Abstract
The median preoptic nucleus (MnPO) receives afferent input from the subfornical organ, a circumventricular organ that has been shown to be necessary in mediating the full chronic hypertensive response to angiotensin II (ANG II) administration. In addition, intravenous ANG II infusion has been shown to cause activation of a number of neurons in both the dorsal and ventral part of MnPO. Taken together, we hypothesized that the MnPO is necessary for the full hypertensive response observed during chronic ANG II-induced hypertension. To test this hypothesis, male Sprague Dawley rats were subjected to either sham (SHAM) or electrolytic lesion of both the dorsal and ventral part of the MnPO (MnPOx). During the same surgery, rats were instrumented with venous catheters, and radiotelemetric transducers for the intravenous administration of ANG II and the measurement of blood pressure and heart rate, respectively. Rats were then given a week recovery period. After 3 days of saline control infusion, ANG II was intravenously infused (10 ng ˙ kg−1˙ min−1) in both sham and MnPOx animals for 10 consecutive days, and followed by 3 recovery days. By day 7 of Ang II infusion, MAP had increased 38 ± 3 mmHg in sham lesion rats (n=6), but MAP of MnPOx rats (>90% MnPO ablated; n=5) had only increased 18 ± 2 mmHg. This trend continued through day 10 of ANG II treatment. These results support the hypothesis that the MnPO is necessary for the chronic hypertensive response to ANG II administration.
Keywords: median preoptic nucleus, sympathetic nervous system, angiotensin II, hypertension
1. Introduction
The central nervous and renin-angiotensin hormonal systems interact with each other in long-term control of blood pressure (Brooks and Osborn, 1995; Dampney et al., 2002; Reid, 1992; Saxena, 1992). Much evidence supports the idea that the central nervous system monitors body fluid and adjusts sympathetic nervous output and hypothalamic-pituitary hormonal secretion based on circulating blood angiotensin II (ANG II) concentration and osmolality (Cerasola et al., 1987; Matsukawa et al., 1991; Ota et al., 1994; Schmitt and Schmitt, 1968; Scrogin et al., 1999; Tobey et al., 1983; Weekley, 1991). Such signals are directly accessible to the brain through specialized brain regions lacking the blood-brain barrier called circumventricular organs (CVOs) (Fink et al., 1987; McKinley et al., 1998). Previously, we have shown that electrolytic lesion of the subfornical organ (SFO), a CVO located on the rostral wall of the third ventricle, attenuated the hypertensive response to chronic infusion of ANG II (Hendel and Collister, 2005). Accordingly, the SFO is likely a CVO which functions as a sensor of ANG II in long-term control of blood pressure. However, how and where this sensory information is further processed downstream within the brain and finally influences activity of sympathetic premotor neurons located in the hindbrain are not fully understood.
The median preoptic nucleus, MnPO, a nuclear group located in the lamina terminalis, has been shown to connect to the SFO both anatomically and functionally (Ciriello and Gutman, 1991; Gutman et al., 1986; Lind et al., 1982; Miselis, 1979, 1982; Saper and Levisohn, 1983). The MnPO receives dense afferent inputs from the SFO (Miselis, 1981; Saper and Levisohn, 1983), and disconnection of the SFO and MnPO disturbs drinking and pressor responses to intravenous ANG II (Miselis, 1982; Lind et al., 1983). Over several decades, a large body of research has implicated the important role of the MnPO on various physiological effects of ANG II believed to be initiated by the actions of ANG II at the SFO. The role of MnPO in a dipsogenic effect of ANG II has been extensively studied, and it has been found that lesions of the MnPO reduce drinking and vasopressin secretion in response to ANG II (Cunningham et al., 1991, 1992; Gardiner and Stricker, 1985a, b; Gutman et al., 1989; Jones, 1988; Lind and Johnson, 1982; Mangiapane et al., 1983). However, few studies have been carried out to assess the role of MnPO in a central hypertensive effect of ANG II and the results have been controversial (Gutman et al., 1989; Jones, 1988; Fink et al., 1986; O'Neill and Brody, 1987).
In the present study, the role of the MnPO in chronic ANG II-induced hypertension was examined. Both the dorsal and ventral portions of the MnPO were electrolytically ablated, and the hypertensive response to a 10-day infusion of ANG II in MnPO lesioned rats was evaluated and compared with those of sham lesioned rats. Our general hypothesis has been that the MnPO is a crucial component of the central sympathoexcitatory pathway following activation of ANG II at the SFO. Specifically, in the present study, we hypothesized that the MnPO is crucial for the full hypertensive response to chronic intravenous administration of ANG II.
2. Results
All rats included in the analyses were healthy and displayed normal behavior throughout the experimental period. Histological examination of the lesions showed that five rats from the MnPOx group adequately met the criteria to be included in the analyses. Data from these MnPOx rats were compared with those from sham lesion rats (n = 6). Representative examples of MnPOx and sham lesion are shown in Figure 1.
Figure 1.
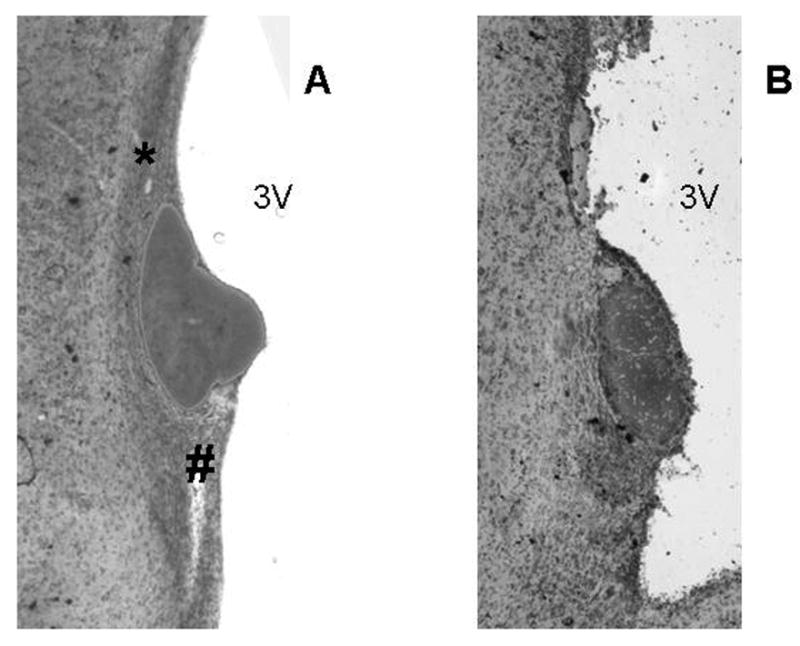
Photomicrographs of 40 μm mid-sagittal sections of the Median preoptic nucleus (MnPO). A. Section from a sham lesion rat demonstrating dorsal and ventral MnPO (* and #, respectively). B. Section from a MnPOx rat demonstrating ablated dorsal and ventral MnPO. 3V = Third ventricle.
2.1 Cardiovascular responses to ANG II infusion
The MAP responses to ANG II in MnPOx and sham lesion rats throughout the experimental protocol are shown in Figure 2 (top panel). T-test analysis revealed no difference in average baseline MAP between the two groups (MnPOx, 99 ± 2 mmHg; sham, 98 ± 3 mmHg). By day 2 of ANG II infusion, MAP in both MnPOx and sham lesion rats were significantly increased from baseline control (MnPOx, 114 ± 3 mmHg; sham, 120 ± 4 mmHg). MAP continued to increase progressively in both groups, but this response was significantly attenuated in MnPOx rats compared to sham lesion rats by day 7 of ANG II infusion (MnPOx, 117 ± 4 mmHg; sham, 136 ± 5 mmHg). This trend continued through the remaining ANG II infusion period. Once ANG II was discontinued, MAP of the two groups decreased gradually and returned to their baseline levels by the end of the recovery period.
Figure 2.
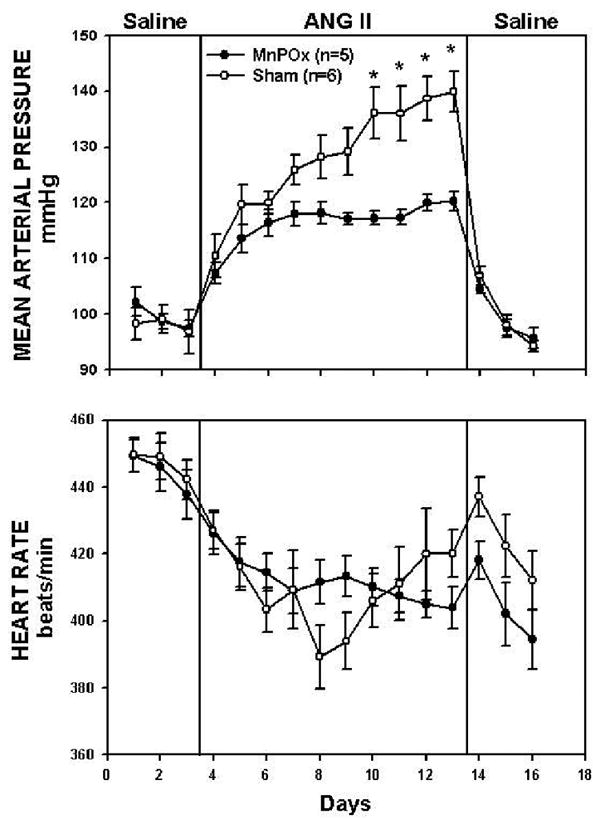
Average 24 h mean arterial pressure (MAP) and heart rate (HR) recorded during control period (3 days of saline), treatment (10 days of ANGII), and recovery (3 days of saline) in MnPOx and sham lesion rats. *P < 0.05 between groups.
Five rats had incomplete lesions of the MnPO and were not included in the final analysis. In these rats, lesions were limited to only 30 to 70% of the MnPO. By day 7 of ANG II infusion, the average MAP in these rats was 131 ± 1 mmHg. Throughout the protocol, there was no significant difference between MAP of these incomplete lesioned rats compared to sham rats.
The HR responses to ANG II are shown in Figure 2 (bottom panel). The average baseline HR in MnPOx rats and sham lesion rats was 444 ± 6 and 447 ± 6 beats/min, respectively. By day 3 of ANG II infusion, HR in both MnPOx and sham were significantly decreased from baseline control (MnPOx, 414.5 ± 5.6 beats/min; sham, 403.3 ± 6.5 beats/min), and this trend continued through the remaining ANG II infusion period. However, HR responses were not different between MnPOx and sham lesion rats throughout the protocol.
2.2 Sodium and Water balance responses
Water balance responses are shown in Figure 3. The average baseline water intake in MnPOx rats was 26 ± 1 ml/day, which was not different from that of the sham lesion rats (26 ± 2 ml/day). Water intake, urine output, and water balance were not different between the two groups throughout the experimental protocol.
Figure 3.
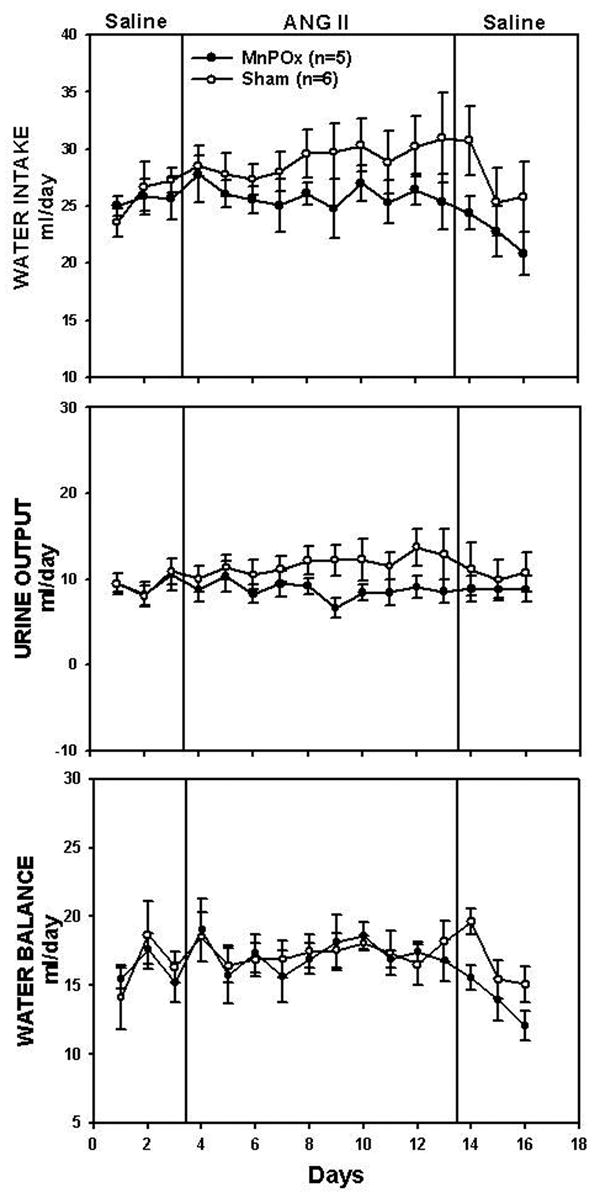
Water intake, urine output, and water balance during control, treatment, and recovery periods in MnPOx and sham lesion rats.
Sodium balance responses are shown in Figure 4. Average baseline sodium intake was 2.9 ± 0.1 mEq/day in MnPOx rats, and 2.9 ± 0.1 mEq/day in sham lesion rats. Average baseline sodium excretion in MnPOx and sham lesion rats was 1.7 ± 0.3 mEq/day and 1.6 ± 0.3 mEq/day, respectively. No significant differences in sodium intake, sodium output, and sodium balance were found between the two groups throughout the protocol.
Figure 4.
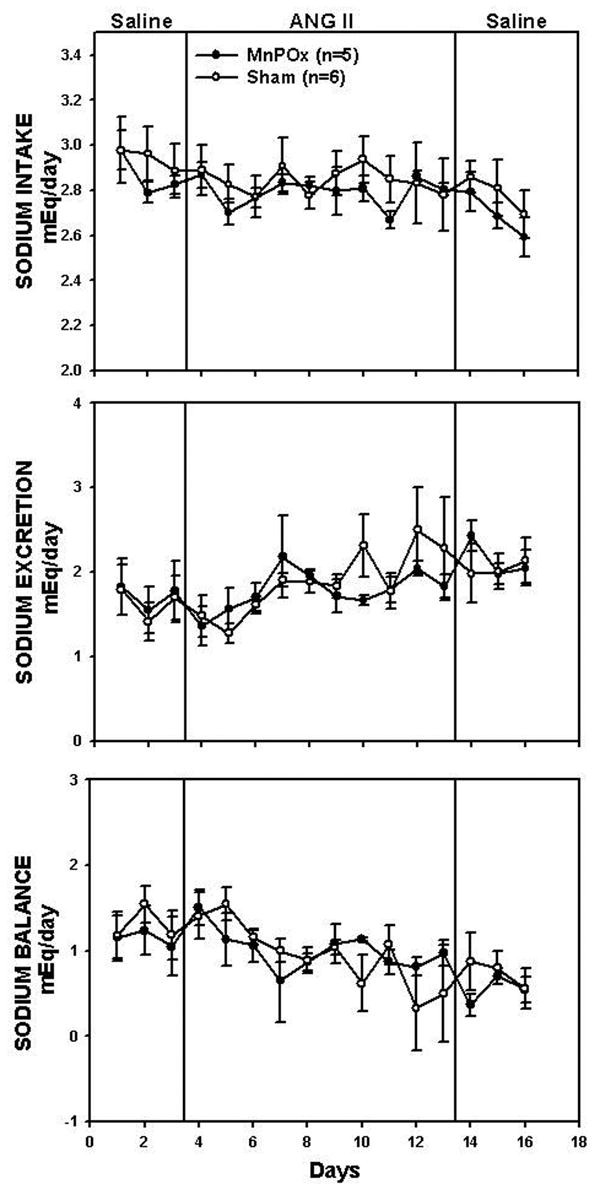
Sodium intake, sodium excretion, and sodium balance during control, treatment, and recovery periods in MnPOx and sham lesion rats.
3. Discussion
The MnPO receives reciprocal inputs from the SFO and other brain regions believed to form the sympathoexcitatory pathway following activation by ANG II at the SFO (Miselis, 1981; Saper and Levisohn, 1983; Sawchenko and Swanson, 1983; Zardetto-Smith et al., 1993). In the present study, we have shown that rats with lesion of the MnPO had a significantly attenuated response to the hypertensive effects of chronic ANG II administration. In fact, we have demonstrated that when MAP reached the steady state from day 7 to 10 of the ANG II infusion, MAP in MnPOx rats had increased from baseline nearly 20 mmHg less than MAP of sham lesion rats. This was approximately a 50% attenuation of the increased MAP seen in sham lesion rats. These findings suggest that the MnPO is one of the crucial components of the central neural circuitry necessary for the central hypertensive response to chronic ANG II administration.
In the present study, HR was decreased significantly from baseline during day 2 to 10 of ANG II infusion in both MnPOx and sham lesion rats. This could be explained by a baroreflex response to the elevated blood pressure induced by ANG II, although many would argue against this as the baroreceptors would reset in response to a sustained stimulus. However, this finding is in contrast to our previous study in which no significant change of HR was observed during chronic ANG II infusion in sham lesion rats and those with lesions of the SFO (Hendel and Collister, 2005). This difference could be explained by the higher level of increased MAP observed in this study compared to that previous study.
It has been shown previously that lesions of the AV3V region, the region surrounding the anteroventral portion of the third cerebral ventricle, attenuate hypertension in a number of rat models including a renin-angiotensin dependent form of hypertension (Brody et al., 1978). AV3V lesions include a medioventral part of the MnPO together with the anterior periventricular nuclei of the hypothalamus, and the OVLT. In the present study, lesions were limited to a more specific region, the MnPO, without damaging the other parts of the AV3V. Therefore, the attenuated response to the chronic hypertensive effect of ANG II in MnPO lesioned rats found in this study suggest that damage of the MnPO may be responsible in part for the attenuated hypertensive response to ANG II in AV3V lesioned animals as well. It also should be noted that although acute adipsia is common in AV3V lesioned animals, rats with MnPO lesions in the present study displayed normal drinking behavior throughout the study protocol, suggesting other areas of the AV3V are responsible for this behavior in the AV3V lesioned rat model.
A role of the MnPO on the central hypertensive effect of ANG II has been investigated in the past. Our findings support those that were conducted under an acute experimental setting, which found that knife cuts of the SFO and MnPO connection, and electrolytic ablation of both the dorsal and ventral part of the MnPO attenuated pressure responses to a low-dose intravenous ANG II infusion (Lind et al., 1983; O'Neill and Brody, 1987). However, although the study by Fink et al (Fink et al., 1986) has shown that chronic MAP response to 5 days of ANG II infusion was not affected by MnPO lesion, the present findings suggest that longer duration of ANG II infusion is required to detect the lesion effect. In fact, in the present study, significant attenuation of the pressure response to ANG II was not observed until day 7 of ANG II infusion, 2 days after the end of ANG II infusion in the previous study. Therefore, the data of this current study is not inconsistent with that of the previous study, but do in fact demonstrate a role of the MnPO in the long-term hypertensive effects of ANG II.
The SFO is one of the CVOs that has been implicated as a primary central target for circulating ANG II. We have previously shown that the SFO is necessary for the full hypertensive response to chronic ANG II infusion (Hendel and Collister, 2005), and this has been shown to be mediated through superoxide production in the SFO (Zimmerman et al., 2004). Also, lesion of the SFO blocks expression of the immediate early gene, c-fos, in many brain regions that have been shown to express fos in response to intravenous infusion of hypertonic saline, and believed to be involved in central sympathoexcitatory pathways, including the MnPO, parvocellular division of the hypothalamic paraventricular nucleus, and the ventrolateral medulla (Hochstenbach and Ciriello, 1996). Part of the neural fibers that emerge from the SFO have been shown to project and synapse directly onto MnPO neurons (Miselis, 1981). Additionally, certain portions of MnPO neurons that project to hypothalamic paraventricular nucleus are activated by ANG II infusion (Stocker and Toney, 2005). One study showed that MnPO lesion diminishes c-fos expression in the paraventricular nucleus of the hypothalamus, but not in the SFO in response to intravenous infusion of ANG II (Xu and Herbert, 1995). This supports the idea that the MnPO is a relay site from the SFO to the paraventricular nucleus. We have demonstrated in the present study the functional importance of the MnPO in the chronic hypertensive effect of ANG II. Taken together, these findings provide strong evidence to support the general hypothesis that the MnPO is part of the central sympathoexcitatory pathway downstream from the SFO and crucial for the central sympathetic response to systemic ANG II.
Neuroanatomical studies have shown that the SFO projects via two main efferent pathways (Miselis, 1981). Efferent fibers from the SFO either synapse directly onto the supraoptic and paraventricular nucleus of the hypothalamus, or relay at the MnPO before reaching these hypothalamic nuclei. Findings that some efferent fibers from the SFO bypass the MnPO may explain why MnPO lesion failed to completely block the hypertensive effects of ANG II in the present study. In addition, the hypertensive response to ANG II observed in MnPOx rats in this study may be partially explained by remaining neuronal pathways originating from other CVOs, particularly the area postrema, that has been shown to be important in the central hypertensive effect of ANG II (Fink et al., 1987). However, results from our recent study appear to diminish the role of the area postrema as a critical mediator of the chronic hypertensive effect of intravenous ANG II in this model (Nahey and Collister, 2007).
Anterograde tracing studies from the SFO (Miselis, 1981) also showed that some of the fibers that travel ventrally toward the MnPO do not actually make a synapse on this nucleus, but pass through or close by the MnPO and eventually terminate at the supraoptic and paraventricular nuclei. Thus, an argument may arise that not only neurons in the MnPO, but also fibers of passage traveling around the periphery or passing through the MnPO were also damaged by the electrolytic ablations performed in this study. Since only MnPOx rats with lesions limited to the MnPO with no or slight damage to the adjacent area were included in the statistical analysis, we believe that neural fibers that pass by the MnPO are less likely responsible for the observed attenuated response to ANG II in this study. In fact, one rat whose lesion was missed and located in the septal diagonal band immediately anterior to the MnPO had blood pressure responses comparable to those of the sham rats (MAP increased 32 mmHg by day 7). Additionally, as a group, the partial lesion rats average MAP was never different from the sham group. Furtheremore, MnPO neurons projecting to the PVN have been shown to increase their activity in response to intravenous ANG II infusion (McKinley et al., 1992; Oldfield et al., 1994; Potts et al., 1999; Rowland et al., 1994; Stocker and Toney, 2005) supporting the idea that electrolytic ablation of the MnPO attenuates blood pressure responses to ANG II by destroying those neurons instead of fibers of passage. However, we cannot reject the possibility that fibers that directly pass through MnPO may play a part in the attenuated response observed. This distinction is critical to our overall understanding of this important pathway, and therefore to further distinguish between the relative importances of MnPO neurons from fibers of passage, current research in our lab using excitotoxin lesion specific to cell bodies of the MnPO along with retrograde tracing technique are being conducted. Nevertheless, our present findings indeed implicate the MnPO as a crucial component of central neural pathway necessary for the full hypertensive response to chronic intravenous ANG II infusion.
In conclusion, the MnPO has long been shown to mediate the central dipsogenic effect of ANG II, but relatively few studies have examined its role in chronic actions of ANG II. The present findings have expanded this knowledge by revealing a crucial role of the MnPO in the chronic hypertensive effect of ANG II. Previously, we and others have demonstrated a role of the SFO in the central hypertensive effects of ANG II. The findings that the SFO is associated with the MnPO both anatomically and functionally, together with the present observations, provide strong evidence to support the general hypothesis that the MnPO is a crucial part in the central sympathoexcitatory pathway following actions of ANG II at the SFO.
4. Experimental procedures
Adult male Sprague-Dawley rats (Charles River Laboratories, Wilmington, Mass) weighing 300-350g were used. All procedures were conducted in accordance with the National Institutes of Health guidelines and approved by the University of Minnesota Institutional Animal Care and Use committee.
4.1 Surgical procedures
Rats were randomly assigned to either a lesion of the median preoptic nucleus (MnPOx) or sham group. Pentobarbital sodium (39 mg/kg, IP) was given as preanesthetic medication, and rats were subsequently injected intramuscularly with a combination of agents (acetylpromazine, 0.2 mg/kg; butorphanol tartrate, 0.15 mg/kg; ketamine, 18.5 mg/kg) to achieve surgical anesthesia. An intramuscular injection of 4 mg tobramycin was given pre-operatively as antimicrobial prophylaxis. Anesthesized rats were then positioned in a Kopf stereotaxic apparatus.
Electrolytic lesion was targeted at both dorsal and ventral parts of the MnPO. A teflon-insulated monopolar tungsten electrode with 1.5 mm exposed at the tip was inserted midline into four predetermined coordinates along two axes: anterior-posterior (AP) relative to Bregma and dorsal-ventral (DV) relative to the surface of the sagittal sinus. The stereotaxic coordinates were selected from the rat brain atlas of Paxinos and Watson (1998). The 4 paired AP and DV coordinates (mm) used were: (−0.25, −7.4), (−0.25, −7.6), (−0.4, −6.1), (−0.4, −7.2). At each coordinate, a current of 1 mA was passed through the electrode for 5 seconds. Sham operated rats underwent an identical procedure as MnPOx rats, except that all DV coordinates were 2 mm less and no current was passed.
In the same surgery, all rats were implanted with radiotelemetry blood pressure transducers (model TA11PA-C40, Data Sciences International, St Paul, MN) and femoral venous catheters for 24 h-sampling of mean blood pressure (MAP) and heart rate (HR), and intravenous infusion of ANG II and saline, respectively. Skin of the inner thigh area was incised to expose the femoral artery and vein, and a femoral venous catheter was implanted. The telemetry unit consists of a fluid-filled catheter attached to the body of the transmitter/transducer. A midline abdominal incision was made in order to insert the body of the transducer into the abdomen. The telemetry catheter was passed through the abdominal wall guided by a 14-gauge stainless steel needle, inserted into the femoral artery, and advanced proximally such that the tip was located within the abdominal aorta. Both catheters were held in place by sutures. The body of the transmitter was secured to the abdominal muscle which was closed with 3/0 silk and the skin was closed with surgical staples. The intravenous catheter was advanced subcutaneously, exteriorized between the scapula through a dacron mesh button tether (Harvard Apparatus Inc, Holliston, MA), and the mesh was sutured to the interscapular muscle. The tether was connected to a spring, which was attached at the other end to a single channel hydraulic swivel above the cage, to which the femoral venous catheter was attached. Following the surgery, rats received a subcutaneous injection of 0.075 mg of butorphanol tartrate for analgesic purposes.
During the first three days after surgery, the rats received daily antimicrobial prophylactic injections of ampicillin (15 mg, IV). The rats were than started on a continuous intravenous infusion of sterile 0.9% saline (7 ml/24 h). They were housed in individual metabolic cages in a housing facility that was maintained at a temperature of approximately 23 °C with a 12 h:12 h light-dark cycle with lights on at 7:00 AM. Rats had free access to 0.4% NaCl diet and distilled water. After the surgery, rats were allowed to recover for at least one week before the experimental protocol was started.
4.2 Experimental protocol
The protocol was divided into three periods: 3 days of baseline control, 10 days of intravenous ANG II infusion (10 ng ˙ kg−1˙ min−1), and 3 days of recovery. ANG II was dissolved in sterile 0.9% saline and given at a rate of 7ml/24hr. During control and recovery periods, all rats received intravenous infusions of normal saline (7ml/24 hr). MAP and HR signals were sampled and recorded at 500 Hz every 1 min for 10 sec. Food intake, water intake, and urine output were measured gravimetrically daily at the same time each day (2:00 PM). Urine samples were collected and urine sodium concentration was measured using a sodium analyzer (NOVA Biomedical, Waltham, MA). Mean daily sodium intake was calculated as the sum of sodium received from the daily infusion (1 mmol/day) and the amount of dietary sodium ingested in 24 hr (0.07 mmol/g diet). Mean daily sodium excretion was calculated as the product of urine flow rate and urine sodium concentration. Mean daily water intake was calculated as the combined intake of drinking water and infusion water (7ml/24h). The daily water and sodium balance were calculated as the difference between intake and urinary excretion.
4.3 MnPO lesion verification
When the experimental protocol was completed, the rats were anesthetized with pentobarbital sodium (78 mg/kg), and perfused intracardially with 140 ml of heparinized saline (20 U/mL heparin in 0.9% saline), followed by 450 ml of 4% paraformaldehyde in phosphate buffer saline (PBS). The brains were then removed, post-fixed at 4°C in 4% paraformaldehyde in PBS overnight, and then transferred to 30% sucrose in PBS at 4°C for 2 days. After fixation, the brains were cut sagittally into 40-μm sections using freezing-microtome (Lipshaw Mfg., Detroit, MI). The sections were mounted on slides, air-dried for 1 day, and then stained with cresyl violet. The location and extent of lesion was determined using a light microscope. MnPOx rats were included in the analyses if they met the following criteria: 1) more than 90% of the MnPO was damaged and 2) no or slight damage to adjacent areas of the MnPO.
4.4 Statistical analysis
Two-way ANOVA with repeated measures was carried out to compare each parameter between MnPOx and sham groups. A Greenhouse-Geisser adjusted P value was used to account for violations of the assumption of compound symmetry that invariably accompany this experimental design. Post-hoc multiple comparisons using Tukey-Kramer test was further conducted if the two-way ANOVA showed significant interaction between two main factors (lesion vs. day). Baseline values were derived from averages over three control days, and the differences of baselines between the two groups were determined using a Student's t-test. A p-value of 0.05 was set as the level of statistical significance for all statistical analyses. Values were presented as mean ± SE. All statistical procedures were performed using NCSS software (NCSS, Kaysville, UT).
Acknowledgments
T.P. is a recipient of a scholarship from the Anandamahidol Foundation. This work was supported by National Heart Lung and Blood Institute grant RO1 HL-072180.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brody MJ, Fink GD, Buggy J, Haywood JR, Gordon FJ, Johnson AK. The role of the anteroventral third ventricle (AV3V) region in experimental hypertension. Circ Res. 1978;43:I-2–I-13. [Google Scholar]
- Brooks VL, Osborn JW. Hormonal-sympathetic interactions in long-term regulation of arterial pressure: An hypothesis. Am J Physiol. 1995;268:R1343–58. doi: 10.1152/ajpregu.1995.268.6.R1343. [DOI] [PubMed] [Google Scholar]
- Cerasola G, Cottone S, D'Ignoto G, Grasso L, Carone MB, Carapelle E, Contorno A. Effects of enalapril maleate on blood pressure, renin-angiotensin-aldosterone system, and peripheral sympathetic activity in essential hypertension. Clin Ther. 1987;9:390–399. [PubMed] [Google Scholar]
- Ciriello J, Gutman MB. Functional identification of central pressor pathways originating in the subfornical organ. Can J Physiol Pharmacol. 1991;69:1035–1045. doi: 10.1139/y91-154. [DOI] [PubMed] [Google Scholar]
- Cunningham JT, Beltz T, Johnson RF, Johnson AK. The effects of ibotenate lesions of the median preoptic nucleus on experimentally-induced and circadian drinking behavior in rats. Brain Res. 1992;580:325–330. doi: 10.1016/0006-8993(92)90961-8. [DOI] [PubMed] [Google Scholar]
- Cunningham JT, Sullivan MJ, Edwards GL, Farinpour R, Beltz TG, Johnson AK. Dissociation of experimentally induced drinking behavior by ibotenate injection into the median preoptic nucleus. Brain Res. 1991;554:153–158. doi: 10.1016/0006-8993(91)90183-v. [DOI] [PubMed] [Google Scholar]
- Dampney RA, Coleman MJ, Fontes MA, Hirooka Y, Horiuchi J, Li YW, Polson JW, Potts PD, Tagawa T. Central mechanisms underlying short- and long-term regulation of the cardiovascular system. Clin Exp Pharmacol Physiol. 2002;29:261–268. doi: 10.1046/j.1440-1681.2002.03640.x. [DOI] [PubMed] [Google Scholar]
- Fink GD, Bruner CA, Mangiapane ML. Area postrema is critical for angiotensin-induced hypertension in rats. Hypertension. 1987;9:355–361. doi: 10.1161/01.hyp.9.4.355. [DOI] [PubMed] [Google Scholar]
- Fink GD, Bruner CA, Mangiapane ML. Median preoptic nucleus ablation does not affect angiotensin II-induced hypertension. Am J Physiol. 1986;251:H148–52. doi: 10.1152/ajpheart.1986.251.1.H148. [DOI] [PubMed] [Google Scholar]
- Gardiner TW, Stricker EM. Impaired drinking responses of rats with lesions of nucleus medianus: Circadian dependence. Am J Physiol. 1985a;248:R224–30. doi: 10.1152/ajpregu.1985.248.2.R224. [DOI] [PubMed] [Google Scholar]
- Gardiner TW, Stricker EM. Hyperdipsia in rats after electrolytic lesions of nucleus medianus. Am J Physiol. 1985b;248:R214–23. doi: 10.1152/ajpregu.1985.248.2.R214. [DOI] [PubMed] [Google Scholar]
- Gutman MB, Jones DL, Ciriello J. Contribution of nucleus medianus to the drinking and pressor responses to angiotensin II acting at subfornical organ. Brain Res. 1989;488:49–56. doi: 10.1016/0006-8993(89)90692-6. [DOI] [PubMed] [Google Scholar]
- Gutman MB, Ciriello J, Mogenson GJ. Electrophysiological identification of forebrain connections of the subfornical organ. Brain Res. 1986;382:119–128. doi: 10.1016/0006-8993(86)90118-6. [DOI] [PubMed] [Google Scholar]
- Hendel MD, Collister JP. Contribution of the subfornical organ to angiotensin II-induced hypertension. Am J Physiol Heart Circ Physiol. 2005;288:H680–5. doi: 10.1152/ajpheart.00823.2004. [DOI] [PubMed] [Google Scholar]
- Jones DL. Kainic acid lesions of the median preoptic nucleus: Effects on angiotensin II induced drinking and pressor responses in the conscious rat. Can J Physiol Pharmacol. 1988;66:1082–1086. doi: 10.1139/y88-176. [DOI] [PubMed] [Google Scholar]
- Lind RW, Ohman LE, Lansing MB, Johnson AK. Transection of subfornical organ neural connections diminishes the pressor response to intravenously infused angiotensin II. Brain Res. 1983;275:361–364. doi: 10.1016/0006-8993(83)90998-8. [DOI] [PubMed] [Google Scholar]
- Lind RW, Johnson AK. Subfornical organ-median preoptic connections and drinking and pressor responses to angiotensin II. J Neurosci. 1982;2:1043–1051. doi: 10.1523/JNEUROSCI.02-08-01043.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind RW, Van Hoesen GW, Johnson AK. An HRP study of the connections of the subfornical organ of the rat. J Comp Neurol. 1982;210:265–277. doi: 10.1002/cne.902100306. [DOI] [PubMed] [Google Scholar]
- Mangiapane ML, Thrasher TN, Keil LC, Simpson JB, Ganong WF. Deficits in drinking and vasopressin secretion after lesions of the nucleus medianus. Neuroendocrinology. 1983;37:73–77. doi: 10.1159/000123518. [DOI] [PubMed] [Google Scholar]
- Matsukawa T, Gotoh E, Minamisawa K, Kihara M, Ueda S, Shionoiri H, Ishii M. Effects of intravenous infusions of angiotensin II on muscle sympathetic nerve activity in humans. Am J Physiol. 1991;261:R690–6. doi: 10.1152/ajpregu.1991.261.3.R690. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Allen AM, Burns P, Colvill LM, Oldfield BJ. Interaction of circulating hormones with the brain: The roles of the subfornical organ and the organum vasculosum of the lamina terminalis. Clin Exp Pharmacol Physiol. 1998;25(Suppl):S61–7. doi: 10.1111/j.1440-1681.1998.tb02303.x. [DOI] [PubMed] [Google Scholar]
- McKinley MJ, Badoer E, Oldfield BJ. Intravenous angiotensin II induces fos-immunoreactivity in circumventricular organs of the lamina terminalis. Brain Res. 1992;594:295–300. doi: 10.1016/0006-8993(92)91138-5. [DOI] [PubMed] [Google Scholar]
- Miselis RR. The subfornical organ's neural connections and their role in water balance. Peptides. 1982;3:501–502. doi: 10.1016/0196-9781(82)90115-2. [DOI] [PubMed] [Google Scholar]
- Miselis RR. The efferent projections of the subfornical organ of the rat: A circumventricular organ within a neural network subserving water balance. Brain Res. 1981;230:1–23. doi: 10.1016/0006-8993(81)90388-7. [DOI] [PubMed] [Google Scholar]
- Miselis RR, Shapiro RE, Hand PJ. Subfornical organ efferents to neural systems for control of body water. Science. 1979;205:1022–1025. doi: 10.1126/science.472723. [DOI] [PubMed] [Google Scholar]
- Oldfield BJ, Badoer E, Hards DK, McKinley MJ. Fos production in retrogradely labelled neurons of the lamina terminalis following intravenous infusion of either hypertonic saline or angiotensin II. Neuroscience. 1994;60:255–262. doi: 10.1016/0306-4522(94)90219-4. [DOI] [PubMed] [Google Scholar]
- O'Neill TP, Brody MJ. Role for the median preoptic nucleus in centrally evoked pressor responses. Am J Physiol. 1987;252:R1165–72. doi: 10.1152/ajpregu.1987.252.6.R1165. [DOI] [PubMed] [Google Scholar]
- Ota M, Crofton JT, Liu H, Festavan G, Share L. Increased plasma osmolality stimulates peripheral and central vasopressin release in male and female rats. Am J Physiol. 1994;267:R923–8. doi: 10.1152/ajpregu.1994.267.4.R923. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press; New York: 1998. [Google Scholar]
- Potts PD, Hirooka Y, Dampney RA. Activation of brain neurons by circulating angiotensin II: Direct effects and baroreceptor-mediated secondary effects. Neuroscience. 1999;90:581–594. doi: 10.1016/s0306-4522(98)00572-7. [DOI] [PubMed] [Google Scholar]
- Reid IA. Interactions between ANG II, sympathetic nervous system, and baroreceptor reflexes in regulation of blood pressure. Am J Physiol. 1992;262:E763–78. doi: 10.1152/ajpendo.1992.262.6.E763. [DOI] [PubMed] [Google Scholar]
- Rowland NE, Li BH, Rozelle AK, Fregly MJ, Garcia M, Smith GC. Localization of changes in immediate early genes in brain in relation to hydromineral balance: Intravenous angiotensin II. Brain Res Bull. 1994;33:427–436. doi: 10.1016/0361-9230(94)90286-0. [DOI] [PubMed] [Google Scholar]
- Saper CB, Levisohn D. Afferent connections of the median preoptic nucleus in the rat: Anatomical evidence for a cardiovascular integrative mechanism in the anteroventral third ventricular (AV3V) region. Brain Res. 1983;288:21–31. doi: 10.1016/0006-8993(83)90078-1. [DOI] [PubMed] [Google Scholar]
- Sawchenko PE, Swanson LW. The organization of forebrain afferents to the paraventricular and supraoptic nuclei of the rat. J Comp Neurol. 1983;218:121–144. doi: 10.1002/cne.902180202. [DOI] [PubMed] [Google Scholar]
- Saxena PR. Interaction between the renin-angiotensin-aldosterone and sympathetic nervous systems. J Cardiovasc Pharmacol. 1992;19 6:S80–8. doi: 10.1097/00005344-199219006-00013. [DOI] [PubMed] [Google Scholar]
- Schmitt H, Schmitt H. Increased activity in sympathetic nerves induced by angiotensin. Rev can Biol. 1968;27:255–257. [PubMed] [Google Scholar]
- Scrogin KE, Grygielko ET, Brooks VL. Osmolality: A physiological long-term regulator of lumbar sympathetic nerve activity and arterial pressure. Am J Physiol. 1999;276:R1579–86. doi: 10.1152/ajpregu.1999.276.6.R1579. [DOI] [PubMed] [Google Scholar]
- Stocker SD, Toney GM. Median preoptic neurones projecting to the hypothalamic paraventricular nucleus respond to osmotic, circulating ang II and baroreceptor input in the rat. J Physiol. 2005;568:599–615. doi: 10.1113/jphysiol.2005.094425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobey JC, Fry HK, Mizejewski CS, Fink GD, Weaver LC. Differential sympathetic responses initiated by angiotensin and sodium chloride. Am J Physiol. 1983;245:R60–8. doi: 10.1152/ajpregu.1983.245.1.R60. [DOI] [PubMed] [Google Scholar]
- Weekley LB. Angiotensin-II acts centrally to alter renal sympathetic nerve activity and the intrarenal renin-angiotensin system. Cardiovasc Res. 1991;25:353–363. doi: 10.1093/cvr/25.5.353. [DOI] [PubMed] [Google Scholar]
- Westerhaus MJ, Loewy AD. Sympathetic-related neurons in the preoptic region of the rat identified by viral transneuronal labeling. J Comp Neurol. 1999;414:361–378. [PubMed] [Google Scholar]
- Xu Z, Herbert J. Regional suppression by lesions in the anterior third ventricle of c-fos expression induced by either angiotensin II or hypertonic saline. Neuroscience. 1995;67:135–147. doi: 10.1016/0306-4522(95)00050-s. [DOI] [PubMed] [Google Scholar]
- Zardto-Smith AM, Thunhorst RL, Cicha MZ, Johnson AK. Afferent signaling and forebrain mechanisms in the behavioral control of extracellular fluid volume. Ann N Y Acad Sci. 1993;689:161–176. doi: 10.1111/j.1749-6632.1993.tb55545.x. [DOI] [PubMed] [Google Scholar]
- Zimmerman MC, Lazartigues E, Sharma RV, Davisson RL. Hypertension caused by angiotensin II infusion involves increased superoxide production in the central nervous system. Circ Res. 2004;95:210–216. doi: 10.1161/01.RES.0000135483.12297.e4. [DOI] [PubMed] [Google Scholar]


