Abstract
The genus Carnobacterium contains nine species, but only C. divergens and C. maltaromaticum are frequently isolated from natural environments and foods. They are tolerant to freezing/thawing and high pressure and able to grow at low temperatures, anaerobically and with increased CO2 concentrations. They metabolize arginine and various carbohydrates, including chitin, and this may improve their survival in the environment. Carnobacterium divergens and C. maltaromaticum have been extensively studied as protective cultures in order to inhibit growth of Listeria monocytogenes in fish and meat products. Several carnobacterial bacteriocins are known, and parameters that affect their production have been described. Currently, however, no isolates are commercially applied as protective cultures. Carnobacteria can spoil chilled foods, but spoilage activity shows intraspecies and interspecies variation. The responsible spoilage metabolites are not well characterized, but branched alcohols and aldehydes play a partial role. Their production of tyramine in foods is critical for susceptible individuals, but carnobacteria are not otherwise human pathogens. Carnobacterium maltaromaticum can be a fish pathogen, although carnobacteria are also suggested as probiotic cultures for use in aquaculture. Representative genome sequences are not yet available, but would be valuable to answer questions associated with fundamental and applied aspects of this important genus.
Keywords: Carnobacterium, natural environment, antimicrobial properties, food spoilage, food safety, probiotics
Introduction
Carnobacteria are ubiquitous lactic acid bacteria (LAB) isolated from cold and temperate environments. More importantly from a practical viewpoint, they also frequently predominate in a range of foods, including fish, meat, and some dairy products. In this regard, they have been extensively studied in the last two decades as protective cultures, to inhibit pathogenic and spoilage microorganisms, and as potential spoilage bacteria in chilled seafood and meat products. In addition, owing to their presence in the aqueous environment, their importance as fish pathogens or probiotic cultures in the aquaculture industry has been examined.
The genus Carnobacterium currently consists of nine species, but only two of these, Carnobacterium divergens and C. maltaromaticum (formerly C. piscicola) are frequently encountered in the environment and in foods (Table 1). This review concerns the importance of these two species in dairy, meat and fish products and in the aquatic environment. The taxonomy and methods for isolation and identification of carnobacteria are not described. It is, however, important to note that older studies frequently used carbohydrate fermentation patterns to distinguish between C. divergens and C. maltaromaticum. These methods are now considered less reliable than molecular methods, and results relying on phenotypic criteria must be interpreted with caution (Hammes & Hertel, 2003; Laursen et al., 2005).
Table 1.
Compilation of Carnobacterium species and known sources*
| Isolated from | |||
|---|---|---|---|
| Species | Isolation frequency | Food products | Environment |
| C. alterfunditum | Very low | Not reported | Live fish, polar lakes/sea, deep sea sediment |
| C. divergens | High | Dairy, meat, fish, shrimp | Intestine of live fish, Sphagnum pond (U)*, alpine permafrost (U) |
| C. funditum | Very low | Not reported | Polar lakes/sea, intestine of live fish, marine sponge |
| C. gallinarum | Low | Meat | Live fish |
| C. inhibens | Very low | Not reported | Atlantic salmon |
| C. maltaromaticum† | High | Dairy, meat, fish, shrimp | Live/diseased fish, moth larval midgut, polar sea, deep sea (U), cold and alkaline tufa columns, Japanese lakes (U), Sphagnum pond (U) |
| C. mobile | Low | Meat, shrimp | Live fish |
| C. pleistocenium | Very low | Not reported | Permafrost tunnel |
| C. viridans | Very low | Meat | Not reported |
| Carnobacterium spp.‡ | NA | See‡ | Andean wetlands (U), Canadian oil sands tailing pond (U), cow rumen (U), deep sea (U), polar areas, pufferfish organs (U), spent mushroom compost, pig effluent-impacted environment (U), watershed polluted with horse manure |
References are quoted in the text except for unpublished (U) studies that include the following accession numbers (EF090688; AM269906; AB248932; AB248935; AB260993; AM711880; EF420230; AY244979; AM111051; DQ778093; DQ337521; DQ337531). In some cases (EF090688, AM269906, AB260993), the source of the sequence was listed as Carnobacterium sp. However, comparison of the 16S rRNA gene sequences with additional carnobacterial 16S rRNA gene sequences in the databases (http://www.ncbi.nlm.nih.gov) allowed us to allocate these sequences to specific carnobacterial species.
Formerly C. pisciciola Mora et al. (2003). Two human clinical isolates of this species are known, Chmelařet al. (2002), Xu et al. (1997); and AF113133. Two clinical isolates of C. divergens have also been described (AY650920).
Examples of habitats described for cultured and noncultured samples. Sequences for samples obtained from foods are, with a few exceptions [e.g. milk (EF204312)], allocated to known species.
NA, not applicable.
Distribution in the natural environment and foods
Natural environment
Among the nine reported species of Carnobacterium, only two species, C. divergens and C. maltaromaticum, are frequently isolated from various sources (Table 1). Four species, C. alterfunditum, C. funditum, C. gallinarum and C. mobile, have been isolated a few times from at most three sources (Table 1). Three species, C. inhibens, C. pleistocenium and C. viridans, have only been isolated from one source (Jöborn et al., 1999; Holley et al., 2002; Pikuta et al., 2005), but it should be noted that Carnobacterium spp. related to, for example, C. inhibens have been described (Simpson et al., 2004). Carnobacterium spp. appear to have both the temperate and polar aquatic environments as habitats including live fish (see also ‘Carnobacteria as pathogenic organisms and/or probiotic cultures’), marine sponges (Li & Liu, 2006), Antarctic lakes (Franzmann et al., 1991; Bratina et al., 1998), Arctic and Antarctic sea water as well as the deep sea (Galkin et al., 1999; Groudieva et al., 2004; Newberry et al., 2004; Toffin et al., 2004; Lauro et al., 2007), aquous alkaline tufa columns from Greenland (Schmidt et al., 2006), and freshwater habitats from the temperate clima zone, including a Sphagnum pond (Leisner et al., unpublished results) and rivers in the northwest region of Spain (González et al., 1999) (Table 1). Although C. maltaromaticum and/or C. divergens have been isolated from tropical fish products, including smoked surubim, a Brazilian tropical freshwater fish (Alves et al., 2005), and from vacuum-packed tuna caught in the Indian Ocean and processed in Sri Lanka (Emborg et al., 2005), it cannot, at least in the latter case, be excluded that this is due to contamination associated with repackaging in Denmark.
The presence of carnobacteria has also been demonstrated in the terrestrial environment, including a field treated with whey (Coombs & Brenchley, 1999), Canadian winter soil (Walker et al., 2006), permafrost ice (Pikuta et al., 2005; Katayama et al., 2007), a compost pile (Ntougias et al., 2004), a collapsed horse manure pile (Simpson et al., 2004), the larval midgut of a moth species (Shannon et al., 2001), and other sources (Table 1). It appears that the temperate/polar aquatic and terrestrial environments are both natural habitats.
Carnobacterium divergens and C. maltaromaticum possess traits that may play a role in their survival in these surroundings. One study has reported that a Carnobacterium sp. soil isolate related to C. maltaromaticum survived 48 serial freeze–thaw cycles better than Escherichia coli and an Enterococcus sp. soil isolate, but worse than a few other soil isolates, including an Acinetobacter sp. (Walker et al., 2006). Indeed, these organisms may survive freezing for considerable periods of time, as witnessed by the isolation of a Carnobacterium sp. preserved in a permafrost ice wedge for 25 000 years (Katayama et al., 2007). Additional data on the abilities of carnobacteria to grow at low temperatures and to survive frozen storage is available for food products. Also, a cold-active β-galactosidase from C. maltaromaticum and a cold-adapted alanine dehydrogenase from a Carnobacterium sp. related to C. alterfunditum have been reported (Coombs & Brenchley, 1999; Galkin et al., 1999). Some carnobacterial isolates originate from natural high-pressure habitats (Lauro et al., 2007), and additional data on resistance to pressure have been reported for high-pressure-processed foods. It has not been described how other physical/chemical parameters such as salt content, atmosphere and pH affect survival and growth of these organisms in the natural environment.
With respect to energy-yielding substrates, one species, C. maltaromaticum, expresses chitinase activity (Leisner & Ingmer, unpublished data). This may facilitate adherence to and survival on zooplankton as suggested for enterococci (Signoretto et al., 2005), and also explain the isolation of this species from the midgut of the larval stage of a species of moth (Shannon et al., 2001). Interestingly, the arginine deiminase pathway is expressed by the most frequently encountered species, C. divergens, C. gallinarum, C. maltaromaticum and C. mobile (e.g. Collins et al., 1987, 2002; Leisner et al., 1994b; Schillinger & Holzapfel, 1995) but not by C. inhibens or C. viridans (Jöborn et al., 1999; Collins et al., 2002; Holley et al., 2002). Arginine is not a substrate that results in growth of C. alterfunditum and C. funditum (Franzmann et al., 1991), and information is not available for C. pleistocenium (Pikuta et al., 2005). This amino acid may represent an additional energy source for growth and survival when carbohydrates are scarce, and it may offer protection against acid stress, as described for Enterococcus faecalis and related species (Marquis et al., 1987). Finally, carnobacteria are able to catabolize a range of carbohydrates, although there are considerable interspecies and intraspecies heterogeneities. Examples of sources of such carbohydrates include animals (e.g. chitin and, to some extent, lactose, although the targets of the lactose hydrolytic activity shown by carnobacteria may instead be byproducts of plant sugar polymers and saccharides adsorbed to humic acid substances in the soil) (Coombs & Brenchley, 2001), plants (e.g. salicin and sucrose), fungi (e.g. chitin and trehalose), prokaryotes (N-acetylglucosamine), and living organisms in general (ribose) (Shaw & Harding, 1984; Lai & Manchester, 2000; Rudi et al., 2004). The importance of these abilities for growth and survival in the environment deserves further study. The genome sizes of C. alterfunditum, C. divergens and C. pleistocenium strains and a Carnobacterium sp. AT7 deep sea isolate have been estimated to be 2.9, 3.2, 3.2 and 2.4 Mb, respectively (Daniel, 1995; Pikuta et al., 2005), and for the AT7 isolate, the data are reported in a database described by Liolios (2006). These sizes are relatively large in comparison to many other LAB, suggesting that carnobacterial genomes may encode a range of genes that makes them well adapted to deal with environmental challenges.
Even if carnobacteria are well adapted to temperate or polar aqueous environments, this does not always provide improved ability to survive as compared to other bacteria. Thus, the actual abilities of strains of C. divergens and C. maltaromaticum isolated from a Sphagnum pond to survive in water from this source were not improved as compared to related gram-positive bacteria originating from other sources (Leisner et al., unpublished data). At present, therefore, the precise mechanisms by which Carnobacterium spp. persist in the natural environment and their underlying genetics are not known. Finally, it should be noted that the environments from which carnobacteria can be isolated are not always as extreme as they appear at first sight. Thus, Carnobacterium spp. isolated from Lake Vanda, Antarctica were found at a depth of 61 m, where the temperature was c. 15–20°C (Bratina et al., 1998).
Dairy, fish and meat products
High concentrations of bacteria (>106–107 CFU g−1) in food are typically required before their activity is sufficient to influence the sensory properties of a product. For this to happen, occurrence and growth kinetics are the key parameters, and databases, including ComBase, are available to determine the effect of food storage conditions and product characteristics on growth of specific bacteria (McMeekin et al., 2006). However, information on carnobacteria has not yet been included in such databases.
Carnobacterium maltaromaticum and another Carnobacterium sp. have been isolated from milk (Miller et al., 1974; EF204312), and they, in addition to C. divergens, have been detected in soft cheeses, including mold-ripened brie (Millière et al., 1994), mozzarella (Morea et al., 1999), Camembert (Cailliez-Grimal et al., 2005) and other types (Millière & Lefebvre, 1994, Cailliez-Grimal et al., 2007). The high concentration of C. divergens in the curd of mozzarella, exposed to 10–37°C during processing, is in agreement with the maximum growth temperature (40°C) of this species (Collins et al., 1987).
The occurrence of Carnobacterium in dairy products (and other foods) is most likely underreported. This is due to the common use of acetate containing media, particularly MRS agar (Oxoid CM361) or Rogosa agar (Oxoid CM0627), for enumeration of LAB (de Man et al., 1960). Growth of Carnobacterium is inhibited by acetate, and both MRS and Rogosa agar media significantly underestimate concentrations in food (Leblanc et al., 1997; Sakala et al., 2002; Hammes & Hertel, 2003; Susiluoto et al., 2003; Chenoll et al., 2007).
Carnobacterium divergens and C. maltaromaticum are present in seafood and are able to grow to high concentrations in different fresh and lightly preserved products. Studies of naturally contaminated products suggest which storage conditions and product characteristics select for carnobacteria as compared to the other bacteria present in seafood. For chilled fresh seafood, we have found no reports where C. divergens and C. maltaromaticum dominated the microbial communities in aerobically stored products, but this has been reported for modified atmosphere-packed (MAP) coalfish, cod, pollack, rainbow trout, salmon, shrimp, swordfish and tuna (Mauguin & Novel, 1994; Leblanc et al., 1997; Emborg et al., 2002, 2005; Franzetti et al., 2003; Rudi et al., 2004). After frozen storage (−20 to −30°C for 5–8 weeks), C. divergens and C. maltaromaticum seem to be particularly prominent in chilled MAP fish, as has been reported for cod, garfish, salmon, and tuna (Guldager et al., 1998; Emborg et al., 2002, 2005; Dalgaard et al., 2006). In addition to freezing, these carnobacteria are relatively resistant to high-pressure processing and are found in high concentrations in vacuum-packed and chilled squid mantle and cold-smoked salmon previously treated with 200–400 MPa for 15–20 min (Paarup et al., 2002; Lakshmanan & Dalgaard, 2004). In vacuum-packed cold-smoked or sugar-salted (‘gravad’) seafood with 3–7% NaCl in the water phase and a pH of 5.8–6.5, high concentrations of C. divergens and C. maltaromaticum are particularly common, as reported for halibut, rainbow trout, salmon, surubim, and tuna (Jeppesen & Huss, 1993; Leisner et al., 1994a; Pilet et al., 1995; Leroi et al., 1998; Lyhs et al., 1998, 2002; Paludan-Müller et al., 1998; Truelstrup Hansen & Huss, 1998; Jørgensen et al., 2000; González-Rodríguez et al., 2002; Alves et al., 2005; Emborg & Dalgaard, 2006). High concentrations of C. maltaromaticum are also reported in salted lumpfish roe, and it has been isolated from frozen, smoked mussels (Basby et al., 1998; Tahiri et al., 2004). Finally, for cooked seafood, high concentrations of C. maltaromaticum have been detected in MAP shrimp after storage at 2–8°C (Mejlholm et al., 2005). Both C. divergens and C. mobile were isolated from cooked and brined MAP shrimps (with NaCl, benzoic acid, citric acid and sorbic acid) after storage at 2–8°C (Dalgaard et al., 2003; Laursen et al., 2005). Clearly, carnobacteria are common in chilled fresh and lightly preserved seafood, but at higher storage temperatures (15–25°C), other species, including Enterococcus spp., more frequently dominate the spoilage microbial community of seafood (Dalgaard et al., 2003).
Carnobacterium divergens and C. maltaromaticum are able to grow in meat products at temperatures as low as 2 to −1.5°C (McMullen & Stiles, 1993; Sakala et al., 2002; Jones, 2004), and they are frequently predominant members (up to 50%C. divergens and up to 26%C. maltaromaticum of the gram-positive or LAB isolates obtained) of the microbial community of raw meat (beef, pork, lamb, and poultry). The two species are found irrespective of whether products have been stored aerobically, vacuum packaged, or subjected to modified atmospheres, including gas compositions of CO2/N2 (%) ranging from 10 : 90 to 80 : 20 (Shaw & Harding, 1984; Grant & Patterson, 1991; McMullen & Stiles, 1993; Barakat et al., 2000; Sakala et al., 2002; Susiluoto et al., 2003; Jones, 2004; Björkroth et al., 2005; Laursen et al., 2005; Vihavainen et al., 2007). One study demonstrated the presence of C. divergens in beef stored in air but not in MAP beef with increased concentrations of O2 (20–40%) in addition to CO2 (40%) (Ercolini et al., 2006). The growth of C. funditum is impaired by oxygen (Franzmann et al., 1991), and it will be of interest to study further whether addition of O2 to the gas composition of modified atmospheres consistently inhibits growth of carnobacteria, as has been observed for some other bacteria (Emborg et al., 2005).
Further studies are needed in order to determine whether differences in the presence of carnobacteria in meat are due to variations in storage conditions or variations in contamination levels at the processing plants. The source of carnobacteria in meat products is most probably the processing plant, as these organisms have not been isolated from the gastrointestinal system or skin of chicken, cattle, pigs or sheep, except for one unpublished study (cow rumen, AY244979). Thus, the source of contamination of broiler carcasses by C. divergens and C. maltaromaticum was shown to be the air in the processing plant and not incoming broiler chickens (Vihavainen et al., 2007). In fact, two studies confirmed that C. divergens and C. maltaromaticum present in the raw material were eliminated or reduced in number by cooking of a processed meat product, ham (Samelis et al., 1998, 2000).
Carnobacterium divergens and C. maltaromaticum have, however, been detected in a variety of processed meat products, including the cured pork product bacon (Shaw & Harding, 1984), ham (Borch & Molin, 1988; Jack et al., 1996), a Danish processed pork product (‘rullepølse’) (Laursen et al., 2005 and unpublished results), various Spanish processed meat products (Chenoll et al., 2007), cooked poultry meat (Barakat et al., 2000), pressure-treated (408–888 MPa) chicken (O'Brien & Marshall, 1996), and irradiated pork and chicken (Grant & Patterson, 1991). On a few occasions, C. maltaromaticum has even been isolated from fermented sausages (Schillinger & Lücke, 1987; Larrouture-Thiveyrat et al., 2003); as noted by Hammes & Hertel (2003), this contrasts with the usual nonaciduric habitats of carnobacteria. Other Carnobacterium spp. isolated from processed meat products include C. gallinarium (irradiated chicken) (Thornley, 1957; Collins et al., 1987), C. mobile (cooked turkey) (Chenoll et al,. 2007) and C. viridans (vacuum-packed Bologna sausage) (Holley et al., 2002). Although these organisms are frequently isolated from processed meat products, it has been suggested that they are rarely present in high numbers, and that terminal spoilage of cooked, cured meats is primarily caused by aciduric LAB (Samelis, 2006; Chenoll et al., 2007).
Finally, a Carnobacterium sp. has also been isolated from egg contents, and it was shown that this isolate had a similar ability to penetrate the eggshell and contaminate the whole egg as various other gram-positive and gram-negative bacteria (De Reu et al., 2006).
Transmission routes for carnobacteria from the natural environment into food-manufacturing plants and further on to dairy, fish and meat products are not known to any extent. Knowledge on this topic is essential to evaluate if species and intraspecific clusters that differ in spoilage ability (as discussed later) also have different colonization abilities. Such knowledge would also illuminate the feasibility of using the production of metabolites, especially tyramine, by C. divergens and C. maltaromaticum as an index of microbial spoilage of specific fish and meat products (Edwards et al., 1987; Dainty & Mackey, 1992; Leisner et al., 1994a; Dainty, 1996; Jørgensen et al., 2000; Emborg et al., 2002; Laursen et al., 2006).
Functional properties of carnobacteria
Bacteriocins and antimicrobial properties
Carnobacteria with the ability to produce antimicrobial peptides, bacteriocins, are commonly encountered in foods (e.g. Lewus et al., 1991; Coventry et al., 1997). The characterized carnobacterial bacteriocins belong to class I and class II [e.g. Drider et al. (2006) for bacteriocin nomenclature]. So far, only one class I bacteriocin (lantibiotic), UI149, has been reported to be produced by Carnobacterium (Stoffels et al., 1992a, b). In contrast, ten amino acid-sequenced bacteriocins belong to class II, and most of these more specifically to class IIa, which comprises small pediocin-like peptides (Table 2). The inhibition spectrum of carnobacterial class IIa bacteriocins includes Listeria, and antimicrobial activity is exerted by pore formation, dissipation of membrane potential, and leakage of internal low molecular weight substances (Suzuki et al., 2005; Drider et al., 2006).
Table 2.
Bacteriocins produced by carnobacteria
| Species | Bacteriocin (class)* | Gene | Unprocessed precursor/ mature chain† | Location of gene | Accession number | References |
|---|---|---|---|---|---|---|
| Cm‡ | Carnobacteriocin A (IIc) | cbnA | 71/53 | Plasmid | P38578 | Worobo et al. (1994) |
| Piscicolin 61§ | Holck et al. (1994) | |||||
| Carnobacteriocin BM1 (IIa) | cbnBM1 | 61/43 | Chromosome | P38579 | Quadri et al. (1994) | |
| Carnobacteriocin B1§ | ||||||
| Piscicocin V1b§ | Bhugaloo-Vial et al. (1996) | |||||
| Carnocin CP51§ | Herbin et al. (1997) | |||||
| Carnobacteriocin B2 (IIa) | cbnB2 | 66/48 | Plasmid | P38580 | Quadri et al. (1994) | |
| Wang et al. (1999) | ||||||
| Carnocin CP52§ | Herbin et al. (1997) | |||||
| A9b§ | Nilsson et al. (2002) | |||||
| Piscicolin 126 (IIa) | pisA | 62/44 | Chromosome | P80569 | Jack et al. (1996) | |
| Piscicocin V1a§ | Bhugaloo-Vial et al. (1996) | |||||
| Gursky et al. (2006) | ||||||
| Piscicocin CS526 (IIa) | ND | X/≥43 | ND | Yamazaki et al. (2005) | ||
| Cd | Divercin V41 (IIa) | dvn41 | 66/43 | Chromosome | CAA11804 | Métivier et al. (1998) |
| Divergicin M35 (IIa) | ND | X/43 | ND | P84962 | Tahiri et al. (2004) | |
| Divergicin A (IIc) | dvnA | 75/46 | Plasmid | AAZ29031 | Worobo et al. (1995) | |
| van Belkum & Stiles (2006) | ||||||
| Divergicin 750 (IIc) | dvn750 | 63/34 | Plasmid | 2209292A | Holck et al. (1996) | |
| Carnobacterium sp. | Carnocin H (II) | ND | Partial sequence¶ | ND | Blom et al. (2001) | |
| Carnocin UI149 (I) | ND | Partial sequence∥ | ND | P36960 | Stoffels et al. (1992b) |
Classification of bacteriocins based on review by Drider et al. (2006).
Numbers of amino acids.
Carnobacterium divergens (Cd), Carnobacterium maltaromaticum (Cm).
Synonyms.
Approximately 75 amino acids.
35–37 amino acids.
ND, not determined.
Class IIa bacteriocins are ribosomally synthesized as inactive prepeptides that are modified by posttranslational cleavage of the N-terminal peptide leader at a double-glycine site in order to release mature and active cationic peptides. Carnobacterial class IIa bacteriocins (Table 2) have similar amino acid sequences, with a YGNGV(X)C(X)4C motif (X denotes any amino acid) near the N-terminus of the mature peptide. Two cysteine residues form a disulfide bond in the N-terminal region, and there is an amphipathic α-helix near the C-terminus. The N-terminal region is relatively hydrophilic and conserved, whereas the C-terminus is hydrophobic and diverse. To establish the structure–activity relationships of carnobacterial bacteriocins, the structures of carnobacteriocin B2 and divercin V41 were modified (Quadri et al., 1997a; Bhugaloo-Vial et al., 1999). Thus, amino acid substitutions closer to the N-terminus in carnobacteriocin B2 drastically reduced or eliminated antimicrobial activity, whereas this was not so for substitutions close to the C-terminal part (Quadri et al., 1997a). Divercin V41 contains a second disulfide bond located in the C-terminal region (Métivier et al., 1998). When the C-terminal region of divercin V41 was separated from the N-terminus by endoproteinase Asp-N, only the C-terminal fragment was active. After trypsin cleavage next to lysine at position 42 or disulfide reduction, the C-terminus lost its inhibitory activity. These results suggested that both hydrophobicity and folding imposed by this second disulfide bond were essential for antilisterial activity of the C-terminal hydrophobic peptide (Bhugaloo-Vial et al., 1999). Chemical oxidation of tryptophan residues by N-bromosuccinimide showed that these residues were crucial for inhibitory activity, as modification of any one of them rendered divercin V41 inactive (Bhugaloo-Vial et al., 1999). The three-dimensional structure of carnobacteriocin B2, its precursor and the corresponding immunity protein have been solved by nuclear magnetic resonance (Wang et al., 1999; Sprules et al., 2004a, b). These are, at present, the only reported carnobacterial protein structures.
Carnobacterial class II bacteriocins that contain a double-glycine-type leader peptide are transported by a dedicated ATP-binding cassette (ABC) transport system (Drider et al., 2006). In contrast, divergicin A does not require a secretion protein, as the transport depend on the general cellular secretion (sec) pathway (Worobo et al., 1995).
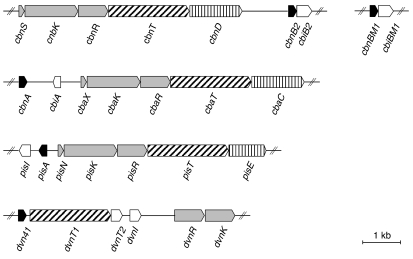
The genes involved in carnobacterial bacteriocin production are generally clustered in operons. In the simplest case, corresponding to the sec-dependent divergicin A, the bacteriocin operon is composed of only the structural gene followed downstream by the gene encoding an immunity protein that protects the cell from is own bacteriocin (Worobo et al, 1995). Production of class IIa bacteriocins requires four genes as a minimum, including genes encoding bacteriocin, immunity protein, ABC of transport protein and its membrane-bound accessory protein. Genes for bacteriocin production may be encoded on the chromosome or on plasmids (Table 2). Carnobacterium maltaromaticum LV17 produces at least three class II bacteriocins. Carnobacteriocins A and B2 are encoded on different but compatible plasmids, pCP49 (72 kb) and pCP40 (61 kb), respectively. The carnobacteriocin BM1 structural gene and its immunity gene are localized on the chromosome. Activation and export of carnobacteriocin BM1 depend on genes located on plasmid pCP40 encoding carnobacteriocin B2 (Quadri et al., 1997b; Rohde & Quadri, 2006). For carnobacteriocins BM1 and B2, a peptide-pheromone dependent quorum-sensing mode caused by the bacteriocin itself or an autoinducer peptide is involved in the regulation of bacteriocin production by two- and three-component signal transduction systems (Saucier et al., 1995; Kleerebezem et al., 2001; Nilsson et al., 2002; Rohde & Quadri, 2006). The components of this regulatory system consist of an induction factor, a histidine protein kinase, and a response regulator. Four carnobacterial bacteriocin operons including genes for immunity proteins and regulatory proteins involved in bacteriocin expression and secretion have been sequenced (Fig. 1).
Fig. 1.
Gene loci involved in carnobacteriocins B2, BM1, A, piscicolin 126 and divercin V41 production and immunity. First line, left: carnobacteriocin B2 locus (plasmid pCP40, accession number L47121). First line, right: carnobacteriocin BM1 chromosomal locus (L29058). Second line: carnobacteriocin A locus (plasmid pCP49, AF207838). Third line: piscicolin 126 chromosomal locus (AF275938). Fourth line: divercin V41 chromosomal locus (AJ224003). Genes cbnB2, cbnBM1, cbnA, pisA and dvn41 encode the precursor bacteriocins (colored in black). Genes cbiB2, cbiBM1, cbiA, pisI, dvnT2 and dvnI encode immunity proteins (colored in white). Genes cbnT, cbaT, pisT and dvnT1 encode ABC transporter (italic hatching). Genes cbnD, cbaC and pisE encode transporter accessory protein (vertical hatching). The loci of carnobacteriocins B2, A and piscicolin 126 contain the cbnS–cbnK–cbnR, cbaX–cbaK–cbaR and pisN–pisK–pisR three-component regulatory system gene clusters, respectively (colored in gray). Only histidine protein kinase and response regulator protein encoded by dvnK–dvnR are found in the divercin locus (colored in gray).
External parameters also affect bacteriocin production. Thus, increasing concentrations of NaCl (2–7%) reduced production of A9b/B2 bacteriocin (Himelbloom et al., 2001; Nilsson et al., 2002), increasing the temperature from below 19 to 25°C inhibited production of piscicolin 126 (Gursky et al., 2006), and reducing the pH from 6.5 to 5.5 inhibited production of carnobacteriocins BM1 and B2 (Ahn & Stiles, 1990). Acetate (A9b bacteriocin) (Nilsson et al., 2002) or the presence of a bacteriocin-sensitive strain belonging to the same genus (Sip et al., 1998) induced bacteriocin production. It is clear that, in order to apply carnobacterial bacteriocins for the biopreservation of foods, detailed knowledge of the factors that influence production of the bacteriocin is necessary. Finally, it should be noted that the bacteriocinogenic activity may be lost, as shown for a C. divergens divercin V41-producing strain that retained only partial activity after being subjected to spray-drying (Silva et al., 2002).
Interest in applying carnobacterial bacteriocins in foods and feeds has been directed towards inhibiting Listeria monocytogenes (Table 3) and spoilage microorganisms, to extend the shelf-life of lightly preserved seafood (Einarsson & Lauzon, 1995). These attempts have generally, but not always [e.g. regarding prevention of spoilage, Einarsson & Lauzon (1995) and Roller et al. (2002)], met with success, although diversity in sensitivity of the target organism has been observed (Brillet et al., 2004). However, the occurrence of resistant L. monocytogenes target organisms has led to the suggestion that bacteriocin-negative LAB may be more suitable for practical use as bioprotective agents against L. monocytogenes in ready-to-eat foods (Nilsson et al., 2004; Vermeiren et al., 2006). Indeed, L. monocytogenes is inhibited by carnobacterial cultures that do not produce bacteriocins, and this is partly due to glucose depletion (Buchanan & Bagi, 1997; Nilsson et al., 1999, 2005). One study also reported a successful application of cultures with no demonstrated bacteriocinogenic activity for extension of the shelf-life of vacuum-packed cold-smoked salmon (Leroi et al., 1996). This effect was not, however, observed for a C. divergens strain that was unable to produce the class IIa divercin V41 (Richard et al., 2003).
Table 3.
Applications of antagonistic strains of Carnobacterium spp. against Listeria monocytogenes* in dairy, meat or fish food and feed products
| Product (Species) | Mechanism | Reference |
|---|---|---|
| Dairy | ||
| UHT milk (Cm) | Bacteriocin, other? | Buchanan & Klawitter (1992) |
| Whole milk (Cm) | Piscicolin 126 | Wan et al. (1997) |
| Camembert cheese (Cm) | Piscicolin 126 | Wan et al. (1997) |
| Fish and shellfish | ||
| Cold-smoked salmon (Cd) | Divercin V41 | Duffes et al. (1999a, b) |
| Connil et al. (2002) | ||
| Cold-smoked salmon (Cm) | Piscocin V1, bacteriocin | Duffes et al. (1999a, b) |
| Cold-smoked salmon (Cm) | Carnobacteriocin B2, other | Nilsson et al. (1999, 2004) |
| Cold-smoked salmon (Cm) | Piscicocin CS526, other? | Yamazaki et al. (2003) |
| Pasteurized crabmeat (Cm) | Bacteriocin, other? | Buchanan & Klawitter (1992) |
| Meat | ||
| Beef steaks (Cm) | Bacteriocinogenic substance | Schöbitz et al. (1999) |
| Canned dog food (Cm) | Bacteriocin, other? | Buchanan & Klawitter (1992) |
| Cooked chicken (Cm) | Bacteriocin, other | Campos et al. (1997) |
| Frankfurters (Cm) | Bacteriocin, other? | Buchanan & Klawitter (1992) |
| Ham paste (Cm) | Piscolin 126 | Jack et al. (1996) |
| Ground meat (Cm) | Piscolin CS526 (freeze dried) | Azuma et al. (2007) |
| Sterile raw ground beef (Cm) | Bacteriocin, other? | Buchanan & Klawitter (1992) |
| Vegetables | ||
| Canned creamed corn (Cm) | Bacteriocin, other? | Buchanan & Klawitter (1992) |
Studies using Listeria innocua as target organism e.g. Roller et al. (2002) have not been included in the table.
Cd, Carnobacterium divergens; Cm, Carnobacterium maltaromaticum.
Resistance of L. monocytogenes to the class IIa divergicin M35 was probably due to modification of the cell wall fatty acid composition (Naghmouchi et al., 2006). Listeria monocytogenes strains resistant to the class IIa divercin V41 also showed substantial differences in protein expressions as compared with the wild type strain (Duffes et al., 2000). A σ54-dependent PTS permease of the mannose family (EIItMan), which belongs to the phosphotransferase system (PTS), is responsible for sensitivity of L. monocytogenes to class IIa bacteriocins. These results suggested that EIItMan encoded by the mptACD operon might be a target molecule for class IIa bacteriocins (Dalet et al., 2001; Gravesen et al., 2002). Recently, two genes coding for a glycerophosphoryl diester phosphodiesterase (GlpQ) and a protein with a putative phosphoesterase function (Pde) were identified as also being involved in the class IIa sensitivity of En. faecalis (Calvez et al., 2007). Finally, it is important to note that the susceptibility of the target strain is affected by various environmental conditions such as NaCl and pH (Lebois et al., 2004).
Another cause of concern when using carnobacteria for biopreservation is that C. divergens and C. maltaromaticum both produce tyramine, as discussed further below. Therefore, it has been suggested that a C. divergens strain in which the tyrosine decarboxylase gene is inactivated by mutagenesis could be used as a protective culture to prevent growth of L. monocytogenes in cold-smoked salmon (Brillet et al., 2006). Currently, no carnobacterial culture, bacteriocin producing or not, is commercially applied for protection against the growth of L. monocytogenes or other bacterial pathogens or spoilage organisms in food.
Effect of catabolic activities on sensory characteristics and safety of foods
As shown in Table 4, the catabolic activities of carnobacteria may result in sensory spoilage of inoculated fish and meat products. Whether this is so for cheese products is not clear. In naturally contaminated products, it seems other members of the bacterial community are typically more important with regard to sensory effects, including spoilage. It is of interest that spoilage was enhanced if moderate-spoilage strains of C. maltaromaticum were inoculated with nonspoilage Vibrio sp. strains or the moderate-spoilage organism Brochothrix thermosphacta into cold-smoked salmon that was subsequently vacuum-packaged (Joffraud et al., 2006). However, this was not so for combinations of C. maltaromaticum and Photobacterium phosphoreum in this product. In addition, a spoilage synergy effect was observed for combinations of B. thermosphacta and C. divergens, C. maltaromaticum or C. mobile in MAP shrimp (Mejlholm et al., 2005; Laursen et al., 2006).
Table 4.
Sensory effect of Carnobacterium spp. inoculated into dairy, fish or meat products
| Food (storage conditions) | Species | Spoilage (sensory effect) | Important metabolites produced | Reference |
|---|---|---|---|---|
| Dairy | ||||
| Skimmed milk | Cm | Spoiled? (Malty aroma/flavor) | Branched alcohols and aldehydes* | Miller et al. (1974) |
| Fish and shellfish | ||||
| Cold-smoked salmon | ||||
| Vacuum, 4–8°C | Cd, Cm | Not or weakly spoiled (cheese/feet in some cases) | Not examined | Brillet et al. (2005) |
| Vacuum, 4–8°C | Cd, Cm | Not spoiled | Not examined | Duffes et al. (1999b) |
| MAP or vacuum, 5°C | Cm | Not spoiled | Not examined | Paludan-Müller et al. (1998) |
| Vacuum, 5°C | Cm | Not spoiled | Not examined | Nilsson et al. (1999) |
| Vacuum, 6°C | Cm | Not spoiled (butter, caramel, sour, fruity) | 2,3-Butanedione, 2,3-pentanedione | Joffraud et al. (2001) |
| Vacuum, 6°C | Cm | Not spoiled [butter/plastic, rubbery and neutral (green/cooked meat)] | Not examined | Stohr et al. (2001) |
| Vacuum, 8°C | Cm | Lightly spoiled (grassy, fruity notes) | Not examined | Joffraud et al. (2006) |
| Shrimp (MAP, 5°C) | Cd, Cm | Spoiled (chlorine, chemical, malty, nutty, sour) | Ornithine, ammonia, acetic acid, alcohols, aldehydes, ketones, 2,4,6-trimethylpyridine | Mejlholm et al. (2005),Laursen et al. (2006) |
| Cm (L)† | Not spoiled (grass/hay, weak chlorine) | Ornithine, ammonia, acetic acid, alcohols, aldehydes, ketones | Laursen et al. (2006) | |
| Cmo | Not spoiled (yogurt-like) | Organic acids, alcohols, ketones | Laursen et al. (2006) | |
| Meat | ||||
| Beef | ||||
| Vacuum, 2°C | Cm | Not spoiled/spoiled‡ | Not examined | Leisner et al. (1995) |
| Vacuum, 4°C, normal pH | Cd | Not indicated (butter, acid) | Not examined | Borch et al. (1996) |
| Vacuum, 4°C, high pH | Cd | Not indicated (acid, slightly sulfurous) | Not examined | Borch et al. (1996) |
| In air, 7°C | Cm | Spoiled | Not examined | Leisner et al. (1995) |
| Cured bologna (4 or 9°C) | Cv | Green discoloration | Presumably by H2O2 | Peirson et al. (2003) |
| Ham, cooked, sliced (vacuum, 5 or 7°C) | Cm | Spoiled | Branched alcohols and aldehydes | Budde et al. (2003) |
| Cooked, minced sausage (24–15°C) | Cm | Sausage/fatty odor | α-Ketoisocaproic acid, hydroxy-α-ketoisocaproic acid, 3-methylbutanoic acid | Larrouture-Thiveyrat et al. (2003) |
2-Methylpropanal, 2-methylpropanol, 3-methylbutanal, 3-methylbutanol (both ham and milk) and 2-methylbutanal (ham). For milk, the production of these compounds was determined in a laboratory medium and not milk per se.
Phenotypical cluster L (Laursen et al., 2005).
Spoilage observed at an initial density of log 4 CFU cm−2 but not at log 2 CFU cm−2.
Cd, Carnobacterium divergens; Cm, Carnobacterium maltaromaticum; Cmo, Carnobacterium mobile; Cv, Carnobacterium viridans.
We will first focus on catabolic reactions with carbohydrates and/or organic acids as substrates and with pyruvic acid as an intermediate metabolite. Respiration might occur in the presence of hematin, as shown for C. maltaromaticum (Meisel et al., 1994), and, indeed, this species consumes substantial proportions of oxygen during exponential growth under aerobic conditions (Borch & Molin, 1989). Carnobacterium spp. are, however, considered to be homofermentative organisms that produce lactic acid from glucose. The presence of the glycolytic pathway in C. divergens has been demonstrated (de Bruyn et al., 1987, 1988). It can be debated whether carnobacteria should instead be considered facultative or atypical heterofermentative organisms, as C. divergens and C. maltaromaticum are able to utilize ribose and gluconic acid as substrates for growth, and may produce acetic acid, formic acid, and CO2 (e.g. Shaw & Harding, 1984; de Bruyn et al., 1988; Borch & Molin, 1989; Leisner, 1992) as end-products of some secondary decarboxylation/dissimilation reactions of pyruvic acid (de Bruyn et al., 1988; Schillinger & Holzapfel, 1995). Indeed, carnobacteria were initially described as heterofermenters (Collins et al., 1987). Acetic acid production by C. divergens and C. maltaromaticum can be substantial during growth in laboratory media under aerobic conditions or in MAP shrimp, and quantitatively it can exceed lactic acid production (Borch & Molin, 1989; Laursen et al., 2006). Production of acetic acid by C. maltaromaticum is also increased relative to lactic acid if glucose is substituted by ribose (Leisner, 1992). Furthermore, C. maltaromaticum can produce large amounts of ethanol from glucose and ribose during growth in a shrimp extract under anaerobic conditions (Leisner, 1992).
Acetoin can be generated by C. maltaromaticum from pyruvic acid (Borch & Molin, 1989), and this reaction is also found for C. divergens and C. gallinarum but not for C. alterfunditum, C. funditum, C. inhibens and C. viridance, according to reactions in the Voges–Proskauer test (Collins et al., 2002; Holley et al., 2002). Carnobacterium mobile gives variable reactions, and information is not available for C. pleistocenium (Collins et al., 1987; Pikuta et al., 2005). The factors affecting acetoin production are not well known, but production is increased by resting cells of C. maltaromaticum in the presence of hematin (Meisel et al., 1994). In addition, two out of four strains of C. divergens and C. maltaromaticum isolated from mozzarella cheese were reported to be able to metabolize citric acid, but the potential sensory role of this reaction, e.g. by production of acetoin and diacetyl, was not examined (Morea et al., 1999). Production of diacetyl and 2,3-pentanedione by C. maltaromaticum during growth in cold-smoked salmon resulted in a butter-like odor but not in spoilage (Joffraud et al., 2001) (Table 4). In conclusion, even, if carbohydrate catabolism by carnobacteria appears to result in a diverse number of metabolites, these have generally a limited effect on the sensory attributes of foods. H2O2 may be produced by C. divergens (Borch & Molin, 1989), and formation of this compound by C. viridans has been associated with spoilage in the form of green discoloration of cured Bologna ham (Table 4).
Whether carnobacteria possess proteolytic activities of potential importance for taste in products such as cheese is not known, and this deserves further investigation. In contrast, metabolites resulting from the degradation of amino acids certainly cause sensory effects in foods (Table 4). Generation of branched alcohols and aldehydes (2-methyl-1-butanal, 2-methyl-1-butanol, 3-methyl-1-butanal, 3-methyl-1-butanol, 2-methylpropanal, and 2-methylpropanol) by transamination, decarboxylation and reduction of the amino acids valine, leucine and isoleucine appears to be particularly important. Production of some of these compounds, such as 3-methyl-1-butanal, is strain or species dependent (Larrouture et al., 2000). Production by a C. maltaromaticum strain of 3-methylbutanal, 3-methylbutanol and 3-methylbutanoic acid from leucine was, in general, increased at pH values of 6.5 or higher and in the presence of increased concentrations of α-ketoisocaproic acid and glucose (Larrouture-Thiveyrat & Montel, 2003). This strain affected the odor and reduced the leucine content of a sausage mince, but a clear causal relationship was not demonstrated (Larrouture-Thiveyrat et al., 2003). Production by C. maltaromaticum of alcohols and aldehydes from valine, leucine and/or isoleucine resulted in a malty, green aroma in skimmed milk and shrimp, and has also caused spoilage of cured ham (Table 4). The association between the presence of these compounds and spoilage is, however, not always straightforward, as production of 3-methyl-1-butanal and/or 3-methyl-1-butanol in shrimp by certain C. maltaromaticum strains did not result in malty off-flavors (Laursen et al., 2006).
Production of NH4+ from arginine as a result of its catabolism catalyzed by the arginine deaminase pathway (Leisner et al., 1994b) may, in theory, cause spoilage of shrimp or squid that contain high levels of free arginine (Asakawa et al., 1981; Hirano et al., 1992). Carnobacterium maltaromaticum and C. divergens but not C. mobile were able to produce NH4+ in MAP shrimp, but this was not the cause of spoilage of this product (Laursen et al., 2006).
Production of indole from the amino acid tryptophan is also a potential cause of spoilage, although C. divergens, C. maltaromaticum and C. mobile are all unable to carry out this reaction in standard laboratory media (Laursen et al., 2006). It has, however, been observed that C. divergens and strains belonging to a major phenotypic cluster of C. maltaromaticum were able to use tryptophan as a substrate during growth in MAP shrimp (Laursen et al., 2006). Whether this resulted in generation of indole was not examined.
Production of tyramine from tyrosine is the only known metabolic reaction by Carnobacterium spp. that constitutes a cause of concern regarding food safety. Not all carnobacterial species possess this ability, as C. mobile does not produce tyramine during growth in shrimp, and variation exists among different strains and phenotypic clusters of C. divergens and C. maltaromaticum for amounts of tyramine produced (Leisner et al., 1994a; Masson et al., 1996; Bover-Cid & Holzapfel, 1999; Laursen et al., 2006). Tyramine production by one C. divergens strain was at its maximum during the stationary growth phase and at a low initial pH in the presence of 0.6% glucose, whereas NaCl (10%) was inhibitory (Masson et al., 1997). Regardless of strain variation and the effects of environmental parameters, tyramine production by C. divergens and/or C. maltaromaticum has been reported in a range of foods, including meat (up to 28 mg kg−1) (Edwards et al., 1987), a meat–fat mixture (up to 121 mg kg−1) (Masson et al., 1999), cold-smoked salmon (up to c. 370 mg kg−1) (Duffes et al., 1999b; Jørgensen et al., 2000; Connil et al., 2002; Brillet et al., 2005), frozen and thawed salmon (up to 40–60 mg kg−1) (Emborg et al., 2002), and shrimp (up to 20–60 mg kg−1) (Laursen et al., 2006). These levels have no adverse effects on most consumers, but for sensitive individuals, e.g. with reduced monoamine oxidase activity due to medication or hereditary deficiency, very little tyramine can cause migraine headaches, and an intake of no more than 5 mg of tyramine per meal has been recommended (McCabe, 1986). Typical consumption of fish and meat products is 50–150 g per meal. As described above, C. divergens and/or C. maltaromaticum are able to form c. 20–370 mg tyramine kg−1 in these products, corresponding to c. 1–55 mg of tyramine per meal. Consequently, tyramine formation by carnobacteria in specific foods can represent a hazard for sensitive individuals who might suffer migraine headaches. This risk for sensitive individuals can be minimized by restricting their consumption of fish and meat to products that are newly processed, i.e. that are not close to the declared shelf-life expiry day.
Growth of Carnobacterium spp. in food products may result in accumulation of a range of volatile alcohols, ketones, hydrocarbons, and other compounds (Laursen et al., 2006). The metabolic reactions leading to these products have not been studied in detail for Carnobacterium, in contrast to the situation for other LAB (Smit et al., 2005), but may be a result of NAD+-generating reactions or nonenzymatic reactions. Several ketones have characteristic odors, but whether they contribute to spoilage off-flavors as a result of carnobacterial metabolic activity is not well understood. With regard to lipids, C. maltaromaticum has been reported to hydrolyze tributyrin, a triglyceride with butyrate as fatty acid (Papon & Talon, 1988), but it is not known whether this lipase activity contributes to flavor changes of dairy, fish and meat products. Carnobacterium divergens and C. maltaromaticum were not able to limit oxidation of linoleic acid during growth (Talon et al., 2000). This indicates that these species may not prevent quality deterioration of meat products by lipid oxidation. Finally, C. maltaromaticum has been shown to cause a softer texture of salmon fillets when inoculated in high numbers (Morzel et al., 1997).
It is worth noting that interspecies and intraspecies differences exist regarding carnobacterial spoilage capacity. Laursen et al. (2006) showed that C. mobile and a minor phenotypic cluster of C. maltaromaticum did not spoil MAP shrimp, in contrast to what was seen with C. divergens and the major phenotypic cluster of C. maltaromaticum. It would be of interest to examine whether similar heterogeneities exist for Carnobacterium spp. growing in other dairy, fish and meat products. As environmental parameters readily affect the metabolism of carbohydrates and amino acids, it is not surprising that product-specific and storage-specific conditions also play important roles. The ability of two C. maltaromaticum strains to relatively rapidly spoil meat during growth under aerobic conditions at 7°C but not or only after a long storage period in vacuum packs at 2°C serves as an example (Table 4) (Leisner et al., 1995).
Carnobacteria as pathogenic organisms and/or probiotic cultures
Two human clinical cases caused by opportunistic infection with C. maltaromaticum and a Carnobacterium sp. have been described (Xu et al., 1997; Chmelar et al., 2002). Two clinical isolates of C. divergens have also been obtained (accession number AY650920). Carnobacteria are not known members of the human gastrointestinal microbial community, unlike several other LAB. The exceptions are an unpublished study reporting an uncultured Carnobacterium sp. clone from cow rumen (AY244976) and two studies demonstrating the presence of carnobacteria in pig and horse effluent-impacted environments (DQ337521; DQ337531) (Simpson et al., 2004). Nevertheless, it is safe to conclude that the presence of Carnobacterium spp. in food does not present a risk factor for human illness, except for the production of the biogenic amine, tyramine, as discussed previously. Neither do Carnobacterium spp. appear to present a risk for nocosomial infections in hospitals and similar institutions, although they have, in one instance, been isolated from contaminated blood plasma (Thornley & Sharpe, 1959).
The presence of virulence factors in carnobacteria is not well documented. Thus C. maltaromaticum strains isolated from diseased fish lacked hemolytic and phospholipolytic activities (Hammes & Hertel, 2003). Carnobacterium viridans shows, however, β-hemolytic activity on sheep blood agar (Holley et al., 2002). Although several isolates of carnobacteria produce bacteriocins, none of these compounds has been reported to exert a cytolytic activity as shown for the En. faecalis class I-related bacteriocin cytolysin (Gilmore et al., 1994). Regarding chemotherapeutic agents, fish pathogenic strains of C. maltaromaticum were resistant to several of the agents widely used in aquaculture, such as oxytetracycline, quinolones, nitrofurans and potentiated sulfonamides, but sensitive to erythromycin (Michel et al., 1986; Baya et al., 1991; Toranzo et al., 1993a). This species is not resistant to lysozyme from salmonid eggs, which supports the idea that it is not vertically transmitted, at least in this fish species (Yousif et al., 1994). Finally, it should be noted that two clinical isolates of C. divergens have been reported to possess a gene encoding a new class of penicillinase (AY650920). It will be of interest to further explore the distribution of this enzyme among carnobacterial species as well as its functionality.
It has been documented that some strains of C. maltaromaticum are pathogenic for several fish species, including Australian salmonids (Humphrey et al., 1987), carp (Michel et al., 1986), rainbow trout (Hiu et al., 1984; Baya et al., 1991; Starliper et al., 1992; Toranzo et al., 1993a, b), striped bass and channel catfish (Baya et al., 1991; Toranzo et al., 1993b), and salmon (Hiu et al., 1984; Michel et al., 1986). Pathologic effects vary, and include, for example, septicemia, peritonitis, exophthalmia, accumulation of ascitic fluid, and hemorrhages. In most (but not all) instances, the virulence appears, however, to be low and only causes problems in fish undergoing severe stress, e.g. due to spawning (Michel et al., 1986; Starliper et al., 1992). It is, therefore, not surprising that C. maltaromaticum and C. maltaromaticum-like strains are also found in the intestine or gills of healthy fish, including Arctic charr (Ringøet al., 1998, 2001; Ringø & Olsen, 1999), Atlantic cod (Seppola et al., 2005; Ringøet al., 2006), Atlantic salmon (Ringø & Holzapfel, 2000; Ringøet al., 2000, 2001), brown bullhead (Baya et al., 1991), trout (González et al., 1999; Spanggaard et al., 2001; Pond et al., 2006), and various freshwater fish (González et al., 1999, 2000). Ringøet al. (2005) also summarize some of these findings.
The presence of other carnobacterial species in healthy fish has also been reported, including C. divergens [Arctic charr (Ringø & Olsen, 1999; Ringøet al., 2002a), Atlantic salmon, Atlantic cod and wolffish (Ringøet al., 2001), trout (Spanggaard et al., 2001; Kim & Austin, 2006b), and freshwater fish (González et al., 2000)], C. alterfunditum-like [rainbow trout (Spanggaard et al., 2001)], C. funditum-like [Arctic charr and Atlantic cod (Ringøet al., 2002b, 2006)], C. gallinarum [Atlantic cod (Seppola et al., 2005)], C. inhibens [Atlantic salmon (Jöborn et al., 1999)], C. mobile-like [Arctic charr and Atlantic cod (Ringø & Olsen, 1999; Ringøet al., 2006)], and Carnobacterium spp. [Arctic charr (Ringøet al., 2001) and carp (Hagi et al., 2004)]. Clearly, further research is necessary to clarify under what circumstances Carnobacterium spp. may be pathogenic for fish and whether the infective capability is species and clone specific.
The reported pathogenicity of C. maltaromaticum has not hindered research into the possibility of using other carnobacteria as probiotic cultures in aquaculture, including Carnobacterium sp. (Irianto & Austin, 2002), C. alterfunditum (Spanggaard et al., 2001), C. divergens (Gildberg et al., 1995, 1997; Spanggaard et al., 2001), and C. inhibens (Jöborn et al., 1997). Isolates of these species, in addition to C. maltaromaticum, exhibit inhibitory activity towards bacterial fish pathogens (Ringø & Holzapfel, 2000; Gram & Ringø, 2005; Ringøet al., 2005). Carnobacterium divergens and C. maltaromaticum enhance the cellular and humoral immune responses and cytokine expression ratios of rainbow trout (Kim & Austin, 2006a, b). Use of carnobacteria as probiotics leads to increased survival of the fish in some instances [larvae of cod fry and Atlantic salmon fry (Gildberg et al., 1995, 1997), rainbow trout (Irianto & Austin, 2002), and salmon (Robertson et al., 2000)], but not in others [larvae of cod fry (Gildberg & Mikkelsen, 1998), and rainbow trout (Spanggaard et al., 2001)]. One study showed that in vitro exposure to C. divergens did not reverse the effects of bacterial pathogens, although these were alleviated (Ringøet al., 2007). Additional research is necessary to validate the usefulness of carnobacteria as probiotic cultures.
Carnobacterium maltaromaticum has also been reported to be pathogenic for the fruit fly, Drosophila melanogaster, upon injection into the thorax (Jensen et al., 2007). This specific case of pathogenesis is probably best understood as a result of opportunistic infection, although C. maltaromaticum (but not C. divergens, C. gallinarum, C. inhibens, C. mobile or C. viridans) exerts chitinolytic acitivity (Leisner & Ingmer, unpublished data), a feature that can be perceived as targeting the chitin-containing exoskeleton of insects. Indeed, insects may serve as a host for this species (Shannon et al., 2001).
Genomics
The complete chromosomes of many LAB species have now been sequenced. Currently, 19 complete genome sequences of streptococci and 18 complete genome sequences of the nonpathogenic LAB representing 14 species from the order Lactobacillales are available (Makarova & Koonin, 2007). However, no genome sequence or physical genetic map is available for Carnobacterium. The LAB have relatively small genomes for nonobligatory bacterial parasites or symbionts, ranging from c. 1.6 to c. 3 Mb. For C. divergens and C. pleistocenium, the genome size was estimated to 3.2 Mb, and for C. alterfunditum pf4T, it was estimated to be 2.9 Mb (Daniel, 1995; Pikuta et al., 2005). At the time of writing (March 2007), there is only one Carnobacterium genome sequencing project. Carnobacterium sp. AT7, a piezophilic strain isolated from the Aleutian trench at a depth of 2500 m, is being sequenced for the Moore Foundation Marine Microbial Genome Sequencing Project with a grant to the J. Craig Venter Institute. The data already available indicate that the Carnobacterium sp. AT7 genome contains 2.4 Mb and encodes 2388 proteins (http://www.genomesonline.org) (Liolios et al., 2006). Carnobacterium sp. AT7 is closely related to C. alterfunditum and C. pleistocenium (Lauro et al., 2007).
To date, knowledge on the genes and DNA sequences of Carnobacterium is comparatively sparse, concerning mostly bacteriocin-related genes in the species C. divergens and C. maltaromaticum (Table 2) and 16S rRNA and 16S–23S rRNA gene intergenic spacer sequences (Kabadjova et al., 2002; Rachman et al., 2004). Genes involved in some important carnobacterial metabolic traits have been sequenced (Table 5). The genetic information obtained is, for most sequences, derived from just one strain, and may therefore be strain specific, as shown for a number of L. monocytogenes genes (Nelson et al., 2004).
Table 5.
Summary of known genes and DNA sequences in Carnobacterium*
| Function | Species/Strain | Gene | Accession number | References† |
|---|---|---|---|---|
| Bacteriocin immunity | ||||
| To carnobacteriocin A | Cm/LV17A | cbiA‡ | AF207838 | Franz et al. (2000) |
| To carnobacteriocin BM1 | Cm/LV17B | cbiBM1 | L29058 | Quadri et al. (1994) |
| To carnobacteriocin B2 | Cm/LV17B, CP52 | cbiB2‡ | L47121 | Quadri et al. (1995) |
| Herbin et al. (1997) | ||||
| To divercin V41 | Cd/V41 | dvnT2, dvnI | AJ224003 | Métivier et al. (1998) |
| To divergicin A | Cd/LV13 | dviA‡ | DQ087597 | Worobo et al. (1995) |
| To piscicolin 126 | Cm/JG126 | pisI | AF275938 | Gibbs et al. (2000) (U)† |
| Regulation of bacteriocin expression/secretion | ||||
| Carnobacteriocin A | Cm/LV17A | cbaX, K, R‡ | AF207838 | Franz et al. (2000) |
| Carnobacteriocin BM1 andB2: three-component regulatory system | Cm/LV17B | cbnS, K,R‡ | L47121 | Quadri et al. (1997b) |
| Kleerebezem et al. (2001) | ||||
| Rohde & Quadri (2006) | ||||
| Divercin regulatory system | Cd/V41 | dvnK, R | AJ224003 | Métivier et al. (1998) |
| Piscicolin 126 regulatory system | Cm/JG126 | pisN, K, R | AF275938 | Gibbs et al. (2000) (U) |
| Bacteriocin ABC transporter | ||||
| Carnobacteriocin A | Cm/LV17A | cbaT,C‡ | AF207838 | Franz et al. (2000) |
| Carnobacteriocin BM1 and B2 | Cm/LV17B | cbnT,D‡ | L47121, L29058 | Quadri et al. (1997) |
| Kleerebezem et al. (2001) | ||||
| Rohde & Quadri (2006) | ||||
| Divercin | Cd/V41 | dvnT1 | AJ224003 | Métivier et al. (1998) |
| Piscicolin 126 | Cm/JG126 | pisT, E | AF275938 | Gibbs et al. (2000) (U) |
| Metabolism | ||||
| Alanine dehydrogenase | Csp/st2 | ald | AF070714 | Galkin et al. (1999) |
| ATP synthase α-subunit | Cd/LMG 9199T | atpA partial | AJ843296 | Naser et al. (2005) |
| Cm/LMG 9839T | atpA partial | AJ843298 | Naser et al. (2005) | |
| Glutamate racemase | Csp/st2 | Glr | AF263927 | Galkin et al. (2000) (U) |
| Glycosyl hydrolases | Cm/BA | agaA | AF376480 | Coombs & Brenchley (2001) |
| bgaC | AF376481 | Coombs & Brenchley (2001) | ||
| bgaB | AF184246 | Coombs & Brenchley (1999) | ||
| Homoserine dehydrogenase and aromatic amino acid aminotransferase | Cm/545 | hdhCP, araTCP | AY029372 | Larrouture-Thiveyrat et al. (2001) (U) |
| Penicillinase | Cd/BM4489 | cad-1 | AY650920 | Perichon et al. (2004) (U) |
| Phenylalanyl-tRNA synthase α-subunit | Cm/LMG 6903T | pheS | AM168425 | Naser et al. (2006) |
| Superoxide dismutase | Cm/3364-01 | sodA, partial | AM490329 | Michel et al. (2007) |
| Cm/NCIMB 2264 | sodA, partial | AM490310 | Michel et al. (2007) | |
| Ci/CIP 106863T | sodA, partial | AM490313 | Michel et al. (2007) | |
| Tyrosine decarboxylase | Cd/V41 | tdc | DQ336701 | Brillet et al. (2005) (U) |
| Coton et al. (2004) | ||||
| Miscellaneous | ||||
| Recombinase A | Cm/LMG 6903T | recA, partial | AJ621690 | Felis et al. (2005) (U) |
| RNAse RH | Csp/st2 | rph, partial | AF263927 | Galkin et al. (2000) (U) |
| Theta-type plasmid | Cd/LV13 | pCD3.4‡ | DQ087597 | Van Belkum & Stiles (2006) |
For bacteriocin structural genes, see Table 2.
U, unpublished sequence (National Center for Biotechnology Information Genome Library, http://www.ncbi.nlm.nih.gov).
The sequences are encoded by plasmids.
Cd, Carnobacterium divergens; Ci, Carnobacterium inhibens; Cm, Carnobacterium maltaromaticum; Csp, Carnobacterium sp.
Clearly, our knowledge of important phenotypes of strains and species of Carnobacterium would benefit from the availability of a complete genome sequence for a strain representative of C. maltaromaticum, which is the species with most importance for the food and aquaculture industries. The most extensive study on phenotypic variation of C. maltaromaticum revealed that the majority of strains studied belonged to one phenotypic cluster (cluster H) (Laursen et al., 2005). Thus, in our opinion, a suitable candidate for a whole genomic sequence could be either C. maltaromaticum LMG 22901 (isolated from pork meat), LMG 22899 (isolated from cod) or LMG 22898 (isolated from salmon), which all belong to this phenotypic cluster. LMG 22901 does not harbor plasmids (Laursen, unpublished), and therefore appears to be a prime candidate for such an endeavor.
Concluding remarks
We have come some way towards understanding important aspects of the presence of carnobacteria in foods and the environment, particularly concerning the genetics of carnobacterial bacteriocins and their application. However, there are important areas where knowledge on these bacteria is limited. These include the following:
Distribution and quantitative microbial ecology: Carnobacterium spp. have not been reported from a number of habitats, such as plants, fermented vegetable foods and the gastrointestinal system of birds and mammals, including humans, that have otherwise been associated with several genera and species of LAB. This may, in some instances, be due to faulty methodology for detecting carnobacteria. Generally, however, our quantitative understanding is limited regarding factors that influence inactivation, survival or growth of carnobacteria in various natural environments. In foods, more detailed information is available, but mathematical models for quantitative prediction of processing, product and storage effects on growth and metabolic activity, including bacteriocin and tyramine formation, awaits further development together with recording of responses in databases. In addition, further studies are needed to elucidate mechanisms underlying the relationship between environmental parameters and kinetic responses of carnobacteria.
Routes of food contamination: To reduce the negative effects of carnobacteria in foods (spoilage metabolites and tyramine), further information on routes of contamination is desirable. In fact, very little research has been performed so far concerning this aspect of carnobacteria in food.
Metabolic activity: It is known that carnobacteria, or at least C. maltaromaticum and C. divergens, have the capacity to produce a wide range of metabolites in chilled food. Further studies on the importance of these metabolites with respect to positive and negative sensory attributes of various foods are, however, needed.
Diversity: We are beginning to appreciate how interspecies and intraspecies variation determine the positive and negative effects of the presence of carnobacteria in food and the environment, but we still do not understand how such variation affects, for instance, their survival in food-processing plants and contamination of foods. The significance of such variation for their potential as fish pathogens also needs to be substantiated. This is also the case for their role as spoilage organisms in meat and fish products, with the exception of a few products such as vacuum-packed smoked salmon and cooked MAP shrimps.
Genomics: Representative genome sequences would offer a very valuable road map in order to answer many of the outstanding questions associated with this important genus.
Statement
Reuse of this article is permitted in accordance with the Creative Commons Deed, Attribution 2.5, which does not permit commercial exploitation.
References
- Ahn C, Stiles ME. Plasmid-associated bacteriocin production by a strain of Carnobacterium piscicola from meat. Appl Environ Microbiol. 1990;56:2503–2510. doi: 10.1128/aem.56.8.2503-2510.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves VF, de Martinis ECP, Destro MT, Vogel BF, Gram L. Antilisterial activity of a Carnobacterium piscicola isolated from Brazilian smoked fish (Surubim [Pseudoplatystoma sp.]) and its activity against a persistant strain of Listeria monocytogenes isolated from Surubim. J Food Prot. 2005;68:2068–2077. doi: 10.4315/0362-028x-68.10.2068. [DOI] [PubMed] [Google Scholar]
- Asakawa A, Yamaguchi K, Konosu S. Taste-active components of the shrimp Pandalus borealis. Nippon Shokuhin Kogyo Gakkaishi. 1981;28:594–599. [Google Scholar]
- Azuma T, Bagenda DK, Yamamoto T, Kawai Y, Yamazaki K. Inhibition of Listeria monocytogenes by freeze-dried piscicocin CS526 fermentate in food. Lett Appl Microbiol. 2007;44:138–144. doi: 10.1111/j.1472-765X.2006.02054.x. [DOI] [PubMed] [Google Scholar]
- Barakat RK, Griffiths MW, Harris LJ. Isolation and characterization of Carnobacterium, Lactococcus, and Enterococcus spp. from cooked, modified atmosphere packaged, refrigerated, poultry meat. Int J Food Microbiol. 2000;62:83–94. doi: 10.1016/s0168-1605(00)00381-0. [DOI] [PubMed] [Google Scholar]
- Basby M, Jeppesen VF, Huss HH. Characterization of the microflora of lightly salted lumpfish (Cyclopterus lumpus) roe stored at 5°C. J Aquat Food Product Technol. 1998;7:35–51. [Google Scholar]
- Baya AM, Toranzo AE, Lupiani B, Li T, Roberson BS, Hetrick FM. Biochemical and serological characterization of Carnobacterium spp. isolated from farmed and natural populations of striped bass and catfish. Appl Environ Microbiol. 1991;57:3114–3120. doi: 10.1128/aem.57.11.3114-3120.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhugaloo-Vial P, Dousset X, Metivier A, Sorokine O, Anglade P, Boyaval P, Marion D. Purification and amino acid sequences of piscicocins V1a and V1b, two class IIa bacteriocins secreted by Carnobacterium piscicola V1 that display significantly different levels of specific inhibitory activity. Appl Environ Microbiol. 1996;62:4410–4416. doi: 10.1128/aem.62.12.4410-4416.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhugaloo-Vial P, Douliez J-P, Mollé D, Dousset X, Boyaval P, Marion D. Delineation of key amino acid side chains and peptide domains for antimicrobial properties of divercin V41, a pediocin-like bacteriocin secreted by Carnobacterium divergens V41. Appl Environ Microbiol. 1999;65:2895–2900. doi: 10.1128/aem.65.7.2895-2900.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkroth J, Ristiniemi M, Vandamme P, Korkeala H. Enterococcus species dominating in fresh modified-atmosphere-packaged, marinated broiler legs are overgrown by Carnobacterium and Lactobacillus species during storage at 6°C. Int J Food Microbiol. 2005;97:267–276. doi: 10.1016/j.ijfoodmicro.2004.04.011. [DOI] [PubMed] [Google Scholar]
- Blom H, Katla T, Nissen H, Holo H. Characterization, production and purification of carnocin H, a bacteriocin produced by Carnobacterium 377. Curr Microbiol. 2001;43:227–231. doi: 10.1007/s002840010292. [DOI] [PubMed] [Google Scholar]
- Borch E, Molin G. Numerical taxonomy of psychrotrophic lactic acid bacteria from prepacked meat and meat products. Anton Leeuw. 1988;54:301–323. doi: 10.1007/BF00393522. [DOI] [PubMed] [Google Scholar]
- Borch E, Molin G. The aerobic growth and product formation of Lactobacillus, Leuconostoc, Brochothrix, and Carnobacterium in batch cultures. Appl Microbiol Biotech. 1989;30:81–88. [Google Scholar]
- Borch E, Kant-Muermans M-L, Blixt Y. Bacterial spoilage of meat and cured meat products. Int J Food Microbiol. 1996;33:103–120. doi: 10.1016/0168-1605(96)01135-x. [DOI] [PubMed] [Google Scholar]
- Bover-Cid S, Holzapfel WH. Improved screening procedure for biogenic amine production by lactic acid bacteria. Int J Food Microbiol. 1999;53:33–41. doi: 10.1016/s0168-1605(99)00152-x. [DOI] [PubMed] [Google Scholar]
- Bratina BJ, Stevenson BS, Green WJ, Schmidt TM. Manganese reduction by microbes from oxic regions of the Lake Vanda (Antarctica) water column. Appl Environ Microbiol. 1998;64:3791–3797. doi: 10.1128/aem.64.10.3791-3797.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brillet A, Pilet M-F, Prevost H, Bouttefroy A, Leroi F. Biodiversity of Listeria monocytogenes sensitivity to bacteriocin-producing Carnobacterium strains and application in sterile cold-smoked salmon. J Appl Microbiol. 2004;97:1029–1037. doi: 10.1111/j.1365-2672.2004.02383.x. [DOI] [PubMed] [Google Scholar]
- Brillet A, Pilet M-F, Prevost H, Cardinal M, Leroi F. Effect of inoculation of Carnobacterium divergens V41, a biopreservative strain against Listeria monocytogenes risk, on the microbiological, chemical and sensory quality of cold-smoked salmon. Int J Food Microbiol. 2005;104:309–324. doi: 10.1016/j.ijfoodmicro.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Brillet A, Matamoros S, Blanchet-Chevrollier C, Leroi F, Prevost H, Pilet M-F. Selection of non-tyramine producing Carnobacterium strains for the biopreservation of cold-smoked salmon. In: Luten J, Jacobsen C, Bekaert K, Sæbø A, Oehlenschlager J, editors. Seafood Research from Fish to Dish. Wageningen, The Netherlands: Wageningen Academic Publishers; 2006. pp. 403–410. [Google Scholar]
- Buchanan RL, Klawitter LA. Effectiveness of Carnobacterium piscicola LK5 for controlling the growth of Listeria monocytogenes Scott A in refrigerated foods. J Food Safety. 1992;12:219–236. [Google Scholar]
- Buchanan RL, Bagi LK. Microbial competition: effect of culture conditions on the suppression of Listeria monocytogenes Scott A by Carnobacterium piscicola. J Food Prot. 1997;60:254–261. doi: 10.4315/0362-028X-60.3.254. [DOI] [PubMed] [Google Scholar]
- Budde BB, Hornbæk T, Jacobsen T, Barkholt V, Koch AG. Leuconostoc carnosum 4010 has the potential for use as a protective culture for vacuum-packed meats: culture isolation, bacteriocin identification, and meat application experiments. Int J Food Microbiol. 2003;83:171–184. doi: 10.1016/s0168-1605(02)00364-1. [DOI] [PubMed] [Google Scholar]
- Cailliez-Grimal C, Miguindou-Mabiala R, Leseine M, Revol-Junelles A-M, Millière J-B. Quantitative polymerase chain reaction used for the rapid detection of Carnobacterium species from French soft cheeses. FEMS Microbiol Lett. 2005;250:163–169. doi: 10.1016/j.femsle.2005.05.037. [DOI] [PubMed] [Google Scholar]
- Cailliez-Grimal C, Edima HC, Revol-Junelles A-M, Millière J-B. Carnobacterium maltaromaticum: the only Carnobacterium species in French ripened soft cheeses as revealed by polymerase chain reaction detection. J Dairy Sci. 2007;90:1133–1138. doi: 10.3168/jds.S0022-0302(07)71599-0. [DOI] [PubMed] [Google Scholar]
- Calvez AR, Auffray Y, Prévost H, Drider D. Identification of new genes associated with intermediate resistance of Enterococcus faecalis to divercin V41, a pediocin-like bacteriocin. Microbiol. 2007;153:1609–1618. doi: 10.1099/mic.0.2006/004812-0. [DOI] [PubMed] [Google Scholar]
- Campos CA, Mazzotta AS, Montville TJ. Inhibition of Listeria monocytogenes by Carnobacterium piscicola in vacuum-packaged cooked chicken at refrigeration temperatures. J Food Safety. 1997;17:151–160. [Google Scholar]
- Chenoll E, Macián MC, Elizaquível P, Aznar R. Lactic acid bacteria associated with vacuum-packed cooked meat product spoilage: population analysis by rDNA-based methods. J Appl Microbiol. 2007;102:498–508. doi: 10.1111/j.1365-2672.2006.03081.x. [DOI] [PubMed] [Google Scholar]
- Chmelař D, Matušek A, Korger J, Durnová E, Steffen M, Chmelařová E. Isolation of Carnobacterium piscicola from human pus – Case report. Folia Microbiol. 2002;47:455–457. doi: 10.1007/BF02818708. [DOI] [PubMed] [Google Scholar]
- Collins MD, Farrow JAE, Phillips BA, Ferusu S, Jones D. Classification of Lactobacillus divergens, Lactobacillus piscicola, and some catalase-negative, asporogenous, rod-shaped bacteria from poultry in a new genus, Carnobacterium. Int J System Bacteriol. 1987;37:310–316. [Google Scholar]
- Collins MD, Hutson RA, Foster G, Falsen E, Weiss N. Isobaculum melis gen. nov., sp. nov., a Carnobacterium-like organism isolated from the intestine of a badger. Int J System Evol Microbiol. 2002;52:207–210. doi: 10.1099/00207713-52-1-207. [DOI] [PubMed] [Google Scholar]
- Connil N, Prévost H, Dousset X. Production of biogenic amines and divercin V41 in cold smoked salmon inoculated with Carnobacterium divergens V41, and specific detection of this strain by multiplex-PCR. J Appl Microbiol. 2002;92:611–617. doi: 10.1046/j.1365-2672.2002.01561.x. [DOI] [PubMed] [Google Scholar]
- Coombs J, Brenchley JE. Characterization of two new glycosyl hydrolases from the lactic acid bacterium Carnobacterium piscicola strain BA. Appl Environ Microbiol. 2001;67:5094–5099. doi: 10.1128/AEM.67.11.5094-5099.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coombs JM, Brenchley JE. Biochemical and phylogenetic analyses of a cold-active β-galactosidase from the lactic acid bacterium Carnobacterium piscicola BA. Appl Environ Microbiol. 1999;65:5443–5450. doi: 10.1128/aem.65.12.5443-5450.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coton M, Coton E, Lucas P, Lonvaud A. Identification of the gene encoding a putative tyrosine decarboxylase of Carnobacterium divergens 508. Development of molecular tools for the detection of tyramine-producing bacteria. Food Microbiol. 2004;21:125–130. [Google Scholar]
- Coventry MJ, Gordon JB, Wilcock A, Harmark K, Davidson BE, Hickey MW, Hillier AJ, Wan J. Detection of bacteriocins of lactic acid bacteria isolated from foods and comparison with pediocin and nisin. J Appl Microbiol. 1997;83:248–258. doi: 10.1046/j.1365-2672.1997.00216.x. [DOI] [PubMed] [Google Scholar]
- Dainty RH. Chemical/biochemical detection of spoilage. Int J Food Microbiol. 1996;33:19–33. doi: 10.1016/0168-1605(96)01137-3. [DOI] [PubMed] [Google Scholar]
- Dainty RH, Mackey BM. The relationship between the phenotypic properties of bacteria from chill-stored meat and spoilage processes. J Appl Bacteriol Symp Suppl. 1992;73:103S–114S. doi: 10.1111/j.1365-2672.1992.tb03630.x. [DOI] [PubMed] [Google Scholar]
- Dalet K, Cenatiempo Y, Cossart P The European Listeria Genome Consortium. Héchard Y. A σ54-dependent PTS permease of the mannose family is responsible for sensitivity of Listeria monocytogenes to mesentericin Y105. Microbiol. 2001;147:3263–3269. doi: 10.1099/00221287-147-12-3263. [DOI] [PubMed] [Google Scholar]
- Dalgaard P, Vancanneyt M, Euras Vilalta N, Swings J, Fruekilde P, Leisner JJ. Identification of lactic acid bacteria from spoilage associations of cooked and brined shrimps stored under modified atmosphere between 0°C and 25°C. J Appl Microbiol. 2003;94:80–89. doi: 10.1046/j.1365-2672.2003.01806.x. [DOI] [PubMed] [Google Scholar]
- Dalgaard P, Madsen HL, Samieian N, Emborg J. Biogenic amine formation and microbial spoilage in chilled garfish (Belone belone belone) – effect of modified atmosphere packaging and previous frozen storage. J Appl Microbiol. 2006;101:80–95. doi: 10.1111/j.1365-2672.2006.02905.x. [DOI] [PubMed] [Google Scholar]
- Daniel P. Sizing of the Lactobacillus plantarum genome and other lactic acid bacteria species by transverse alternating field electrophoresis. Curr Microbiol. 1995;30:243–246. [Google Scholar]
- de Bruyn I, Holzapfel WH, Wisser L, Louw AI. Glucose metabolism by Lactobacillus divergens. J Gen Microbiol. 1988;134:2103–2109. doi: 10.1099/00221287-134-8-2103. [DOI] [PubMed] [Google Scholar]
- de Bruyn IN, Louw AI, Visser L, Holzapfel WH. Lactobacillus divergens is a homofermentative organism. Syst Appl Microbiol. 1987;9:173–175. [Google Scholar]
- de Man JC, Rogosa M, Sharpe ME. A medium for the cultivation of Lactobacilli. J Appl Bacteriol. 1960;23:130–135. [Google Scholar]
- De Reu K, Grijspeerdt K, Messens W, Heyndrickx M, Uyttendaele M, Debevere J, Herman L. Eggshell factors influencing eggshell penetration and whole egg contamination by different bacteria, including Salmonella enteritidis. Int J Food Microbiol. 2006;112:253–260. doi: 10.1016/j.ijfoodmicro.2006.04.011. [DOI] [PubMed] [Google Scholar]
- Drider D, Firmland G, Héchard Y, McMullen LM, Prévost H. The continuing story of class IIa bacteriocins. Microbiol Mol Biol Rev. 2006;70:564–582. doi: 10.1128/MMBR.00016-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffes F, Leroi F, Boyaval P, Dousset X. Inhibition of Listeria monocytogenes by Carnobacterium spp. strains in a simulated cold smoked fish system stored at 4°C. Int J Food Microbiol. 1999a;47:33–42. doi: 10.1016/s0168-1605(98)00206-2. [DOI] [PubMed] [Google Scholar]
- Duffes F, Corre C, Leroi F, Dousset X, Boyaval P. Inhibition of Listeria monocytogenes by in situ produced and semipurified bacteriocins of Carnobacterium spp. on vacuum-packed, refrigerated cold-smoked salmon. J Food Prot. 1999b;62:1394–1403. doi: 10.4315/0362-028x-62.12.1394. [DOI] [PubMed] [Google Scholar]
- Duffes F, Jenoe P, Boyaval P. Use of two-dimensional electrophoresis to study differential protein expression in divercin V41-resistant and wild-type strains of Listeria monocytogenes. Appl Environ Microbiol. 2000;66:4318–4324. doi: 10.1128/aem.66.10.4318-4324.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RA, Dainty RH, Hibbard CM, Ramantanis SV. Amines in fresh beef of normal pH and the role of bacteria in changes in concentration observed during storage in vacuum packs at chill temperatures. J Appl Bacteriol. 1987;63:427–434. doi: 10.1111/j.1365-2672.1987.tb04864.x. [DOI] [PubMed] [Google Scholar]
- Einarsson H, Lauzon HL. Biopreservation of brined shrimps (Pandalus borealis) by bacteriocins from lactic acid bacteria. Appl Environ Microbiol. 1995;61:669–676. doi: 10.1128/aem.61.2.669-676.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emborg J, Dalgaard P. Formation of histamine and biogenic amines in cold-smoked tuna: an investigation of psychrotolerant bacteria from samples implicated in cases of histamine fish poisoning. J Food Prot. 2006;69:897–906. doi: 10.4315/0362-028x-69.4.897. [DOI] [PubMed] [Google Scholar]
- Emborg J, Laursen BG, Rathjen T, Dalgaard P. Microbial spoilage and formation of biogenic amines in fresh and thawed modified atmosphere-packed salmon (Salmo salar) at 2°C. J Appl Microbiol. 2002;92:790–799. doi: 10.1046/j.1365-2672.2002.01588.x. [DOI] [PubMed] [Google Scholar]
- Emborg J, Laursen BG, Dalgaard P. Significant histamine formation in tuna (Thunnus albacares) at 2°C – effect of vacuum- and modified atmosphere-packaging on psychrotolerant bacteria. Int J Food Microbiol. 2005;101:263–279. doi: 10.1016/j.ijfoodmicro.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Ercolini D, Russo F, Torrieri E, Masi P, Villani F. Changes in the spoilage-related microbiota of beef during refrigerated storage under different packaging conditions. Appl Environ Microbiol. 2006;72:4663–4671. doi: 10.1128/AEM.00468-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz CMAP, van Belkum MJ, Worobo RW, Vederas JC, Stiles ME. Characterization of the genetic locus responsible for production and immunity of carnobacteriocin A: the immunity gene confers cross-protection to enterocin B. Microbiology. 2000;146:621–631. doi: 10.1099/00221287-146-3-621. [DOI] [PubMed] [Google Scholar]
- Franzetti L, Scarpellini M, Mora D, Galli A. Carnobacterium spp. in seafood packaged in modified atmosphere. Ann Microbiol. 2003;53:189–198. [Google Scholar]
- Franzmann PD, Höpfl P, Weiss N, Tindall BJ. Psychrotrophic, lactic acid-producing bacteria from anoxic waters in Ace Lake, Antartica; Carnobacterium funditum sp. nov. and Carnobacterium alterfunditum sp. nov. Archiv Microbiol. 1991;156:255–262. doi: 10.1007/BF00262994. [DOI] [PubMed] [Google Scholar]
- Galkin A, Kulakova L, Ashida H, Sawa Y, Esaki N. Cold-adapted alanine dehydrogenases from two Antarctic bacterial strains: gene cloning, protein characterization, and comparison with mesophilic amd thermophilic counterparts. Appl Environ Microbiol. 1999;65:4014–4020. doi: 10.1128/aem.65.9.4014-4020.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gildberg A, Mikkelsen H. Effects of supplementing the feed to Atlantic cod (Gadus morhua) fry with lactic acid bacteria and immuno-stimulating peptides during a challenge trial with Vibrio anguillarum. Aquaculture. 1998;167:103–113. [Google Scholar]
- Gildberg A, Johansen A, Bøgwald J. Growth and survival of Atlantic salmon (Salmo salar) fry given diets supplemented with fish protein hydrolysate and lactic acid bacteria during a challenge trial with Aeromonas salmonicida. Aquaculture. 1995;138:23–34. [Google Scholar]
- Gildberg A, Mikkelsen H, Sandaker E, Ringø E. Probiotic effect of lactic acid bacteria in the feed on growth and survival of fry of Atlantic cod (Gadus morhua) Hydrobiologia. 1997;352:279–285. [Google Scholar]
- Gilmore MS, Segarra RA, Booth MC, Bogie CP, Hall LR, Clewell DB. Genetic structure of Enterococcus faecalis plasmid pAD1-encoded cytolytic toxin system and its relationship to lantibiotic determinants. J Bacteriol. 1994;176:7335–7344. doi: 10.1128/jb.176.23.7335-7344.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González C-J, López-Díaz T-M, García-López M-L, Prieto M, Otero A. Bacterial microflora of wild brown trout (Salmo trutta), wild pike (Esox lucius), and aquacultured rainbow trout (Oncorhynchus mykiss) J Food Prot. 1999;62:1270–1277. doi: 10.4315/0362-028x-62.11.1270. [DOI] [PubMed] [Google Scholar]
- González CJ, Encinas JP, García-López ML, Otero A. Characterization and identification of lactic acid bacteria from freshwater fishes. Food Microbiol. 2000;17:383–391. [Google Scholar]
- González-Rodríguez M-N, Sanz J-J, Santos J-Á, Otero A, García-López M-L. Numbers and types of microorganisms in vacuum-packed cold-smoked freshwater fish at the retail level. Int J Food Microbiol. 2002;77:161–168. doi: 10.1016/s0168-1605(02)00048-x. [DOI] [PubMed] [Google Scholar]
- Gram L, Ringø E. Prospects of fish probiotics. In: Holzapfel WH, Naughton PJ, editors. Microbial Ecology in Growing Animals. Edinburgh: Elsevier; 2005. pp. 379–417. [Google Scholar]
- Grant IR, Patterson MF. A numerical taxonomic study of lactic acid bacteria isolated from irradiated pork and chicken packaged under various gas atmospheres. J Appl Bacteriol. 1991;70:302–307. doi: 10.1111/j.1365-2672.1991.tb02940.x. [DOI] [PubMed] [Google Scholar]
- Gravesen A, Ramnath M, Rechinger KB, Andersen N, Jänsch L, Héchard Y, Hastings JW, Knøchel S. High-level resistance to class IIa bacteriocins is associated with one general mechanism in Listeria monocytogenes. Microbiol. 2002;148:2361–2369. doi: 10.1099/00221287-148-8-2361. [DOI] [PubMed] [Google Scholar]
- Groudieva T, Kambourova M, Yusef H, Royter M, Grote R, Trinks H, Antranikian G. Diversity and cold-active hydrolytic enzymes of culturable bacteria associated with Arctic sea ice, Spitzbergen. Extremophiles. 2004;8:475–488. doi: 10.1007/s00792-004-0409-0. [DOI] [PubMed] [Google Scholar]
- Guldager HS, Bøknæs N, Østerberg C, Nielsen J, Dalgaard P. Thawed cod fillets spoil less rapidly than unfrozen fillets when stored under modified atmosphere at 2°C. J Food Prot. 1998;61:1129–1136. doi: 10.4315/0362-028x-61.9.1129. [DOI] [PubMed] [Google Scholar]
- Gursky LJ, Martin NI, Derksen DJ, van Belkum MJ, Kaur K, Vederas JC, Stiles ME, McMullen LM. Production of piscicolin 126 by Carnobacterium maltaromaticum UAL26 is controlled by temperature and induction peptide concentration. Arch Microbiol. 2006;186:317–325. doi: 10.1007/s00203-006-0147-z. [DOI] [PubMed] [Google Scholar]
- Hagi T, Tanaka D, Iwamura Y, Hoshino T. Diversity and seasonal changes in lactic acid bacteria in the intestinal tract of cultured freshwater fish. Aquaculture. 2004;234:335–346. [Google Scholar]
- Hammes WP, Hertel C. The genera Lactobacillus and Carnobacterium. In: Dworkin M, editor. The Procaryotes: An Evolving Electronic Resource for the Microbiological Community. New York: Springer-Verlag; 2003. pp. 320–403. [Google Scholar]
- Herbin S, Mathieu F, Brulé F, Branlant C, Lefebvre G, Lebrihi A. Characteristics and genetic determinants of bacteriocin activities produced by Carnobacterium piscicola CP5 isolated from cheese. Curr Microbiol. 1997;35:319–326. doi: 10.1007/s002849900262. [DOI] [PubMed] [Google Scholar]
- Himelbloom B, Nilsson L, Gram L. Factors affecting production of an antilisterial bacteriocin by Carnobacterium piscicola strain A9b in laboratory media and model fish systems. J Appl Microbiol. 2001;91:506–513. doi: 10.1046/j.1365-2672.2001.01401.x. [DOI] [PubMed] [Google Scholar]
- Hirano T, Yamaguchi M, Shirai T, Suzuki T, Suyama M. Free amino acids, trimethylamine oxide, and betaines of the raw and boiled meats of mantis shrimp Oratosquilla oratolia. Nippon Suisan Gakkaishi. 1992;58:973. [Google Scholar]
- Hiu SF, Holt RA, Sriranganathan N, Seidler RJ, Fryer JL. Lactobacillus piscicola, a new species from salmonid fish. Int J System Bacteriol. 1984;34:393–400. [Google Scholar]
- Holck AL, Axelsson L, Schillinger U. Purification and cloning of piscicolin-61, a bacteriocin from Carnobacterium piscicola LV61. Curr Microbiol. 1994;29:63–68. doi: 10.1007/BF01575750. [DOI] [PubMed] [Google Scholar]
- Holck A, Axelsson L, Schillinger U. Divergicin 750, a novel bacteriocin produced by Carnobacterium divergens 750. FEMS Microbiol Lett. 1996;136:163–168. doi: 10.1111/j.1574-6968.1996.tb08043.x. [DOI] [PubMed] [Google Scholar]
- Holley RA, Guan TY, Peirson M, Yost CK. Carnobacterium viridans sp. nov., an alkaliphilic, facultative anaerobe isolated from refrigerated, vacuum-packed bologna sausage. Int J System Evol Microbiol. 2002;52:1881–1885. doi: 10.1099/00207713-52-5-1881. [DOI] [PubMed] [Google Scholar]
- Humphrey JD, Lancaster CE, Gudkovs N, Copland JW. The disease status of Australian salmonids: bacteria and bacterial diseases. J Fish Dis. 1987;10:403–410. [Google Scholar]
- Irianto A, Austin B. Probiotics in aquaculture. J Fish Dis. 2002;25:633–642. [Google Scholar]
- Jack RW, Wan J, Gordon J, Harmark K, Davidson BE, Hillier AJ, Wettenhall REH, Hickey MW, Coventry MJ. Characterization of the chemical and antimicrobial properties of piscicolin 126, a bacteriocin produced by Carnobacterium piscicola JG126. Appl Environ Microbiol. 1996;62:2897–2903. doi: 10.1128/aem.62.8.2897-2903.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen RL, Pedersen KS, Loeschcke V, Ingmer H, Leisner JJ. Limitations in the use of Drosophila melanogaster as a model host for Gram positive bacterial infection. Lett Appl Microbiol. 2007;44:218–223. doi: 10.1111/j.1472-765X.2006.02040.x. [DOI] [PubMed] [Google Scholar]
- Jeppesen VF, Huss HH. Characteristics and antagonistic activity of lactic acid bacteri isolated from chilled fish products. Int J Food Microbiol. 1993;18:305–320. doi: 10.1016/0168-1605(93)90153-8. [DOI] [PubMed] [Google Scholar]
- Jöborn A, Olsson JC, Westerdahl A, Conway PL, Kjelleberg S. Colonization in the fish intestinal tract and production of inhibitory substances in intestinal mucus and faecal extracts by Carnobacterium sp. strain K1. J Fish Dis. 1997;20:383–392. [Google Scholar]
- Jöborn A, Dorsch M, Olsson JC, Westerdahl A, Kjelleberg S. Carnobacterium inhibens sp. nov., isolated from the intestine of Atlantic salmon (Salmo salar) Int J System Bacteriol. 1999;49:1891–1898. doi: 10.1099/00207713-49-4-1891. [DOI] [PubMed] [Google Scholar]
- Joffraud JJ, Leroi F, Roy C, Berdagué JL. Characterisation of volatile compounds produced by bacteria isolated from the spoilage flora of cold-smoked salmon. Int J Food Microbiol. 2001;66:175–184. doi: 10.1016/s0168-1605(00)00532-8. [DOI] [PubMed] [Google Scholar]
- Joffraud J-J, Cardinal M, Cornet J, Chasles J-S, Léon S, Gigout F, Leroi F. Effect of bacterial interactions on the spoilage of cold-smoked salmon. Int J Food Microbiol. 2006;112:51–61. doi: 10.1016/j.ijfoodmicro.2006.05.014. [DOI] [PubMed] [Google Scholar]
- Jones RJ. Observations on the succession dynamics of lactic acid bacteria populations in chill-stored vacuum-packaged beef. Int J Food Microbiol. 2004;90:273–282. doi: 10.1016/s0168-1605(03)00310-6. [DOI] [PubMed] [Google Scholar]
- Jørgensen LV, Huss HH, Dalgaard P. The effect of biogenic amine production by single bacterial cultures and metabiosis on cold-smoked salmon. J Appl Microbiol. 2000;89:920–934. doi: 10.1046/j.1365-2672.2000.01196.x. [DOI] [PubMed] [Google Scholar]
- Kabadjova P, Dousset X, Le Cam V, Prevost H. Differentiation of closely related Carnobacterium food isolates based on 16S–23S ribosomal DNA intergenic spacer region polymorphism. Appl Environ Microbiol. 2002;68:5358–5366. doi: 10.1128/AEM.68.11.5358-5366.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama T, Tanaka M, Moriizumi J, Nakamura T, Brouchkov A, Douglas TA, Fukuda M, Tomita F, Asano K. Phylogenetic analysis of bacteria preserved in a permafrost ice wedge for 25 000 years. Appl Environ Microbiol. 2007;73:2360–2363. doi: 10.1128/AEM.01715-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D-H, Austin B. Cytokine expression in leucocytes and gut cells of rainbow trout, Oncorhynchus mykiss Walbaum, induced by probiotics. Vet Immun Immunopath. 2006a;114:297–304. doi: 10.1016/j.vetimm.2006.08.015. [DOI] [PubMed] [Google Scholar]
- Kim D-H, Austin B. Innate immune responses in rainbow trout (Oncorhynchus mykiss, Walbaum) induced by probiotics. Fish Shellfish Immunol. 2006b;21:513–524. doi: 10.1016/j.fsi.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Kleerebezem M, Kuipers OP, de Vos WM, Stiles ME, Quadri LEN. A two-component signal-transduction cascade in Carnobacterium piscicola LV17B: two signaling peptides and one sensor-transmitter. Peptides. 2001;22:1597–1601. doi: 10.1016/s0196-9781(01)00494-6. [DOI] [PubMed] [Google Scholar]
- Lai S, Manchester LN. Numerical phenetic study of the genus Carnobacterium. Anton Leeuw. 2000;78:73–85. doi: 10.1023/a:1002723609675. [DOI] [PubMed] [Google Scholar]
- Lakshmanan R, Dalgaard P. Effects of high-pressure processing on Listeria monocytogenes, spoilage microflora and multiple compound quality indices in chilled cold-smoked salmon. J Appl Microbiol. 2004;96:398–408. doi: 10.1046/j.1365-2672.2004.02164.x. [DOI] [PubMed] [Google Scholar]
- Larrouture C, Ardaillon V, Pépin M, Montel MC. Ability of meat starter cultures to catabolize leucine and evaluation of the degradation products by using an HPLC method. Food Microbiol. 2000;17:563–570. [Google Scholar]
- Larrouture-Thiveyrat C, Montel M-C. Effects of environmental factors on leucine catabolism by Carnobacterium piscicola. Int J Food Microbiol. 2003;81:177–184. doi: 10.1016/s0168-1605(02)00227-1. [DOI] [PubMed] [Google Scholar]
- Larrouture-Thiveyrat C, Pepin M, Leroy-Sétrin S, Montel M-C. Effect of Carnobacterium piscicola on aroma formation in sausage mince. Meat Sci. 2003;63:423–426. doi: 10.1016/s0309-1740(02)00083-9. [DOI] [PubMed] [Google Scholar]
- Lauro FM, Chastain RA, Blankenship LE, Yayanos AA, Bartlett DH. The unique 16S rRNA genes of piezophiles reflect both phylogeny and adaptation. Appl Environ Microbiol. 2007;73:838–845. doi: 10.1128/AEM.01726-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen BG, Bay L, Cleenwerck I, Vancanneyt M, Swings J, Dalgaard P, Leisner JJ. Carnobacterium divergens and Carnobacterium maltaromaticum as spoilers or protective cultures in meat and seafood: phenotypic and genotypic characterization. Syst Appl Microbiol. 2005;28:151–164. doi: 10.1016/j.syapm.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Laursen BG, Leisner JJ, Dalgaard P. Carnobacterium species: effect of metabolic activity and interaction with Brochothrixthermosphacta on sensory characteristics of modified atmosphere packed shrimp. J Agric Food Chem. 2006;54:3604–3611. doi: 10.1021/jf053017f. [DOI] [PubMed] [Google Scholar]
- Leblanc EL, Stiles ME, McMullen LM, Leisner JJ. Characterization of the prevailing flora of Carnobacterium ssp. growing on CO2 modified atmosphere packaged rainbow trout (Oncorhynchus mykiss) fillets. In: Luten JB, Børresen T, Oehlenschläger J, editors. Seafood from Producer to Consumer. Integrated Approach to Quality. Amsterdam: Elsevier; 1997. pp. 445–454. [Google Scholar]
- Lebois M, Connil N, Onno B, Prévost H, Dousset X. Effects of divercin V41 combined to NaCl content, phenol (liquid smoke) concentration and pH on Listeria monocytogenes Scott A growth in BHI broth by an experimental design approach. J Appl Microbiol. 2004;96:931–937. doi: 10.1111/j.1365-2672.2004.02221.x. [DOI] [PubMed] [Google Scholar]
- Leisner J. Copenhagen, Denmark: Royal Veterinary and Agricultural University; 1992. Characterisation of lactic acid bacteria isolated from lightly preserved fish products and their ability to metabolise various carbohydrates and amino acids. Ph.D. thesis. [Google Scholar]
- Leisner JJ, Millan JC, Huss HH, Larsen LM. Production of histamine and tyramine by lactic acid bacteria isolated from vacuum-packed sugar-salted fish. J Appl Bacteriol. 1994a;76:417–423. doi: 10.1111/j.1365-2672.1994.tb01097.x. [DOI] [PubMed] [Google Scholar]
- Leisner JJ, Tidemand J, Larsen LM. Catabolism of arginine by Carnobacterium spp. isolated from vacuum-packed sugar-salted fish. Curr Microbiol. 1994b;29:95–99. [Google Scholar]
- Leisner JJ, Greer GG, Dilts BD, Stiles ME. Effect of growth of selected lactic acid bacteria on storage life of beef stored under vacuum and in air. Int J Food Microbiol. 1995;26:231–243. doi: 10.1016/0168-1605(94)00133-q. [DOI] [PubMed] [Google Scholar]
- Leroi F, Arbey N, Joffraud J-J, Chevalier F. Effect of inoculation with lactic acid bacteria on extending the shelf-life of vacuum-packed cold smoked salmon. Int J Food Sci Technol. 1996;31:497–504. [Google Scholar]
- Leroi F, Joffraud J-J, Chevalier F, Cardinal M. Study of the microbial ecology of cold-smoked salmon during storage at 8°C. Int J Food Microbiol. 1998;39:111–121. doi: 10.1016/s0168-1605(97)00126-8. [DOI] [PubMed] [Google Scholar]
- Lewus CB, Kaiser A, Montville TJ. Inhibition of food-borne bacterial pathogens by bacteriocins from lactic acid bacteria isolated from meat. Appl Environ Microbiol. 1991;57:1683–1688. doi: 10.1128/aem.57.6.1683-1688.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z-Y, Liu Y. Marine sponge Craniella austrialiensis-associated bacterial diversity revelation based on 16S rDNA library and biologically active Actinomycetes screening, phylogenetic analysis. Lett Appl Microbiol. 2006;43:410–416. doi: 10.1111/j.1472-765X.2006.01976.x. [DOI] [PubMed] [Google Scholar]
- Liolios K, Tavernarakis N, Hugenholtz P, Kyrpides N. The genomes on line database (GOLD) v.2: a monitor of genome projects worldwide. Nucl Acids Res. 2006;34:D332–D334. doi: 10.1093/nar/gkj145. (Database issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyhs U, Björkroth J, Hyytiä E, Korkeala H. The spoilage flora of vacuum-packaged, sodium nitrite or potassium nitrate treated, cold-smoked rainbow trout stored at 4°C or 8°C. Int J Food Microbiol. 1998;45:135–142. doi: 10.1016/s0168-1605(98)00160-3. [DOI] [PubMed] [Google Scholar]
- Lyhs U, Korkeala H, Björkroth J. Identification of lactic acid bacteria from spoiled, vacuum-packaged ‘gravad’ rainbow trout using ribotyping. Int J Food Microbiol. 2002;72:147–153. doi: 10.1016/s0168-1605(01)00634-1. [DOI] [PubMed] [Google Scholar]
- Makarova KS, Koonin EV. Evolutionary genomics of lactic acid bacteria. J Bacteriol. 2007;189:1199–1208. doi: 10.1128/JB.01351-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis RE, Bender GR, Murray DR, Wong A. Arginine deiminase system and bacterial adaptation to acid environments. Appl Environ Microbiol. 1987;53:198–200. doi: 10.1128/aem.53.1.198-200.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson F, Talon R, Montel MC. Histamine and tyramine production by bacteria from meat products. Int J Food Microbiol. 1996;32:199–207. doi: 10.1016/0168-1605(96)01104-x. [DOI] [PubMed] [Google Scholar]
- Masson F, Lebert A, Talon R, Montel MC. Effects of physico-chemical factors influencing tyramine production by Carnobacterium divergens. J Appl Microbiol. 1997;83:36–42. doi: 10.1046/j.1365-2672.1997.00163.x. [DOI] [PubMed] [Google Scholar]
- Masson F, Johansson G, Montel MC. Tyramine production by a strain of Carnobacterium divergens inoculated in meat – fat mixture. Meat Sci. 1999;52:65–69. doi: 10.1016/s0309-1740(98)00149-1. [DOI] [PubMed] [Google Scholar]
- Mauguin S, Novel G. Characterization of lactic acid bacteria isolated from seafood. J Appl Bacteriol. 1994;76:616–625. [Google Scholar]
- McCabe BJ. Dietary tyramine and other pressor amines in MAOI regimes: a review. J Am Diet Assoc. 1986;86:1059–1064. [PubMed] [Google Scholar]
- McMeekin TA, Baranyi J, Bowman J, Dalgaard P, Kirk M, Ross T, Schmid S, Zwietering MH. Information systems in food safety management. Int J Food Microbiol. 2006;112:181–194. doi: 10.1016/j.ijfoodmicro.2006.04.048. [DOI] [PubMed] [Google Scholar]
- McMullen LM, Stiles ME. Microbial ecology of fresh pork stored under modified atmosphere at −1°C, 4.4°C and 10°C. Int J Food Microbiol. 1993;18:1–14. doi: 10.1016/0168-1605(93)90002-x. [DOI] [PubMed] [Google Scholar]
- Meisel J, Wolf G, Hammes WP. Heme-dependent cytochrome formation in Lactobacillus maltaromicus. Syst Appl Microbiol. 1994;17:20–23. [Google Scholar]
- Mejlholm O, Bøknæs N, Dalgaard P. Shelf life and safety aspects of chilled cooked and peeled shrimps (Pandalus borealis) in modified atmosphere packaging. J Appl Microbiol. 2005;99:66–76. doi: 10.1111/j.1365-2672.2005.02582.x. [DOI] [PubMed] [Google Scholar]
- Métivier A, Pilet M-F, Dousset X, Sorokine O, Anglade P, Zagorec M, Piard J-C, Marion D, Cenatiempo Y, Fremaux C. Divercin V41, a new bacteriocin with two disulphide bonds produced by Carnobacterium divergens V41: primary structure and genomic organization. Microbiology. 1998;144:2837–2844. doi: 10.1099/00221287-144-10-2837. [DOI] [PubMed] [Google Scholar]
- Michel C, Faivre B, Kerouault B. Biochemical identification of Lactobacillus piscicola strains from France and Belgium. Dis Aquat Org. 1986;2:27–30. [Google Scholar]
- Michel C, Pelletier C, Boussaha M, Douet D-G, Lautraite A, Tailliez P. Diversity of lactic acid bacteria associated with fish and the fish farm environment, established by amplified rDNA gene restriction analysis. Appl Environ Microbiol. 2007;73:2947–2955. doi: 10.1128/AEM.01852-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A, III, Morgan ME, Libbey LM. Lactobacillus maltaromicus, a new species producing a malty aroma. Int J System Bacteriol. 1974;24:346–354. [Google Scholar]
- Millière JB, Lefebvre G. Carnobacterium piscicola, a common species of French soft cheeses from cow's raw milk. Neth Milk Dairy J. 1994;48:19–30. [Google Scholar]
- Millière JB, Michel M, Mathieu F, Lefebvre G. Presence of Carnobacterium spp. in French surface mould-ripened soft-cheese. J Appl Bacteriol. 1994;76:264–269. [Google Scholar]
- Mora D, Scarpellini M, Franzetti L, Colombo S, Galli A. Reclassification of Lactobacillus maltaromicus (Miller et al., 1974) DSM 20342T and DSM 20344 and Carnobacterium piscicola (Collins et al., 1987) DSM 20730T and DSM 20722 as Carnobacterium maltaromaticum comb. nov. Int J System Evol Microbiol. 2003;53:675–678. doi: 10.1099/ijs.0.02405-0. [DOI] [PubMed] [Google Scholar]
- Morea M, Baruzzi F, Cocconcelli PS. Molecular and physiological characterization of dominant bacterial populations in traditional Mozzarella cheese processing. J Appl Microbiol. 1999;87:574–582. doi: 10.1046/j.1365-2672.1999.00855.x. [DOI] [PubMed] [Google Scholar]
- Morzel M, Fitzgerald GF, Arendt EK. Fermentation of salmon fillets with a variety of lactic acid bacteria. Food Res Int. 1997;30:777–785. [Google Scholar]
- Naghmouchi K, Drider D, Kheadr E, Lacroix C, Prévost H, Fliss I. Multiple characterizations of Listeria monocytogenes sensitive and insensitive variants to divergicin M35, a new pediocin-like bacteriocin. J Appl Microbiol. 2006;100:29–39. doi: 10.1111/j.1365-2672.2005.02771.x. [DOI] [PubMed] [Google Scholar]
- Naser S, Thompson FL, Hoste B, Gevers D, Vandemeulebroecke K, Cleenwerck I, Thompson CC, Vancanneyt M, Swings J. Phylogeny and identification of enterococci by atpA gene sequence analysis. J Clin Microbiol. 2005;43:2224–2230. doi: 10.1128/JCM.43.5.2224-2230.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naser SM, Vancanneyt M, Hoste B, Snauwaert C, Swings J. Lactobacillus cypricasei Lawson et al. 2001 is a later heterotypic synonym of Lactobacillus acidipiscis Tanasupawat et al. 2000. Int J System Evol Microbiol. 2006;56:1681–1683. doi: 10.1099/ijs.0.64229-0. [DOI] [PubMed] [Google Scholar]
- Nelson KE, Fouts DE, Mongodin EF, et al. Whole genome comparisons of serotype 4b and 1/2a strains of the food-borne pathogen Listeria monocytogenes reveal new insights into the core genome components of this species. Nucleic Acids Res. 2004;32:2386–2395. doi: 10.1093/nar/gkh562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry CJ, Webster G, Cragg BA, Parkes RJ, Weightman AJ, Fry JC. Diversity of prokaryotes and methanogenesis in deep subsurface sediments from the Nankai Trough, Ocean Drilling Program Leg 190. Environ Microbiol. 2004;6:274–287. doi: 10.1111/j.1462-2920.2004.00568.x. [DOI] [PubMed] [Google Scholar]
- Nilsson L, Gram L, Huss HH. Growth control of Listeria monocytogenes on cold-smoked salmon using a competitive lactic acid bacteria flora. J Food Prot. 1999;62:336–342. doi: 10.4315/0362-028x-62.4.336. [DOI] [PubMed] [Google Scholar]
- Nilsson L, Nielsen MK, Ng Y, Gram L. Role of acetate in production of an autoinducible class IIa bacteriocin in Carnobacterium piscicola A9b. Appl Environ Microbiol. 2002;68:2251–2260. doi: 10.1128/AEM.68.5.2251-2260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson L, Ng YY, Christiansen JN, Jørgensen BL, Grótinum D, Gram L. The contribution of bacteriocin to inhibition of Listeria monocytogenes by Carnobacterium piscicola strains in cold-smoked salmon systems. J Appl Microbiol. 2004;96:133–143. doi: 10.1046/j.1365-2672.2003.02129.x. [DOI] [PubMed] [Google Scholar]
- Nilsson L, Hansen TB, Garrido P, Buchrieser C, Glaser P, Knøchel S, Gram L, Gravesen A. Growth inhibition of Listeria monocytogenes by a nonbacteriocinogenic Carnobacterium piscicola. J Appl Microbiol. 2005;98:172–183. doi: 10.1111/j.1365-2672.2004.02438.x. [DOI] [PubMed] [Google Scholar]
- Ntougias S, Zervakis GI, Kavroulakis N, Ehaliotis C, Kapadopoulou KK. Bacterial diversity in spent mushroom compost assessed by amplified rDNA restriction analysis and sequencing of cultivated isolates. Syst Appl Microbiol. 2004;27:746–754. doi: 10.1078/0723202042369857. [DOI] [PubMed] [Google Scholar]
- O'Brien JK, Marshall RT. Microbiological quality of raw ground chicken processed at high isostatic pressure. J Food Prot. 1996;59:146–150. doi: 10.4315/0362-028X-59.2.146. [DOI] [PubMed] [Google Scholar]
- Paarup T, Sanchez JA, Peláez C, Moral A. Sensory, chemical and bacteriological changes in vacuum-packed pressurised squid mantle (Todaropsis eblanae) stored at 4°C. Int J Food Microbiol. 2002;74:1–12. doi: 10.1016/s0168-1605(01)00701-2. [DOI] [PubMed] [Google Scholar]
- Paludan-Müller C, Dalgaard P, Huss HH, Gram L. Evaluation of the role of Carnobacterium piscicola in spoilage of vacuum- and modified-atmosphere-packed cold-smoked salmon stored at 5°C. Int J Food Microbiol. 1998;39:155–166. doi: 10.1016/s0168-1605(97)00133-5. [DOI] [PubMed] [Google Scholar]
- Papon M, Talon R. Factors affecting growth and lipase production by meat lactobacilli strains and Brochothrix thermosphacta. J Appl Bacteriol. 1988;64:107–115. doi: 10.1111/j.1365-2672.1988.tb02729.x. [DOI] [PubMed] [Google Scholar]
- Peirson MD, Guan TY, Holley RA. Aerococci and carnobacteria cause discoloration in cooked cured bologna. Food Microbiol. 2003;20:149–158. [Google Scholar]
- Pikuta EV, Marsic D, Bej A, Tang J, Krader P, Hoover RB. Carnobacterium pleistocenium sp. nov., a novel psychrotolerant, facultative anerobe isolated from permafrost of the Fox tunnel in Alaska. Int J System Evol Microbiol. 2005;55:473–478. doi: 10.1099/ijs.0.63384-0. [DOI] [PubMed] [Google Scholar]
- Pilet M-F, Dousset X, Barré R, Novel G, Desmazeaud M, Piard J-C. Evidence for two bacteriocins produced by Carnobacterium piscicola and Carnobacterium divergens isolated from fish and active against Listeria monocytogenes. J Food Prot. 1995;58:256–262. doi: 10.4315/0362-028X-58.3.256. [DOI] [PubMed] [Google Scholar]
- Pond MJ, Stone DM, Alderman DJ. Comparison of conventional and molecular techniques to investigate the intestinal microflora of rainbow trout (Oncorhynchus mykiss) Aquaculture. 2006;261:194–203. [Google Scholar]
- Quadri LEN, Sailer M, Roy KL, Vederas JC, Stiles ME. Chemical and genetic characterization of bacteriocins produced by Carnobacterium piscicola LV17B. J Biol Chem. 1994;269:12204–12211. [PubMed] [Google Scholar]
- Quadri LEN, Sailer M, Terebiznik MR, Roy KL, Vederas JC, Stiles ME. Characterization of the protein conferring immunity to the antimicrobial peptide carnobacteriocin B2 and expression of carnobacteriocins B2 and BM1. J Bacteriol. 1995;177:1144–1151. doi: 10.1128/jb.177.5.1144-1151.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quadri LEN, Yan LZ, Stiles ME, Vederas JC. Effect of amino acid substitutions on the activity of carnobacteriocin B2. J Biol Chem. 1997a;272:3384–3388. doi: 10.1074/jbc.272.6.3384. [DOI] [PubMed] [Google Scholar]
- Quadri LEN, Kleerebezem M, Kuipers OP, de Vos WM, Roy KL, Vederas JC, Stiles ME. Characterization of a locus from Carnobacterium piscicola LV17B involved in bacteriocin production and immunity: evidence for global inducer-mediated transcriptional regulation. J Bacteriol. 1997b;179:6163–6171. doi: 10.1128/jb.179.19.6163-6171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachman C, Kabadjova P, Valcheva R, Prévost H, Dousset X. Identification of Carnobacterium species by restriction fragment length polymorphism of the 16S–23S rRNA gene intergenic spacer region and species-specific PCR. Appl Environ Microbiol. 2004;70:4468–4477. doi: 10.1128/AEM.70.8.4468-4477.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard C, Brillet A, Pilet MF, Prévost H, Drider D. Evidence on inhibition of Listeria monocytogenes by divercin V41 action. Lett Appl Microbiol. 2003;36:288–292. doi: 10.1046/j.1472-765x.2003.01310.x. [DOI] [PubMed] [Google Scholar]
- Ringø E, Olsen RE. The effect of diet on aerobic bacterial flora associated with intestine of Arctic charr (Salvelinus alpinus L.) J Appl Microbiol. 1999;86:22–28. doi: 10.1046/j.1365-2672.1999.00631.x. [DOI] [PubMed] [Google Scholar]
- Ringø E, Holzapfel W. Identification and characterization of carnobacteria associated with the gills of Atlantic salmon (Salmo salar L.) Syst Appl Microbiol. 2000;23:523–527. doi: 10.1016/s0723-2020(00)80026-0. [DOI] [PubMed] [Google Scholar]
- Ringø E, Bendiksen HR, Grausen SJ, Sundsfjord A, Olsen RE. The effect of dietary fatty acids on lactic acid bacteria associated with the epithelial mucosa and from faecalia of Arctic charr, Salvelinus alpinus (L.) J Appl Microbiol. 1998;85:855–864. doi: 10.1046/j.1365-2672.1998.00595.x. [DOI] [PubMed] [Google Scholar]
- Ringø E, Bendiksen HR, Wesmajervi MS, Olsen RE, Jansen PA, Mikkelsen H. Lactic acid bacteria associated with the digestive tract of Atlantic salmon (Salmo salar L.) J Appl Microbiol. 2000;89:317–322. doi: 10.1046/j.1365-2672.2000.01116.x. [DOI] [PubMed] [Google Scholar]
- Ringø E, Wesmajervi MS, Bendiksen HR, Berg A, Olsen RE, Johnsen T, Mikkelsen H, Seppola M, Strøm E, Holzapfel W. Identification and characterization of carnobacteria isolated from fish intestine. Syst Appl Microbiol. 2001;24:183–191. doi: 10.1078/0723-2020-00020. [DOI] [PubMed] [Google Scholar]
- Ringø E, Seppola M, Berg A, Olsen RE, Schillinger U, Holzapfel W. Characterization of Carnobacterium divergens strain 6251 isolated from intestine of Arctic charr (Salvelinus alpinus L.) Syst Appl Microbiol. 2002a;25:120–129. doi: 10.1078/0723-2020-00080. [DOI] [PubMed] [Google Scholar]
- Ringø E, Lødemel JB, Mykleburst R, Jensen L, Lund V, Mayhew TM, Olsen RE. The effect of soybean, linseed and marine oils on aerobic gut microflora of Arctic charr (Salvelinus alpinus L.) before and after challenge with Aeromonas salmonicida spp. salmonicida. Aquaculture Res. 2002b;33:591–606. [Google Scholar]
- Ringø E, Schillinger U, Holzapfel WH. Antimicrobial activity of lactic acid bacteria isolated from aquatic animals and the use of lactic acid bacteria in aquaculture. In: Holzapfel WH, Naughton PJ, editors. Microbial Ecology in Growing Animals. Edinburgh: Elsevier; 2005. pp. 418–453. [Google Scholar]
- Ringø E, Sperstad S, Mykleburst R, Refstie S, Krogdahl Å. Characterisation of the microbiota associated with intestine of Atlantic cod (Gadus morhua L.). The effect of fish meal, standard soybean meal and a bioprocessed soybean meal. Aquaculture. 2006;261:829–841. [Google Scholar]
- Ringø E, Salinas I, Olsen RE, Nyhaug A, Mykleburst R, Mayhew TM. Histological changes in intestine of Atlantic salmon (Salmo salar L.) following in vitro exposure to pathogenic and probiotic bacterial strains. Cell Tissue Res. 2007;328:109–116. doi: 10.1007/s00441-006-0323-0. [DOI] [PubMed] [Google Scholar]
- Robertson PAW, O'Dowd C, Burrels C, Williams P, Austin B. Use of Carnobacterium sp. as a probiotic for Atlantic salmon (Salmo salar L.) and rainbow trout (Oncorhynchus mykiss, Walbaum) Aquaculture. 2000;185:235–243. [Google Scholar]
- Rohde BH, Quadri LEN. Functional characterization of a three-component regulatory system involved in quorum sensing-based regulation of peptide antibiotic production in Carnobacterium maltaromaticum. BMC Microbiol. 2006;6:93. doi: 10.1186/1471-2180-6-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roller S, Sagoo S, Board R, O'Mahony T, Caplice E, Fitzgerald G, Fogden M, Owen M, Fletcher H. Novel combinations of chitosan, carnocin and sulphite for the preservation of chilled pork sausages. Meat Sci. 2002;62:165–177. doi: 10.1016/s0309-1740(01)00243-1. [DOI] [PubMed] [Google Scholar]
- Rudi K, Maugesten T, Hannevik SE, Nissen H. Explorative multivariate analyses of 16S rDNA gene data from microbial communities in modified-atmosphere-packed salmon and coalfish. Appl Environ Microbiol. 2004;70:5010–5018. doi: 10.1128/AEM.70.8.5010-5018.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakala RM, Hayashidani H, Kato Y, Hirata T, Makino Y, Fukushima A, Yamada T, Kaneuchi C, Ogawa M. Change in the composition of the microflora on vacuum-packaged beef during chiller storage. Int J Food Microbiol. 2002;74:87–99. doi: 10.1016/s0168-1605(01)00732-2. [DOI] [PubMed] [Google Scholar]
- Samelis J. Managing microbial spoilage in the meat industry. In: Blackburn C de W., editor. Food Spoilage Microorganisms. Cambridge, UK: Woodhead Publishing Limited; 2006. pp. 213–286. [Google Scholar]
- Samelis J, Kakouri A, Georgiadou KG, Metaxopoulos J. Evaluation of the extent and type of bacterial contamination at different stages of processing of cooked ham. J Appl Microbiol. 1998;84:649–660. doi: 10.1046/j.1365-2672.1998.00392.x. [DOI] [PubMed] [Google Scholar]
- Samelis J, Kakouri A, Rementzis J. The spoilage microflora of cured, cooked turkey breasts prepared commercially with or without smoking. Int J Food Microbiol. 2000;56:133–143. doi: 10.1016/s0168-1605(99)00190-7. [DOI] [PubMed] [Google Scholar]
- Saucier L, Poon A, Stiles ME. Induction of bacteriocin in Carnobacterium piscicola LV17. J Appl Bacteriol. 1995;78:684–690. [Google Scholar]
- Schillinger U, Lücke F-K. Identification of lactobacilli from meat and meat products. Food Microbiol. 1987;4:199–208. [Google Scholar]
- Schillinger U, Holzapfel WH. The genus Carnobacterium. In: Wood BJB, Holzapfel WH, editors. The Genera of Lactic Acid Bacteria. London: Blackie Academic & Professional; 1995. pp. 307–326. [Google Scholar]
- Schmidt M, Priemé A, Stougaard P. Bacterial diversity in permanently cold and alkaline ikaite columns from Greenland. Extremophiles. 2006;10:551–562. doi: 10.1007/s00792-006-0529-9. [DOI] [PubMed] [Google Scholar]
- Schöbitz R, Zaror T, León O, Costa M. A bacteriocin from Carnobacterium piscicola for the control of Listeria monocytogenes in vacuum-packaged meat. Food Microbiol. 1999;16:249–255. [Google Scholar]
- Seppola M, Olsen RE, Sandaker E, Kanapathippillai P, Holzapfel W, Ringø E. Random amplification of polymorphic DNA (RAPD) typing of carnobacteria isolated from hindgut chamber and large intestine of Atlantic cod (Gadus morhua l) Syst Appl Microbiol. 2005;29:131–137. doi: 10.1016/j.syapm.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Shannon AL, Attwood G, Hopcroft DH, Christeller JT. Characterization of lactic acid bacteria in the larval midgut of the keratinophagous lepidopteran Hofmannophila pseudospretella. Lett Appl Microbiol. 2001;32:36–41. doi: 10.1046/j.1472-765x.2001.00854.x. [DOI] [PubMed] [Google Scholar]
- Shaw BG, Harding CD. A numerical taxonomic study of lactic acid bacteria from vacuum-packed beef, pork, lamb and bacon. J Appl Bacteriol. 1984;56:25–40. doi: 10.1111/j.1365-2672.1984.tb04693.x. [DOI] [PubMed] [Google Scholar]
- Signoretto C, Burlacchini G, Pruzzo C, Canepari P. Persistence of Enterococcus faecalis in aquatic environments via surface interactions with copepods. Appl Environ Microbiol. 2005;71:2756–2761. doi: 10.1128/AEM.71.5.2756-2761.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva J, Carvalho AS, Teixeira P, Gibbs PA. Bacteriocin production by spray-dried lactic acid bacteria. Lett Appl Microbiol. 2002;34:77–81. doi: 10.1046/j.1472-765x.2002.01055.x. [DOI] [PubMed] [Google Scholar]
- Simpson JM, Domingo JWS, Reasoner DJ. Assessment of equine fecal contamination: the search for alternative bacterial source-tracking targets. FEMS Microbiol Ecol. 2004;47:65–75. doi: 10.1016/S0168-6496(03)00250-2. [DOI] [PubMed] [Google Scholar]
- Sip A, Grajek W, Boyaval P. Enhancement of bacteriocin production by Carnobacterium divergens AS7 in the presence of a bacteriocin-sensitive strain Carnobacterium piscicola. Int J Food Microbiol. 1998;42:63–69. doi: 10.1016/s0168-1605(98)00062-2. [DOI] [PubMed] [Google Scholar]
- Smit G, Smit BA, Engels WJM. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiol Rev. 2005;29:591–610. doi: 10.1016/j.femsre.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Spanggaard B, Huber I, Nielsen J, Sick EB, Pipper CB, Martinussen T, Slierendrecht J, Gram L. The probiotic potential against vibriosis of the indigenous microflora of rainbow trout. Environ Microbiol. 2001;3:755–765. doi: 10.1046/j.1462-2920.2001.00240.x. [DOI] [PubMed] [Google Scholar]
- Sprules T, Kawulka KE, Gibbs AC, Wishart DS, Vederas JC. NMR solution structure of the precursor for carnobacteriocin B2, an antimicrobial peptide from Carnobacterium piscicola. Implications of the α-helical leader section for export and inhibition of type IIa bacteriocin activity. Eur J Biochem. 2004a;271:1748–1756. doi: 10.1111/j.1432-1033.2004.04085.x. [DOI] [PubMed] [Google Scholar]
- Sprules T, Kawulka KE, Vederas JC. NMR solution structure of imB2, a protein conferring immunity to antimicrobial activity of the type IIa bacteriocin, carnobacteriocin B2. Biochemistry. 2004b;43:11740–11749. doi: 10.1021/bi048854+. [DOI] [PubMed] [Google Scholar]
- Starliper CE, Shotts EB, Brown J. Isolation of Carnobacterium piscicola and an unidentified Gram-positive bacillus from sexually mature and post-spawning rainbow trout Oncorhynchus mykiss. Dis Aquat Org. 1992;13:181–187. [Google Scholar]
- Stoffels G, Nes IF, Gudmundsdottir A. Isolation and properties of a bacteriocin-producing Carnobacterium piscicola isolated from fish. J Appl Bacteriol. 1992a;73:309–316. doi: 10.1111/j.1365-2672.1992.tb04982.x. [DOI] [PubMed] [Google Scholar]
- Stoffels G, Nissen-Meyer J, Gudmundsdottir A, Sletten K, Holo H, Nes IF. Purification and characterization of a new bacteriocin isolated from a Carnobacterium sp. Appl Environ Microbiol. 1992b;58:1417–1422. doi: 10.1128/aem.58.5.1417-1422.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohr V, Joffraud JJ, Cardinal M, Leroi F. Spoilage potential and sensory profile associated with bacteria isolated from cold-smoked salmon. Food Res Int. 2001;34:797–806. [Google Scholar]
- Susiluoto T, Korkeala H, Björkroth KJ. Leuconostoc gasicomitatum is the dominating lactic acid bacterium in retail modified-atmosphere-packaged marinated broiler meat strips on sell-by-day. Int J Food Microbiol. 2003;80:89–97. doi: 10.1016/s0168-1605(02)00123-x. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Yamamoto T, Kawai Y, Inoue N, Yamazaki K. Mode of action of piscicocin CS526 produced by Carnobacterium piscicola CS526. J Appl Microbiol. 2005;98:1146–1151. doi: 10.1111/j.1365-2672.2005.02546.x. [DOI] [PubMed] [Google Scholar]
- Tahiri I, Desbiens M, Benech R, Kheadr E, Lacroix C, Thibault S, Oullet D, Fliss I. Purification, characterization and amino acid sequencing of divergicin M35: a novel class IIa bacteriocin produced by Carnobacterium divergens M35. Int J Food Microbiol. 2004;97:123–136. doi: 10.1016/j.ijfoodmicro.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Talon R, Walter D, Montel MC. Growth and effect of staphylococci and lactic acid bacteria on unsaturated free fatty acids. Meat Sci. 2000;54:41–47. doi: 10.1016/s0309-1740(99)00068-6. [DOI] [PubMed] [Google Scholar]
- Thornley MJ. Observations on the microflora of minced chicken meat irradiated with 4 MeV cathode rays. J Appl Bacteriol. 1957;20:286–298. [Google Scholar]
- Thornley MJ, Sharpe ME. Micro-organisms from chicken meat related to both lactobacilli and aerobic sporeformers. J Appl Bacteriol. 1959;22:368–376. [Google Scholar]
- Toffin L, Webster G, Weightman AJ, Fry JC, Prieur D. Molecular monitoring of culturable bacteria from deep-sea sediment of the Nankai Trough, Leg 190 Ocean Drilling Program. FEMS Microbiol Ecol. 2004;48:357–367. doi: 10.1016/j.femsec.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Toranzo AE, Romalde JL, Nuñez S, Figueras A, Barja JL. An epizootic in farmed, market-size rainbow trout in Spain caused by a strain of Carnobacterium piscicola of unusual virulence. Dis Aquat Org. 1993a;17:87–99. [Google Scholar]
- Toranzo AE, Novoa B, Baya AM, Hetrick FM, Barja JL, Figueras A. Histopathology of rainbow trout, Oncorhynchus mykiss (Walbaum), and striped bass, Morone saxatilis (Walbaum), experimentally infected with Carnobacterium piscicola. J Fish Dis. 1993b;16:261–267. [Google Scholar]
- Truelstrup Hansen L, Huss HH. Comparison of the microflora isolated from spoiled cold-smoked salmon from three smokehouses. Food Res Int. 1998;31:703–711. [Google Scholar]
- van Belkum MJ, Stiles ME. Characterization of the theta-type plasmid pCD3.4 from Carnobacterium divergens, and modulation of its host range by repA mutation. Microbiology. 2006;152:171–178. doi: 10.1099/mic.0.28294-0. [DOI] [PubMed] [Google Scholar]
- Vermeiren L, Devlieghere F, Vandekinderen I, Debevere J. The interaction of the non-bacteriocinogenic Lactobacillus sakei 10A and lactocin S producing Lactobacillus sakei 148 towards Listeia monocytogenes on a model cooked ham. Food Microbiol. 2006;23:511–518. doi: 10.1016/j.fm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Vihavainen E, Lundström H-S, Susiluoto T, Koort J, Paulin L, Auvinen P, Björkroth KJ. Role of broiler carcasses and processing plant air in contamination of modified-atmosphere-packaged broiler products with psychrotrophic lactic acid bacteria. Appl Environ Microbiol. 2007;73:1136–1145. doi: 10.1128/AEM.01644-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker VK, Palmer GR, Voordouw G. Freeze – thaw tolerance and clues to the winter survival of a soil community. Appl Environ Microbiol. 2006;72:1784–1792. doi: 10.1128/AEM.72.3.1784-1792.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J, Harmark K, Davidson BE, Hillier AJ, Gordon JB, Wilcock A, Hickey MW, Coventry MJ. Inhibition of Listeria monocytogenes by piscicolin 126 in milk and Camembert cheese manufactured with a thermophilic starter. J Appl Microbiol. 1997;82:273–280. doi: 10.1046/j.1365-2672.1997.00349.x. [DOI] [PubMed] [Google Scholar]
- Wang Y, Henz ME, Gallagher NLF, Chai S, Gibbs AC, Yan LZ, Stiles ME, Wishart DS, Vederas JC. Solution structure of carnobacteriocin B2 and implications for structure–activity relationships among type IIa bacteriocins from lactic acid bacteria. Biochemistry. 1999;38:15438–15447. doi: 10.1021/bi991351x. [DOI] [PubMed] [Google Scholar]
- Worobo RW, Henkel T, Sailer M, Roy KL, Vederas JC, Stiles ME. Characteristics and genetic determinant of a hydrophobic peptide bacteriocin, carnobacteriocin A, produced by Carnobacterium piscicola LV17A. Microbiology. 1994;140:517–526. doi: 10.1099/00221287-140-3-517. [DOI] [PubMed] [Google Scholar]
- Worobo RW, van Belkum MJ, Sailer M, Roy KL, Vederas JC, Stiles ME. A signal peptide secretion-dependent bacteriocin from Carnobacterium divergens. J Bacteriol. 1995;177:3143–3149. doi: 10.1128/jb.177.11.3143-3149.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Yang H, Lai X, Fu X, Wu J, Huang L, Yu X, Wu Y, Wu Y, Liu B. Etiological study for a case of multi-bacterial synergistic gangrene. Chinese Sci Bull. 1997;42:511–517. [Google Scholar]
- Yamazaki K, Suzuki M, Kawai Y, Inoue N, Montville TJ. Inhibition of Listeria monocytogenes in cold-smoked salmon by Carnobacterium piscicola CS526 isolated from frozen surimi. J Food Prot. 2003;66:1420–1425. doi: 10.4315/0362-028x-66.8.1420. [DOI] [PubMed] [Google Scholar]
- Yamazaki K, Suzuki M, Kawai Y, Inoue N, Montville TJ. Purification and characterization of a novel class IIa bacteriocin, piscicocin CS526, from surimi-associated Carnobacterium piscicola CS526. Appl Environ Microbiol. 2005;71:554–557. doi: 10.1128/AEM.71.1.554-557.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousif AN, Albright LJ, Evelyn TPT. In vitro evidence for the antibacterial role of lysozyme in salmonid eggs. Dis Aquat Org. 1994;19:15–19. [Google Scholar]