Abstract
Hearing loss in mammals is irreversible because cochlear neurons and hair cells do not regenerate. To determine whether we could replace neurons lost to primary neuronal degeneration, we injected EYFP-expressing embryonic stem cell–derived mouse neural progenitor cells into the cochlear nerve trunk in immunosuppressed animals 1 week after destroying the cochlear nerve (spiral ganglion) cells while leaving hair cells intact by ouabain application to the round window at the base of the cochlea in gerbils. At 3 days post transplantation, small grafts were seen that expressed endogenous EYFP and could be immunolabeled for neuron-specific markers. Twelve days after transplantation, the grafts had neurons that extended processes from the nerve core toward the denervated organ of Corti. By 64–98 days, the grafts had sent out abundant processes that occupied a significant portion of the space formerly occupied by the cochlear nerve. The neurites grew in fasciculating bundles projecting through Rosenthal’s canal, the former site of spiral ganglion cells, into the osseous spiral lamina and ultimately into the organ of Corti, where they contacted hair cells. Neuronal counts showed a significant increase in neuronal processes near the sensory epithelium, compared to animals that were denervated without subsequent stem cell transplantation. The regeneration of these neurons shows that neurons differentiated from stem cells have the capacity to grow to a specific target in an animal model of neuronal degeneration.
Keywords: regeneration, stem cell, transplantation, neuron, auditory, axon
INTRODUCTION
Replacement of degenerated peripheral or central neurons by stem cell–derived neurons requires differentiation of the stem cell to the appropriate phenotype and directed growth of projections to reestablish functional neural circuits. Neural regeneration from stem cells presents challenges in addition to the initial differentiation, such as overcoming apoptotic cell death, preventing rejection, and encouraging new neurons to grow processes from the cell to the host target. While differentiation of stem cells to neurons and survival of these cells after engraftment in a host have been demonstrated, it has been more difficult to demonstrate growth of neuronal processes from these neurons to defined targets.
Transplantation of adult and embryonic stem (ES) cells has led to proposals for new therapies for neurodegenerative disease (Lie et al., 2004; McKay, 2004) both within a species and across species (Deacon et al., 1994; Edge et al., 1998; Edge, 2000; Isacson et al., 2003). Growth of stem-cell–derived neurons to remake connections lost to degeneration is the next important step in realizing the capacity of these cells for regeneration and establishment of lost neural circuits. This will be necessary for the differentiation of neurons from stem cells to be translated into a cell therapy.
After degeneration of cochlear neurons, there are few options available that lead to regeneration. Sensorineural hearing loss can be caused by primary degeneration of spiral ganglion neurons, the sensory fibers connecting cochlear sensory cells (hair cells) with the brain (Varga et al., 2003). Cochlear neurons do not regenerate to any clinically significant extent (Carnicero et al., 2002; Sekiya et al., 2003), and even if the cell bodies and central axons survive, loss of hearing can be caused by degeneration of peripheral processes (Nadol, 1997). New protocols for regeneration of hair cells in patients with sensorineural hearing loss would also require an intact afferent innervation for function (Izumikawa et al., 2005). Stem cells are present in the inner ear and in spiral ganglion tissue (Li et al., 2003; Rask-Andersen et al., 2005) and are capable of giving rise to hair cells and neurons, but regeneration may not occur because the stem cell population decreases after birth (Oshima et al., 2006). Therefore, cell transplantation may be the best option for regeneration. Indeed, in in vitro experiments, spiral ganglion neurons, transplanted into the organ of Corti after degeneration of afferent neurons, form connections with hair cells and express synaptic markers (Martinez-Monedero et al., 2006).
In this article, we show that neural progenitors derived from murine ES cells, transplanted into the cochlea in a gerbil model with nearly complete loss of the afferent innervation of cochlear hair cells, survived and differentiated into neurons that appeared to respond to cues guiding neurite outgrowth by sending out processes toward denervated hair cells in the organ of Corti.
MATERIALS AND METHODS
Animals and Groups
Female gerbils, aged 8–20 weeks, were used. In each animal, the right ear was denervated by ouabain application to the round window (RW) niche under sterile conditions, while the left ear served as an untreated control. Animals were then divided into two groups. In one group (“grafted”; 12 animals), a second surgical approach to the inner ear was made 8 days after the denervation surgery, and neural progenitor cells were transplanted into the cochlear nerve trunk. The second group (“denervated”; 3 animals) did not undergo the cell injections. Both groups underwent cochlear function tests [compound action potentials (CAPs) and distortion product otoacoustic emissions (DPOEs)] before and immediately after the ouabain application. The “grafted” group underwent a second round of functional testing immediately before the cell injections. Grafted animals (3 groups of 4) were allowed to survive for 3, 12–24, or 64–98 days before harvesting cochlear tissues. All procedures were approved by the IACUC of the Massachusetts Eye and Ear Infirmary.
Cochlear Denervation
In gerbils, brief application of ouabain to the RW niche destroys most cochlear neurons without damage to hair cells (Schmiedt et al., 2002). For surgical procedures, gerbils were anesthetized with ketamine (25 mg/kg) and nembutal (40 mg/kg, intraperitoneal). Additional doses were given intramuscularly at half the starting dose when needed. Atropine (0.2 mg/kg, intramuscular) was given to reduce secretions. The gerbils were placed in a snout clamp in a heated room at 39°C. A postero-inferior skin incision was made in the retroauricular area of the right ear. The underlying muscles and the facial nerve were separated by blunt dissection to expose the middle compartment of the bulla. Fine forceps were used to make a small opening in the bulla, which was expanded with a hemostatic forcep to expose the RW niche. A 10 μL Hamilton syringe, filled with 1 mM ouabain, was directed toward the RW niche. The oubain solution (3–5 μL) was applied for 1 h, with exchange-refill at 30 min, and then removed by absorption and rinse. The bulla was covered with the underlying muscle and fascia, the incision was closed with nonabsorbable suture, and the animal was transferred to a homeothermic blanket at 39°C for the recovery period.
Cochlear Function Tests
Functional testing included measurement of DPOAEs and CAPs. DPOAEs are distortions created and amplified by the healthy inner ear, when two primary tones (f1 and f2) are presented, which can be recorded with a sensitive microphone in the ear canal. Here, we measure the distortion component at 2f1–f2. Anesthesia was performed as for the surgery. Detailed techniques are described elsewhere (Kujawa and Liberman, 1997; Maison et al., 2003). DPOAEs were run as amplitude vs level functions in 5 dB steps for test frequencies from 2.0 to 32 kHz (in half octave steps). Thresholds were defined as the stimulus level required to produce a response at 0 dB SPL. CAPs represent the summed activity of the cochlear nerve fibers, as recorded with a fine silver wire on the RW membrane in response to short tone pips (0.5 ms rise fall with cos2 shaping). CAP thresholds are defined as the sound pressure level required to produce a response of 10 μV, as determined by a computer-driven algorithm.
Neural Progenitor Cells for Transplant
Neural progenitor cells derived from murine ES cell YC5/EYFP were used for transplantation. YC5/EYFP cells (Hadjantonakis et al., 1998), a cell-line derived from the totipotent cell line R1 (Nagy et al., 1993), carry the gene for enhanced yellow fluorescent protein (EYFP) under control of a promoter composed of a cytomegalovirus enhancer coupled to the β-actin promoter. The ES cells were converted to neural progenitor cells by a procedure that expands the neural progenitors as adherent cultures on tissue culture dishes (Ying et al., 2003). The ES cells were cultured on adhesive culture dishes in 50%DMEM/50%F12 supplemented with N2 and B27, ampicillin and bFGF to form enriched progenitor cells. The YC5 cells remained fluorescent under these conditions. On the day of transplant, cells were dissociated by incubation with trypsin–EDTA for 5–10 min to single cells and small aggregates, and were then resuspended in DMEM/F12 with N2 and B27 supplements and ampicillin at 3–4 × 107/mL and stored on ice until transplantation.
Cell Injections
The RW niche was reexposed as described earlier, and a microdissecting needle (RS-6130 Roboz Surgical) used to expose the cochlear nerve trunk via a 200 μm opening in the bone separating the nerve from the floor of the RW niche. A 10 μL Hamilton syringe coupled to a micropipet (tip diameter: 100 μm) was placed in a micromanipulator and inserted 1.5 mm into the opening. A volume of 5 μL, containing 175,000 cells, was injected: half at the 1.5 mm depth and the rest after retracting the electrode by 1 mm. The incision was closed as above and the animal transferred to the homeothermic blanket. To prevent cell rejection, cyclosporine A (10 mg/kg subcutaneous) was given daily from the day of transplantation until the end of the experiment.
Histological Processing
Animals were cardio-perfused with either 4% paraformaldehyde in PBS (for immunostaining) or with 2.5% glutaraldehyde and 1.5% paraformaldehyde (for plastic embedding). The cochlea was dissected and, after opening the round and oval windows, postfixed overnight. Cochleas were then decalcified in 0.12 M EDTA at room temperature for 1 week. For plastic embedding, cochleas were incubated with 1% osmium tetroxide for 60 min, then dehydrated, embedded in araldite and sectioned at 40 μm on a Historange.
Cochleas to be immunostained were transferred to 30% sucrose at 4°C overnight, and embedded in OTC for cryo-sectioning. Serial sections (16 μm) were mounted on Ultra-Stick Gold Seal glass slides (Becton, Dickson), and stored at −80°C. After an initial wash with PBS, the sections were incubated with PBT1 (PBS, 0.1% Triton-100, 1%BSA (w/v), 5% heat-inactivated goat serum) for 5 min, followed by incubation with primary antibodies in PBT1 overnight at 4°C. Concentrations used were 1:500 for monoclonal antibody to β-III tubulin (TuJ antibody, Covance); 1:200 for rabbit polyclonal antibody to neurofilament M (145 kD) (Chemicon International); 1:500 for rabbit polyclonal antibody to glial fibrillary acidic protein (Sigma); 1:50 for monoclonal antibody to oligodendrocyte marker O4 (Chemicon); 1:10 for rat monoclonal antibody M2 (Pollack et al., 1992) (Developmental Studies Hybridoma Bank, Iowa City, IA); and 1:2000 for rabbit polyclonal antibody to parvalbumin 3 (Heller et al., 2002). The parvalbumin 3 antibody is specific for the chick protein but reacts with oncomodulin in mouse hair cells and a parvalbumin of lower molecular weight in spiral ganglion neurons that can be distinguished on Western blots (Heller et al., 2002). Sections were then washed 2 times for 15 min each with PBT1 followed by 2 washes with PBT2 (PBS, 0.1% Triton-100, 0.1% BSA (w/v)). FITC, TRITC, and Cy5 conjugated anti-rabbit and anti-mouse secondary antibodies (Jackson ImmunoResearch) were used to detect primary antibodies. Cell nuclei were stained by exposure to 4,6-diamidino-2-phenylindole with Vectashield (Vector Laboratories). Specimens were examined on a Zeiss Axioskop 2 MOT fluorescence microscope or by confocal microscopy (TCS; Leica). Quantification of neurons in the cochlear sections was performed in sections that covered the cochlea by counting neurons in the basal, middle, and apical turns in every fifth section (~25 sections per ear) in the control, denervated, and grafted groups.
RESULTS
Removal of Spiral Ganglion Neurons
We used a local application of ouabain to remove the afferent innervation of the cochlea in the gerbil (Schmiedt et al., 2002; Lang et al., 2005). Under optimal conditions, ouabain treatment destroyed the type I spiral ganglion neurons in the basal, middle, and apical turns of the cochlea. The spiral ganglion neuron cell bodies and dendrites were stained by neurofilament antibody [Fig. 1(a)], and the hair cells and neuron cell bodies were stained by parvalbumin 3 antibody [Fig. 1(b)] in the untreated cochlea, whereas the spiral ganglion was devoid of type I cells in the treated cochlea [Fig. 1(d,e)]. The remaining cells were type II spiral ganglion cells as described for this model (Schmiedt et al., 2002; Lang et al., 2005). Inner and outer hair cells were left intact, as shown by parvalbumin 3 staining in both control and denervated gerbils [Fig. 1(b,e)]. Osmium-stained sections showed an empty space in the area normally occupied by the nerve (see Supplementary Material).
Figure 1.
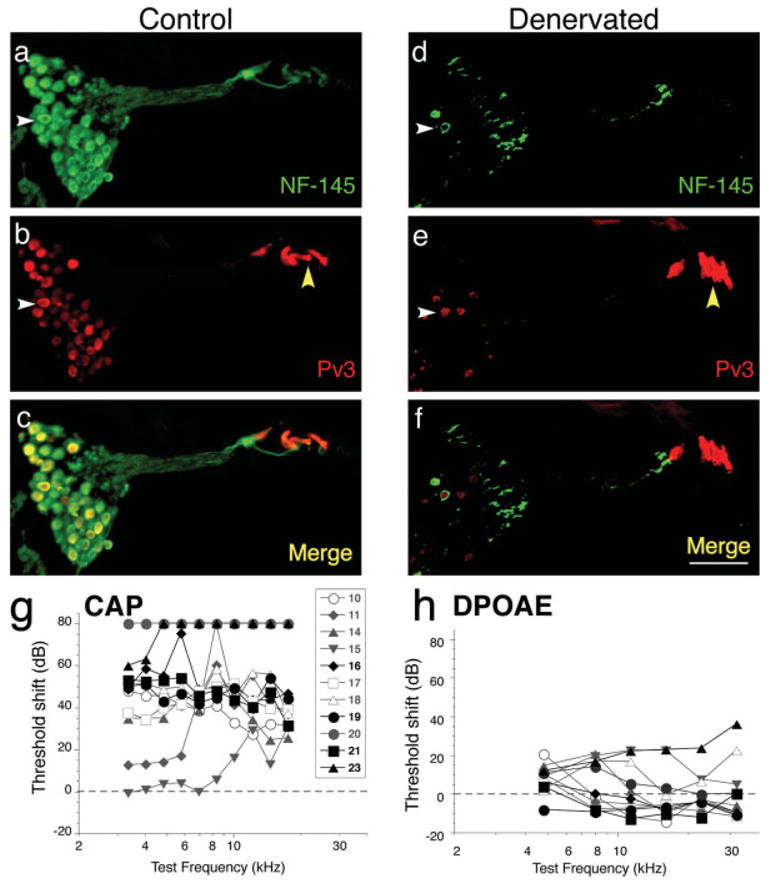
Denervation of the gerbil cochlea. Cochlear sections from a control ear (a–c) and a ouabain-treated ear (d–f; referred to as denervated in all figures) show primary neuronal degeneration 8 days after treatment. Sections were stained by immunohistochemistry for neurofilament (green) and parvalbumin 3 (red). Neurofilament-positive spiral ganglion neurons are plentiful in Rosenthal’s canal in the control ear (white arrowhead in a) but are almost completely absent in the denervated ear (white arrowhead in d). Parvalbumin 3 also stains most neuronal cell bodies (white arrowhead in b), as well as the hair cells (yellow arrowhead in b). In the section from the denervated ear, hair cells remain intact (yellow arrowhead in e) but the spiral ganglion neurons are almost completely absent (white arrowhead in e). Merged images are shown (c and f) and the scale bar in f is 80 μm and applies to a–f. (g and h): Compound action potential (CAP) thresholds (g) were significantly elevated, consistent with massive loss of spiral ganglion cells, whereas distortion product otoacoustic emissions (DPOAEs) were close to normal (h), indicating that cochlear hair cells remained healthy. Data from eleven cases are shown; the symbol key in g also applies to h. Highlighted black symbols in g and h are the cases that received long-term grafts. Threshold shift for each measure was computed by subtracting the data from each case from the mean values for all ears obtained before the ouabain treatment. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
The selective neural effects of the ouabain treatment were confirmed by measurement of DPOAEs and CAPs from gerbils before and after treatment [Fig. 1(g,h)]. DPOAEs are acoustic distortions, measurable in the ear canal when two stimulus tones are presented. These DPOAEs are created and amplified by the hair cells in the normal inner ear. After ouabain treatment, the DPOAE thresholds were nearly unchanged, consistent with maintenance of normal hair cell function. By contrast, the CAP thresholds were always elevated after ouabain treatment. The CAP represents the summed sound-evoked activity of spiral ganglion cells, and the observed 40–60 dB threshold elevations are consistent with massive loss of cochlear neurons (Kujawa and Liberman, 1997). Smaller threshold shifts in two of the animals were seen at low frequency and correlated with incomplete removal of neurons in the apical turn in those cases. These animals were not used for the analysis of neural progenitor engraftment shown in the figures or for the quantitative analysis of engraftment.
Transplantation of Neural Progenitors and Survival of Grafts
The progenitor cells obtained from YC5/EYFP cells were positive for neural lineage markers, Sox1 and nestin (Lee et al., 2000; Ying et al., 2003). An aliquot of cells from each batch used for transplantation was differentiated, in vitro, into cells that were positive for neuronal (β-III tubulin), glial (GFAP), and oligodendrocyte (O4) markers (Ying et al., 2003) as a test for their pluripotency.
The undifferentiated neural progenitors were transplanted into the denervated cochleas 8 days after ouabain treatment. Four animals were sacrificed at 3 days after transplantation to examine short-term grafts for survival of cells and integration into the host tissue. In all animals, cells expressing EYFP were found in the expected position in the cochlear nerve trunk near the basal or middle turns of the cochlea [Fig. 2(a)]. Graft identification was unambiguous, both because the endogenous EYFP expression in the murine-cell implants is not shared by any endogenous cells of the gerbil cochlea, and because nerve cell clusters are not normally present in this distal region of the cochlear nerve trunk in the gerbil. The recovery of cells in the grafts was an average of 13.6% of the transplanted cells. Short neurites that were labeled by an antibody to the neuronal marker, β-III tubulin (4.8% of EYFP positive cells), were elaborated by the grafts at this time point [Fig. 2(b)].
Figure 2.
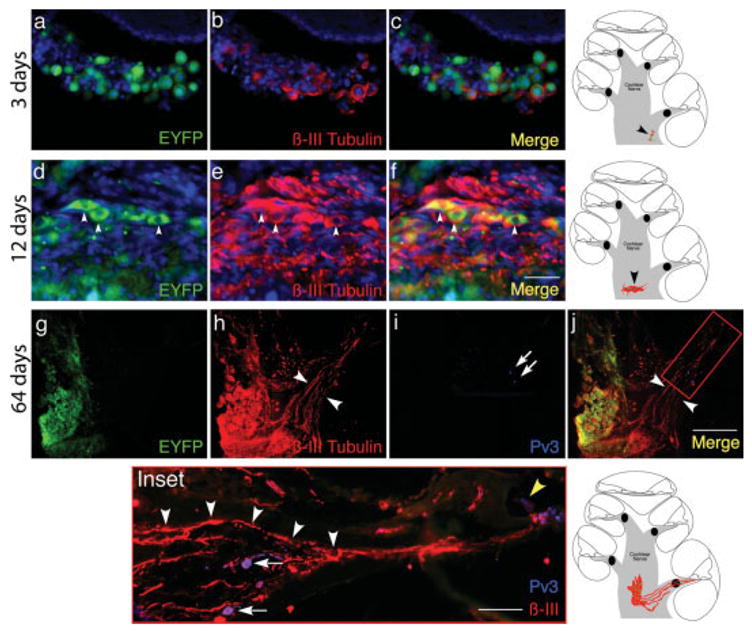
Grafted neural progenitors in the gerbil cochlea at 3, 12, and 64 days after transplantation. Neural progenitor cells were engrafted into a denervated ear 8 days after ouabain treatment. In a–c the ear is shown 3 days after transplantation. The grafted cells, identified by endogenous fluorescence (EYFP; green), are surrounded by a small number of βIII-tubulin positive neurites (red). The graft was detected near the basal turn, at a position indicated (black arrowhead) in the schematic at right of c. Nuclei were visualized by DAPI staining (a–f; blue). Scale bar is 10 μm and applies to a–f. In d, mouse neural progenitor cells engrafted in the gerbil cochlea are shown at 12 days after transplantation. The cells expressing endogenous fluorescence (arrowheads in d; EYFP) and neuronal markers (arrowheads in e; βIII tubulin) remain in the nerve trunk, where they were injected, surrounded by abundant βIII tubulin immunopositive neurites. In the merged image (f), the cell bodies appear yellow because of overlap between EYFP and βIII tubulin staining (arrowheads in f). The position of the graft in the cochlear nerve trunk is indicated (black arrowhead) in the schematic to the right of f. New neurites stream from the EYFP-positive graft, through Rosenthal’s canal (white arrowheads in h and j) and the osseous spiral lamina and into the organ of Corti after 64 days (g–j) in low-power images of the graft region near the basal turn (immunostaining for each channel as indicated). The Inset shows a high-power view of the area indicated in the merged image: two surviving spiral ganglion cells (Pv3-positive; white arrows in i and Inset) can be seen, surrounded by radially directed neurites (Inset, white arrowheads), which do not connect to ganglion cell bodies, and appear directed to the organ of Corti where they appear to contact the hair cells (yellow arrowhead in Inset). The position of the graft is shown in the schematic at right of Inset. Scale bar in the merged image (200 μm) applies to g–j; in the inset, the scale bar is 50 μm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Grafts in four other denervated gerbils were examined at 12–24 days post implantation (average recovery of 10.3% of transplanted cells). Implanted cells in all four cases were β-III tubulin positive (12.5% of EYFP positive cells), suggesting they had differentiated to neurons [Fig. 2(e)] and the β-III tubulin-positive processes had lengths up to 32.8 μm whereas the longest process measured at 3 days was 19.6 μm. The grafted cells could be identified by their ectopic position in the cochlear nerve trunk and by their EYFP expression [Fig. 2(f)]. The outgrowth of neurites was most pronounced in areas of the graft adjacent to the osseous spiral lamina, where they extended away from the graft toward the organ of Corti [as shown in the schematic diagram to Fig. 2(f)].
Neuronal Processes Extended from the Graft into the Organ of Corti at 64–98 days
Substantial neural differentiation was observed in the ears transplanted with neural progenitors after 64 days. The grafts were found in the cochlear nerve trunk near the basal and/or middle turns of the cochlea [Fig. 2(g–j)] in four animals. Most strikingly, the abundant processes had a clear preferred direction of growth, with the bundles projecting towards Rosenthal’s canal, the bony channel which previously encased the spiral ganglion cells. These neural processes were derived from numerous EYFP-positive graft cells that had sprouted and sent out several branches of β-III tubulin-positive fasciculating neurites that grew in the direction of the organ of Corti, exiting the modiolus to enter Rosenthal’s canal [Fig. 2(h)] and had processes with continuous β-III-tubulin staining for as long as 431 μm. The neurites traversed Rosenthal’s canal, where few spiral ganglion cell bodies remained [Fig. 2(j)], and continued growing through the osseous spiral lamina toward the hair cells of the organ of Corti (Fig. 2 Inset, white arrowheads). It appeared that the projections followed the pathways remaining from degenerated neurons and responded to cues that guided them to the organ of Corti, possibly emanating from the surviving supporting cells or hair cells seen in Figure 1.
To confirm that the neurites emanating from EYFP-positive cell bodies in the graft were of mouse origin (because the processes lost EYFP staining during differentiation) the neurites were examined by staining with mouse-specific antibody M2 [Fig. 3(a)]. The anti-body was negative on control gerbil tissue and positive on control mouse tissue (see Supplementary Material). Neurons staining for both β-III-tubulin and M2 were observed in sections from the cochlea [Fig. 3(a)] that were directly adjacent to the section containing the graft shown in Figure 2(h). The cell bodies in the nerve trunk that had been identified as grafted neurons and the fibers emanating from them [Fig. 2(g,h)] were clearly of mouse origin [Fig. 3(c)] based on staining with this antibody. Cell bodies positive for both mouse M2 and β-III-tubulin were identified in orthogonal projections of the confocal image [Fig. 3(f)]. These mouse progenitor cell-derived neurons and their processes were positive for expression of peripherin [Fig. 3(g–i)], a marker of sensory neurons that indicated appropriate differentiation of the cells.
Figure 3.
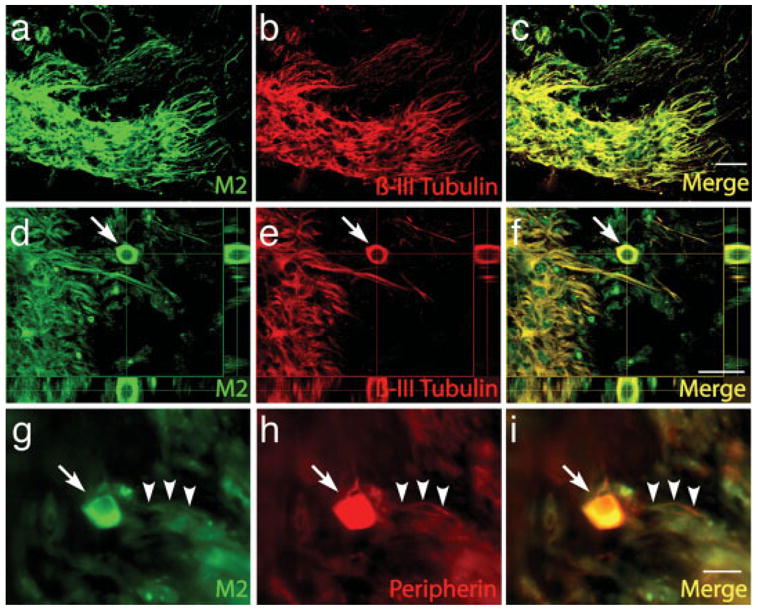
Immunostaining with M2 antibody demonstrates mouse origin of neurons in the nerve trunk. To identify mouse cells in the graft from a gerbil at 64 days after transplantation that had been analyzed for βIII-tubulin [Fig. 2(h)], sections of cochlea adjacent to the graft were immunostained with mouse-specific antibody M2 (a). Neurons in the graft stained for both βIII-tubulin (b) and M2 which positively identified the neurons as grafted cells derived from mouse progenitor cells. Costaining with both antibodies is shown in c. Orthogonal projections (d–f) of confocal images show cell bodies (arrow) labeled with both M2 (d) and βIII-tubulin antibody (e). Costaining of neurons for M2 and peripherin (g–i), a sensory marker, shows that cell bodies (arrow) and fibers (arrowheads) are stained with both antibodies. Scale bars are 20μm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
In one case examined at 98 days post transplantation, the graft was examined by osmium-stained plastic sections (see Fig. 4) which showed that the graft filled a large part of the cochlear nerve trunk [Fig. 4(a–c)]. In serial sections, we followed the graft from the base to the middle turns of the cochlea [Fig. 4(a–c)], and neurites could be seen extending toward the middle turn [Fig. 4(b)]. The grafted cells remained in the site of the former nerve trunk; none appeared to have migrated into Rosenthal’s canal. The neuronal processes from the grafts could be seen to extend out of the graft in the nerve trunk [Fig. 4(b1,c1)] and into the osseous spiral lamina toward the organ of Corti. No evidence of cellular infiltrate in the vicinity of the graft was seen.
Figure 4.
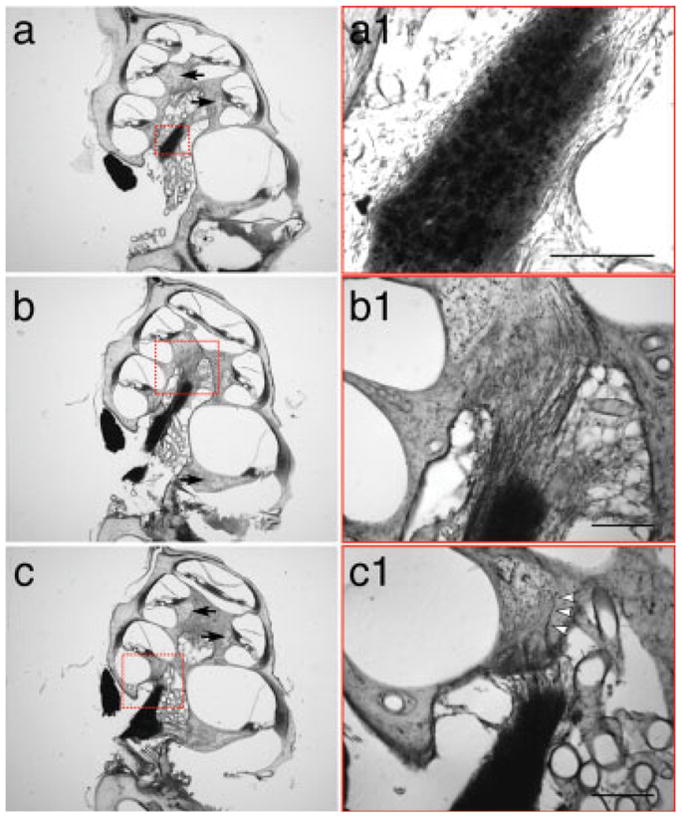
Morphology of grafted mouse cells in the denervated gerbil cochlea 98 days after injection. Each row shows a pair of images: low-power at the left; higher power view of the area indicated on the right. In osmium-stained sections, the cell bodies of the grafted neurons are visible in the middle of the nerve trunk, at 98 days post transplantation (a and a1), while neuronal processes are seen streaming into Rosenthal’s canal of the basal turn (c and c1) and the middle turn (b and b1). Individual fibers can be seen penetrating the osseous spiral lamina (arrowheads in c1). The low power views show that there are almost no ganglion cells remaining in Rosenthal’s canal in any of the cochlear turns (black arrows in a–c). Each column shows a different section through the graft. Scale bar in insets a1–c1 are 200 μm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
We attempted to remeasure the thresholds for DPOAEs and CAPs in all these long-surviving grafted animals. Thresholds for both measures were extremely high in all cases. Subsequent gross dissection revealed that thick scar tissue in the RW niche at the injection site had grown over the RW membrane, thus impeding sound transmission into the cochlear fluids.
Increased Projections in the Cochleas of Grafted Animals
In denervated cases with 64–98 day survival, analysis of cochlear sections stained with β-III tubulin or neurofilament antibodies or osmium revealed a significant increase in the numbers of axons projecting to the organ of Corti in grafted vs. denervated animals. In midcochlear sections through the ganglion, control ears showed abundant cell bodies [Fig. 5(a)], and denervated ears showed few [Fig 5(b)]. Grafted ears also had few cell bodies [Fig. 5(c)], but there was a clear increase in the number of radially directed axonal processes. These processes must originate from the ectopic grafts in the cochlear nerve trunk, pass through the ganglion region without making connections to other cells and continue on toward the hair cells. In midcochlear sections through the osseous spiral lamina, denervated ears had very few fibers [Fig. 5(e)] compared to controls [Fig. 5(d)], whereas, in grafted ears, a large number of neurites appeared to course toward the organ of Corti [Fig. 5(f)]. In the cochlear sections shown in Figure 5(g–i), fibers in the osseous spiral lamina are cut in cross section. Examination of these “tangential” sections also showed abundant neuronal processes in control ears [Fig. 5(g)], sharply reduced numbers in denervated ears [Fig. 5(h)] and an increase in the grafted cases [Fig. 5(i)].
Figure 5.
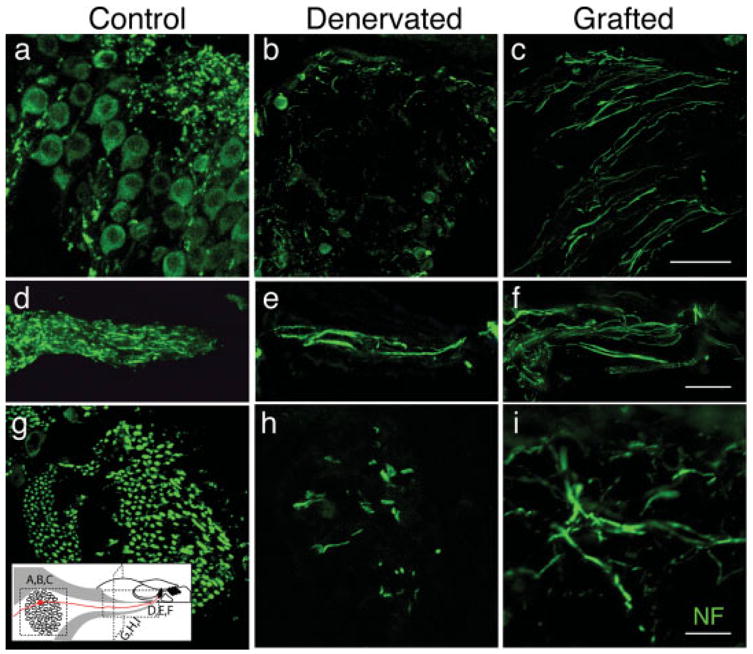
Immunostaining of sections from control ears (Control), denervated ears (Denervated), and denervated ears grafted with mouse neural progenitors (Grafted). Sections were from three sites in the osseous spiral lamina and Rosenthal’s canal of the basal turn and were immunostained for neurofilament (green) to visualize neuronal processes. a–c show the spiral ganglion in midcochlear sections, where it is cut in cross section. d–f show the osseous spiral lamina in midcochlear sections, where axons are cut longitudinally. g–i show tangential sections through the cochlear spiral where the axons in the osseous spiral lamina are normally cut in cross section. Section orientations are indicated in the schematic at the lower left. Although reduced compared to controls, grafted ears (64 days posttransplant) show a clear increase in fibers as compared to denervated ears in spiral ganglion cross sections (c vs b), in longitudinal view (f vs e) and in tangential sections (i vs h). Scale bars are 20 μm. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Using immunostained midcochlear sections, we counted neuronal processes in apical, middle and basal turns from three control, three denervated, and four grafted ears (64–98 days posttransplant). The quantitative analysis (see Fig. 6) showed (1) that the initial chemical denervation eliminated 85–95% of axons exiting from the organ of Corti, and (2) the subsequent grafting of neural progenitors increased axonal numbers in the basal turn by more than 8-fold and in middle and apical and turns by ~2-fold. These increases in mean axonal numbers between denervated and grafted ears were highly significant (p < 0.01). The smaller increases in mean neuronal counts in middle and apical turns were not statistically significant. The enhanced reinnervation of the basal turn correlated with the position of most of the cell implants in the basal turn (see Fig. 2). Using tangential cochlear sections, we rechecked the axonal counts for the basal turn and found a ~4-fold increase in neuronal counts in the grafted ears, which was highly significant (data not shown).
Figure 6.
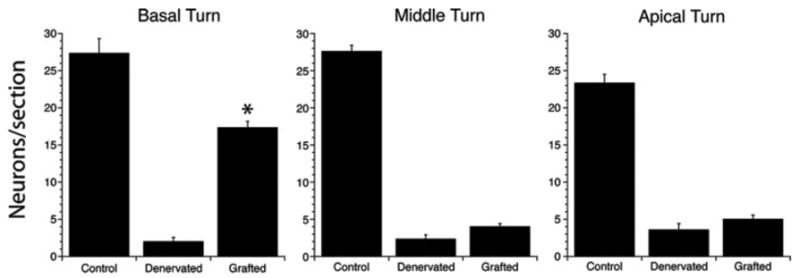
Counts of neurites in the engrafted animals as compared to control and denervated animals without grafts. The counts are from the osseous spiral lamina in antineurofilament (NF) stained sections, one of which is shown for each group in Figure 5(d–f). In each of 3 untreated ears (Control), 3 denervated/ungrafted ears (Denervated), and 4 denervated/grafted ears (Grafted), all NF-positive fibers were counted in the basal, middle and apical turns. Group means and standard errors are shown. Group differences that were statistically significant are indicated by asterisks. Neurite counts show striking increases in the basal turn in grafted compared to denervated animals. The denervated ears were assessed at 8 and 106 days post-ouabain and gave similar counts at each time point; the grafted ears were implanted with cells 8 days postouabain and analyzed 64 and 98 days postgraft.
DISCUSSION
We have investigated in this study whether ES cell–derived neurons could survive and regrow neural processes to the sensory epithelium of the organ of Corti after transplantation into animals in which the cochlea’s afferent innervation from spiral ganglion neurons had been removed. Neurons developed from neural progenitor cells engrafted in the animals, and neurites from these cells projected into the organ of Corti. The regrowth appeared to be directed toward the original neuronal targets in the sensory epithelium and may have been directed by factor(s) from the denervated sensory epithelium or the degenerated spiral ganglion neurons.
The neuronal targeting may be attributed to the environment of the inner ear, which could provide both differentiation signals and guidance cues to the developing neural progenitors. The injected cells are derived from ES cells, and are injected at a progenitor stage, having committed to becoming neural cells, but prior to taking on the characteristics of differentiated neurons. Their ability to integrate into the ear and respond to the denervated cochlear environment suggests that the neural progenitors that we used were at a developmental stage that retained the plasticity to respond to these potential signals from the immediate environment. These cells are more likely to be responsive to cues in the host ear than differentiated neurons (Hu et al., 2005a) that did not grow neurites toward hair cells when transplanted into the inner ear and neural stem cells that did not show evidence of neurite outgrowth toward the hair cells in the inner ear of guinea pigs (Hu et al., 2005b). The cues guiding axon growth might also be interpreted best by progenitor cells rather than mature neurons since molecules that guide axon growth by binding to receptors on growth cones are expressed in the embryo (Brors et al., 2003; Tessarollo et al., 2004). Our attempts to assess functional recovery in the grafted ears were unsuccessful due to scar tissue at the site of the surgery, and we plan to use an antiinflammatory agent in combination with immunosuppressive drugs to allow functional recordings of engrafted cochleas in future studies.
Directed growth of a neuron to a defined target is a sought-after goal in transplantation therapies, in addition to the necessary cell survival and differentiation and lack of rejection. The ability of grafted ES-cell–derived progenitors to survive in the inner ear and send neurites into the organ of Corti demonstrated that neurons derived from these cells could grow toward a defined target. Growth of motor axons after conversion of mouse ES cells to neurons and implantation into the spinal cord of embryonic chick was shown to be specific to muscle targets where the cells formed nerve terminals (Wichterle et al., 2002), but this type of demonstration of targeted growth from ES cells has generally been difficult to achieve. With dopaminergic neurons, numerous demonstrations of neuronal survival (Deacon et al., 1997; Bjorklund et al., 2003) and functional improvement in Parkinson’s disease models have been achieved, and have included use of ES cells as precursors, but this has usually been without demonstration of precise regrowth of neurons to reconstitute the original path to the striatal targets (Edge et al., 1998; Baker et al., 2000; Isacson et al., 2003; Baker and Mendez, 2005). In the retina, immature neurons display targeted growth in a transplant model (Kinouchi et al., 2003), but ES-cell–derived neurons have not been shown to regenerate specific pathways. The inner ear provides an anatomical situation that is well-suited for the visualization of neuronal growth to a target by a defined route, and our observation of directed growth of these neurites to the organ of Corti suggests that these cells could be responding to environmental cues, although this remains to be determined experimentally.
The cell bodies of the engrafted neurons were within the track normally occupied by spiral ganglion central processes, where they come together to form the auditory nerve trunk. Neuronal cell bodies are not normally found in the nerve trunk, but, rather, peripheral to the nerve trunk, in Rosenthal’s canal, where the spiral ganglion is formed by the somata of these neurons. The ectopic location of the grafts in combination with the EYFP and the mouse-specific antibody that stained the neurons made the identification of the transplanted cells straightforward and allowed us to trace the path subsequently taken by the nerve fibers from the grafted cells. The new fibers grew to the site of the original cell bodies and continued from there toward the hair cells, and, therefore, the neurites were not following a path used in the developing animal. The growth of processes from the cell bodies in an apical direction along the axis of the cochlear nerve tract, and in fasciculating bundles of neurites that left the nerve trunk and grew in a radial direction to Rosenthal’s canal, was apparent in four of four animals examined at the longest time points. It is likely that these neurites responded to a tropic factor emanating from hair cells after first passing through Rosenthal’s canal. The structure imposed by the osseous spiral lamina could also play a role in selecting a path for the neurons as they exit the bone into the organ of Corti.
Replacement of spiral ganglion neurons and regeneration of synapses between the new neurons and hair cells is a key first step toward functional repair of primary neuronal degeneration in the cochlea. Replacement of the neurons is the best option for recovery of these connections because of the lack of regeneration by endogenous cells, and this is consistent with the finding that stem cell numbers in the spiral ganglion decrease after birth (Oshima et al., 2006). We have recently shown that grafted neurons grow to hair cells and express synaptic markers at points of contact with hair cells in cultures of the organ of Corti in vitro (Martinez-Monedero et al., 2005). That result is encouraging for attempts to replace auditory neurons because it shows that synaptogenesis with hair cells is possible after regrowth of neuronal processes. Our current results are the first to show this regrowth in vivo and augments the in vitro observation of growth toward hair cells (Kondo et al., 2005; Martinez-Monedero et al., 2005; Matsumoto et al., 2005) and formation of contacts with expression of synaptic markers (Martinez-Monedero et al., 2005). The next step will be to examine extensions to the brainstem and to determine whether the regeneration of synaptic contacts at the hair cell and in the cochlear nucleus results in a functional improvement in animals deafened by ouabain-induced primary neuronal degeneration.
Supplementary Material
Acknowledgments
Contract grant sponsor: National Institute on Deafness and Other Communicative Disorders (NIDCD); contract grant numbers: F33 DC006789, RO1 DC007174, DC006167, DC00188, P30 DC05209; Hamilton H. Kellogg and Mildred H. Kellogg Charitable Trust and the Falk Trust.
Footnotes
This article contains supplementary material available via the Internet at http://www.interscience.wiley.com/jpages/0022-3034/suppmat.
References
- Baker KA, Mendez I. Long distance selective fiber outgrowth of transplanted hNT neurons in white matter tracts of the adult rat brain. J Comp Neurol. 2005;486:318–330. doi: 10.1002/cne.20477. [DOI] [PubMed] [Google Scholar]
- Baker KA, Sadi D, Hong M, Mendez I. Simultaneous intrastriatal and intranigral dopaminergic grafts in the parkinsonian rat model: Role of the intranigral graft. J Comp Neurol. 2000;426:106–116. [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB, Brundin P, Stoessl AJ, Freed CR, Breeze RE, Levivier M, et al. Neural transplantation for the treatment of Parkinson’s disease. Lancet Neurol. 2003;2:437–445. doi: 10.1016/s1474-4422(03)00442-3. [DOI] [PubMed] [Google Scholar]
- Brors D, Bodmer D, Pak K, Aletsee C, Schafers M, Dazert S, Ryan AF. EphA4 provides repulsive signals to developing cochlear ganglion neurites mediated through ephrin-B2 and -B3. J Comp Neurol. 2003;462:90–100. doi: 10.1002/cne.10707. [DOI] [PubMed] [Google Scholar]
- Carnicero E, Knipper M, Tan J, Alonso MT, Schimmang T. Herpes simplex virus type 1-mediated transfer of neurotrophin-3 stimulates survival of chicken auditory sensory neurons. Neurosci Lett. 2002;321:149–152. doi: 10.1016/s0304-3940(01)02501-0. [DOI] [PubMed] [Google Scholar]
- Deacon T, Schumacher J, Dinsmore J, Thomas C, Palmer P, Kott S, Edge A, et al. Histological evidence of fetal pig neural cell survival after transplantation into a patient with Parkinson’s disease. Nat Med. 1997;3:350–353. doi: 10.1038/nm0397-350. [DOI] [PubMed] [Google Scholar]
- Deacon TW, Pakzaban P, Burns LH, Dinsmore J, Isacson O. Cytoarchitectonic development, axon-glia relationships, and long distance axon growth of porcine striatal xenografts in rats. Exp Neurol. 1994;130:151–167. doi: 10.1006/exnr.1994.1194. [DOI] [PubMed] [Google Scholar]
- Edge AS. Current applications of cellular xenografts. Transplant Proc. 2000;32:1169–1171. doi: 10.1016/s0041-1345(00)01170-2. [DOI] [PubMed] [Google Scholar]
- Edge AS, Gosse ME, Dinsmore J. Xenogeneic cell therapy: Current progress and future developments in porcine cell transplantation. Cell Transplant. 1998;7:525–539. doi: 10.1177/096368979800700603. [DOI] [PubMed] [Google Scholar]
- Hadjantonakis AK, Gertsenstein M, Ikawa M, Okabe M, Nagy A. Generating green fluorescent mice by germline transmission of green fluorescent ES cells. Mech Dev. 1998;76:79–90. doi: 10.1016/s0925-4773(98)00093-8. [DOI] [PubMed] [Google Scholar]
- Heller S, Bell AM, Denis CS, Choe Y, Hudspeth AJ. Parvalbumin 3 is an abundant Ca2+ buffer in hair cells. J Assoc Res Otolaryngol. 2002;3:488–498. doi: 10.1007/s10162-002-2050-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z, Ulfendahl M, Olivius NP. NGF stimulates extensive neurite outgrowth from implanted dorsal root ganglion neurons following transplantation into the adult rat inner ear. Neurobiol Dis. 2005a;18:184–192. doi: 10.1016/j.nbd.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Hu Z, Wei D, Johansson CB, Holmstrom N, Duan M, Frisen J, Ulfendahl M. Survival and neural differentiation of adult neural stem cells transplanted into the mature inner ear. Exp Cell Res. 2005b;302:40–47. doi: 10.1016/j.yexcr.2004.08.023. [DOI] [PubMed] [Google Scholar]
- Isacson O, Bjorklund LM, Schumacher JM. Toward full restoration of synaptic and terminal function of the dopaminergic system in Parkinson’s disease by stem cells. Ann Neurol. 2003;53(Suppl 3):S135–S146. S146–S138. doi: 10.1002/ana.10482. [DOI] [PubMed] [Google Scholar]
- Izumikawa M, Minoda R, Kawamoto K, Abrashkin KA, Swiderski DL, Dolan DF, Brough DE, et al. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat Med. 2005;11:271–276. doi: 10.1038/nm1193. [DOI] [PubMed] [Google Scholar]
- Kinouchi R, Takeda M, Yang L, Wilhelmsson U, Lundkvist A, Pekny M, Chen DF. Robust neural integration from retinal transplants in mice deficient in GFAP and vimentin. Nat Neurosci. 2003;6:863–868. doi: 10.1038/nn1088. [DOI] [PubMed] [Google Scholar]
- Kondo T, Johnson SA, Yoder MC, Romand R, Hashino E. Sonic hedgehog and retinoic acid synergistically promote sensory fate specification from bone marrow-derived pluripotent stem cells. Proc Natl Acad Sci USA. 2005;102:4789–4794. doi: 10.1073/pnas.0408239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa SG, Liberman MC. Conditioning-related protection from acoustic injury: Effects of chronic deefferentation and sham surgery. J Neurophysiol. 1997;78:3095–3106. doi: 10.1152/jn.1997.78.6.3095. [DOI] [PubMed] [Google Scholar]
- Lang H, Schulte BA, Schmiedt RA. Ouabain induces apoptotic cell death in type I spiral ganglion neurons, but not type II neurons. J Assoc Res Otolaryngol. 2005;6:63–74. doi: 10.1007/s10162-004-5021-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Lumelsky N, Studer L, Auerbach JM, McKay RD. Efficient generation of midbrain and hindbrain neurons from mouse embryonic stem cells. Nat Biotechnol. 2000;18:675–679. doi: 10.1038/76536. [DOI] [PubMed] [Google Scholar]
- Li H, Liu H, Heller S. Pluripotent stem cells from the adult mouse inner ear. Nat Med. 2003;9:1293–1299. doi: 10.1038/nm925. [DOI] [PubMed] [Google Scholar]
- Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: New strategies for central nervous system diseases. Annu Rev Pharmacol Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- Maison SF, Emeson RB, Adams JC, Luebke AE, Liberman MC. Loss of α-CGRP reduces sound-evoked activity in the cochlear nerve. J Neurophysiol. 2003;90:2941–2949. doi: 10.1152/jn.00596.2003. [DOI] [PubMed] [Google Scholar]
- Martinez-Monedero R, Corrales CE, Cuajungco M, Heller S, Edge ASB. Reinnervation of hair cells by auditory neurons after selective removal of spiral ganglion neurons. J Neurobiol. 2006;66:319–331. doi: 10.1002/neu.20232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Nakagawa T, Higashi T, Kim TS, Kojima K, Kita T, Sakamoto T, et al. Innervation of stem cell-derived neurons into auditory epithelia of mice. Neuroreport. 2005;16:787–790. doi: 10.1097/00001756-200505310-00001. [DOI] [PubMed] [Google Scholar]
- McKay RD. Stem cell biology and neurodegenerative disease. Philos Trans R Soc Lond B Biol Sci. 2004;359:851–856. doi: 10.1098/rstb.2004.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadol JB., Jr Patterns of neural degeneration in the human cochlea and auditory nerve: Implications for cochlear implantation. Otolaryngol Head Neck Surg. 1997;117:220–228. doi: 10.1016/s0194-5998(97)70178-5. [DOI] [PubMed] [Google Scholar]
- Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci USA. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima K, Corrales CE, Grimm C, Senn P, Martinez-Monedero R, Geleoc GSG, Edge A, et al. Differential distribution of stem cells in the auditory and vestibular organs of the inner ear. 2006 doi: 10.1007/s10162-006-0058-3. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack IF, Lee LH, Zhou HF, Lund RD. Long-term survival of mouse corpus callosum grafts in neonatal rat recipients, and the effect of host sensitization. J Neurosci Res. 1992;31:33–45. doi: 10.1002/jnr.490310106. [DOI] [PubMed] [Google Scholar]
- Rask-Andersen H, Bostrom M, Gerdin B, Kinnefors A, Nyberg G, Engstrand T, Miller JM, et al. Regeneration of human auditory nerve. In-vitro/in video demonstration of neural progenitor cells in adult human and guinea pig spiral ganglion. Hear Res. 2005;203:180–191. doi: 10.1016/j.heares.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Schmiedt RA, Okamura HO, Lang H, Schulte BA. Ouabain application to the round window of the gerbil cochlea: A model of auditory neuropathy and apoptosis. J Assoc Res Otolaryngol. 2002;3:223–233. doi: 10.1007/s1016200220017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya T, Shimamura N, Yagihashi A, Suzuki S. Effect of topically applied basic fibroblast growth factor on injured cochlear nerve. Neurosurgery. 2003;52:900–907. 907. doi: 10.1227/01.neu.0000053509.98561.16. [DOI] [PubMed] [Google Scholar]
- Tessarollo L, Coppola V, Fritzsch B. NT-3 replacement with brain-derived neurotrophic factor redirects vestibular nerve fibers to the cochlea. J Neurosci. 2004;24:2575–2584. doi: 10.1523/JNEUROSCI.5514-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga R, Kelley PM, Keats BJ, Starr A, Leal SM, Cohn E, Kimberling WJ. Non-syndromic recessive auditory neuropathy is the result of mutations in the otoferlin (OTOF) gene. J Med Genet. 2003;40:45–50. doi: 10.1136/jmg.40.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Ying QL, Stavridis M, Griffiths D, Li M, Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat Biotechnol. 2003;21:183–186. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


