Abstract
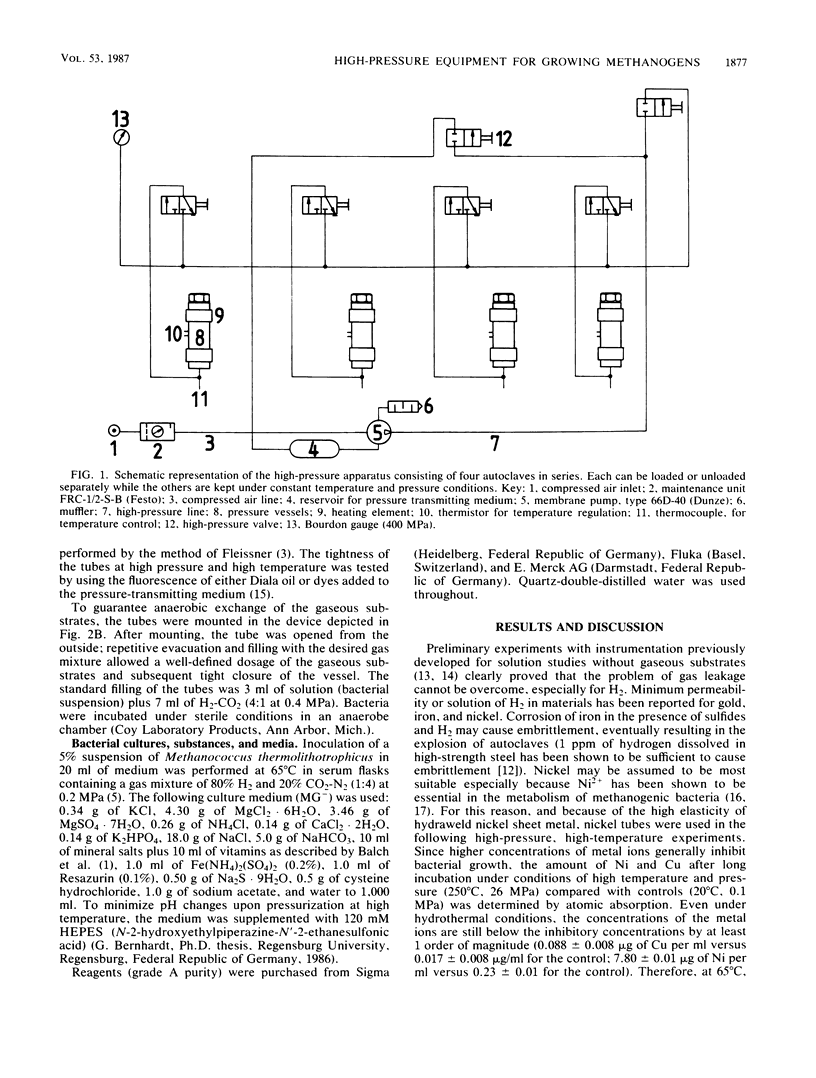
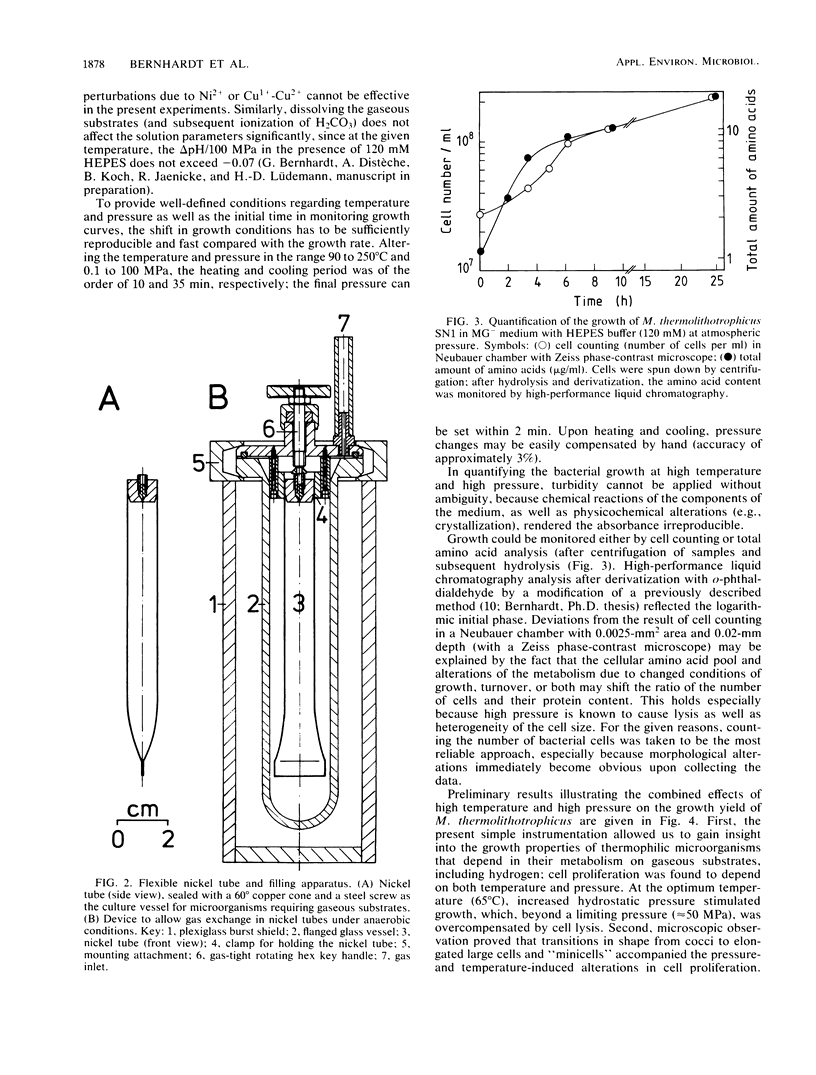
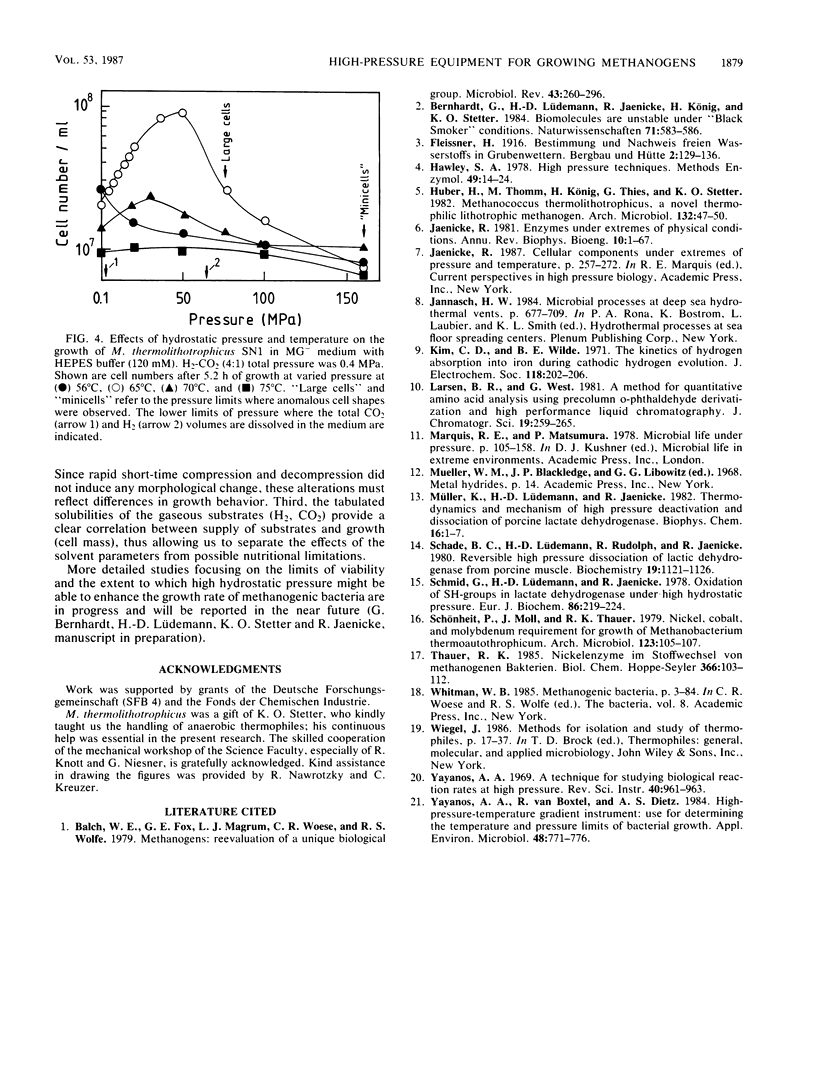
High-pressure, high-temperature investigations on thermophilic microorganisms that grow on hydrogen or other gaseous substrates require instrumentation which provides sufficient substrate for cell proliferation up to 2 × 108 to 3 × 108 cells per ml under isothermal and isobaric conditions. To minimize H2 leakage and to optimize reproducibility at high pressure and high temperature, 10-ml nickel tubes with a liquid/gas ratio of 1:2 were used in a set of autoclaves connected in series. By applying a hydraulic pump and a 2.5-kW heating device, fast changes in temperature (up to 400°C) and pressure (up to 400 MPa) can be accomplished within less than 10 min. To quantify bacterial growth, determinations of cell numbers per unit volume yielded optimum accuracy. Preliminary experiments with the thermophilic, methanogenic archaebacterium Methanococcus thermolithotrophicus showed that bacterial growth depends on both temperature and pressure. At the optimum temperature, increased hydrostatic pressure up to 50 MPa enhanced the growth yield; at a pressure of >75 MPa, cell lysis dominated. Changes in cell proliferation were accompanied by changes in morphology.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balch W. E., Fox G. E., Magrum L. J., Woese C. R., Wolfe R. S. Methanogens: reevaluation of a unique biological group. Microbiol Rev. 1979 Jun;43(2):260–296. doi: 10.1128/mr.43.2.260-296.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley S. A. High-pressure techniques. Methods Enzymol. 1978;49:14–24. doi: 10.1016/s0076-6879(78)49004-4. [DOI] [PubMed] [Google Scholar]
- Jaenicke R. Enzymes under extremes of physical conditions. Annu Rev Biophys Bioeng. 1981;10:1–67. doi: 10.1146/annurev.bb.10.060181.000245. [DOI] [PubMed] [Google Scholar]
- Müller K., Lüdemann H. D., Jaenicke R. Thermodynamics and mechanism of high-pressure deactivation and dissociation of porcine lactic dehydrogenase. Biophys Chem. 1982 Aug;16(1):1–7. doi: 10.1016/0301-4622(82)85001-1. [DOI] [PubMed] [Google Scholar]
- Schade B. C., Rudolph R., Lüdemann H. D., Jaenicke R. Reversible high-pressure dissociation of lactic dehydrogenase from pig muscle. Biochemistry. 1980 Mar 18;19(6):1121–1126. doi: 10.1021/bi00547a013. [DOI] [PubMed] [Google Scholar]
- Schmid G., Lüdemann H. D., Jaenicke R. Oxidation of sulfhydryl groups in lactate dehydrogenase under high hydrostatic pressure. Eur J Biochem. 1978 May;86(1):219–224. doi: 10.1111/j.1432-1033.1978.tb12302.x. [DOI] [PubMed] [Google Scholar]
- Schönheit P., Moll J., Thauer R. K. Nickel, cobalt, and molybdenum requirement for growth of Methanobacterium thermoautotrophicum. Arch Microbiol. 1979 Oct;123(1):105–107. doi: 10.1007/BF00403508. [DOI] [PubMed] [Google Scholar]
- Thauer R. K. Nickelenzyme im Stoffwechsel von methanogenen Bakterien. Vortrag anlässlich der Verleihung der Otto-Warburg-Medaille am 18. September 1984. Biol Chem Hoppe Seyler. 1985 Feb;366(2):103–112. [PubMed] [Google Scholar]
- Yayanos A. A. A technique for studying biological reaction rates at high pressure. Rev Sci Instrum. 1969 Jul;40(7):961–963. doi: 10.1063/1.1684124. [DOI] [PubMed] [Google Scholar]
- Yayanos A. A., van Boxtel R., Dietz A. S. High-pressure-temperature gradient instrument: use for determining the temperature and pressure limits of bacterial growth. Appl Environ Microbiol. 1984 Oct;48(4):771–776. doi: 10.1128/aem.48.4.771-776.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]


