Abstract
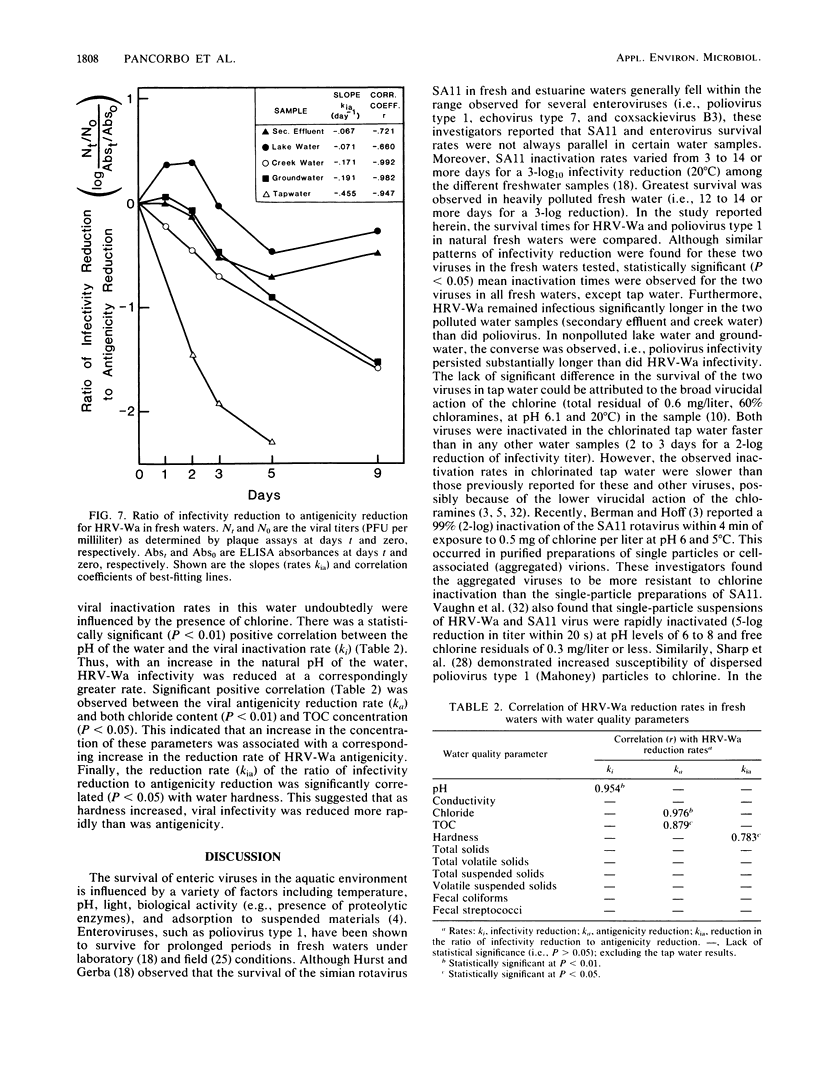
The rates of inactivation of human rotavirus type 2 (strain Wa) (HRV-Wa) and poliovirus type 1 (strain CHAT) were compared in polluted waters (creek water and secondary effluent before chlorination) and nonpolluted waters (lake water, groundwater, and chlorinated tap water). Viral infectivity titers were determined by plaque assays, while HRV-Wa antigenicity also was monitored by an enzyme-linked immunosorbent assay. Both viruses persisted longest in lake water and shortest in tap water. The actual inactivation times (i.e., times required for two-log10 reductions of initial viral titers) for the two viruses were significantly different in all waters except tap water. With the exception of the groundwater and secondary effluent results, the HRV-Wa inactivation times in the fresh waters tested were significantly different. Owing perhaps to aggregation, HRV-Wa appeared less susceptible to the effects of chlorine than previously reported for this virus and for the simian rotavirus SA11. HRV-Wa displayed prolonged survival in lake water and groundwater exceeding that previously reported for the SA11 virus. The HRV-Wa infectivity reduction rate (ki) was significantly correlated with the water pH (i.e., as pH increased, ki increased). The water pH may have influenced viral aggregation and thereby HRV-Wa susceptibility to other virucidal factors in the water. Enzyme-linked immunosorbent assay results showed similar inactivation patterns with the most significant reduction in HRV-Wa antigenicity occurring in polluted waters and tap water. In all waters, particularly tap water, infectivity declined at a faster rate than antigenicity. It is proposed that HRV-Wa can be used as a model for future studies of rotaviral persistence in the aquatic environment.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez M. E., O'Brien R. T. Effects of chlorine concentration on the structure of poliovirus. Appl Environ Microbiol. 1982 Jan;43(1):237–239. doi: 10.1128/aem.43.1.237-239.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman D., Hoff J. C. Inactivation of simian rotavirus SA11 by chlorine, chlorine dioxide, and monochloramine. Appl Environ Microbiol. 1984 Aug;48(2):317–323. doi: 10.1128/aem.48.2.317-323.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J. A., Middleton P. J., Szymanski M. T., Huber J., Petric M. Fatal rotavirus gastroenteritis: an analysis of 21 cases. Am J Dis Child. 1978 May;132(5):477–479. doi: 10.1001/archpedi.1978.02120300037006. [DOI] [PubMed] [Google Scholar]
- Churn C. C., Bates R. C., Boardman G. D. Mechanism of chlorine inactivation of DNA-containing parvovirus H-1. Appl Environ Microbiol. 1983 Dec;46(6):1394–1402. doi: 10.1128/aem.46.6.1394-1402.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esparza J., Gorziglia M., Gil F., Römer H. Multiplication of human rotavirus in cultured cells: an electron microscopic study. J Gen Virol. 1980 Apr;47(2):461–472. doi: 10.1099/0022-1317-47-2-461. [DOI] [PubMed] [Google Scholar]
- Farrah S. R., Goyal S. M., Gerba C. P., Conklin R. H., Smith E. M. Comparison between adsorption of poliovirus and rotavirus by aluminum hydroxide and activated sludge flocs. Appl Environ Microbiol. 1978 Feb;35(2):360–363. doi: 10.1128/aem.35.2.360-363.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyd R., Sharp D. G. Viral aggregation: buffer effects in the aggregation of poliovirus and reovirus at low and high pH. Appl Environ Microbiol. 1979 Sep;38(3):395–401. doi: 10.1128/aem.38.3.395-401.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamblet F. E., Hill W. F., Akin E. W. Effect of plaque assay diluent upon enumeration of poliovirus type 1. Appl Microbiol. 1967 Jan;15(1):208–208. doi: 10.1128/am.15.1.208-.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hejkal T. W., Smith E. M., Gerba C. P. Seasonal occurrence of rotavirus in sewage. Appl Environ Microbiol. 1984 Mar;47(3):588–590. doi: 10.1128/aem.47.3.588-590.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins R. S., Gaspard G. B., Williams F. P., Jr, Karlin R. J., Cukor G., Blacklow N. R. A community waterborne gastroenteritis outbreak: evidence for rotavirus as the agent. Am J Public Health. 1984 Mar;74(3):263–265. doi: 10.2105/ajph.74.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst C. J., Gerba C. P. Stability of simian rotavirus in fresh and estuarine water. Appl Environ Microbiol. 1980 Jan;39(1):1–5. doi: 10.1128/aem.39.1.1-5.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morens D. M., Zweighaft R. M., Vernon T. M., Gary G. W., Eslien J. J., Wood B. T., Holman R. C., Dolin R. A waterborne outbreak of gastroenteritis with secondary person-to-person spread. Association with a viral agent. Lancet. 1979 May 5;1(8123):964–966. doi: 10.1016/s0140-6736(79)91734-3. [DOI] [PubMed] [Google Scholar]
- Murphy A. M., Grohmann G. S., Christopher P. J., Lopez W. A., Davey G. R., Millsom R. H. An Australia-wide outbreak of gastroenteritis from oysters caused by Norwalk virus. Med J Aust. 1979 Oct 6;2(7):329–333. doi: 10.5694/j.1326-5377.1979.tb104133.x. [DOI] [PubMed] [Google Scholar]
- O'Brien R. T., Newman J. S. Inactivation of polioviruses and coxsackieviruses in surface water. Appl Environ Microbiol. 1977 Feb;33(2):334–340. doi: 10.1128/aem.33.2.334-340.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien R. T., Newman J. Structural and compositional changes associated with chlorine inactivation of polioviruses. Appl Environ Microbiol. 1979 Dec;38(6):1034–1039. doi: 10.1128/aem.38.6.1034-1039.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao V. C., Seidel K. M., Goyal S. M., Metcalf T. G., Melnick J. L. Isolation of enteroviruses from water, suspended solids, and sediments from Galveston Bay: survival of poliovirus and rotavirus adsorbed to sediments. Appl Environ Microbiol. 1984 Aug;48(2):404–409. doi: 10.1128/aem.48.2.404-409.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp D. G., Young D. C., Floyd R., Johnson J. D. Effect of ionic environment on the inactivation of poliovirus in water by chlorine. Appl Environ Microbiol. 1980 Mar;39(3):530–534. doi: 10.1128/aem.39.3.530-534.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley J. A., Beards G. M., Thouless M. E., Flewett T. H. The influence of divalent cations on the stability of human rotavirus. Arch Virol. 1981;67(1):1–9. doi: 10.1007/BF01314596. [DOI] [PubMed] [Google Scholar]
- Smith E. M., Gerba C. P. Development of a method for detection of human rotavirus in water and sewage. Appl Environ Microbiol. 1982 Jun;43(6):1440–1450. doi: 10.1128/aem.43.6.1440-1450.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann J. Detection of rotavirus in sewage. Appl Environ Microbiol. 1981 Apr;41(4):1043–1045. doi: 10.1128/aem.41.4.1043-1045.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn J. M., Chen Y. S., Thomas M. Z. Inactivation of human and simian rotaviruses by chlorine. Appl Environ Microbiol. 1986 Feb;51(2):391–394. doi: 10.1128/aem.51.2.391-394.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R. L., Knowlton D. R., Winston P. E. Mechanism of inactivation of enteric viruses in fresh water. Appl Environ Microbiol. 1986 Sep;52(3):450–459. doi: 10.1128/aem.52.3.450-459.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woode G. N., Bridger J. C., Jones J. M., Flewett T. H., Davies H. A., Davis H. A., White G. B. Morphological and antigenic relationships between viruses (rotaviruses) from acute gastroenteritis of children, calves, piglets, mice, and foals. Infect Immun. 1976 Sep;14(3):804–810. doi: 10.1128/iai.14.3.804-810.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R. G., Greenberg H. B., James W. D., Pittman A. L., Kalica A. R., Flores J., Chanock R. M., Kapikian A. Z. Definition of human rotavirus serotypes by plaque reduction assay. Infect Immun. 1982 Jul;37(1):110–115. doi: 10.1128/iai.37.1.110-115.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R. G., James W. D., Bohl E. H., Theil K. W., Saif L. J., Kalica A. R., Greenberg H. B., Kapikian A. Z., Chanock R. M. Human rotavirus type 2: cultivation in vitro. Science. 1980 Jan 11;207(4427):189–191. doi: 10.1126/science.6243190. [DOI] [PubMed] [Google Scholar]
- Yates M. V., Gerba C. P., Kelley L. M. Virus persistence in groundwater. Appl Environ Microbiol. 1985 Apr;49(4):778–781. doi: 10.1128/aem.49.4.778-781.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]



