Abstract
Background
Visceral adipose tissue (VAT), which is linked with the metabolic consequences of obesity, is usually characterized by measuring VAT area at the L4–L5 vertebral interspace. However, the location of the slice with the strongest relation to VAT volume is not established.
Objective
We sought to investigate the relations between cross-sectional VAT areas at different anatomic locations and VAT volume in a large, diverse sample of healthy subjects.
Design
VAT volume was derived from slice areas taken at 5-cm intervals from magnetic resonance images in 121 healthy men [x̄ ± SD age: 41.9 ± 15.8 y; body mass index (BMI; in kg/m2): 26.0 ± 3.2; VAT: 2.7 ± 1.8 L] and 198 healthy women (age: 48.1 ± 18.7 y; BMI: 27.0 ± 5.4; VAT: 1.7 ± 1.2 L). Regression models were developed to identify the best single slice for estimating VAT volume.
Results
The VAT area 10 cm above L4–L5 (A+10) in men (R2 = 0.932, P < 0.001) and 5 cm above L4–L5 (A+5) in women (R2 = 0.945, P <0.001) had the highest correlation with abdominal VAT. R2 increased by only 3.8% in men and 0.5% in women with adjustment for age, race, scanning position, BMI, and waist circumference. Studies using A+10 in men and A+5 in women will require 14% and 9% fewer subjects, respectively, than those using slices at L4–L5 and will have equivalent power.
Conclusion
Measurement of slice areas at A+10 in men and A+5 in women provides greater power for the detection of VAT volume differences than does measurement at L4–L5.
Keywords: Volume prediction, magnetic resonance imaging, computed tomography, body composition, L4-L5
INTRODUCTION
Over the past 2 decades, the availability of high-resolution cross-sectional imaging methods, such as computerized axial tomography (CT) and magnetic resonance imaging (MRI), has provided important opportunities for quantifying whole-body and regional adipose tissue in vivo. These technologies afford the means by which adiposity can be linked with health risks and outcomes. Specifically, the relatively small visceral adipose tissue (VAT) compartment is now widely recognized as conveying the highest health risks of the adipose tissue compartments that are currently measurable in humans (1–5).
Whereas multislice volume imaging is generally considered the reference for measuring total and regional adipose tissue volumes (6–8), application is limited by the radiation exposure associated with CT and by the relatively high cost of image analyses obtained by using MRI. Accordingly, most investigators use a single cross-sectional image as a representative measure of VAT as a compromise between accuracy and cost in their research (9–16). However, the accuracy of a single cross-sectional image area in estimating or representing VAT volume is not well established. Early studies of small subject samples showed a good correlation (r = 0.95–0.99) between VAT area measured in a single slice and VAT volume measured by using multiple slices (7, 17, 18). In other studies, either lower correlations (r = 0.82 to ≈0.83) of single-slice areas with VAT volume were found (19, 20), or the investigators questioned the usefulness of single CT or MRI images in estimating VAT volume (21).
A related concern is that there has been no systematic investigation to identify the anatomic location of a single slice whose area is most highly correlated with VAT volume or to ascertain whether such an optimum location is generalizable across age and race groups. A high intrasubject between-slice variability in abdominal adiposity was reported by Greenfield et al (22), and the inference was drawn that single-image slices are of limited utility for between-subject comparisons. In another recent study, Sumner et al (23) found that comparisons that used single-slice CT methods to measure adipose tissue areas failed to detect the well-known differences in VAT volume between men and women. The accuracy of a single-image slice in depicting VAT volume is of particular importance to future studies using a single-image slice for phenotyping subjects and to evaluations of the large number of published reports over the past 2 decades that rely on a single slice to represent VAT volume.
The aim of the present study was to investigate the relations between single cross-sectional image areas and the volumes of abdominal and abdominopelvic VAT in a large sample of healthy subjects who completed whole-body MRI studies. Our focus was to identify the slice location with the strongest association with VAT volume across age and race groups.
SUBJECTS AND METHODS
Protocol and design
The study was carried out by evaluating the relations between single cross-sectional image areas and the volumes of abdominal and abdominopelvic VAT in healthy subjects. Subjects were established as healthy on the basis of a medical history, physical examination, and screening blood studies. All subjects with body mass index (BMI; in kg/m2) ≥ 18.5 who underwent standardized whole-body MRI scanning were included in the present study, and additional measurements, including weight, height, age, and waist circumference, were taken. Race was self-reported by the subjects.
Subjects
Two hundred seventy-seven healthy subjects were selected from among those available in the Image Analysis Laboratory database of the New York Obesity Research Center at St Luke’s–Roosevelt Hospital. The database was compiled from studies carried out between 1995 and 2002. The present study also included 42 subjects who had participated in 2 earlier studies at the School of Dietetics and Human Nutrition of McGill University (24, 25). The total selected sample included 121 men and 198 women. The subjects’ characteristics are summarized in Table 1.
TABLE 1.
Subject characteristics
| Men (n = 121) | Women (n = 198) | |
|---|---|---|
| Race or ethnicity (n) | ||
| White | 66 | 86 |
| African American | 29 | 91 |
| Hispanic | 14 | 13 |
| Asian1 | 10 | 8 |
| Age (y) | 41.9 ± 15.8 (20.0–83.0)2 | 48.1 ± 18.73 (18.0–94.0) |
| Weight (kg) | 81.8 ± 11.4 (56.8–108.2) | 70.9 ± 15.04 (44.0–111.3) |
| Height (cm) | 177.2 ± 6.9 (159.9–191.5) | 162.1 ± 7.04 (143.1–182.6) |
| BMI (kg/m2) | 26.0 ± 3.2 (19.4–33.3) | 27.0 ± 5.43 (18.5–40.9) |
| Normal-weight, BMI 18.5–24.9 (n) | 48 | 87 |
| Overweight, BMI 25.0–29.9 (n) | 57 | 48 |
| Obese, BMI > 30.0 (n) | 16 | 63 |
| Waist circumference (cm)5 | 87.9 ± 9.8 (69.5–116.2) | 82.9 ± 14.23 (57.7–117.0) |
| Visceral adipose tissue (L) | 2.7 ± 1.8 (0.2–8.4) | 1.7 ± 1.24 (0.2–5.9) |
A multigenerational mixture of Chinese, Indian, Korean, and Japanese.
x̄ ± SD; range in parentheses (all such values).
Significantly different from men (Student’s t test):3P < 0.05,4P < 0.001.
Data available for 97 men and 180 women.
Anthropometric measurements
Body weight was measured to the nearest 0.1 kg and height to the nearest 0.1 cm with the use of appropriately calibrated scales and stadiometers. Waist circumference (WC) measurements were available for all subjects at the New York Obesity Research Center, including 97 men and 180 women. Waist circumference was measured while the subjects were wearing only undergarments and standing with their heels together. Minimum WC was measured between the lower rib margin and the iliac crest.
Magnetic resonance imaging
Whole-body MRI was carried out as previously reported by our group (26, 27). At the New York Obesity Research Center, MRI scans were performed by using a 1.5 T system (6X Horizon; General Electric, Milwaukee); at the School of Dietetics and Human Nutrition, scans were performed by using a 1.5 T Siemens system (Magnetom; Siemens, Mississauga, Canada). All subjects were scanned by using a T1-weighted, spin-echo sequence with a 210-ms repetition time, a 17-ms echo time, a 48-cm field of view, and a 256 × 256 matrix. The protocol involved acquisition of ≈40 axial images of 10-mm thickness at 40-mm intervals from fingers to toes while the subject was either prone or supine by using the L4 –L5 intervertebral space as the point of origin. After image acquisition, the VAT was segmented by trained and quality-controlled technicians with the use of image analysis software (SLICEOMATIC, version 4.0; Tomovision Inc, Montreal). The intraclass correlation coefficient for volume rendering of VAT by different technicians at our center is 0.95. The VAT volume was calculated as:
where V is volume, Ai is each scan’s cross-sectional area, h is the between-slice interval, t is the thickness of each slice, and N is the number of total slices. Abdominopelvic VAT volumes were calculated by using all slices between the dome of the liver and the bottom of the pelvis (abdominopelvic region), whereas abdominal VAT volumes were calculated by using all slices between the dome of the liver to one slice below L4 –L5. Abdominal and abdominopelvic VAT were chosen in this study because they were the most frequently measured compartments in previous studies. Shen et al (28) has provided an extended critical review of VAT definitions and the use of VAT estimations in clinical research.
Statistical analysis
Group data are presented as means ± SDs. Variation in VAT area among slices at different locations is expressed as the intrasubject between-slice CV, both for 4 slices, as reported by Green-field et al (22), and for all 8 available slices. The correlations among single-slice VAT areas and those between single-slice VAT areas and VAT volume were calculated for each slice, and the results were used to identify the slice location with the highest correlation with abdominal and abdominopelvic VAT. Because most previous investigations reported abdominal rather than abdominopelvic VAT (3, 29–32), we simplified our presentation of results by providing illustrative examples mainly for abdominal VAT, although we carried out analyses for both compartments of VAT and had similar results.
The slice showing the highest correlation between VAT area and VAT volume was chosen as the best slice for use in regression models. A simple regression model was then applied to ascertain separately the coefficients for the observed relations between VAT volume and VAT area for the selected slice and for the slice at L4–L5. The variance of the residuals from the regression of L4–L5 and that of residuals obtained by using the best single slice were compared with the use of Pitman’s test for correlated variances (33). Differences between correlation coefficients from correlated samples were tested by using the method of Steiger (34).
Multiple regression models were applied to establish whether the relation between the VAT volume and the VAT area of the selected slice is modified by age, ethnicity, scanning body position (ie, prone or supine), body mass index (BMI) or waist circumference. Interactions of the selected slice VAT area with other variables were explored. Two-way and three-way interactions among covariates were also tested. The validity of the equation when used in samples other than the one from which it was derived was estimated by calculating the cross-validation SD of the prediction error. This involves deriving a regression equation from N − 1 cases, and applying the derived equation to predict the dependent variable in the excluded case, the leave-one-out method (35).
A polynomial curve was fitted to describe the smoothed relation of correlation strength for slice area and VAT volume over the range of different anatomic locations for men and women separately. A similar polynomial was fitted to describe the relation between slice area and different anatomic locations. The location of the peak of the polynomial curve was taken as an estimate of the anatomic location of the single slice that would have the highest correlation between VAT area and volume.
All statistical analyses were carried out with the use of SPSS for Windows software (version 11.0; SPSS Inc, Chicago). Two-tailed (α = 0.05) tests of significance were used.
RESULTS
Single-slice VAT area with the highest correlation with VAT volume
The single-slice VAT area having the highest correlation with abdominal VAT volume in men was located 10 cm above L4–L5 (r = 0.966), and that having the highest correlation with abdominal volume in women was located 5 cm above L4–L5 (r = 0.972) (Table 2). The highest correlation coefficients are significantly greater in both men and women than those at L4–L5. A scatter plot of the relation between the single-slice area at 10 cm above L4–L5 and abdominal VAT in men and that between the single-slice area at 5 cm above L4–L5 and abdominal VAT in women is shown in Figure 1. Overall, the correlations between single-slice VAT areas and abdominopelvic VAT volume were slightly lower than those between the same single-slice areas and abdominal VAT (Table 2). The single-slice area showing the highest correlation with abdominopelvic VAT was also 10 cm above L4–L5 in men (r = 0.961) and 5 cm above L4–L5 in women (r = 0.964).
TABLE 2.
Pearson’s correlation coefficients for visceral adipose tissue (VAT) volume and VAT areas for individual transverse slices below (−) or above (+) the L4–L5 location1
| Correlation coefficients
|
||||||
|---|---|---|---|---|---|---|
| −10 cm | −5 cm | L4–L5 | +5 cm | +10 cm | +15 cm | |
| Men | ||||||
| Abdominal VAT | 0.843 | 0.899 | 0.951 | 0.966 | 0.924 | |
| Abdominopelvic VAT | 0.857 | 0.889 | 0.919 | 0.950 | 0.961 | 0.917 |
| Women | ||||||
| Abdominal VAT | 0.908 | 0.929 | 0.972 | 0.961 | 0.822 | |
| Abdominopelvic VAT | 0.856 | 0.930 | 0.936 | 0.964 | 0.9512 | 0.821 |
All correlation coefficients are significant at P <0.001; all coefficients in the +5 cm and +10 cm columns are significantly greater than the corresponding correlations in the L4–L5 column, P < 0.05, except where noted otherwise.
Greater than correlations at L4–L5, P = 0.07.
FIGURE 1.
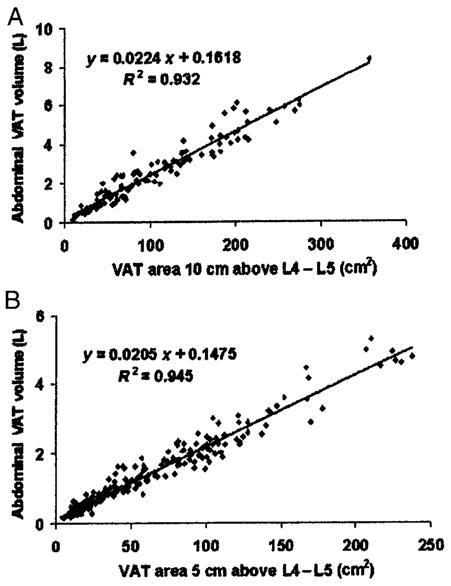
Relation between abdominal visceral adipose tissue (VAT) volumes and VAT area 10 cm above L4–L5 in men (A) and 5 cm above L4–L5 in women (B). Both the coefficient for VAT area and the intercept of the equation are significantly different from 0 (P < 0.001). Note that the scales of both axes differ between the 2 panels of the figure.
Between-slice variation in VAT
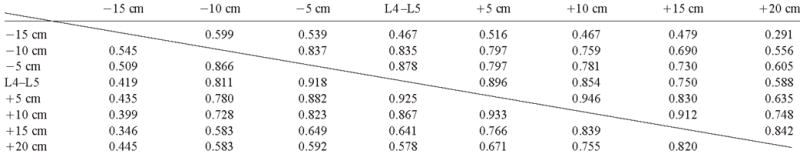
The intrasubject CVs, both for 4 slices and for all 8 slices, were 11.7%–39.6% (Table 3). The correlations among the evaluated VAT areas of these slices are presented in Table 4, and the r value of adjacent slices in the middle portion of the abdomen was ≈0.9.
TABLE 3.
Intrasubject variability between slices of visceral adipose tissue1
| CV
|
||
|---|---|---|
| 4 slices | 8 slices | |
| % | ||
| Men | 11.7 ± 8.1 | 32.3 ± 23.2 |
| Women | 25.3 ± 15.8 | 39.6 ± 18.8 |
All values are x̄ ± SD.
TABLE 4.
Correlation coefficients among single-slice areas of visceral adipose tissue at different anatomic locations below (−) or above (+) the L4–L5 location1
|
Values above the diagonal are for men; values below the diagonal are for women.
Single-slice area with the strongest relation to VAT
The VAT area 10 cm above L4–L5 (A+10 in cm2) in men and 5 cm above L4–L5 (A+5 in cm2) in women was selected for inclusion in the multiple regression models as the independent variable, and abdominal VAT volume was used as the dependent variable. The coefficients for the developed regression equations are shown in Table 5. The SD of the prediction error derived by the leave-one-out method was 0.448 L for men and 0.258 L for women. The SD of the prediction error of the regression model was 0.443 L for men and 0.255 L for women. The small size of the difference between these 2 errors indicates that the developed regression equation would have a high validity when applied to other samples similar to the one from which it was developed.
TABLE 5.
Regression models linking visceral adipose tissue (VAT) areas 10 cm above L4–L5 with abdominal VAT volume in men and 5 cm above L4–L5 with abdominal VAT volume in women1
| Men | R2 | Women | R2 | |
|---|---|---|---|---|
| Equation with VAT area | V = 0.0224 × A+10 + 0.162 | 0.932 | V = 0.0205 × A+5 + 0.147 | 0.945 |
| + Covariates2 | Race3 | 0.941 | — | — |
| BMI4 | 0.936 | BMI3 | 0.949 | |
| WC3 | 0.958 | WC4 | 0.950 | |
| + Interactions2,5 | A+10 × age × WC4 | 0.963 | A+5 × age × BMI6 | 0.950 |
| A+5 × race × age6 | 0.949 |
V, volume (in L); A+10, area 10 cm above L4–L5 (in cm2); A+5, area 5 cm above L4–L5 (in cm2); WC, waist circumference.
Additional control for covariates or interactions that contributed significantly to model.
P < 0.001.
P < 0.01.
These three-way interactions include all two-way interactions in the model.
P < 0.05.
The contributions of age, race, scanning body position (ie, prone or supine), BMI, and WC as significant covariates to the developed model are shown in Table 5. Although some of these factors were significant either as main effects or in two-way and three-way interactions, together they improved R2 only in the range of 0.004–0.031. When all of the significant factors were entered either as main effects or as interactions, the R2 increased from 0.932 to 0.970 in men and from 0.945 to 0.950 in women.
With the equation using only the VAT area 10 cm above L4–L5 as the independent variable for men and that 5 cm above L4–L5 for women, the SE for an individual subject’s estimated VAT is 0.445 L for men and 0.256 L for women. Imaging of a single appropriate slice can thus be used to distinguish reliably between subjects who differ in VAT volume by ≥1.0 L (women) and ≥1.7 L (men). With the equation that uses the best slice as the independent variable and covariates of age, race, prone or supine body position, BMI, and WC, the SE for an individual subject’s abdominal VAT volume is ≈ 0.261 L for men and ≈ 0.231 L for women.
The best slice was also tested for inclusion in the multiple regression models with abdominopelvic VAT volume as the dependent variable. The results are similar to those for abdominal VAT volume: abdominopelvic VAT has a slightly lower R2 (0.923 in men and 0.929 in women) than does abdominal VAT. The improvement in R2 is 2.7% for men and 0.4% for women with all significant covariates and interactions entered into the model (data not shown).
Estimation of VAT at L4–L5
The VAT area at L4–L5 was investigated with multiple regression modeling that used abdominal and abdominopelvic VAT volumes as the dependent variables. The SE for an individual subject’s abdominal VAT volume is ≈ 0.750 L for men and ≈ 0.404 L for women. Pitman’s test for correlated variances confirmed that the variance for residuals in the L4–L5 model was greater than that in the models using the best slice (P < 0.001). This indicates that the errors of predictions based on the L4–L5 area were significantly larger than those based on an area 10 cm above L4–L5 in men and 5 cm above L4–L5 in women.
Power estimates for different anatomic locations
If VAT volume as measured by the multiple-slice protocol is taken as the “true” value, and if the squared correlations between single-slice locations and VAT volume are considered to be estimates of the reliability of the single slice as a measure of VAT volume, then we can calculate the relative loss of power when a single slice is used (36). To achieve equivalent power, a study that would require N subjects when multiple-slice measures of VAT volume were used as the dependent variable would require 7% more men if a single-slice area is measured 10 cm above L4–L5 and 6% more women if a single-slice area is measured 5 cm above L4–L5. In contrast, 24% more men and 16% more women will be required to achieve equivalent power if a single-slice area is measured at L4–L5.
Polynomial interpolation
Because contiguous slices were not available in this data set, we estimated the anatomic location of maximum correlation by using polynomial interpolation. Fourth-order polynomials were fitted to the 5 correlation coefficients describing the relations between VAT area and abdominal VAT volume and to single-slice VAT areas and anatomic locations (equations not shown; Figure 2). The maximums of the fitted curves suggest that the strongest association between VAT area and volume is found when slices are located at 9.0 cm and 7.1 cm above L4–L5 in men and women, respectively. The maximums of the polynomials relating correlation coefficients to anatomic location and single-slice area to anatomic location occur at different anatomic locations, which suggests that the slice with the largest area does not necessarily have the strongest association with VAT volume.
FIGURE 2.
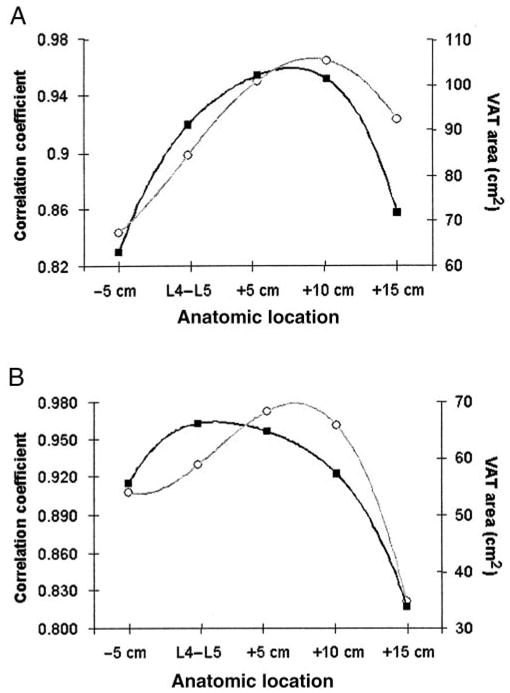
Relations between correlation coefficients (○) and anatomic location and between visceral adipose tissue (VAT) slice areas (■) and anatomic location. The data were fitted with 4th-order polynomial equations in men (A), and women (B). L4–L5 is taken as the zero point, and slices are identified as being taken from below (−) or above (+) this location.
DISCUSSION
The present study, carried out in a large sample and including subjects who varied in race, age, and BMI, generally confirms the previously reported association between VAT area and volume at L4–L5 (r: 0.82 to ≈0.99; 7, 17, 19, 20, 37). In addition, we observed the highest correlation between VAT area and volume ≈5–10 cm above L4–L5. Studies using slices from this higher location will require ≈9%–14% fewer subjects for equivalent power than will studies using VAT slices from the L4–L5 location.
When the effects of subject age, race, scanning body position, BMI, and WC on the strength of the associations between the identified optimum anatomic location and VAT volume were examined, WC and its interactions with age were found to improve R2 ≈3% in men; all other covariate and their interactions increased R2 by only <1.5%. Thus, an anatomic location ≈10 cm above L4–L5 in men and ≈5 cm above L4–L5 in women may be used to represent VAT volume across a wide range of healthy subjects, as evaluated in the present study. Several previous small-scale studies (n < 50 subjects) reported the highest correlation between VAT area and volume at either L4–L5 (17) or L2–L3 (18, 38). However, some of those studies are not comparable to the present investigation because only the middle portion of the abdomen was investigated to predict VAT volume (18, 39). Some previous studies adopted other landmarks, such as L2–L3 (18), which is ≈10 cm above L4–L5 but which varies in location with subject stature. The degree of correspondence between a single slice at L2–L3 and a slice 5 or 10 cm above L4–L5 cannot be evaluated in the current study.
The SE for predicting VAT volume in an individual subject from a single-slice area is 0.256–0.445 L. Therefore, the use of a single-slice VAT area estimate has limited application to estimations of VAT volume in an individual subject. The cutoff point suggested previously for diagnosing excessive VAT volume (ie, 130 cm2 at L4–L5; 40) will therefore be imprecise and should be used cautiously in individual subjects. However, in group studies, the use of an appropriate single-slice area would require only 7% more men and 6% more women than would the use of a multiple-slice volume protocol, which indicates that an appropriately selected single slice can be used for group studies.
A noteworthy finding in this study is that the slice area with the highest association with VAT volume was not the slice with the largest VAT area, especially in women (Figure 2). A possible explanation for this unanticipated finding is that VAT has 2 compartments, intraperitoneal and extraperitoneal adipose tissue (IPAT and EPAT, respectively; 28). Whereas EPAT components serve primarily as mechanical cushions for organs such as kidneys, rectum, uterus, and bladder, IPAT depots are of high metabolic activity, and thus they may account for a large proportion of the intersubject variation in observed VAT volume. Consequently, a slice that contains mostly IPAT may show the highest correlation between slice area and VAT volume even though the slice does not have the largest VAT area. A previous study of diet- and exercise-induced weight loss found that the relative loss in VAT area 15 cm above L4–L5 was significantly larger than that at other abdominal slice areas (41). This observation supports the hypothesis that slices above L4–L5 contain more metabolically active VAT, and it is consistent with the observation that IPAT is located primarily in the upper abdomen (Unpublished segmentation of Visible Man; Visible Man; National Library of Medicine, Bethesda, MD; Internet: http://www.nlm.nih.gov/research/visible/visible_human.html).
The L4–L5 VAT area has served as a measure of VAT in almost all studies over the past 2 decades, including those investigating physiologic processes and pathological states (9–16) and method validations or optimizations (42–44). The use of the transverse L4–L5 slice to represent the entire abdominal compartment can be dated back to the earliest studies of body-composition imaging methods (45). In the initial CT studies, Kvist et al observed that the highest correlations between a single-slice area and VAT volume (from 22 scans) of both total-body adipose tissue (37) and VAT (17) were at L4–L5 (r = 0.991). Although both studies were carried out in a small number of subjects (ie, 8 and 26) and the latter included women who had undergone colectomy, many studies thereafter adopted the L4–L5 anatomic location for studies of VAT and cited the study of Kvist et al (17) for this approach (16, 42, 46–52).
Previous investigations favored L4–L5 or its approximation, the umbilical location, for a variety of reasons. First, those locations offer ease in detecting the landmarks including L4–L5 intervertebral disc, umbilicus, or iliac crest (14, 39, 53). Second, they are the same location as the WC measurement site (54). Third, they offer the maximum ratio of adipose tissue to total tissue area (39). Fourth, the percentage of total tissue as VAT found at the umbilical location cross-section is closer to the mean value for all slices combined than for any other single slice (39). Subsequent reports cited those earlier investigations (13, 54) or did not give a reason for choosing L4–L5 as a measure of VAT volume (44,55–58). Whereas the choice of a selected slice for VAT measurement may have merit, depending on the aims of the study, the location at which the slice area has the highest correlation with VAT volume is the most appropriate choice.
The high intrasubject between-slice variability in VAT area recently report by Greenfield et al (22) was also noted by investigators almost 2 decades ago (59). Despite the high intrasubject between-slice variability in VAT area also observed in the present study (Table 3), the correlation between a single-slice area and VAT volume reached an r value of 0.966 in men and of 0.972 in women. We can thus conclude that a high regional intrasubject between-slice variability in VAT area does not necessarily imply a poor correlation between a single-slice area and VAT volume. In other words, although there is considerable variation between single-slice VAT areas, that does not imply that a single slice cannot be used to estimate VAT volume, especially for group comparisons.
There are several limitations of the present study. Although we found that VAT areas 5 and 10 cm above L4–L5 have the highest correlations with VAT volume, we do not have continuous scans, the exact location of the slice with the highest correlation cannot be identified precisely, and we cannot study landmarks such as L2–L3 or L3–L4. In the present study, we used a polynomial curve to estimate the location of the slice area with the highest correlation with VAT volume. Our currently available data cannot be used to differentiate between IPAT and EPAT, and therefore we cannot identify the slice that has the highest correlation with the proposed highly active IPAT compartment. Further studies are needed to confirm our findings in longitudinal data sets and in patients with health disorders. Another limitation is that the cohort of the present study includes few Hispanics and Asians, and thus our findings should be applied cautiously in racial groups other than whites and blacks.
Conclusions
Single-slice VAT areas 5–10 cm above L4–L5 have a higher correlation with VAT volume than does the VAT area at the traditional L4–L5 location. The influence of age, race, prone or supine body position, BMI, and WC on the relations between VAT areas and VAT volume is relatively small. The present study results indicate that statistically more powerful group comparisons of VAT volume may be carried out by using a single-slice VAT area located 10 cm above L4–L5 in men and 5 cm above L4–L5 in women rather than by using a slice from the L4–L5 location.
Acknowledgments
We acknowledge the supportive role in the study played by Peter JH Jones of McGill University.
Footnotes
Supported by National Institutes of Health Grants NIDDK 42618 and 1 R21 DK66360-01, R29-AG14715, F32-AJ05679, M01 RR00645, R01 DK40414, P30 DK26687.
WS, SBH, and SH designed the study; WS and SH analyzed the data; WS, SBH, and SH wrote the manuscript; DG, MPS, and JA collected data; WS and MP organized data; ZM, DG, MPS, and JA provided advice; and MP, SBH, and SH provided administrative support. None of the authors had any financial or personal interest in any company or organization sponsoring the research, including advisory board affiliations.
References
- 1.Faria AN, Ribeiro Filho FF, Gouveia Ferreira SR, Zanella MT. Impact of visceral fat on blood pressure and insulin sensitivity in hypertensive obese women. Obes Res. 2002;10:1203–6. doi: 10.1038/oby.2002.164. [DOI] [PubMed] [Google Scholar]
- 2.Rissanen J, Hudson R, Ross R. Visceral adiposity, androgens, and plasma lipids in obese men. Metabolism. 1994;43:1318–23. doi: 10.1016/0026-0495(94)90229-1. [DOI] [PubMed] [Google Scholar]
- 3.Ross R, Rissanen J, Pedwell H, Clifford J, Shragge P. Influence of diet and exercise on skeletal muscle and visceral adipose tissue in men. J Appl Physiol. 1996;81:2445–55. doi: 10.1152/jappl.1996.81.6.2445. [DOI] [PubMed] [Google Scholar]
- 4.Phillips GB, Jing T, Heymsfield SB. Relationships in men of sex hormones, insulin, adiposity, and risk factors for myocardial infarction. Metabolism. 2003;52:784–90. doi: 10.1016/s0026-0495(03)00072-6. [DOI] [PubMed] [Google Scholar]
- 5.Björntorp P. Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis. 1990;10:493–6. [PubMed] [Google Scholar]
- 6.Ross R. Magnetic resonance imaging provides new insights into the characterization of adipose and lean tissue distribution. Can J Physiol Pharmacol. 1996;74:778–85. [PubMed] [Google Scholar]
- 7.Ross R, Leger L, Morris D, de Guise J, Guardo R. Quantification of adipose tissue by MRI: relationship with anthropometric variables. J Appl Physiol. 1992;72:787–95. doi: 10.1152/jappl.1992.72.2.787. [DOI] [PubMed] [Google Scholar]
- 8.Heymsfield SB, Wang Z, Baumgartner RN, Ross R. Human body composition: advances in models and methods. Annu Rev Nutr. 1997;17:527–58. doi: 10.1146/annurev.nutr.17.1.527. [DOI] [PubMed] [Google Scholar]
- 9.Gray DS, Fujioka K, Colletti PM, et al. Magnetic-resonance imaging used for determining fat distribution in obesity and diabetes. Am J Clin Nutr. 1991;54:623–7. doi: 10.1093/ajcn/54.4.623. [DOI] [PubMed] [Google Scholar]
- 10.Leenen R, van der Kooy K, Seidell JC, Deurenberg P. Visceral fat accumulation measured by magnetic resonance imaging in relation to serum lipids in obese men and women. Atherosclerosis. 1992;94:171–81. doi: 10.1016/0021-9150(92)90242-9. [DOI] [PubMed] [Google Scholar]
- 11.Despres JP, Moorjani S, Ferland M, et al. Adipose tissue distribution and plasma lipoprotein levels in obese women. Importance of intra-abdominal fat. Arteriosclerosis. 1989;9:203–10. doi: 10.1161/01.atv.9.2.203. [DOI] [PubMed] [Google Scholar]
- 12.Pouliot MC, Despres JP, Nadeau A, et al. Visceral obesity in men. Associations with glucose tolerance, plasma insulin, and lipoprotein levels. Diabetes. 1992;41:826–34. doi: 10.2337/diab.41.7.826. [DOI] [PubMed] [Google Scholar]
- 13.Stallone DD, Stunkard AJ, Wadden TA, Foster GD, Boorstein J, Arger P. Weight loss and body fat distribution: a feasibility study using computed tomography. Int J Obes. 1991;15:775–80. [PubMed] [Google Scholar]
- 14.Hendler RG, Welle SL, Statt MC, Barnard R, Amatruda JM. The effects of weight reduction to ideal body weight on body fat distribution. Metabolism. 1995;44:1413–6. doi: 10.1016/0026-0495(95)90139-6. [DOI] [PubMed] [Google Scholar]
- 15.Anderson PJ, Chan JC, Chan YL, et al. Visceral fat and cardiovascular risk factors in Chinese NIDDM patients. Diabetes Care. 1997;20:1854–8. doi: 10.2337/diacare.20.12.1854. [DOI] [PubMed] [Google Scholar]
- 16.Goodpaster BH, Kelley DE, Wing RR, Meier A, Thaete FL. Effects of weight loss on regional fat distribution and insulin sensitivity in obesity. Diabetes. 1999;48:839–47. doi: 10.2337/diabetes.48.4.839. [DOI] [PubMed] [Google Scholar]
- 17.Kvist H, Chowdhury B, Grangard U, Tylen U, Sjostrom L. Total and visceral adipose-tissue volumes derived from measurements with computed tomography in adult men and women: predictive equations. Am J Clin Nutr. 1988;48:1351–61. doi: 10.1093/ajcn/48.6.1351. [DOI] [PubMed] [Google Scholar]
- 18.Han TS, Kelly IE, Walsh K, Greene RM, Lean ME. Relationship between volumes and areas from single transverse scans of intra-abdominal fat measured by magnetic resonance imaging. Int J Obes Relat Metab Disord. 1997;21:1161–6. doi: 10.1038/sj.ijo.0800530. [DOI] [PubMed] [Google Scholar]
- 19.Ross R, Shaw KD, Martel Y, de Guise J, Avruch L. Adipose tissue distribution measured by magnetic resonance imaging in obese women. Am J Clin Nutr. 1993;57:470–5. doi: 10.1093/ajcn/57.4.470. [DOI] [PubMed] [Google Scholar]
- 20.Ross R, Shaw KD, Rissanen J, Martel Y, de Guise J, Avruch L. Sex differences in lean and adipose tissue distribution by magnetic resonance imaging: anthropometric relationships. Am J Clin Nutr. 1994;59:1277–85. doi: 10.1093/ajcn/59.6.1277. [DOI] [PubMed] [Google Scholar]
- 21.Thomas EL, Saeed N, Hajnal JV, et al. Magnetic resonance imaging of total body fat. J Appl Physiol. 1998;85:1778–85. doi: 10.1152/jappl.1998.85.5.1778. [DOI] [PubMed] [Google Scholar]
- 22.Greenfield JR, Samaras K, Chisholm DJ, Campbell LV. Regional intrasubject variability in abdominal adiposity limits usefulness of computed tomography. Obes Res. 2002;10:260–5. doi: 10.1038/oby.2002.35. [DOI] [PubMed] [Google Scholar]
- 23.Sumner AE, Farmer NM, Tulloch-Reid MK, et al. Sex differences in visceral adipose tissue volume among African Americans. Am J Clin Nutr. 2002;76:975–9. doi: 10.1093/ajcn/76.5.975. [DOI] [PubMed] [Google Scholar]
- 24.St-Onge MP, Bourque C, Jones PJ, Ross R, Parsons WE. Medium-versus long-chain triglycerides for 27 days increases fat oxidation and energy expenditure without resulting in changes in body composition in overweight women. Int J Obes Relat Metab Disord. 2003;27:95–102. doi: 10.1038/sj.ijo.0802169. [DOI] [PubMed] [Google Scholar]
- 25.St-Onge MP, Ross R, Parsons WD, Jones PJ. Medium-chain triglycerides increase energy expenditure and decrease adiposity in overweight men. Obes Res. 2003;11:395–402. doi: 10.1038/oby.2003.53. [DOI] [PubMed] [Google Scholar]
- 26.Gallagher D, Belmonte D, Deurenberg P, et al. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am J Physiol. 1998;275:E249–58. doi: 10.1152/ajpendo.1998.275.2.E249. [DOI] [PubMed] [Google Scholar]
- 27.Heymsfield SB, Gallagher D, Kotler DP, Wang Z, Allison DB, Heshka S. Body-size dependence of resting energy expenditure can be attributed to nonenergetic homogeneity of fat-free mass. Am J Physiol Endocrinol Metab. 2002;282:E132–8. doi: 10.1152/ajpendo.2002.282.1.E132. [DOI] [PubMed] [Google Scholar]
- 28.Shen W, Wang ZM, Punyanita M, et al. Adipose tissue quantification by imaging methods: a proposed classification. Obes Res. 2003;11:5–16. doi: 10.1038/oby.2003.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yanovski JA, Yanovski SZ, Filmer KM, et al. Differences in body composition of black and white girls. Am J Clin Nutr. 1996;64:833–9. doi: 10.1093/ajcn/64.6.833. [DOI] [PubMed] [Google Scholar]
- 30.Baumgartner RN, Heymsfield SB, Roche AF, Bernardino M. Abdominal composition quantified by computed tomography. Am J Clin Nutr. 1988;48:936–45. doi: 10.1093/ajcn/48.4.936. [DOI] [PubMed] [Google Scholar]
- 31.Rice B, Janssen I, Hudson R, Ross R. Effects of aerobic or resistance exercise and/or diet on glucose tolerance and plasma insulin levels in obese men. Diabetes Care. 1999;22:684–91. doi: 10.2337/diacare.22.5.684. [DOI] [PubMed] [Google Scholar]
- 32.Janssen I, Ross R. Effects of sex on the change in visceral, subcutaneous adipose tissue and skeletal muscle in response to weight loss. Int J Obes Relat Metab Disord. 1999;23:1035–46. doi: 10.1038/sj.ijo.0801038. [DOI] [PubMed] [Google Scholar]
- 33.Snedecor GW, Cochran WG. Statistical methods. 8. Ames, IA: Iowa State University Press; 1989. [Google Scholar]
- 34.Steiger JH. Tests for comparing elements of a correlation matrix. Psychol Bull. 1980;87:245–61. [Google Scholar]
- 35.Efron B. Estimating the error rate of a prediction rule; improvements on crossvalidation. J Am Statist Assoc. 1983;78:316–31. [Google Scholar]
- 36.Muller MJ, Szegedi A. Effects of interrater reliability of psychopathologic assessment on power and sample size calculations in clinical trials. J Clin Psychopharmacol. 2002;22:318–25. doi: 10.1097/00004714-200206000-00013. [DOI] [PubMed] [Google Scholar]
- 37.Kvist H, Sjostrom L, Tylen U. Adipose tissue volume determinations in women by computed tomography: technical considerations. Int J Obes. 1986;10:53–67. [PubMed] [Google Scholar]
- 38.Abate N, Garg A, Coleman R, Grundy SM, Peshock RM. Prediction of total subcutaneous abdominal, intraperitoneal, and retroperitoneal adipose tissue masses in men by a single axial magnetic resonance imaging slice. Am J Clin Nutr. 1997;65:403–8. doi: 10.1093/ajcn/65.2.403. [DOI] [PubMed] [Google Scholar]
- 39.Borkan GA, Gerzof SG, Robbins AH, Hults DE, Silbert CK, Silbert JE. Assessment of abdominal fat content by computed tomography. Am J Clin Nutr. 1982;36:172–7. doi: 10.1093/ajcn/36.1.172. [DOI] [PubMed] [Google Scholar]
- 40.Williams MJ, Hunter GR, Kekes-Szabo T, et al. Intra-abdominal adipose tissue cut-points related to elevated cardiovascular risk in women. Int J Obes Relat Metab Disord. 1996;20:613–7. [PubMed] [Google Scholar]
- 41.Ross R, Rissanen J. Mobilization of visceral and subcutaneous adipose tissue in response to energy restriction and exercise. Am J Clin Nutr. 1994;60:695–703. doi: 10.1093/ajcn/60.5.695. [DOI] [PubMed] [Google Scholar]
- 42.Clasey JL, Bouchard C, Wideman L. The influence of anatomical boundaries, age, and sex on the assessment of abdominal visceral fat. Obes Res. 1997;5:395–401. doi: 10.1002/j.1550-8528.1997.tb00661.x. [DOI] [PubMed] [Google Scholar]
- 43.Terry JG, Hinson WH, Evans GW, Schreiner PJ, Hagaman AP, Crouse JR., 3rd Evaluation of magnetic resonance imaging for quantification of intra-abdominal fat in human beings by spin-echo and inversion-recovery protocols. Am J Clin Nutr. 1995;62:297–301. doi: 10.1093/ajcn/62.2.297. [DOI] [PubMed] [Google Scholar]
- 44.Elbers JM, Haumann G, Asscheman H, Seidell JC, Gooren LJ. Reproducibility of fat area measurements in young, non-obese subjects by computerized analysis of magnetic resonance images. Int J Obes Relat Metab Disord. 1997;21:1121–9. doi: 10.1038/sj.ijo.0800525. [DOI] [PubMed] [Google Scholar]
- 45.Tokunaga K, Matsuzawa Y, Ishikawa K, Tarui S. A novel technique for the determination of body fat by computed tomography. Int J Obes. 1983;7:437–45. [PubMed] [Google Scholar]
- 46.Ferland M, Despres JP, Tremblay A, et al. Assessment of adipose tissue distribution by computed axial tomography in obese women: association with body density and anthropometric measurements. Br J Nutr. 1989;61:139–48. doi: 10.1079/bjn19890104. [DOI] [PubMed] [Google Scholar]
- 47.Schoen RE, Schragin J, Weissfeld JL, et al. Lack of association between adipose tissue distribution and IGF-1 and IGFBP-3 in men and women. Cancer Epidemiol Biomarkers Prev. 2002;11:581–6. [PubMed] [Google Scholar]
- 48.Yoshizumi T, Nakamura T, Yamane M, et al. Abdominal fat: standardized technique for measurement at CT. Radiology. 1999;211:283–6. doi: 10.1148/radiology.211.1.r99ap15283. [DOI] [PubMed] [Google Scholar]
- 49.Takami R, Takeda N, Hayashi M, et al. Body fatness and fat distribution as predictors of metabolic abnormalities and early carotid atherosclerosis. Diabetes Care. 2001;24:1248–52. doi: 10.2337/diacare.24.7.1248. [DOI] [PubMed] [Google Scholar]
- 50.Lemieux I, Pascot A, Lamarche B, et al. Is the gender difference in LDL size explained by the metabolic complications of visceral obesity? Eur J Clin Invest. 2002;32:909–17. doi: 10.1046/j.1365-2362.2002.01092.x. [DOI] [PubMed] [Google Scholar]
- 51.Sjostrand M, Gudbjornsdottir S, Holmang A, Lonn L, Strindberg L, Lonnroth P. Delayed transcapillary transport of insulin to muscle interstitial fluid in obese subjects. Diabetes. 2002;51:2742–8. doi: 10.2337/diabetes.51.9.2742. [DOI] [PubMed] [Google Scholar]
- 52.Hernandez-Ono A, Monter-Carreola G, Zamora-Gonzalez J, et al. Association of visceral fat with coronary risk factors in a population-based sample of postmenopausal women. Int J Obes Relat Metab Disord. 2002;26:33–9. doi: 10.1038/sj.ijo.0801842. [DOI] [PubMed] [Google Scholar]
- 53.Snel YE, Brummer RJ, Doerga ME, et al. Adipose tissue assessed by magnetic resonance imaging in growth hormone-deficient adults: the effect of growth hormone replacement and a comparison with control subjects. Am J Clin Nutr. 1995;61:1290–4. doi: 10.1093/ajcn/61.6.1290. [DOI] [PubMed] [Google Scholar]
- 54.Seidell JC, Oosterlee A, Thijssen MA, et al. Assessment of intra-abdominal and subcutaneous abdominal fat: relation between anthropometry and computed tomography. Am J Clin Nutr. 1987;45:7–13. doi: 10.1093/ajcn/45.1.7. [DOI] [PubMed] [Google Scholar]
- 55.Seidell JC, Bakker CJ, van der Kooy K. Imaging techniques for measuring adipose-tissue distribution–a comparison between computed tomography and 1.5-T magnetic resonance. Am J Clin Nutr. 1990;51:953–7. doi: 10.1093/ajcn/51.6.953. [DOI] [PubMed] [Google Scholar]
- 56.Dixon AK. Abdominal fat assessed by computed tomography: sex difference in distribution. Clin Radiol. 1983;34:189–91. doi: 10.1016/s0009-9260(83)80303-1. [DOI] [PubMed] [Google Scholar]
- 57.Weits T, van der Beek EJ, Wedel M, Ter Haar Romeny BM. Computed tomography measurement of abdominal fat deposition in relation to anthropometry. Int J Obes. 1988;12:217–25. [PubMed] [Google Scholar]
- 58.Ashwell M, Cole TJ, Dixon AK. Obesity: new insight into the anthropometric classification of fat distribution shown by computed tomography. Br Med J (Clin Res Ed) 1985;290:1692–4. doi: 10.1136/bmj.290.6483.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grauer WO, Moss AA, Cann CE, Goldberg HI. Quantification of body fat distribution in the abdomen using computed tomography. Am J Clin Nutr. 1984;39:631–7. doi: 10.1093/ajcn/39.4.631. [DOI] [PubMed] [Google Scholar]


