Abstract
Background:
Exercise-induced bronchoconstriction (EIB) is a common cause of symptoms in a subgroup of asthmatic subjects. The pathobiology that makes this group of asthmatic subjects susceptible to bronchoconstriction after a brief period of exercise remains poorly understood.
Objective:
We sought to determine whether there are differences in lower airway inflammation and production of cytokines and eicosanoids between asthmatic subjects with and without EIB.
Methods:
Two distinct groups of asthmatic subjects based on a priori definitions were identified, one with moderate-to-severe EIB and the other without significant bronchoconstriction after exercise challenge. Both groups met the definition of asthma on the basis of bronchodilator response, bronchial hyperresponsiveness, or both. A comparative immunopathology study was conducted by using induced sputum to identify differences in lower airway inflammation and production of cytokines and eicosanoids.
Results:
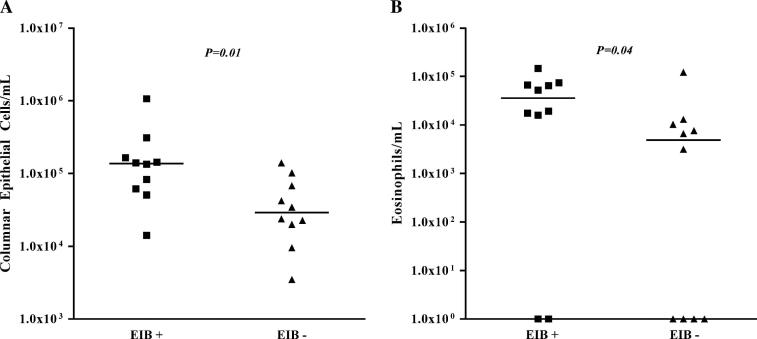
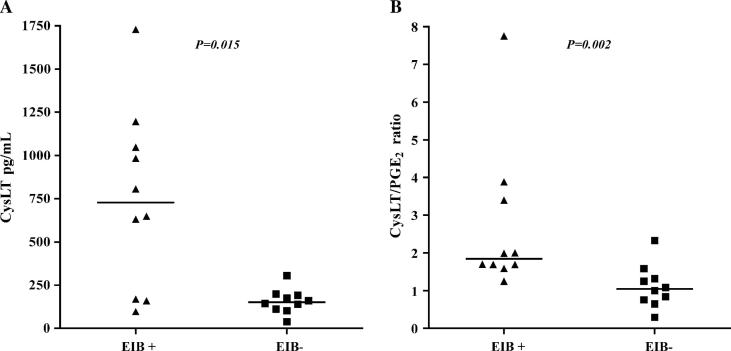
The groups had similar baseline lung function and bronchodilator response and did not have any asthma exacerbations within the prior year. The concentration of columnar epithelial cells was markedly higher in the group with EIB (1.4 × 105 vs 2.9 × 104 cells/mL, P = .01). The concentration of eosinophils was higher in the group with EIB (3.6 × 104 vs 4.9 × 103 cells/mL P = .04). Cysteinyl leukotrienes (CysLTs; 727.7 vs 151.9 pg/mL, P = .01) and the ratio of CysLTs to prostaglandin E2 (1.85 vs 1.04, P = .002) in the airways were higher in the group with EIB.
Conclusion:
Injury to the airway epithelium, overexpression of CysLTs, relative underproduction of prostaglandin E2, and greater airway eosinophilia are distinctive immunopathologic features of asthma with EIB. (J Allergy Clin Immunol 2005;116:586-93.)
Keywords: Asthma, exercise-induced bronchoconstriction, epithelial cell, leukotriene, prostaglandin, eosinophil, mast cell
Asthma is a complex syndrome with a number of clinical phenotypes.1 Exercise-induced bronchoconstriction (EIB) is a highly prevalent but discrete clinical phenotype that shares common features with other measures of indirect bronchial hyperresponsiveness (BHR).2 Among triggers of indirect BHR, exercise is particularly important because it generally cannot be avoided. Recent studies indicate that EIB occurs in less than half of asthmatic subjects tested with a standardized exercise challenge.3,4 Compared with other features of asthma, EIB has distinct pathophysiology. There is no relationship between baseline lung function and severity of EIB.4 Only a weak relationship exists between direct BHR and severity of EIB.5,6 The presence of EIB in children might precede the development of other features of asthma, representing an early stage of the disease.7 Some asthmatic subjects susceptible to EIB have severe bronchoconstriction and hypoxemia after a brief period of exercise.8 Furthermore, asthma triggered during sports is a common cause of sports-related deaths in children.9
The pathobiology that makes this group of asthmatic subjects susceptible to bronchoconstriction after a short period of exercise remains poorly understood. Because the exercise challenge test used to identify EIB is the maximum stimulus for EIB,10 asthmatic subjects with and without EIB can be readily identified by threshold responses to a standardized exercise challenge test.10 The stimulus for EIB involves drying and cooling of the intrathoracic airways as a result of increased ventilation during exercise.11 After exercise challenge in human subjects with EIB, increased levels of cysteinyl leukotrienes (CysLTs; leukotriene E4)12,13 are detected in the urine, although these findings have not been seen in all studies.14 In a canine model of EIB, epithelial disruption occurs during dry air challenge, which is correlated with the amount of bronchoconstriction and release of CysLTs into the airways.15,16 Airway eosinophilia is correlated with the severity of EIB in asthmatic subjects with EIB.6 To determine whether there are differences in lower airway inflammation and production of cytokines and eicosanoids between asthmatic subjects with and without EIB, we identified 2 distinct groups of asthmatic subjects on the basis of a priori definitions, one with moderate-to-severe EIB and the other without significant bronchoconstriction after exercise challenge. Both groups met the definition of asthma on the basis of bronchodilator response, BHR, or both.17 We conducted a comparative immunopathology study by using induced sputum. The primary objective of the study was to determine whether asthmatic subjects with EIB have epithelial injury, leading to airway eosinophilia and release of CysLTs into airway fluid.
METHODS
Subjects
The University of Washington Institutional Review Board approved the study protocol, and written informed consent was obtained from all participants. Subjects 18 to 59 years of age were recruited who had a physician's diagnosis of asthma for 1 year or longer and used only an inhaled β2-agonist for asthma treatment. In accordance with a priori definitions, asthmatic subjects with EIB (EIB+ group) were identified with a 30% or greater decrease in FEV1 after exercise challenge, and asthmatic control subjects without EIB (EIB− group) were identified with a 7% or less decrease in FEV1 after exercise challenge. A diagnosis of asthma was established in the EIB− group on the basis of either a change in FEV1 of 12% or greater after the administration of a β2-agonist or a methacholine PC20 of 8 mg/mL or less. Potential participants were excluded if baseline FEV1 was 65% of predicted value or less, if there was history of smoking cigarettes within the prior year or a 7 pack-years or longer smoking history, if the patient was treated for acute asthma within the prior month, if the patient was hospitalized for asthma within the prior 3 months, or if the patient had a history of life-threatening asthma. Participants were excluded if they had used an inhaled corticosteroid, leukotriene modifier, long-acting antihistamine, cromone, or long-acting β2-agonist in the 30 days before the study.
Study protocol
The first visit consisted of a physical examination, spirometry, and exercise challenge to determine eligibility for the study. Participants with a 30% or greater maximum decrease in FEV1 after exercise challenge were enrolled in the EIB+ group. Participants with a 7% or smaller maximum decrease in FEV1 after exercise challenge were eligible for inclusion in the EIB− group. On the second visit, conducted at the same time of day 4 to 10 days later, all participants had spirometry before and 15 minutes after administration of 180 μg of albuterol through a metered-dose inhaler, followed by induced sputum. Participants in the EIB− group with less than a 12% improvement in FEV1 after the administration of albuterol had a third study visit for a methacholine challenge 4 to 10 days later. Subjects were asked not to exercise, not to use short-acting antihistamines for 48 hours, and not to use β2-agonists and caffeinated beverages for 6 hours before each study visit. All participants in the EIB+ group were enrolled in a subsequent study.18
Spirometry, exercise, and methacholine challenge
Spirometry, exercise, and methacholine challenges were conducted in accordance with American Thoracic Society standards.10,19 Exercise challenge was performed on a motorized treadmill such that each subject sustained 85% or more of their maximum heart rate for the final 6 minutes of exercise.10 Subjects wore nose clips and breathed dry air (0% relative humidity, 22°C) delivered from a weather balloon reservoir through a 1-way valve (Hans Rudolph, Kansas City, Mo) during exercise. Spirometry was conducted 20 and 5 minutes before each exercise challenge and repeated at 0, 3, 6, 10, 15, and 30 minutes after the end of exercise. The better of at least 2 FEV1 maneuvers within 5% of each other was recorded at each time point. Methacholine challenge was conducted with a dosimeter.10
Sputum induction
Induced sputum was conducted with 3% hypertonic saline administered through an ultrasonic nebulizer (DeVilbiss, Somerset, Pa), as previously described.20 At 2-minute intervals, subjects were asked to clear saliva from their mouth and then expectorate sputum. Sputum was collected over 12 minutes and was pooled into a single sample container. The induced sputum was placed on ice immediately and processed within 30 minutes of collection. Samples were coded with a subject number, visit number, and date. The link between the clinical characteristics of the participants and the coded label was maintained in a separate file by the principal investigator. Total and differential cell counts were performed by an investigator (MWM) who was blinded to the clinical characteristics of each participant. The levels of histamine, CysLTs, leukotriene B4, prostaglandin (PG) E2, IL-4, IL-5, IL-8, IL-13, TNF-α, vascular endothelial growth factor, and RANTES were determined in induced sputum supernatants. Details of induced sputum processing and analysis are presented in the Online Repository in the online version of this article at www.mosby.com/jaci.
Statistical analysis
The sample size was based on the percentage of eosinophils because of the relationship between the percentage of eosinophils in induced sputum and the severity of EIB.6 The sample size in this study was selected to detect a 2% difference in the percentage of eosinophils with 95% power on the basis of data from Crimi et al.21 The characteristics of the study participants were compared with unpaired t tests for continuous variables and χ2 tests for categoric variables. The area under the FEV1-time curve (AUC)22 quantified the severity of EIB over a 30-minute period after exercise (AUC30). Comparison of the severity of EIB between the groups was made with an unpaired t test. The relationship between lung function and bronchodilator response and the severity of EIB was assessed within each group with the Pearson correlation coefficient. The medians of differential cell counts and concentrations of cellular constituents and inflammatory mediators were compared between different groups with the Mann-Whitney U test. On the basis of the study by Szefler et al,23 indicating that age might be a factor in the response to the CysLT1 antagonists, a regression analysis (see the Online Repository in the online version of this article at www.mosby.com/jaci) was also performed to account for the possible effects of age and sex on the levels of cellular constituents and inflammatory mediators in induced sputum.
RESULTS
Subject characteristics
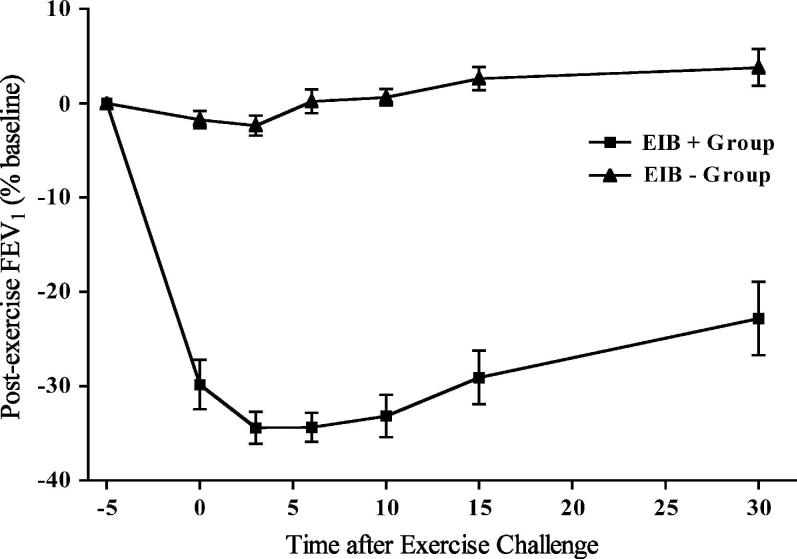
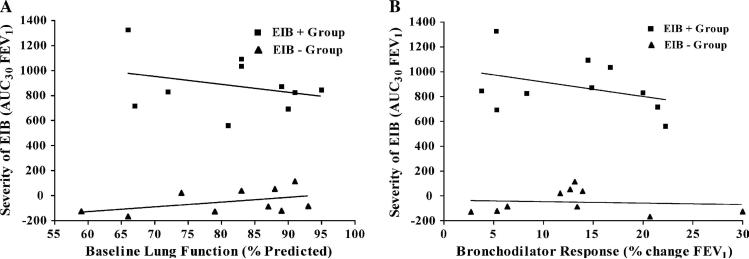
The characteristics of each of the groups are listed in Table I. The diagnosis of asthma was confirmed in the EIB− group by a 12% or greater bronchodilator response in 4 participants and by a methacholine PC20 of 8 mg/mL or less (mean, 2.8 mg/mL; range, 0.25-8 mg/mL) in 6 participants. There were no differences in baseline lung function or response to the administration of a bronchodilator between the 2 groups. None of the patients in either group reported an asthma-related emergency department visit, acute-care visit, or hospitalization within the prior year. Both groups reported asthma symptoms primarily during activity, including 4 of 10 from each group who had no asthma symptoms except during activity. There were no differences in the rate of allergic rhinitis or prior use of inhaled corticosteroids between the groups (Table I). A past history of cigarette smoking was present in none of the EIB+ group and 3 of the EIB− group (range, 1-5 pack-years; P = .21). The reduction in FEV1 after exercise challenge was much greater in the EIB+ group compared with that seen in the EIB− group (P < .001, Fig 1). The relationship between baseline lung function and the severity of EIB was assessed separately within each group, showing that there was no relationship between baseline FEV1 and severity of EIB assessed on the basis of the AUC30 (EIB+, r2 = 0.087, P = .41; EIB−, r2 = 0.201, P = .19; Fig 2, A). Similarly, there was no relationship between the response to the administration of a bronchodilator at baseline and the severity of EIB in either group (EIB+, r2 = 0.139, P = .29; EIB−, r2 = 0.009, P = .79; Fig 2, B).
Table I.
Characteristics of study participants*
| Asthma |
|||
|---|---|---|---|
| Characteristic | EIB+ (n = 10) | EIB− (n = 10) | P value |
| Age (y) | |||
| Mean | 26.6 | 32.5 | .21 |
| Range | 20-28 | 20-54 | |
| Sex (% male) | 60 | 30 | .18 |
| Race | |||
| White | 9 | 8 | |
| African American | 0 | 1 | |
| Asian | 1 | 0 | |
| Hispanic | 0 | 1 | |
| Clinical features | |||
| Asthma Exacerbations/y | 0 | 0 | NS |
| Prior ICS use (%) | 50 | 40 | .50 |
| Allergic rhinitis (%) | 40 | 20 | .31 |
| Baseline | |||
| FEV1 (%) | 81.7 ± 10.3 | 80.9 ± 11.3 | .87 |
| FVC (%) | 99.1 ± 10.9 | 97.8 ± 10.1 | .79 |
| FEV1/FVC | 0.7 ± 0.1 | 0.7 ± 0.1 | .96 |
| FEF25-75 (%) | 54.0 ± 16.5 | 55.0 ± 14.7 | .89 |
| After bronchodilator | |||
| Δ FEV1 (%) | 13.3 ± 7.1 | 14.3 ± 11.3 | .81 |
| Δ FVC (%) | 3.7 ± 9.0 | 2.4 ± 7.7 | .72 |
| Δ FEV1/FVC (%) | 11.0 ± 6.8 | 11.8 ± 7.7 | .79 |
| Δ FEF25-75 (%) | 38.8 ± 20.4 | 32.9 ± 20.9 | .53 |
| After exercise | |||
| Maximum decrease in FEV1 | −37.2 ± 5.3 | −3.1 ± 3.1 | <.001 |
| Area under FEV1 curve† | −879.3 ± 220.6 | 48.5 ± 96.1 | <.001 |
FVC, Forced vital capacity; FEF25-75, forced expiratory flow, midexpiratory phase.
Values are reported as means ± SD unless otherwise specified.
Area under the FEV1 curve over the first 30 minutes after exercise (percentage change × min).
FIG 1.
Comparison of lung function response after exercise challenge in asthmatic subjects with EIB and an asthmatic control group without EIB. The severity of EIB measured on the basis of the AUC30 was markedly greater in the EIB+ group (P < .001).
FIG 2.
Relationship between lung function and severity of EIB measured on the basis of the AUC30 (A) and lung function response to a short-acting β2-agonist and severity of EIB (B). No relationship was identified between either of these 2 physiologic parameters and the severity of EIB.
Comparison of lower airway cellular constituents
The induced sputum volume was higher in the EIB+ group compared with that in the EIB− group (7.7 vs 3.4 mL, P = .003). The concentration of columnar epithelial cells was higher in the EIB+ group (1.4 × 105 vs 2.9 × 104 cells/mL, P = .01; Fig 3, A). The concentration of eosinophils was higher in the group with EIB (3.6 × 104 vs 4.9 × 103 cells/mL, P = .04; Fig 3, B). There were no differences in the concentrations of lymphocytes, macrophages, or neutrophils between the groups (Table II). There was a trend toward a higher concentration of inflammatory cells in induced sputum in the group with EIB (1.3 × 106 vs 7.8 × 105 cells/mL, P = .14). There were no differences in the percentage of inflammatory cells in the lower airways between the 2 groups, but there was a trend toward higher percentages of eosinophils and columnar epithelial cells (Table II). A regression analysis accounting for the possible effects of age and sex on the levels of columnar epithelial cells and eosinophils is presented in the Online Repository in the online version of this article at www.mosby.com/jaci. Addition of age and sex had no significant effect on the observed differences in columnar epithelial cells and eosinophils between the groups.
FIG 3.
Comparison of the concentration of columnar epithelial cells (A) and the concentration of eosinophils (B) in induced sputum between asthmatic subjects with EIB and asthmatic control subjects without EIB. The median concentration of columnar epithelial cells and eosinophils was higher in the group with EIB.
Table II.
Cellular findings in induced sputum*
| Asthma |
|||
|---|---|---|---|
| EIB+ (n = 10) | EIB− (n = 10) | P value† | |
| Percentage | |||
| Eosinophils‡ | 2.07 (0.58-3.49) | 0.44 (0.00-1.49) | .123 |
| Lymphocytes‡ | 1.50 (0.78-4.36) | 1.20 (0.88-1.74) | .353 |
| Macrophages‡ | 36.89 (26.02-51.44) | 43.80 (16.14-52.22) | 1.000 |
| Neutrophils‡ | 36.20 (24.40-56.41) | 35.29 (28.50-64.86) | .579 |
| Columnar epithelial cells‡ | 7.07 (3.65-14.35) | 2.43 (1.40-10.16) | .143 |
| Squamous epithelial cells§ | 18.51 (10.41-25.70) | 34.99 (14.46-46.49) | .190 |
| Other cells¶ | 4.77 (3.15-8.42) | 7.26 (3.56-10.14) | .393 |
| Concentration (× 104) | |||
| Eosinophils‡ | 3.58 (12.06-68.71) | 0.49 (0.00-1.10) | .043 |
| Lymphocytes‡ | 2.73 (0.90-7.38) | 0.99 (0.63-2.27) | .105 |
| Macrophages‡ | 49.63 (23.18-119.01) | 22.59 (15.05-65.88) | .123 |
| Neutrophils‡ | 45.33 (23.60-127.58) | 44.03 (14.35-69.73) | .247 |
| Columnar epithelial cells‡ | 13.70 (5.91-20.13) | 2.91 (1.78-7.65) | .011 |
| Squamous epithelial Cells | 32.42 (20.43-62.47) | 38.62 (15.15-70.03) | .912 |
| Other cells¶ | 6.68 (3.52-17.62) | 5.60 (3.67-10.05) | .579 |
Data are expressed as medians (interquartile ranges).
Mann-Whitney U test.
Inflammatory cells and columnar epithelial cells expressed as the percentage of nonsquamous epithelial cells.
Squamous epithelial cells expressed as the percentage of total cells.
Cells that could not be classified.
Comparison of airway inflammatory mediators
The concentration of CysLTs in the airways was higher in the EIB+ group compared with in the EIB− group (727.7 vs 151.9 pg/mL, P = .01; Fig 4, A), and the ratio of CysLTs to PGE2 was higher in the EIB+ group compared with that seen in the EIB− group (1.85 vs 1.04, P = .002; Fig 4, B). There were no differences in the levels of histamine, LTB4, PGE2, IL-5, IL-8, and vascular endothelial growth factor between the groups (Table III). The levels of IL-4, IL-13, TNF-α, and RANTES were less than the level of detection. A regression analysis accounting for the possible effects of age and sex on the levels of CysLTs and the CysLT/PGE2 ratio is presented in the Online Repository in the online version of this article at www.mosby.com/jaci. Addition of age and sex did not affect the differences in CysLTs and the CysLT/PGE2 ratio between the groups.
FIG 4.
Comparison of the levels of CysLTs (A) and the ratio of CysLT to PGE2 (B) in the airways of asthmatic subjects with EIB compared with those seen in asthmatic control subjects without EIB. The median concentrations of CysLT and the CysLT/PGE2 ratio were higher in the group with EIB.
Table III.
Mediator concentrations in induced sputum*
| Asthma |
|||
|---|---|---|---|
| EIB+ (n = 10) | EIB− (n = 10) | P value† | |
| Histamine (ng/mL) | 24.2 (13.9-200.3) | 28.4 (12.1-74.8) | .853 |
| CysLT (pg/mL) | 727.7 (164.0-1122.0) | 151.9 (106.5-195.0) | .015 |
| LTB4 (pg/mL) | 1050.0 (520.0-3369.0) | 1564.0 (176.0-3249.0) | .912 |
| PGE2 (pg/mL) | 225.8 (77.1-225.8) | 125.2 (58.6-231.9) | .218 |
| IL-5 (pg/mL) | 1.9 (1.9-7.5) | 1.9 (1.9-1.9) | .143 |
| IL-8 (pg/mL) | 882.3 (424.0-2389.0) | 550.9 (227.0-1066.0) | .353 |
| VEGF (pg/mL) | 342.2 (175.0-712.0) | 502.1 (160.0-991.0) | .631 |
VEGF, Vascular endothelial growth factor.
Data are expressed as medians (interquartile ranges).
Mann-Whitney U test.
DISCUSSION
Asthma is a phenotypically diverse syndrome that is defined in part by the presence of increased BHR.1 The degree and type of BHR is variable among patients with asthma. The severity of direct BHR is related to baseline lung function,24 possibly indicating structural remodeling of the lung. In contrast, indirect BHR is measured by the response to stimuli, such as exercise, hypertonic aerosols, and AMP, and is thought to act indirectly through the release of inflammatory mediators into the airways.2 Indirect BHR is not related to baseline lung function4,24 and might indicate greater airway inflammation.25 There is limited information about the distinct immunopathology that leads to indirect BHR. The present study examined EIB, a measure of BHR that overlaps with other measures of indirect BHR and is a distinct phenotypic manifestation of asthma that is only weakly associated with severity of direct BHR5,6,26 and is not associated with baseline lung function.4 Several cross-sectional studies have shown that the severity of EIB and methacholine BHR are not strongly related. In 27 asthmatic children there was no relationship between the methacholine PC20 and the maximum decrease in FEV1 after exercise (r = −0.2, P = .40).5 Another cross-sectional study showed no relationship between methacholine PC20 and maximum decrease in FEV1 after exercise (r = −0.2, P = .31) in 21 young adults with asthma.6 Only 9 of 25 elite athletes with positive results on eucapnic voluntary hyperpnea challenge, a surrogate for exercise challenge, had a positive methacholine challenge result.27 Inhaled corticosteroid treatment causes a progressive improvement in methacholine BHR over 24 weeks that is greater with high-dose than with moderate-dose inhaled corticosteroid treatment, whereas the maximum inhibition of EIB during inhaled corticosteroid treatment occurs after 3 weeks and is the same for high- and moderate-dose inhaled corticosteroid treatment.26 The results of this study show that the airway immunopathology associated with asthma with EIB includes greater injury to the airway epithelium, overexpression of CysLTs, relative underproduction of PGE2, and greater airway eosinophilia.
The amount of epithelial disruption identified in studies of asthma has been inconsistent, leading to controversy over the role of epithelial disruption in asthma pathogenesis.28 Disruption of the airway epithelium has been indirectly associated with EIB in previous studies. Sputum albumin level, a marker of permeability, was associated with the severity of EIB.29 Similarly, a population-based study showed that another marker of epithelial permeability, serum surfactant levels, was associated with the presence of EIB in schoolchildren.30 This alteration in epithelial permeability might be responsible for the higher volume of induced sputum in asthmatic subjects with EIB. Disruption of the epithelium also increases the transit of granulocytes into the airways,31 which might be responsible for the higher concentration of eosinophils in asthmatic subjects with EIB.
High levels of ventilation alone might be sufficient to injure the epithelium, leading to EIB. Dry air delivered repeatedly to canine lower airways injures the airway epithelium leading to a process similar to EIB.15 Isocapnic hyperpnea in human subjects with asthma leads to the release of columnar epithelial cells into bronchoalveolar lavage fluid.32 Winter athletes exposed to recurrent episodes of high ventilation with dry cold air, leading to the development of BHR, have increased epithelial disruption and metaplasia on airway biopsy specimens.33
Injury to the airway epithelium might increase the susceptibility to EIB through several mechanisms. Studies in isolated airways show that the contractile responses to a number of stimuli, such as acetylcholine and histamine, are increased in areas in which the epithelium is disrupted.34 Although this partly represents a reduction in the barrier function of epithelium, the predominant mechanism is an inhibition of the production of epithelial derived substances, such as PGE2, that are inhibitory to smooth muscle contraction.35 The airway epithelium is a key regulator of airway surface liquid osmolarity,36 a critical factor in the initiation of EIB.11 Disruption of the airway epithelium alters the normal regulation of water loss, which, under normal circumstances, is regulated by changes in epithelial cell volume and water flow through the columnar cell basolateral membrane and tight junctions between apical cells.36 In addition, mast cells, which have been implicated in the pathogenesis of EIB, infiltrate areas of epithelial injury and persist after repeated dry air challenges to the lower airways in the canine model of EIB, even after other abnormalities resolve.15
PGE2 is the predominant eicosanoid synthesized by the airway epithelium. Epithelial-derived PGE2 has important bronchodilatory, anti-inflammatory, and antifibrotic effects.37 Injury to the airway epithelium decreases the production of PGE2.37 The alteration in the ratio of CysLTs to PGE2 identified in the present study is indicative of airway epithelial injury. Because of transcellular metabolism, relative underproduction of epithelial PGE2 leads to an overproduction of CysLTs by leukocytes, such as bronchial macrophages.37 Alteration in this ratio leads to a predominance of the effects of CysLTs, which are counteracted in part by PGE2 under normal circumstances. A similar alteration in the ratio of CysLTs to PGE2 has been identified in aspirin-sensitive asthma. Taken together with the epithelial disruption identified in the asthmatic subjects with EIB, these data implicate epithelial injury, relative underproduction of PGE2, and overproduction of CysLTs as important factors in the development of EIB.
Higher levels of CysLTs in asthmatic subjects with EIB compared with asthmatic subjects without EIB were demonstrated in induced sputum in the present study and in exhaled breath condensate in another recent study.38 Overproduction of CysLTs might increase the susceptibility to EIB through a number of proinflammatory mechanisms. CysLTs mediate airway smooth muscle constriction, mucus release, and increased vascular permeability.39 The pathogenesis of EIB involves the release of CysLTs, demonstrated by the release of CysLTs into the airways,18,40 an increase in leukotriene E4 in the urine,13 and the inhibition of EIB pharmacologically by drugs that antagonize CysLTs.22 CysLTs increase direct BHR to histamine in patients with asthma but not in nonasthmatic individuals.41 Eosinophils, which have been associated with the severity of EIB,41 have increased survival induced by CysLTs through antiapoptic effects.42 Smooth muscle proliferation is increased in vitro in response to CysLTs in the presence of epidermal growth factor or TGF-β,37 an effect that can be inhibited in a mouse model by treatment with a CysLT1 receptor antagonist.43 CysLTs also potentiate the release of neuropeptides from airway sensory nerves, which modulate bronchoconstriction in the canine model of EIB.44
Several limitations should be considered when interpreting the results of this study. Because of the small sample size, the study had 95% power to detect 2-fold differences in airway immunopathology, such as that identified for columnar epithelial cells, eosinophils, and CysLTs, but was insufficiently powered to detect smaller differences, such as the difference in levels of IL-8. Although the groups were matched for lung function and bronchodilator response, there might have been differences in the distribution of age and sex between the groups. To account for these differences, we performed regression analysis (see the Online Repository in the online version of this article at www.mosby.com/jaci) and found the same results after accounting for the possible effects of age and sex. Because the study did not include a group of asthmatic subjects with frequent exacerbations or frequent asthma symptoms, we cannot state specifically how the findings in this study relate to poor asthma control.
In summary, EIB is an important component of the asthma syndrome that is correlated with other measures of indirect BHR. EIB is a distinct phenotype that is not related to baseline lung function, bronchodilator response, or frequency of exacerbations, indicating that the susceptibility to EIB is regulated independently from these other features of asthma. By comparing persons with asthma with and without EIB, the present study identified injury to the airway epithelium, overexpression of CysLTs, relative underproduction of PGE2, and greater airway eosinophilia as distinctive immunopathology leading to EIB. These data suggest that treatments within this subgroup should target epithelial disruption and overproduction of CysLTs. Because EIB has been identified in children before the development of other features of asthma,7 treatments targeting theses features of asthma could influence the persistence of asthma.
Acknowledgments
We greatly appreciate the technical assistance of Linda Deller, Patricia McDowell, Peter Meyer, John Smith, and Jon Rudzinski. We thank Dr Lianne Sheppard for her guidance on the biostatistics.
Supported by a National Institutes of Health grant HL04231 (TSH) and an American Lung Association Clinical Research Grant (TSH).
Abbreviations used
- AUC
Area under the FEV1-time curve
- BHR
Bronchial hyperresponsiveness
- CysLT
Cysteinyl leukotriene
- EIB
Exercise-induced bronchosontriction
- PG
Prostaglandin
Footnotes
Disclosure of potential conflict of interest: Dr Hallstrand has given lectures (2001-2004) in sessions at regional and national meetings, which were funded in part by Merck & Co, Inc, and received research support from a Merck & Co Medical School grant awarded to the University of Washington in 2000. Mr Moody does not have a financial relationship with a commercial entity that has an interest in the subject of this article. Dr Aitken does not have a financial relationship with a commercial entity that has an interesting the subject of this article. Dr Henderson has given lectures (2001-2004) in sessions at regional, national, and international meetings, which were funded in part by Merck & Co, Inc, and received research support from a Merck & Co Medical School grant awarded to the University of Washington in 2002.
REFERENCES
- 1.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344:350–62. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 2.Joos GF, O'Connor B, Anderson SD, Chung F, Cockcroft DW, Dahlen B, et al. Indirect airway challenges. Eur Respir J. 2003;21:1050–68. doi: 10.1183/09031936.03.00008403. [DOI] [PubMed] [Google Scholar]
- 3.Hallstrand TS, Curtis JR, Koepsell TD, Martin DP, Schoene RB, Sullivan SD, et al. Effectiveness of screening examinations to detect unrecognized exercise-induced bronchoconstriction. J Pediatr. 2002;141:343–8. doi: 10.1067/mpd.2002.125729. [DOI] [PubMed] [Google Scholar]
- 4.Cabral AL, Conceicao GM, Fonseca-Guedes CH, Martins MA. Exercise-induced bronchospasm in children: effects of asthma severity. Am J Respir Crit Care Med. 1999;159:1819–23. doi: 10.1164/ajrccm.159.6.9805093. [DOI] [PubMed] [Google Scholar]
- 5.Freezer NJ, Croasdell H, Doull IJ, Holgate ST. Effect of regular inhaled beclomethasone on exercise and methacholine airway responses in school children with recurrent wheeze. Eur Respir J. 1995;8:1488–93. [PubMed] [Google Scholar]
- 6.Yoshikawa T, Shoji S, Fujii T, Kanazawa H, Kudoh S, Hirata K, et al. Severity of exercise-induced bronchoconstriction is related to airway eosinophilic inflammation in patients with asthma. Eur Respir J. 1998;12:879–84. doi: 10.1183/09031936.98.12040879. [DOI] [PubMed] [Google Scholar]
- 7.Jones A. Screening for asthma in children. Br J Gen Pract. 1994;44:179–83. [PMC free article] [PubMed] [Google Scholar]
- 8.Marotel C, Natali F, Heyraud JD, Vaylet F, L'Her P, Bonnet D, et al. Severe forms of effort-induced asthma. Allerg Immunol (Paris) 1989;21:61–4. [PubMed] [Google Scholar]
- 9.Becker JM, Rogers J, Rossini G, Mirchandani H, D'Alonzo GE., Jr Asthma deaths during sports: report of a 7-year experience. J Allergy Clin Immunol. 2004;113:264–7. doi: 10.1016/j.jaci.2003.10.052. [DOI] [PubMed] [Google Scholar]
- 10.Crapo RO, Casaburi R, Coates AL, Enright PL, Hankinson JL, Irvin CG, et al. Guidelines for methacholine and exercise challenge testing-1999. Am J Respir Crit Care Med. 2000;161:309–29. doi: 10.1164/ajrccm.161.1.ats11-99. [DOI] [PubMed] [Google Scholar]
- 11.Anderson SD, Daviskas E. The mechanism of exercise-induced asthma is…. J Allergy Clin Immunol. 2000;106:453–9. doi: 10.1067/mai.2000.109822. [DOI] [PubMed] [Google Scholar]
- 12.Mickleborough TD, Murray RL, Ionescu AA, Lindley MR. Fish oil supplementation reduces severity of exercise-induced bronchoconstriction in elite athletes. Am J Respir Crit Care Med. 2003;168:1181–9. doi: 10.1164/rccm.200303-373OC. [DOI] [PubMed] [Google Scholar]
- 13.Reiss TF, Hill JB, Harman E, Zhang J, Tanaka WK, Bronsky E, et al. Increased urinary excretion of LTE4 after exercise and attenuation of exercise-induced bronchospasm by montelukast, a cysteinyl leukotriene receptor antagonist. Thorax. 1997;52:1030–5. doi: 10.1136/thx.52.12.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson SD, Brannan JD. Exercise-induced asthma: is there still a case for histamine? J Allergy Clin Immunol. 2002;109:771–3. doi: 10.1067/mai.2002.123644. [DOI] [PubMed] [Google Scholar]
- 15.Davis MS, Schofield B, Freed AN. Repeated peripheral airway hyper-pnea causes inflammation and remodeling in dogs. Med Sci Sports Exerc. 2003;35:608–16. doi: 10.1249/01.MSS.0000058660.88987.A0. [DOI] [PubMed] [Google Scholar]
- 16.Freed AN, Davis MS. Hyperventilation with dry air increases airway surface fluid osmolality in canine peripheral airways. Am J Respir Crit Care Med. 1999;159:1101–7. doi: 10.1164/ajrccm.159.4.9802072. [DOI] [PubMed] [Google Scholar]
- 17.Pauwels RA, Pedersen S, Busse WW, Tan WC, Chen YZ, Ohlsson SV, et al. Early intervention with budesonide in mild persistent asthma: a randomised, double-blind trial. Lancet. 2003;361:1071–6. doi: 10.1016/S0140-6736(03)12891-7. [DOI] [PubMed] [Google Scholar]
- 18.Hallstrand TS, Henderson WR, Jr, Aitken ML. The role of airway inflammation in exercise-induced bronchoconstriction [abstract] Am J Respir Crit Care Med. 2003;167:A354. [Google Scholar]
- 19.American Thoracic Society Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152:1107–36. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 20.Fahy JV, Boushey HA, Lazarus SC, Mauger EA, Cherniack RM, Chinchilli VM, et al. Safety and reproducibility of sputum induction in asthmatic subjects in a multicenter study. Am J Respir Crit Care Med. 2001;163:1470–5. doi: 10.1164/ajrccm.163.6.9901105. [DOI] [PubMed] [Google Scholar]
- 21.Crimi E, Balbo A, Milanese M, Miadonna A, Rossi GA, Brusasco V. Airway inflammation and occurrence of delayed bronchoconstriction in exercise-induced asthma. Am Rev Respir Dis. 1992;146:507–12. doi: 10.1164/ajrccm/146.2.507. [DOI] [PubMed] [Google Scholar]
- 22.Leff JA, Busse WW, Pearlman D, Bronsky EA, Kemp J, Hendeles L, et al. Montelukast, a leukotriene-receptor antagonist, for the treatment of mild asthma and exercise-induced bronchoconstriction. N Engl J Med. 1998;339:147–52. doi: 10.1056/NEJM199807163390302. [DOI] [PubMed] [Google Scholar]
- 23.Szefler SJ, Phillips BR, Martinez FD, Chinchilli VM, Lemanske RF, Strunk RC, et al. Characterization of within-subject responses to fluticasone and montelukast in childhood asthma. J Allergy Clin Immunol. 2005;115:233–42. doi: 10.1016/j.jaci.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 24.De Meer G, Heederik D, Postma DS. Bronchial responsiveness to adenosine 5′-monophosphate (AMP) and methacholine differ in their relationship with airway allergy and baseline FEV1. Am J Respir Crit Care Med. 2002;165:327–31. doi: 10.1164/ajrccm.165.3.2104066. [DOI] [PubMed] [Google Scholar]
- 25.Van Den Berge M, Meijer RJ, Kerstjens HA, de Reus DM, Koeter GH, Kauffman HF, et al. PC20 adenosine 5′-monophosphate is more closely associated with airway inflammation in asthma than PC20 methacholine. Am J Respir Crit Care Med. 2001;163:1546–50. doi: 10.1164/ajrccm.163.7.2010145. [DOI] [PubMed] [Google Scholar]
- 26.Hofstra WB, Neijens HJ, Duiverman EJ, Kouwenberg JM, Mulder PG, Kuethe MC, et al. Dose-responses over time to inhaled fluticasone propionate treatment of exercise- and methacholine-induced bronchoconstriction in children with asthma. Pediatr Pulmonol. 2000;29:415–23. doi: 10.1002/(sici)1099-0496(200006)29:6<415::aid-ppul1>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 27.Holzer K, Anderson SD, Douglass J. Exercise in elite summer athletes: challenges for diagnosis. J Allergy Clin Immunol. 2002;110:374–80. doi: 10.1067/mai.2002.127784. [DOI] [PubMed] [Google Scholar]
- 28.Fahy JV. Remodeling of the airway epithelium in asthma. Am J Respir Crit Care Med. 2001;164(suppl):S46–51. doi: 10.1164/ajrccm.164.supplement_2.2106066. [DOI] [PubMed] [Google Scholar]
- 29.Kanazawa H, Asai K, Hirata K, Yoshikawa J. Vascular involvement in exercise-induced airway narrowing in patients with bronchial asthma. Chest. 2002;122:166–70. doi: 10.1378/chest.122.1.166. [DOI] [PubMed] [Google Scholar]
- 30.Bernard A, Carbonnelle S, Michel O, Higuet S, De Burbure C, Buchet JP, et al. Lung hyperpermeability and asthma prevalence in school-children: unexpected associations with the attendance at indoor chlorinated swimming pools. Occup Environ Med. 2003;60:385–94. doi: 10.1136/oem.60.6.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erjefalt JS, Uller L, Malm-Erjefalt M, Persson CG. Rapid and efficient clearance of airway tissue granulocytes through transepithelial migration. Thorax. 2004;59:136–43. doi: 10.1136/thorax.2003.004218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pliss LB, Ingenito EP, Ingram RH, Jr, Pichurko B. Assessment of bronchoalveolar cell and mediator response to isocapnic hyperpnea in asthma. Am Rev Respir Dis. 1990;142:73–8. doi: 10.1164/ajrccm/142.1.73. [DOI] [PubMed] [Google Scholar]
- 33.Karjalainen EM, Laitinen A, Sue-Chu M, Altraja A, Bjermer L, Laitinen LA. Evidence of airway inflammation and remodeling in ski athletes with and without bronchial hyperresponsiveness to methacholine. Am J Respir Crit Care Med. 2000;161:2086–91. doi: 10.1164/ajrccm.161.6.9907025. [DOI] [PubMed] [Google Scholar]
- 34.Gao Y, Vanhoutte PM. Removal of the epithelium potentiates acetylcholine in depolarizing canine bronchial smooth muscle. J Appl Physiol. 1988;65:2400–5. doi: 10.1152/jappl.1988.65.6.2400. [DOI] [PubMed] [Google Scholar]
- 35.Stuart-Smith K, Vanhoutte PM. Arachidonic acid evokes epithelium-dependent relaxations in canine airways. J Appl Physiol. 1988;65:2170–80. doi: 10.1152/jappl.1988.65.5.2170. [DOI] [PubMed] [Google Scholar]
- 36.Matsui H, Davis CW, Tarran R, Boucher RC. Osmotic water permeabilities of cultured, well-differentiated normal and cystic fibrosis airway epithelia. J Clin Invest. 2000;105:1419–27. doi: 10.1172/JCI4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holgate ST, Peters-Golden M, Panettieri RA, Henderson WR., Jr Roles of cysteinyl leukotrienes in airway inflammation, smooth muscle function, and remodeling. J Allergy Clin Immunol. 2003;111(suppl):S18–34. doi: 10.1067/mai.2003.25. [DOI] [PubMed] [Google Scholar]
- 38.Carraro S, Corradi M, Zanconato S, Alinovi R, Pasquale MF, Zacchello F, et al. Exhaled breath condensate cysteinyl leukotrienes are increased in children with exercise-induced bronchoconstriction. J Allergy Clin Immunol. 2005;115:764–70. doi: 10.1016/j.jaci.2004.10.043. [DOI] [PubMed] [Google Scholar]
- 39.Hallstrand TS, Henderson WR., Jr Leukotriene modifiers. Med Clin North Am. 2002;86:1009–33. doi: 10.1016/s0025-7125(02)00091-3. [DOI] [PubMed] [Google Scholar]
- 40.Freed AN, Wang Y, McCulloch S, Myers T, Suzuki R. Mucosal injury and eicosanoid kinetics during hyperventilation-induced bronchoconstriction. J Appl Physiol. 1999;87:1724–33. doi: 10.1152/jappl.1999.87.5.1724. [DOI] [PubMed] [Google Scholar]
- 41.Arm JP, Spur BW, Lee TH. The effects of inhaled leukotriene E4 on the airway responsiveness to histamine in subjects with asthma and normal subjects. J Allergy Clin Immunol. 1988;82:654–60. doi: 10.1016/0091-6749(88)90979-7. [DOI] [PubMed] [Google Scholar]
- 42.Lee E, Robertson T, Smith J, Kilfeather S. Leukotriene receptor antagonists and synthesis inhibitors reverse survival in eosinophils of asthmatic individuals. Am J Respir Crit Care Med. 2000;161:1881–6. doi: 10.1164/ajrccm.161.6.9907054. [DOI] [PubMed] [Google Scholar]
- 43.Henderson WR, Jr, Tang LO, Chu SJ, Tsao SM, Chiang GK, Jones F, et al. A role for cysteinyl leukotrienes in airway remodeling in a mouse asthma model. Am J Respir Crit Care Med. 2002;165:108–16. doi: 10.1164/ajrccm.165.1.2105051. [DOI] [PubMed] [Google Scholar]
- 44.Freed AN, McCulloch S, Meyers T, Suzuki R. Neurokinins modulate hyperventilation-induced bronchoconstriction in canine peripheral airways. Am J Respir Crit Care Med. 2003;167:1102–8. doi: 10.1164/rccm.200201-055OC. [DOI] [PubMed] [Google Scholar]