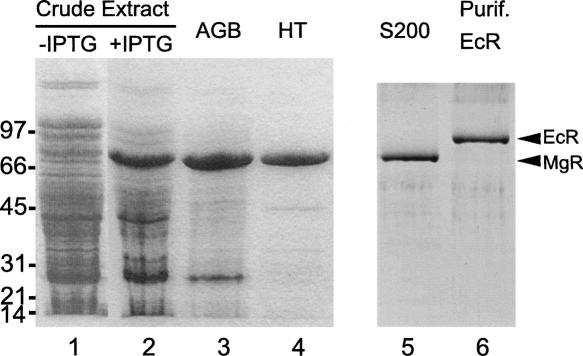
FIGURE 1.
Purification of recombinant M. genitalium RNase R (MgR) from E. coli. About 5 μg of protein from each fraction were denatured, separated on a 4%–15% SDS-polyacrylamide gel, and stained with Coomassie blue. (lane 1) Crude cell extract from culture of Rosetta-gami (DE3)/pLysS harboring pETmgR before IPTG induction; (lane 2) crude extract from culture after IPTG induction; (lane 3) pooled Affi-gel Blue (AGB) peak fractions; (lane 4) flowthrough of hydroxyapatite (HT) column; (lane 5) peak fractions of Sephadex S200 column. In addition, E. coli RNase R (EcR) was purified as described previously (Cheng and Deutscher 2002; Zuo et al. 2006) and is shown in lane 6. The migration positions of molecular mass standards are shown on the left (in kilodaltons). The positions of MgR and EcR are indicated by arrows on the right.