has investigated how different types of detritus affect properties of communities (e.g., species richness) within tree holes.
We used artificial tree holes to test predictions of the MIH. Because natural tree hole communities are often resource limited (Kitching 2000, 2001), we predicted that increasing detritus-based productivity would support greater species richness of invertebrates. We also tested for differences in richness (S), abundance (N), and abundance per species (N/S) between plant- versus animal-based tree holes. Based on the MIH, we predicted that increased productivity should result in increased invertebrate abundance (or abundance per species) and that as a consequence of this greater abundance, richness also should increase. We also tested Srivastava and Lawton’s (1998) prediction that abundance should increase as a linear function of productivity, whereas richness should increase as either a power or logarithmic function of productivity, and added the prediction that abundance per species should increase with increasing productivity. Finally, we used path analysis to test the prediction that changes in abundance with increasing productivity are the cause of changes in richness. Path analysis, unlike typical regression techniques, enables the researcher to make statistical comparisons of alternative casual relationships among productivity, abundance, and richness (Mitchell 2001).
Methods
Artificial tree holes (hereafter, microcosms) were located within ParkLands Foundation Merwin Preserve, Lexington, Illinois (40°39′10″N, 88°52′21″W). This preserve is an upland deciduous forest dominated by oak, hickory, elm, and maple trees and contains natural tree holes that provide a source pool of invertebrate colonists. We constructed microcosms out of 10-cm diameter PVC pipe cut into 25-cm segments and sealed at the bottom with a plastic cover and rubber cap to make them water tight. Each microcosm had a lid with a 4-cm opening to decrease evaporation and uncontrolled additions of detritus. Microcosm water levels were maintained at or above 1800 ml. Because we used containers of a standard size, the volume of water in any one microcosm necessarily decreased as the amount of detritus increased. We placed microcosms in wire mesh cages (1.3 × 1.3-cm openings) attached to trees (≥ 30 cm in diameter), approximately 1 m off the ground and ≥ 10 m apart. The cages minimized uncontrolled additions of detritus and disturbance by mammals.
We used two types of detritus: leaves and dead crickets. The leaf detritus consisted of a mix of equal parts of senescent leaves of three tree species common at this site: white oak (Quercus alba), sugar maple (Acer saccharum), and American elm (Ulmus americana). We collected senescent leaves in the fall of 2003 from the ParkLands Preserve, stored them dry at room temperature, and then cut them into approximately 1.25-cm2 pieces after the petioles had been removed. Decorated crickets (Gryllodes sigillatus) were obtained from colonies within the Department of Biological Sciences, Illinois State University. Whenever possible, the mass of detritus needed for a treatment comprised whole crickets. We chose crickets as a readily available, large-bodied terrestrial arthropod that was similar in size to some of the taxa that form animal detritus in natural tree holes (e.g., roaches, spiders, beetles; see Yee 2006). The crickets were cold-killed and dried at 50°C for 48 h before being added to the microcosms.
We used seven levels of leaf litter: 0.50, 1.00, 2.00, 4.00, 8.00, 16.00, and 24.00 g. We sampled 16 natural tree holes at ParkLands for total detritus (including all sediment) and found a mean ± 1 SD wet mass of detritus during May and June 2003 of 27.6 ± 13.6 g (median: 13.3 g, range: 1.6–232.3 g). Assuming that dry detritus would be approximately 0.33-fold the mass of wet detritus, we estimated that each tree hole had a mean of 9.2 g dry mass of leaves (range: 0.50–75.0 g). Thus, our series of dry detritus amounts would have ranged from approximately twofold the mean detritus amount encountered in a natural tree hole to the lowest amount. For animal-based microcosms, we used three levels of detritus: 0.25, 0.50, and 1.00 g. We chose these lower amounts because the dominant tree hole consumer (O. triseriatus) attains similar survivorship, growth, and development rates with animal detritus at 1/25 the amount of leaf detritus (Yee and Juliano 2006). Thus, our highest amounts of leaf and animal detritus should produce similar mosquito performance. In addition, animal detritus amounts into natural tree holes are much less than leaf additions (Yee 2006). We randomly assigned each detritus amount and type to three microcosms for a total of 30 microcosms.
We established microcosms with detritus in October 2003, after the end of the invertebrate active season. To ensure colonization by microorganisms, the microcosms remained open for 1 month. At that time, we removed 500 ml of fluid from each microcosm and stored it frozen until April 2004. We discarded the remaining fluid, leaving the detritus, and covered each microcosm with a solid lid. Liquid removal was necessary to prevent the PVC from cracking during winter freezing. Establishing microcosms the previous fall enabled us to age the fluid and to the allow microorganism colonization necessary for invertebrate colonization the following spring. Prior to invertebrate activity in 2004 (early April), we uncovered each microcosm, returned the 500 ml of frozen fluid, and refilled each microcosm with additional deionized water. Conductivity (μM HOS cm−1), which measures total ion concentration, did not differ significantly between November and May (paired t-test: t = 1.83, df = 29, P = 0.08).
We sampled microcosms four times at approximately 5-week intervals: 19 May, 15 June, 22 July, and 16 September 2004. At each time, the contents of each microcosm were removed and taken to a laboratory where all invertebrates were identified to species or morphospecies level and counted, following which the fluid, detritus, and all invertebrates were returned to field microcosms within 24 h of collection. Because mosquito pupae are fragile and difficult to identify to species, they were not included in the analyses of richness, but they were used to calculate the total abundance of all invertebrates for each container.
We measured several productivity surrogates: conductivity, the metabolic rates of microorganisms (MR), and the production of new bacterial biomass measured as protein synthesis (PS). In addition, we used the type and initial total amount of detritus (g) as measures of productivity (Srivastava and Lawton 1998). These productivity surrogates characterize both available essential nutrients (i.e., total detritus, conductivity) and rates of energy utilization by microorganisms that are the base of the decomposer food chain (i.e., MR, PS) (Rosenzweig 1995). Because they are measures of rates of energy use, MR (μl O2 h−1) and PS (nmol ml−1 h−1) are consistent with our definition of productivity (Rosenzweig 1995). All productivity surrogate measurements were conducted on a 20-ml sample of fluid removed from each microcosm before invertebrate removal. We measured PS by measuring the incorporation of tritiated leucine (for details see Kirchman 1993). We added a solution of 3.9:1 unlabeled: 3H-labeled leucine to suspended samples of bacteria, incubated for 30 min, and quantified 3H-leucine incorporation (nmol ml−1 h−1) into protein as a measure of the rate of production of new bacterial biomass. We determined the amount of labeled protein using a Beckman LS-6500 scintillation counter (Beckman Coulter, Fullerton, Calif.). Microorganism community MRs (expressed in microliters of O2 consumed per hour for an 8-ml sample for each microcosm at each time), which represent respiration rates of the lowest trophic levels (i.e., bacteria, fungi, and protozoa), were measured in darkness using a Gilson Differential Respirometer (GDR) (Gilson, Middleton, Wis.). Both MR and PS values for individual microcosms were obtained using the mean microcosm temperature at each collection time. All productivity surrogates were measured in each month, with the exception of PS, which was only measured in May and June.
Statistical analyses
We tested for differences in richness, total abundance, and abundance per species between animal and leaf-based microcosms using ANCOVA (PROC GLM; SAS Institute 1990) with detritus type as the single factor and log-transformed detritus amount as the covariate. Our main focus was on the overall effects of leaf and animal detritus on invertebrate richness, abundance, or per species abundance, and thus we limited the analysis to the final sampling time (September).
We used principal components analysis (PROC FACTOR; SAS Institute 1990; Hatcher and Stepanski 1994) to reduce the total number of productivity surrogates (MR, PS, conductivity, detritus amount, detritus type) and to obtain uncorrelated descriptors of productivity. We retained principal components (PCs) with eigenvalues ≥ 1.0 (Hatcher 1994). Principal component analysis was conducted on the mean values for MR, PS, and conductivity across all of the months these were measured and on the initial detritus amount and type (coded as leaf = 0, animal = 1). We used PC1, with the largest proportion variance in the original productivity surrogates explained, as the measure of productivity. By calculating our estimate of productivity using surrogate variables averaged over time, we are estimating the productivity of each microcosm as a whole, ignoring monthly variation in productivity measures.
The MIH predicts that richness should increase with productivity as a power or logarithmic function and that total abundance should increase linearly with productivity (Srivastava and Lawton 1998). We used regression (PROC REG; SAS Institute 1990) to determine whether a power, logarithmic, or linear function best fit the relationship between richness, total abundance, or abundance per species (dependent) and productivity (independent) for each month. The best model was determined based on the highest R2, as all models have the same degrees of freedom.
Path analysis has been used to test the predictions of the MIH in unmanipulated tree holes (Yee 2006). Path analysis is superior to regression or correlation techniques in that it allows for a statistical comparison of a set of hypothesized relationships (i.e., paths) among independent and dependent variables (Mitchell 2001). Path coefficients, which are equivalent to standardized regression coefficients, quantify direct effects on a dependent variable caused by variation in an independent variable, while removing effects of other independent variables (Hatcher 1994; Mitchell 2001). After constructing a full model, the importance of a particular path, including all of its indirect effects, can be tested by assessing the fit of reduced models in which one or more paths have been removed (Hatcher 1994; Mitchell 2001). Our full model included direct links between productivity, abundance, and richness, and an indirect path from productivity to richness via abundance (Fig. 1). PC1, our measure of productivity, was used as the exogenous variable (Mitchell 2001) in the path diagrams. In order to test predictions of the MIH, we constructed two reduced path diagrams and compared their fit to that of the full model using a goodness-of-fit χ2 test (PROC CALIS, SAS 1990; Hatcher 1994). In the first model, we removed the direct paths from productivity to richness (Fig. 1; Reduced Model 1). If this reduced model yielded no significant decrease in fit, then the direct effect of productivity on richness was unimportant, a result consistent with the MIH. Alternatively, if Reduced Model 1 results in significant lack of fit, productivity affects richness directly, by some mechanism that is beyond the scope of the MIH. In the second model, we removed the indirect effect of productivity on richness via abundance by removing the direct link between abundance and richness (Fig. 1; Reduced Model 2). If this reduced model yielded no significant decrease in fit, then the indirect effect via abundance was statistically unimportant, providing evidence against the MIH.
Fig. 1.
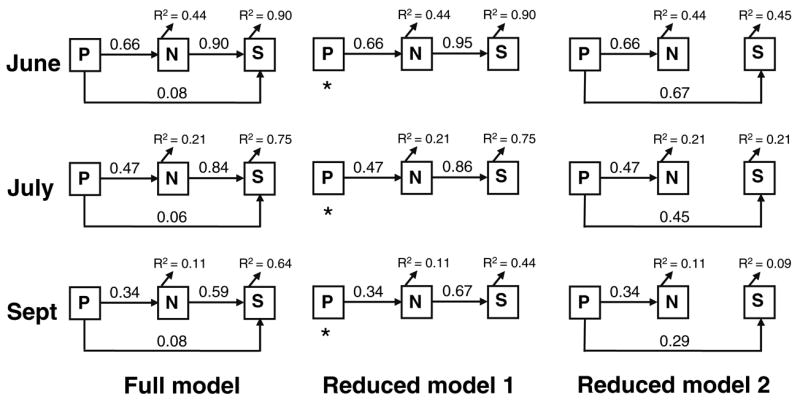
Proposed path diagrams testing the more individuals hypothesis for invertebrates colonizing artificial tree holes during June, July, and September 2004 (ParkLands Preserve, Lexington, Ill.). Full model: (1) direct effects of productivity [P; PC1 from Principal Component Analysis (PCA)] and abundance (N) on species richness (S); (2) indirect effects of productivity on richness through abundance. Reduced Model 1: no direct effects of productivity on richness. Reduced Model 2: no indirect effects of productivity on richness via abundance. Values for R2 are provided next to each variable. An asterisk appears next to the most parsimonious model based on χ2 tests
Results
Colonization
Because of poor colonization in May (only 11 of the 30 microcosms colonized), we restricted our analysis to the final three samples (June, July, September). There also was consistently poor colonization of microcosms with 0.50 g (one individual in one replicate in June) and 1.00 g (18 individuals of one species in one replicate in June) leaf litter, and thus it is likely that these amounts of leaf litter were too low to attract invertebrates. Microcosms with greater amounts of leaf litter and all amounts of animal detritus were consistently colonized after May (Table 1). Species that colonized these microcosms were representative of those encountered in natural tree holes (D.A. Yee, personal observation). Mosquitoes were the dominant family, comprising >50% of all individuals. The most common taxa were the mosquitoes Ochlerotatus triseriatus and Culex restuans, and a morphospecies of syrphid fly (Table 1). There was seasonal variation, with several species reaching peak abundance in June or July and declining thereafter (Table 1). Orthopodymia signifera and Telmatoscopus albipunctatus increased in abundance toward the end of sampling (Table 1).
Table 1.
Invertebrates colonizing artificial tree holes during 2004a
| Family | Species | May | June | July | September | Total |
|---|---|---|---|---|---|---|
| Culicidae | Culex restuans | 471 | 270 | 50 | 0 | 791 |
| Ochlerotatus triseriatus | 0 | 0 | 529 | 311 | 840 | |
| Orthopodomyia signifera | 0 | 0 | 0 | 286 | 286 | |
| Ochlerotatus hendersoni | 0 | 0 | 141 | 1 | 142 | |
| Anopheles barberi | 0 | 1 | 77 | 6 | 84 | |
| Syrphidae | Morphospecies 1 | 363 | 308 | 83 | 8 | 762 |
| Mallota posticata | 0 | 9 | 8 | 9 | 26 | |
| Ceratopogonidae | Culicoides sp. | 0 | 14 | 264 | 133 | 411 |
| Psychodidae | Telmatoscopus albipunctatus | 0 | 3 | 8 | 227 | 238 |
| Stratiomyidae | Morphospecies 1 | 0 | 2 | 1 | 0 | 3 |
| Scirtidae | Helodes sp. | 0 | 0 | 0 | 3 | 3 |
| Total | 834 | 607 | 1,161 | 984 | 3,586 |
Numbers of individuals during each of four surveys (May, June, July, and September) and cumulative numbers in all months and treatments (n = 30)
Responses to leaf and animal detritus
The interaction between detritus type and amount was not significant for richness (S; F1,26 = 0.81, P = 0.374), abundance (N; F1,26 = 0.44, P = 0.514), or for N/S (F1,26 = 3.27, P = 0.082) for the final month, indicating parallel slopes (Fig. 2a–c). Richness (F1,27 = 10.57, P = 0.003), abundance (F1,27 = 11.58, P = 0.002), and N/S (F1,27 = 8.17, P = 0.008) all increased significantly with detritus amount. For a given detritus amount, species richness (P = 0.071), N/S (P = 0.105), and abundance (P = 0.034) were all greater in animal versus plant-based microcosms (Fig. 2).
Fig. 2.

Effect of animal (filled circle) or leaf (open circle) detritus and detritus amount (g) on species (a) richness, abundance (b), and abundance (c) divided by richness in microcosms simulating tree holes during the final month of this study (September 2004). Regression lines are significant (P < 0.05) based on ANCOVA. Values on the x-axis are presented on a log scale
Relationships to productivity
Principal component analysis of productivity surrogates yielded two PCs that explained 69% of the variation in productivity variables (Table 2). PC1 was positively related to the initial amount and type of detritus, mean MR, mean conductivity, and to the binary variable detritus type. PC2 was positively related to values for detritus type and mean PS values.
Table 2.
Results of principal component analysis (PCA) for productivity variables
| PC1a | PC2a | |
|---|---|---|
| Eigen value | 2.22 | 1.23 |
| Proportion variance | 0.44 | 0.25 |
| Cumulative proportion variance | 0.69 | |
| Variableb | ||
| Detritus type | 55c | 64 |
| Initial detritus (g) | 84 | 15 |
| Conductivity (μM HOS cm−1) | 67 | −29 |
| Metabolic rate (MR) | 83 | −9 |
| Protein synthesis (PS) | −27 | 84 |
Principal components (PCs) with eigen values ≥1.0 are shown
Measurements of protein synthesis of bacteria from May and June only were used. Mean values for MR and conductivity were used across all months (May–September)
Large (≥40) loading values from rotated factor patterns are listed in boldface
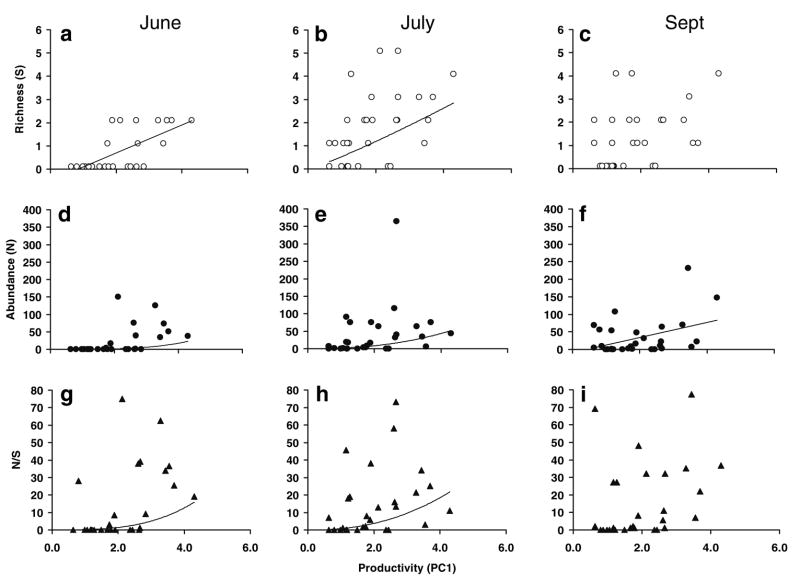
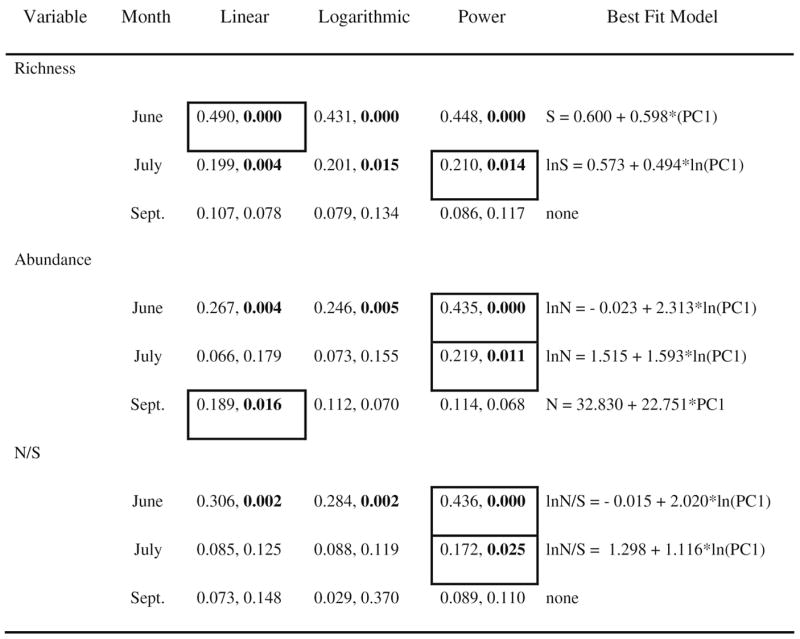
There were significant relationships between richness and productivity in two of the months (Fig. 3a–I) (Table 3). All three functions were significant for richness in June, although the linear function yielded the highest R2 (Table 3; Fig. 3a). In July, all functions were significant and produced a similar fit, although the power function had the highest R2 (Table 3; Fig. 3b). In September, no model was significant (Table 3; Fig. 3c). For abundance in June, linear, logarithmic, and power functions were significant, with the power function having the best fit (Table 3; Fig. 3d). In July, only the power function was significant (Fig. 3e), whereas only a linear function was significant for September (Table 3; Fig. 3f). The relationships between productivity and abundance per species was best described by a power function in June and July (Table 3; Fig. 3g–I).
Fig. 3.
The relationship between productivity and species richness, total abundance, and abundance divided by richness (N/S) for artificial tree holes in June (a, d, g), July (b, e, h) and September (c, f, i) 2004. Productivity (PC1) is defined in Table 2. The x-axis has been shifted (PC1 + 2) to facilitate fitting the functions. Equations for regressions and the best-fit models are presented in Table 3
Table 3.
Results from regressions between species richness (S), abundance (N), or abundance per species (N/S) and productivity (PC1) for microcosms. For each model, the R2 and P value are listed. For each month, best-fit models (i.e., greatest variance explained) are marked by a box, and equations are presented
Path analysis
Because most relationships between productivity, N, S, and N/S were best described by a power function (Table 2), we ran separate path analyses assuming a power or linear relationship among variables. As the results of these analyses did not differ (i.e., consistent model selection outcome based on χ2 tests, analyses not shown), we chose to include the analysis based on power functions. During all months, the saturated model explained from 11–44% of the variation in abundance, and 64–90% of the variation in richness (Fig. 1). The path coefficients from abundance to richness were moderate to large and positive (0.59–0.90). For June, removal of the direct effect of productivity on richness resulted in no significant decrease in fit (Reduced Model 1: χ2 = 1.01, P = 0.315), whereas removal of the indirect effect of productivity on richness via abundance yielded significantly poorer fit (Reduced Model 2: χ2 = 50.43, P < 0.001) and lowered the variance explained for richness considerably (Fig. 1). For July, removal of the direct effect of productivity on richness resulted in no significant decrease in fit (Reduced Model 1: χ2 = 0.35, P = 0.555), whereas the indirect effects model resulted in significant lack of fit (Reduced Model 2: χ2 = 32.45, P < 0.001). Reduced Model 2 in July resulted in a large decrease in variance explained for richness compared to either the full model or Reduced Model 1 (Fig. 1). Thus, the Reduced Model 1 model was the best explanation of the relationships among productivity, abundance, and richness (Fig. 1). For September, removal of the direct path from productivity to richness did not produce a significant reduction in fit (Reduced Model 1: χ2 = 0.27, P = 0.603), but removing the indirect link from productivity to richness via abundance resulted in a significant decrease in fit (Reduced Model 2: χ2 = 14.69, P = 0.008). Very little variance in richness was explained in September once the indirect path from productivity to richness was removed (Fig. 1). Because Reduced Model 1 adequately explained the relationships among variables in June, July, and September, it is the most parsimonious causal model for the pathways by which productivity affects invertebrate richness (Hatcher 1994).
Discussion
Our results from microcosms simulating tree holes show that productivity is an important determinant of species richness and total abundance. Although other abiotic factors, such as tree hole volume (Paradise 2004), opening direction (Barrera 1988), and disturbance (Jenkins et al. 1992) may influence species richness in natural tree holes, our microcosms of standard size, shape, and position enabled us to isolate the effects of productivity on richness. Our results are similar to those of Jenkins et al. (1992), who demonstrated that 10- to 100-fold increases in leaf litter inputs into artificial tree holes in Australia had significant effects on species richness and the abundance of the dominant mosquito consumer. Jenkins et al. (1992) suggested that increased leaf litter may have a direct effect on nutrient availability, which could enhance the growth of microorganisms. Our experiment provides direct support for this suggestion, as increased detritus increased mean microorganism metabolic rates. The importance of microorganisms to growth and the development of the dominant animals in tree holes (i.e., mosquitoes) is well documented (Clements 1992; Merritt et al. 1992). Other arthropod groups also appeared to benefit from increased microorganism activity. Even though the mass of detritus remaining in the container likely decreased though the experiment, the fact that other dynamic measures of productivity (MR, conductivity) were correlated with initial detritus amount (PC1, Table 2) indicates that there were long-term effects of initial conditions on the tree hole communities.
During the final month of the experiment, animal detritus yielded a greater number of species (Fig. 2a) and individuals per species (Fig. 2c) than did an equal amount of plant detritus. In addition, animal-based microcosms supported significantly more individuals that did leaf-based microcosms (Fig. 2b). Plant material is a relatively poor-quality resource, containing a relatively high ratio of carbon to nitrogen (Garman 1992). Animal detritus appears to benefit consumers through a rapid release of nutrients, ease of direct ingestion by consumers (Yee and Juliano 2006), and greater concentration of nutrients (Garman 1991; Cloe and Garman 1996; Nakano et al. 1999; Henschel et al. 2001). These results suggest that animal detritus is indeed a higher quality resource for invertebrates. The mechanism for the observed effect of animal detritus on abundance may be via a greater attractiveness to ovipositing females or via a greater survival of larvae, although neither of these mechanisms has been tested.
The relationships between abundance and productivity, and richness and productivity were variable over time (Fig. 3; Table 3). Consistent with the MIH, we found that richness in July was best fit by a power function of productivity. In June, however, a linear function explained the greatest variance, although it should be noted that all models explained a similar amount of variance in June (Table 3). The form of the function that best fit productivity–abundance relationships varied from month to month (June = power, July = power, September = linear). The fact that richness increased with a power function and abundance increased as a linear function of productivity in some months of the experiment supports one premise of the MIH (i.e., energy limitation; see Wright 1983) and is further evidence of the mechanistic connection of productivity to richness (Srivastava and Lawton 1998). Abundance per species (N/S) was best explained by a power function of productivity in 2 months and approached significance in the third month (Table 3; Fig. 3g–i), indicating that the addition of individuals with increasing productivity was increasing at a faster rate than the addition of new species. This fact is consistent with the sampling effect (Kaspari et al. 2003; Evans et al. 2005), because we might expect that sampling more individuals should affect richness at a decelerating rate. Regardless, an increasing relationship between N/S and productivity is consistent with our prediction and further suggests a strong relationship between abundance and richness with increasing productivity. Under a similar experimental design, the MIH was not supported for artificial tree hole communities in England, where Srivastava and Lawton (1998) found that although the richness of invertebrates increased with productivity, the abundance of invertebrates was not significantly related to productivity. Differences in methodologies (e.g., shorter productivity gradient and different measures of productivity used by Srivastava and Lawton 1998) or type and number of potential invertebrate colonists (lower in England; see Srivastava and Lawton 1998) may have led to differences in the degree of support for the MIH between our study and that of Srivastava and Lawton (1998).
The prediction that a power function would best describe the relationship between richness and productivity arises from premises similar to the well-known species-area relationship (Preston 1962; MacArthur and Wilson 1967). The cause of a power relationship of abundance to productivity in our experiment is unknown, although it is clear that container opening size and volume were not determinants of abundance and richness, as all containers were of standard dimensions. Hurlbert (2004) demonstrated that habitat complexity could lead to greater richness of North American birds along a productivity gradient, and that changes in abundance along this gradient did not by itself explain changes in richness, as predicted by the MIH. In our study, microcosms with greater productivity had more detritus than did containers with lower productivity, and this difference may have increased habitat complexity in high detritus containers, leading to changes in abundance of some species, and more importantly, increases in species richness that are not products of productivity per se. Because microcosms with higher detritus and productivity in our study also provided more complex habitats for invertebrates, we cannot rule out habitat complexity as an explanation for changes in richness.
A central postulate of the MIH is that increased abundance results in increased richness because the former lowers the extinction risk of rare species. This could be tested with correlations between richness and abundance, but such correlations could be spurious, as these two variables could appear to be correlated because of shared positive relationships to productivity (Srivastava and Lawton 1998). Path analysis enables us to test specific predictions about the importance of causal pathways among these variables. Path analyses unequivocally support the prediction of the MIH of an important indirect effect of productivity on richness via total abundance, and they also show that direct effects of productivity on richness were relatively unimportant (Reduced Models, Table 3). This demonstrates a strong link between the number of individuals and the number of species in our experiment and probably provides the best evidence possible for the MIH. Srivastava and Lawton (1998) determined that there were power or logarithmic relationships between productivity and species richness for invertebrates colonizing artificial tree holes in England, although there was on occasion very little difference among models in fit (e.g., R2 linear = 0.22, logarithmic = 0.23, power = 0.24). Because it is not possible to test among these relationships statistically to determine the best fit, choosing the best relationship between productivity and richness or abundance based on variance explained is somewhat arbitrary (Table 3). As was true for Srivastava and Lawton (1998), differences in fit among models for richness or abundance versus productivity were often small (e.g., richness in June), making testing predictions of MIH via regression uncertain. Path analysis testing the importance of specific causal paths seems to be preferable to the comparison of different regression functions for testing the MIH.
Invertebrates in natural tree holes in Illinois show evidence for both direct and indirect effects of productivity on richness (Yee 2006). In natural tree holes, although the same indirect effect of productivity on richness via abundance was present (Yee 2006), other processes beyond productivity and total abundance (e.g., tree hole volume, age, complexity, history) may partially obscure the effect of the number of individuals on species richness. Richness also may respond to factors beyond productivity that are unique to this system (e.g., colonization decisions of ovipositing adults, disturbance, species interactions). The results presented in this study illustrate that direct manipulations of productivity can affect abundance and richness in the manner predicted by the MIH. Identifying other processes that may interact with abundance and productivity to affect richness in natural tree holes will be one of the research challenges for this model community.
Our path analyses show that communities of invertebrates in artificial tree holes in Illinois conform to predictions of the MIH. Although the regressions of S, N, and N/S versus productivity produced equivocal results, they do indicate that productivity has a positive effect on communities and populations. More importantly, we have provided evidence for a causal indirect link between productivity and richness through abundance, as predicted by the MIH. In our study, best functions describing the relationships of abundance or richness to productivity varied among months and were not uniformly consistent with detailed MIH predictions of functional form. These results imply that effects of productivity on richness and abundance may be more complex than current hypotheses suggest.
Acknowledgments
Discussions with M.R. Willig regarding the MIH improved this study. We thank M. Kaufman for guidance with leucine methodologies. We thank S. Hohm, S. Harrell Yee, H. Les, K. Costanzo, B. Kesavaraju, and C. Villanueva who provided assistance in the field, three anonymous referees who provided insightful and helpful suggestions on an earlier version of this manuscript, The ParkLands Foundation for access to their property to conduct this experiment, and the Sakaluk lab, ISU, for providing us with crickets. This work was supported by grants to D.A. Yee from the ISU Department of Biological Sciences and the Phi Sigma Biological Society, and to S. A. Juliano and D. A. Yee from the National Institute of Allergy and Infectious Disease (R15 AI-051374).
Footnotes
Communicated by Nathan Sanders.
References
- Abrams PA. Monotonic or unimodal diversity-productivity gradients: what does competition theory predict? Ecology. 1995;76:2019–2027. [Google Scholar]
- Anderson JM, Macfayden A, editors. The role of terrestrial and aquatic organisms in decomposition processes. Blackwell; Oxford: 1976. [Google Scholar]
- Barrera R. PhD thesis. The Pennsylvania State University; University Park, Pa: 1988. Multiple factors and their interactions on structuring the community of aquatic insects of treeholes. [Google Scholar]
- Carpenter SR. Resource limitation of larval treehole mosquitoes subsisting on beech detritus. Ecology. 1983;64:219–223. [Google Scholar]
- Chase JM, Leibold MA. Spatial scale dictates the productivity–biodiversity relationship. Science. 2002;416:427–430. doi: 10.1038/416427a. [DOI] [PubMed] [Google Scholar]
- Clements AN. The biology of mosquitoes. Vol. 1. Chapman & Hall; London: 1992. [Google Scholar]
- Cloe WW, III, Garman GC. The energetic importance of terrestrial arthropod inputs to three warm-water streams. Freshw Biol. 1996;36:105–114. [Google Scholar]
- Evans KL, Warren PH, Gaston KJ. Species–energy relationships at the macroecological scale: a review of the mechanisms. Biol Rev. 2005;80:1–25. doi: 10.1017/s1464793104006517. [DOI] [PubMed] [Google Scholar]
- Garman GC. Use of terrestrial arthropod prey by a stream-dwelling cyprinid fish. Environ Biol Fishes. 1991;30:325–331. [Google Scholar]
- Garman GC. Fate and potential significance of postspawning anadromous fish carcasses in an Atlantic costal river. Trans Am Fish Soc. 1992;121:390–394. [Google Scholar]
- Groner E, Novoplansky A. Reconsidering diversity–productivity relationship: directness of productivity estimate matters. Ecol Lett. 2003;6:695–699. [Google Scholar]
- Hatcher L. A step-by-step approach to using the SAS system for factor analysis and structural equation modeling. SAS Institute; Cary, N.C: 1994. [Google Scholar]
- Hatcher L, Stepanski EJ. A step-by-step approach to using the SAS system for univariate and multivariate statistics. SAS Institute; Cary, N.C: 1994. [Google Scholar]
- Henschel JR, Mahsberg D, Stumpf H. Allochthonous aquatic insects increase predation and decrease herbivory in river shore food webs. Oikos. 2001;93:429–438. [Google Scholar]
- Hurlbert AH. Species–energy relationship and habitat complexity in bird communities. Ecol Lett. 2004;7:714–720. [Google Scholar]
- Hurlbert AH. Linking species–area and species–energy relationships in Drosophila microcosms. Ecol Lett. 2006;9:287–294. doi: 10.1111/j.1461-0248.2005.00870.x. [DOI] [PubMed] [Google Scholar]
- Jenkins B, Kitching RL, Pimm SL. Productivity, disturbance and food web structure at a local spatial scale in experimental container habitats. Oikos. 1992;65:249–255. [Google Scholar]
- Kaspari M, Yuan M, Alonso L. Spatial grain and the causes of regional diversity gradients in ants. Am Nat. 2003;161:459–477. doi: 10.1086/367906. [DOI] [PubMed] [Google Scholar]
- Kirchman DL. Leucine incorporation as a measure of biomass production by heterotrophic bacteria. In: Kemp PL, Sherr BF, Sherr EB, Cole JJ, editors. Handbook of methods in aquatic microbiology. Lewis; Boca Raton: 1993. pp. 509–512. [Google Scholar]
- Kitching RL. An ecological study of water-filled tree-holes and theirposition in the woodland ecosystem. J Anim Ecol. 1971;40:281–302. [Google Scholar]
- Kitching RL. The natural history and ecology of phytotelmata. Cambridge University Press; England: 2000. Food webs and container habitats. [Google Scholar]
- Kitching RL. Food webs in phytotelmata: “bottom-up” and “top-down” explanations for community structure. Annu Rev Entomol. 2001;46:729–760. doi: 10.1146/annurev.ento.46.1.729. [DOI] [PubMed] [Google Scholar]
- Kneitel JM, Miller TE. Resource and top-predator regulation in the pitcher plant Sarracenia purpurea inquiline community. Ecology. 2002;83:680–688. [Google Scholar]
- Léonard PM, Juliano SA. Effect of leaf litter and density on fitness and populations performance of the tree hole mosquito Aedes triseriatus. Ecol Entomol. 1995;20:125–136. [Google Scholar]
- Mason CF, MacDonald SM. The input of terrestrial invertebrates from tree canopies to a stream. Freshw Biol. 1982;12:305–311. [Google Scholar]
- MacArthur RH, Wilson EO. The theory of island biogeography. Harvard University Press; Cambridge: 1967. [Google Scholar]
- Merritt RW, Dadd RH, Walker ED. Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annu Rev Entomol. 1992;37:349–376. doi: 10.1146/annurev.en.37.010192.002025. [DOI] [PubMed] [Google Scholar]
- Mitchell RJ. Path analysis: pollination. In: Schiner SM, Gurevitch J, editors. Design and analysis of ecological experiments. Oxford University Press; Oxford: 2001. pp. 211–231. [Google Scholar]
- Mittelbach GG, Steiner CF, Scheiner SM, Gross KL, Reynolds HL, Waide RB, Willig MR, Dodson SI, Gough L. What is the observed relationship between species richness and productivity? Ecology. 2001;82:2381–2396. [Google Scholar]
- Moore JC, Berlow EL, Coleman DC, Ruiter PC, Dong Q, Hastings A, Johnson NC, McCann KS, Melville K, Morin PJ, Nadelhoffer K, Rosemond AD, Post DM, Sabo JL, Scow KM, Vanni MJ, Wall DH. Detritus, trophic dynamics and biodiversity. Ecol Lett. 2004;7:584–600. [Google Scholar]
- Nakano S, Miyasaka H, Kuhara N. Terrestrial–aquatic linkages: riparian arthropod inputs alter trophic cascades in a stream food web. Ecology. 1999;80:2435–2441. [Google Scholar]
- O’Neill RV, Reichle DA. Dimensions of ecosystem theory. In: Waring RH, editor. Forests: fresh perspectives from ecosystem analysis. Oregon State University Press; Corvallis: 1980. pp. 11–26. [Google Scholar]
- Paradise CJ. Relationship of water and leaf litter variability to insect inhabiting treeholes. J North Am Bentho Soc. 2004;23:793–805. [Google Scholar]
- Preston FW. The canonical distribution of commonness and rarity. Ecology. 1962;43:185–215. [Google Scholar]
- Rosenzweig ML. Paradox of enrichment: destabilization of exploitation ecosystems in ecological time. Science. 1971;171:385–387. doi: 10.1126/science.171.3969.385. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ML. Species diversity in space and time. Cambridge University Press; Cambridge: 1995. [Google Scholar]
- Rosenzweig ML, Abramsky Z. Species diversity gradients: we know more or less than we thought. J Mammal. 1993;73:715–730. [Google Scholar]
- SAS Institute. SAS/STAT users guide, version 6. 4. 1 and 2. SAS Institute; Cary, N.C: 1990. [Google Scholar]
- Srivastava DS, Lawton JH. Why more productive sites have more species: an experimental test of theory using tree-hole communities. Am Nat. 1998;152:510–529. doi: 10.1086/286187. [DOI] [PubMed] [Google Scholar]
- Waide RB, Willig MR, Steiner CF, Mittelbach GG, Gough L, Dodson SI, Juday P, Parmenter R. The relationship between productivity and species diversity. Annu Rev Ecol Syst. 1999;30:257–300. [Google Scholar]
- Walker ED, Lawson DL, Merritt RW, Morgan WT, Klug MJ. Nutrient dynamics, bacterial populations, and mosquito productivity in tree hole ecosystems and microcosms. Ecology. 1991;72:1529–1546. [Google Scholar]
- Walker ED, Kaufman MG, Ayres MP, Riede MH, Merritt RW. Effects of variation in quality of leaf detritus on growth of the eastern tree-hole mosquito, Aedes triseriatus (Diptera: Culicidae) Can J Zool. 1997;75:706–718. [Google Scholar]
- Weiher E. The combined effects of scale and productivity on species richness. J Ecol. 1999;87:1005–1011. [Google Scholar]
- Wright DH. Species–energy theory: an extension of species–area theory. Oikos. 1983;41:496–506. [Google Scholar]
- Yanoviak SP. Predation, resource availability, and community structure in Neotropical water-filled tree holes. Oecologia. 2001;126:125–133. doi: 10.1007/s004420000493. [DOI] [PubMed] [Google Scholar]
- Yee DA. PhD thesis. Illinois State University, Ill; 2006. Effects of species interactions and productivity on aquatic macroinvertebrate diversity and community composition in tree holes: patterns and mechanisms. [Google Scholar]
- Yee DA, Juliano SA. Consequences of detritus type in an aquatic microsystems: assessing water quality, microorganisms, and the performance of the dominant consumer. Freshw Biol. 2006;51:448–459. doi: 10.1111/j.1365-2427.2005.01504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]