Abstract
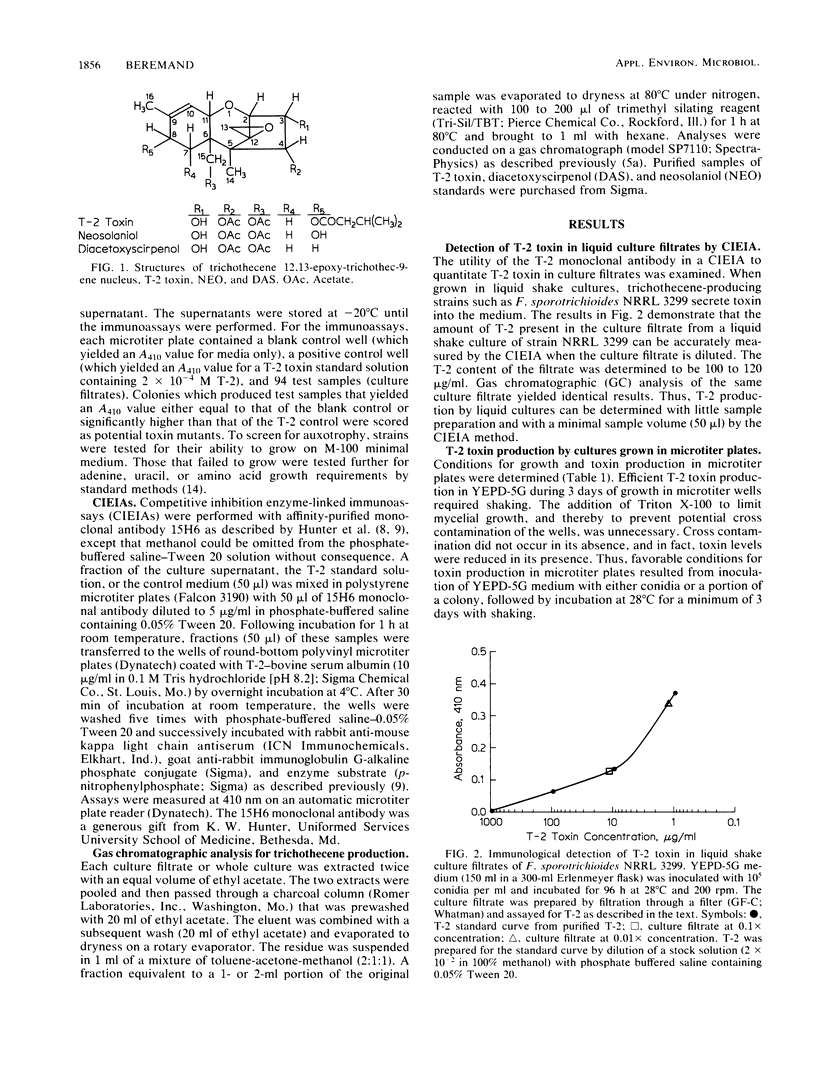
Mutants of Fusarium sporotrichioides NRRL 3299 that were blocked or altered in the biosynthesis of the trichothecene T-2 toxin were generated by UV treatment and identified by a rapid screen in which monoclonal antibodies to T-2 were used. Three stable mutants were isolated and chemically characterized. Two mutants accumulated diacetoxyscirpenol, which suggests that they were defective in the step required for the addition of a hydroxyl group to the C-8 position in the trichothecene core structure. The third mutant appeared to be partially blocked at an early step or regulatory point in the pathway. This represents the first isolation of mutants in a trichothecene biosynthetic pathway.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achilladelis B. A., Adams P. M., Hanson J. R. Studies in terpenoid biosynthesis. 8. The formation of the trichothecane nucleus. J Chem Soc Perkin 1. 1972;11:1425–1428. doi: 10.1039/p19720001425. [DOI] [PubMed] [Google Scholar]
- Avalos J., Casadesús J., Cerdá-Olmedo E. Gibberella fujikuroi mutants obtained with UV radiation and N-methyl-N'-nitro-N-nitrosoguanidine. Appl Environ Microbiol. 1985 Jan;49(1):187–191. doi: 10.1128/aem.49.1.187-191.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen D., Smalley E. B., Dimond R. L. Heterokaryosis in Fusarium tricinctum and F. sporotrichioides. J Gen Microbiol. 1983 Oct;129(10):3035–3041. doi: 10.1099/00221287-129-10-3035. [DOI] [PubMed] [Google Scholar]
- Desjardins A. E., Plattner R. D., Vanmiddlesworth F. Trichothecene Biosynthesis in Fusarium sporotrichioides: Origin of the Oxygen Atoms of T-2 Toxin. Appl Environ Microbiol. 1986 Mar;51(3):493–497. doi: 10.1128/aem.51.3.493-497.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontelo P. A., Beheler J., Bunner D. L., Chu F. S. Detection of T-2 toxin by an improved radioimmunoassay. Appl Environ Microbiol. 1983 Feb;45(2):640–643. doi: 10.1128/aem.45.2.640-643.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter K. W., Jr, Brimfield A. A., Miller M., Finkelman F. D., Chu S. F. Preparation and characterization of monoclonal antibodies to the trichothecene mycotoxin T-2. Appl Environ Microbiol. 1985 Jan;49(1):168–172. doi: 10.1128/aem.49.1.168-172.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter K. W., Jr, Lenz D. E., Brimfield A. A., Naylor J. A. Quantification of the organophosphorus nerve agent soman by competitive inhibition enzyme immunoassay using monoclonal antibody. FEBS Lett. 1982 Nov 22;149(1):147–151. doi: 10.1016/0014-5793(82)81091-0. [DOI] [PubMed] [Google Scholar]
- MACDONALD K. D., HUTCHINSON J. M., GILLETT W. A. ISOLATION OF AUXOTROPHS OF PENICILLIUM CHRYSOGENUM AND THEIR PENICILLIN YIELDS. J Gen Microbiol. 1963 Dec;33:365–374. doi: 10.1099/00221287-33-3-365. [DOI] [PubMed] [Google Scholar]
- Srikrai S., Robbers J. E. Methods for mutation and selection of the ergot fungus. Appl Environ Microbiol. 1983 Apr;45(4):1165–1169. doi: 10.1128/aem.45.4.1165-1169.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno Y., Sawano M., Ishii K. Production of trichothecene mycotoxins by Fusarium species in shake culture. Appl Microbiol. 1975 Jul;30(1):4–9. doi: 10.1128/am.30.1.4-9.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



