Abstract
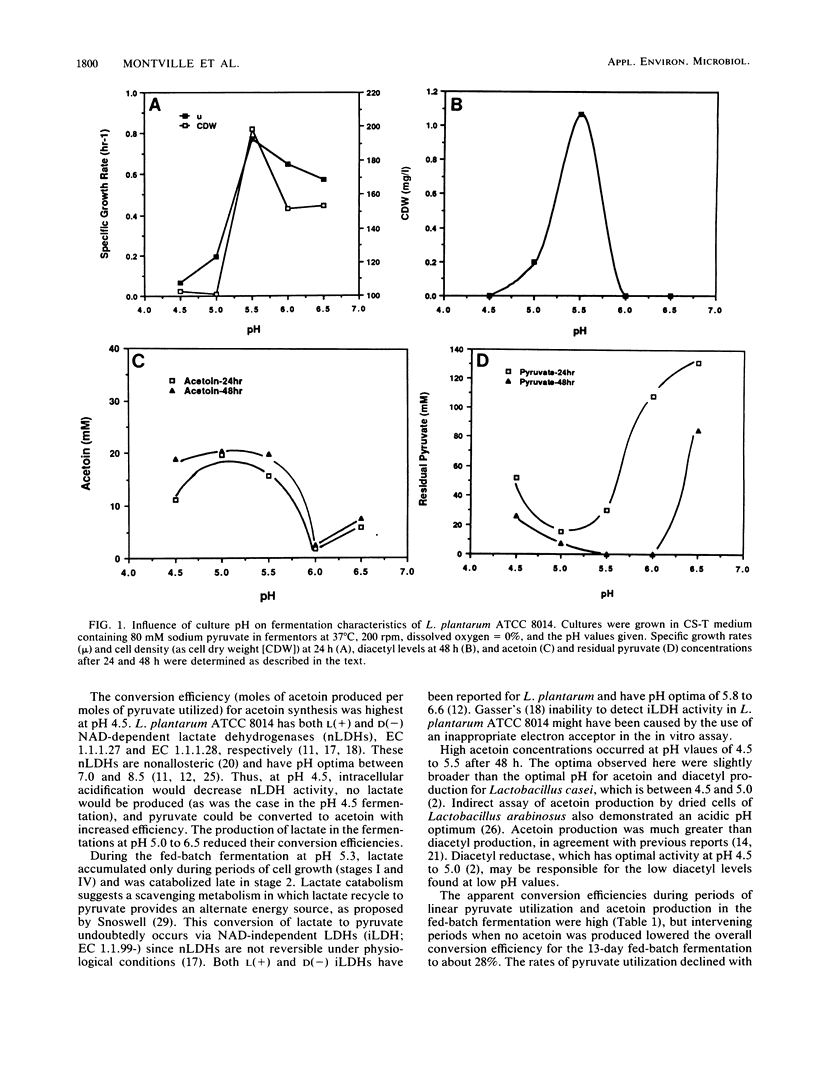
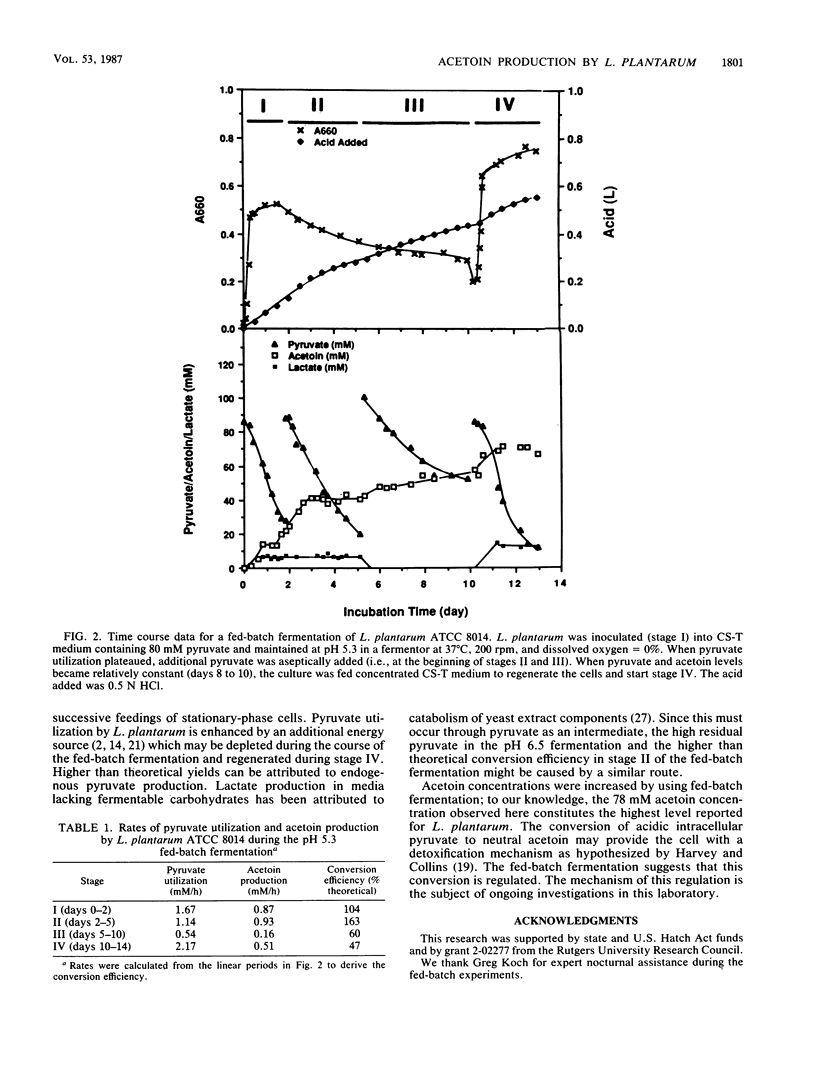
The influence of pH on the type and concentration of metabolites produced from pyruvate by Lactobacillus plantarum ATCC 8014 was examined in pH-controlled fermentors at pH values of 4.5 to 6.5. Specific growth rates, cell dry weights, and diacetyl concentrations were highest at pH 5.5, with values of 0.78 h−1, 190 mg/liter, and 1.2 mM, respectively. While the conversion efficiency (millimoles of acetoin formed per millimoles of pyruvate utilized) was highest (94.6%) at pH 4.5, acetoin levels were similar (20 mM) between pH 4.5 and 5.5. Feeding stationary-phase cells exogenous pyruvate increased acetoin levels to 78 mM.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branen A. L., Keenan T. W. Diacetyl reductase of Lactobacillus casei. Can J Microbiol. 1970 Oct;16(10):947–951. doi: 10.1139/m70-162. [DOI] [PubMed] [Google Scholar]
- CHRISTENSEN M. D., PEDERSON C. S. Factors affecting diacetyl production by lactic acid bacteria. Appl Microbiol. 1958 Sep;6(5):319–322. doi: 10.1128/am.6.5.319-322.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CRAIG J. A., SNELL E. E. The comparative activities of pantethine, pantothenic acid, and coenzyme A for various microorganisms. J Bacteriol. 1951 Mar;61(3):283–291. doi: 10.1128/jb.61.3.283-291.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins E. B., Bruhn J. C. Roles of acetate and pyruvate in the metabolism of Streptococcus diacetilactis. J Bacteriol. 1970 Sep;103(3):541–546. doi: 10.1128/jb.103.3.541-546.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins E. B., Speckman R. A. Influence of acetaldehyde on growth and acetoin production by Leuconostoc citrovorum. J Dairy Sci. 1974 Dec;57(12):1428–1431. doi: 10.3168/jds.S0022-0302(74)85084-8. [DOI] [PubMed] [Google Scholar]
- DENNIS D., KAPLAN N. O. D- and L-lactic acid dehydrogenases in Lactobacillus plantarum. J Biol Chem. 1960 Mar;235:810–818. [PubMed] [Google Scholar]
- Demain A. L., Jackson M., Trenner N. R. Thiamine-dependent accumulation of tetramethylpyrazine accompanying a mutation in the isoleucine-valine pathway. J Bacteriol. 1967 Aug;94(2):323–326. doi: 10.1128/jb.94.2.323-326.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doelle H. W. Nicotinamide adenine dinucleotide-dependent and nicotinamide adenine dinucleotide-independent lactate dehydrogenases in homofermentative and heterofermentative lactic acid bacteria. J Bacteriol. 1971 Dec;108(3):1284–1289. doi: 10.1128/jb.108.3.1284-1289.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordyce A. M., Crow V. L., Thomas T. D. Regulation of product formation during glucose or lactose limitation in nongrowing cells of Streptococcus lactis. Appl Environ Microbiol. 1984 Aug;48(2):332–337. doi: 10.1128/aem.48.2.332-337.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvie E. I. Bacterial lactate dehydrogenases. Microbiol Rev. 1980 Mar;44(1):106–139. doi: 10.1128/mr.44.1.106-139.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasser F. Electrophoretic characterization of lactic dehydrogenases in the genus Lactobacillus. J Gen Microbiol. 1970 Aug;62(2):223–239. doi: 10.1099/00221287-62-2-223. [DOI] [PubMed] [Google Scholar]
- Hasan N., Durr I. F. Induction of beta-galactosidase in Lactobacillus plantarum. J Bacteriol. 1974 Oct;120(1):66–73. doi: 10.1128/jb.120.1.66-73.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensel R., Mayr U., Fujiki H., Kandler O. Comparative studies of lactate dehydrogenases in lactic acid bacteria. Amino-acid composition of an active-site region and chemical properties of the L-lactate dehydrogenase of Lactobacillus casei, Lactobacillus curvatus, Lactobacillus plantarum, and Lactobacillus acidophilus. Eur J Biochem. 1977 Oct 17;80(1):83–92. doi: 10.1111/j.1432-1033.1977.tb11859.x. [DOI] [PubMed] [Google Scholar]
- Hickey M. W., Hillier A. J., Jago G. R. Metabolism of pyruvate and citrate in lactobacilli. Aust J Biol Sci. 1983;36(5-6):487–496. doi: 10.1071/bi9830487. [DOI] [PubMed] [Google Scholar]
- MOAT A. G., LICHSTEIN H. C. Factors affecting the formation of acetylmethylcarbinol by lactobacillus arabinosus. J Bacteriol. 1953 Sep;66(3):324–327. doi: 10.1128/jb.66.3.324-327.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D., Ellwood D. C., Longyear V. M. Change from homo- to heterolactic fermentation by Streptococcus lactis resulting from glucose limitation in anaerobic chemostat cultures. J Bacteriol. 1979 Apr;138(1):109–117. doi: 10.1128/jb.138.1.109-117.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]


