Abstract
p16INK4A (p16) binds to cyclin-dependent kinase 4/6 and negatively regulates cell growth. Recent studies have led to an understanding of additional biologic functions for p16; however, the detailed mechanisms involved are still elusive. In this article, we show an unexpected expression of anion exchanger 1 (AE1) in the cytoplasm in poorly and moderately differentiated gastric and colonic adenocarcinoma cells and in its interaction with p16, thereby sequestrating the protein in the cytoplasm. Genetic alterations of p16 and AE1 were not detectable. Forced expression of AE1 in these cells sequestrated more p16 in the cytoplasm, whereas small interfering RNA-mediated silencing of AE1 in the cells induced the release of p16 from the cytoplasm to the nucleus, leading to cell death and growth inhibition of tumor cells. By analyzing tissue samples obtained from patients with gastric and colonic cancers, we found that 83.33% of gastric cancers and 56.52% of colonic cancers coexpressed AE1 and p16 in the cytoplasm. We conclude that AE1 plays a crucial role in the pathogenesis of gastric and colonic adenocarcinoma and that p16 dysfunction is a novel pathway of carcinogenesis.
Keywords: Anion exchanger 1, p16, gastric adenocarcinoma, colonic adenocarcinoma, siRNA
Introduction
Prior research has demonstrated that p16 expression is associated with cell-cycle arrest at G1 checkpoint [1,2]. Several factors are involved in the mechanism of the functional inactivation of p16 in human malignancies, such as homozygous deletions, intragenic mutations, and transcriptional silencing due to promoter hypermethylation [3–5]. Hence, expression of p16 has been proposed as a biomarker for the identification of cancer.
Apart from cell-cycle control, a number of studies have led to a better understanding of additional biologic functions for p16 [6–8]. The molecular basis for the regulation of expression, function, and localization in subfractions remains poorly understood, although regulation is important for understanding the physiological and pathophysiological significance of p16. Our previous work has shown that there are direct interaction and functional synergy between p16 and anion exchanger 1 (AE1) [9].
Human AE is a transmembrane protein that facilitates the electroneutral exchange of Cl- and across the plasma membrane. To date, three different genes, AE1, AE2, and AE3, have been identified. Each of these genes encodes for a different polypeptide, depending on splicing and transcription initiation site [10,11]. AE proteins can be divided into three distinct domains: 1) an N-terminal cytoplasmic domain; 2) a C-terminal membrane-spanning domain of 500 amino acids comprising 14 helices; and 3) an acidic, short C-terminal domain (∼ 40 amino acids) in the cytoplasm. The isoforms differ primarily in their cytoplasmic and membrane-spanning domains, but show great similarities in their role in anion translocation through the plasma membrane [12,13].
AE1 has been shown to be a unique AE member that is abundantly expressed in erythrocytes. The protein makes up 25% of the total erythrocyte membrane protein, and up to 50% of the intrinsic membrane protein. Its N-terminal-truncated variant is expressed in renal cortical and medullary collecting ducts, which lacks N-terminal [65 (human) or 79 (mouse)] amino acid residues of erythrocyte AE1 (kAE1) [14,15]. AE2 is primarily expressed in gastric mucosa, choroid plexus, and intestine [16,17]. Although mRNA transcripts have been reported for AE1 in spleen, heart, lungs, and liver, the actual protein remains undetectable, except in erythrocytes [18]. In this study, we report that AE1 protein is abundantly expressed in gastric and colonic cancer tissues and is closely related to human gastric and colonic carcinogenesis due to p16 sequestration in the cytoplasm.
Materials and Methods
Cell Lines
The following cell lines were used: human chronic myeloid leukemic cell line K562; hepatoma cell line HepG2; human pancreatic cancer cell line Patu8988; human breast cancer cell line T47D; human gastric adenocarcinoma cell lines MKN45, MKN28, SGC7901, and AGS; and colon adenocarcinoma cell line SW1116 (Cell Bank of Shanghai Institutes for Biological Sciences, Shanghai, China).
Cell Fractionation
For fractionations of the nucleus and cytoplasm, 1 x 107 cells were incubated in 400 µl of lysis buffer for 10 minutes, then supplemented with NP-40 and protease inhibitor cocktail (Sigma, St. Louis, MO) for 1 minute on ice. Lysates were centrifuged for 1 minute at 2300g. Supernatants were collected for cytoplasmic protein extracts. Pellets were washed with lysis buffer without NP-40, resuspended in 150 µl of extraction buffer, and incubated for 20 minutes on ice. The samples were centrifuged at 12,000g for 10 minutes, and the supernatants were collected as nuclear protein extracts.
Coimmunoprecipitation of Endogenous p16 and AE1 in MKN45 and SW1116 Cells
After ultrasonic vibration, cell samples were cleared of insoluble debris by centrifugation for 15 minutes at 10,000g and were incubated with anti-AE1 (Sigma) or p16 (Santa Cruz Biotechnology, Santa Cruz, CA) antibodies and protein A-Sepharose beads (Amersham Biosciences, Piscataway, NJ) overnight on a rotator at 4°C. The protein A-Sepharose beads were washed thrice with lysis buffer and quenched with 35 ml of SDS sample buffer. Coimmunoprecipitates were resolved by 9% SDS-PAGE and analyzed by Western blot analysis.
Immunocytochemistry
For endogenous AE1 and AE2, MKN45 and SW1116 cells were grown on glass coverslips, and the proteins were detected with anti-AE1 or anti-AE2 antibodies (Alpha Diagnostics International, San Antonio, TX). Secondary anti-AE1 antibody was coupled to Texas red, and anti-AE2 antibody was coupled to fluorescein isothiocyanate. Coverslips were then mounted on slides and inspected with a fluorescence microscope.
siRNA Vector Construction and Transfections
Three-target sequences of siRNA were selected: P1, 5′-GGG TAC TGT TCT CCT AGA C-3′; P2, 5′-CGG TCT AGA GTA CAT CGT G-3′; and P3, 5′-GGC AAC CTT TGA TGA GGA G-3′. These sequences were synthesized and inserted into the pSilencer 3.1-H1 neo vector in accordance with the manufacturer's instructions (Ambion, Austin, TX). For stable transfection, cells were transfected with P2 vector or scrambled vector and placed under G418 selection for 4 weeks. Surviving colonies were isolated and expanded.
Western Blot Analysis
Cell lysates or tissue protein extracts were equally loaded into an 8% acrylamide gel, electrophoresed, and transferred to nitrocellulose membranes. After blocking with 5% nonfat milk in PBS, the membranes were probed with mouse monoclonal anti-AE1 antibody (Sigma) or rabbit polyclonal antip16 (Santa Cruz Biotechnology), followed by horseradish peroxidase-linked secondary antibodies (Cell Signaling, Danvers, MA).
Immunohistochemistry
For paraffin sections, human tissues were fixed with 4% paraformaldehyde in 0.1 M cacodylate buffer and postfixed in a similar fixative, with the exception that 3% paraformaldehyde was used in a 1-hour postfixation. The use of human tissues had prior approval of the National Ethical Committee. Paraffin-embedded tissue sections were dewaxed in xylene and rehydrated with ethanol. After a 30-minute incubation in 0.3% H2O2 in methanol to block endogenous peroxidase activity, rehydration was completed with ethanol. Sections were incubated overnight at 4°C with anti-AE1 and anti-p16 antibodies diluted in 10 mM PBS (pH 7.4) containing 0.1% Triton X-100 and 0.1% BSA.
Results
Two Gastrointestinal Cancer Cells Abnormally Expressed Cytoplasmic AE1 and p16
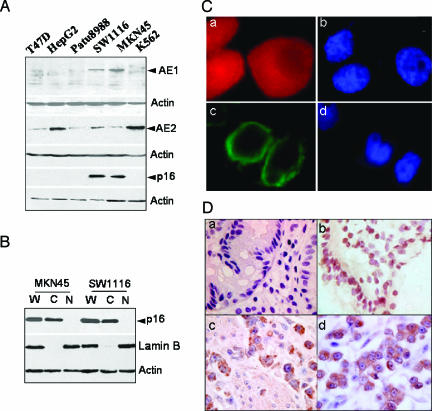
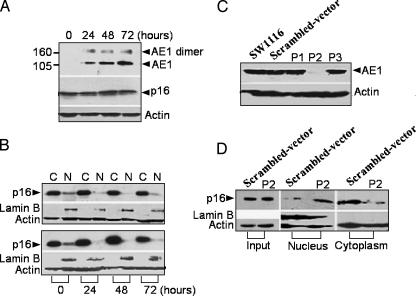
All cell lines tested normally express AE2 protein. However, the gastrointestinal tumor cell lines MKN45 and SW1116 unconventionally expressed AE1 protein, which is absent in normal gastric or colonic tissue. Because of the interaction of AE1 with p16, as we have previously reported [9], we subsequently investigated the expression of p16 protein in these cells. Western blot analysis showed that p16 is expressed in the cytoplasm in MKN45 and SW1116 cells and is absent in the other four cell lines (Figure 1A). Investigation of p16 localization in cellular subfractions indicated that p16 protein is not expressed in the nucleus, but largely in the cytoplasm of MKN45 and SW1116 cells (Figure 1B). We thus proceeded to analyze the localization of AE1 and AE2 proteins in the two cell lines with an immunofluorescent assay. Unlike AE2 protein, which is normally localized on cell plasma membrane, AE1 had no expression on the cell surface of the cell lines, but was diffuse within the cytoplasm (Figure 1C).
Figure 1.
Cytoplasmic expression of AE1 and p16. (A) The expression of AE1, AE2, and p16 in tumor cell lines was identified by Western blot analysis. AE2 was expressed in all cells as indicated. Positive staining of AE1 and p16 was observed in MKN45 and SW1116 cells. (B) The proportion of p16 in the nucleus and cytoplasm of MKN45 and SW1116 cells was identified, and the cytoplasmic distribution of p16 was dramatically observed. W, whole-cell extract; C, cytoplasmic fraction; N, nuclear fraction. (C) Immunochemistry of endogenous AE1 and AE2 in MKN45 (a) and SW1116 (c) cells. Secondary Texas red - or fluorescein isothiocyanate-labeled antibodies were added to the samples before mounting the coverslips on slides. Blue: Nuclear staining of the cells (b to a; d to c). (D) The expression of AE1 and p16 in human gastric adenocarcinoma tissues. Sample slices were probed with primary anti-AE1 or anti-p16 antibodies. (a) Negative staining of AE1 in noncancer tissue. (b) Nuclear staining of p16 in noncancer tissue (brown). (c) Cytoplasmic staining of AE1 in gastric adenocarcinoma (brown). (d) Cytoplasmic expression of p16 in gastric adenocarcinoma (brown).
In the next phase of the analysis, 66 paraffin-embedded human gastric cancer samples and 27 colonic cancer samples were assessed for the expression of AE1 and p16 proteins in parallel with normal human tissue control samples. Fifty-five of 66 cases of gastric cancer, and 15 of 27 cases of colonic cancer positively stained for AE1, which was distributed in the cytoplasm in different grades, and p16 was also stained in the cytoplasm in those samples. A typical staining of AE1 (a and c) and p16 (b and d) in human gastric noncancer (a and b) and adenocarcinoma (c and d) tissues is shown in Figure 1D. In contrast to cancer tissues, noncancer human gastric and colonic (data not shown) tissues stained negative for AE1 and the nuclear expression of p16. Immunoreactivities of whole-tissue specimens were semiquantified independently by two individuals into four degrees (0 = none, 1 = mild, 2 = moderate, 3 = abundant, and 4 = strong). The correlation of expression of AE1 with p16 was statistically analyzed using Pearson's correlation coefficient (Table 1). Together, the results strongly support the notion that AE1 expression in gastric and colonic adenocarcinoma is closely related to the cytoplasmic expression of p16 (gastric cancer AE1 vs cytoplasmic p16, r = 0.93; colonic cancer AE1 vs cytoplasmic p16, r = 0.99).
Table 1.
Correlation of AE1 and p16 Expressions in Gastric and Colonic Adenocarcinoma.
| Grade | Gastric Adenocarcinoma* (n = 55) | Colonic Adenocarcinoma† (n = 15) | ||
| AE1 (%) | p16 (%) | AE1(%) | p16 (%) | |
| 1 | 30.3 | 27.3 | 54.5 | 45.5 |
| 2 | 36.4 | 30.3 | 27.3 | 27.3 |
| 3 | 18.2 | 24.2 | 0 | 9.0 |
| 4 | 15.1 | 18.2 | 18.2 | 18.2 |
Grade 1, mild; grade 2, moderate; grade 3, abundant; grade 4, strongly abundant.
r = 0.93.
r = 0.99 (AE1 vs p16 expression).
AE1 and p16 Proteins Interacted with Each Other in MKN45 and SW1116 Cells
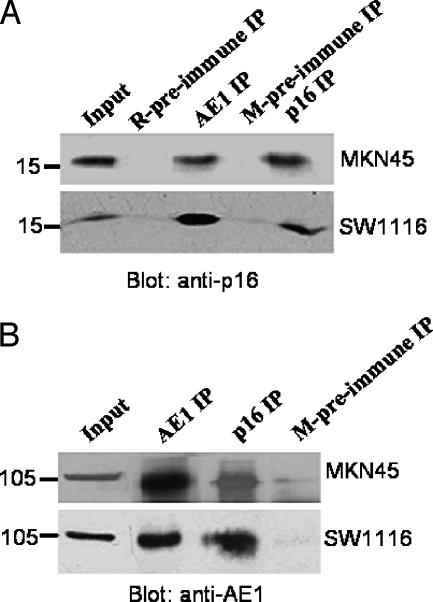
To investigate whether endogenous AE1 and p16 proteins interacted with each other in MKN45 and SW1116 cells, a coimmunoprecipitation (Co-IP) experiment was carried out. As shown in Figure 2, anti-AE1 and anti-p16 antibodies, but not preimmune mouse serum, could precipitate both AE1 and p16, indicating the specificity of the Co-IP experiment. More importantly, anti-AE1 coprecipitated p16 protein, whereas antip16 antibody precipitated AE1 protein, strongly confirming the interaction of AE1 and p16 proteins in the two tumor cells.
Figure 2.
AE1 and p16 interaction. Whole-cell lysates from MKN45 or SW1116 cells were immunoprecipitated with mouse anti-human AE1 or rabbit anti-human p16 antibodies, followed by immunoblotting for associated p16 (A) or AE1 (B) antibodies. Mouse and rabbit preimmune sera were used as negative controls.
Distribution of p16 in Cellular Subfractions Was Not pH-Dependent
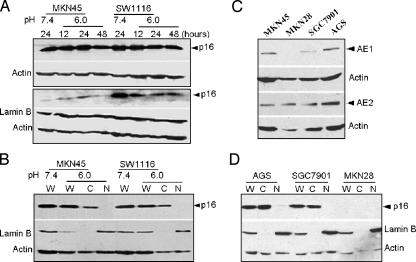
Both AE1 and AE2 facilitated Cl- and exchange and regulated cellular pH homeostasis in cells in which they were abundantly expressed. We reasoned that aberrant expression and localization of AE1 protein in tumor cells interrupt the physiological function of AE2 protein and thereby change the intracellular pH (pHi) of the tumor cells. To test this hypothesis, the pHi of single MKN45 or SW1116 cells was recorded by spectrofluorimetric monitoring with the pHsensitive fluorescent dye BCECF, using an Electrophysiology Analysis System (ORCA AG; Hamamatsu, Japan). The resting pHi is higher than that of the cell that did not express AE1 (data not shown), suggesting that the regulation of pHi is abnormal in the two tumor cells. This result raised the question as to whether the cytoplasmic distribution of p16 protein was induced by an elevated pHi. To determine whether the aberrant p16 distribution in cellular subfractions was pH-dependent, the two tumor cells were cultured in a low-pH medium (pH stable at 6.0), the cells were harvested, and nuclear-cytoplasmic fractionation was performed after the culture for 12, 24, and 48 hours, respectively. The expression of p16 in whole MKN45 cells was upregulated at 12 and 24 hours, but was downregulated at 48 hours after the culture pH was decreased from 7.4 to 6.0. Upregulation of p16 was not observed in SW1116 cells (Figure 3A, upper panel). The movement of p16 from the cytoplasm to the nucleus was not visualized by dynamic detection of p16 expression in the nucleus of MKN45 and SW1116 cells (Figure 3A, lower panel). This was further confirmed by comparing the expression of p16 protein in the nucleus and cytoplasm in the same sample (Figure 3B). The results demonstrated that the distribution of p16 in cellular subfractions is not pH-dependent, despite the possibility that aberrant AE1 expression may be responsible for the elevated pHi in the two tumor cells.
Figure 3.
The cytoplasmic distribution of p16 in MKN45 and SW1116 cells was not affected by the low pH of the culture, and expression occurred in the three other gastric adenocarcinoma cell lines. (A) p16 expression in whole-cell extract (upper panel) and nucleus (lower panel) from two tumor cell lines cultured at pH 6.0 (for 12, 24, and 48 hours) and pH 7.4. (B) Direct comparison of the nuclear-cytoplasmic distribution of p16 in the two tumor cell lines cultured at pH 6.0 and 7.4. (C) The expression of AE1 and AE2 in the three other gastric adenocarcinoma cell lines. (D) Cytoplasmic distribution of p16 in the three other gastric adenocarcinoma cell lines. W, whole-cell extract; C, cytoplasmic fraction; N, nuclear fraction. MKN28, AGS, and SGC7901 represent well, moderately, and poorly differentiated gastric adenocarcinoma cell lines, respectively.
siRNA-Mediated Silencing of AE1 in the Cells Induced p16 Release from the Cytoplasm to the Nucleus
To better understand the relationship between AE1 and p16, we detected the expression of AE1 and the cytoplasmic distribution of p16 in the three other gastric adenocarcinoma cell lines, MKN28 (well-differentiated), AGS (moderately differentiated), and SGC7901 (poorly differentiated). AGS and SGC7901 cells expressed AE1 and cytoplasmic p16, whereas neither AE1 nor p16 was expressed in MKN28 cells. Nevertheless, all cell lines normally expressed AE2 (Figure 3, C and D).
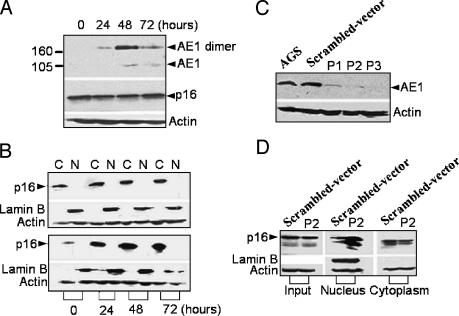
Taken together, the results demonstrate that AE1 is the factor responsible for p16 cytoplasmic sequestration. We suppose that overexpression of AE1 could sequester more p16 in the cytoplasm and, conversely, blocking the expression of AE1 in these cells could lead to the release of p16 from the cytoplasm to the nucleus. We therefore transfected AE1 constructs into AGS and SW1116 cells. The results showed that the level of p16 expression in whole-cell lysates of the two tumor cells was not regulated by forced expression of AE1 (Figures 4A and 5A). However, cytoplasmic p16 was upregulated in a time-dependent manner along with the expression of AE1 (Figures 4B and 5B, lower panel) compared with empty vector transfection (Figures 4B and 5B, upper panel), indicating that overexpression of AE1 in the two tumor cells sequestered more p16 in the cytoplasm.
Figure 4.
Forced expression or knockdown of AE1 in AGS cells. (A) Forced expression of AE1 in AGS cells at 0 to 72 hours posttransfection of the pcDNA3-AE1 construct. The expression of endogenous p16 in whole-cell lysates was synchronously detected as indicated. (B) Cytoplasmic sequestration of p16 in AGS cells transfected with pcDNA3-AE1 (lower panel), compared with the transfection of an empty vector (upper panel). C, cytoplasmic fraction; N, nuclear fraction. (C) The expression of endogenous AE1 in AGS cells transiently transfected with P1-pSilencer, P2-pSilencer, and P3-pSilencer 3.1-H1 neo constructs for 48 hours; AE1 was detected by immunoblots. (D) Movement of p16 from the cytoplasm to the nucleus after the transfection of P2-pSilencer 3.1-H1 neo constructs.
Figure 5.
Forced expression or blockage of AE1 in SW1116 cells. The experimental design was the same as that in Figure 4.
Next, we attempted to block the expression of the AE1 gene in AGS and SW1116 cells by siRNA. Three single-stranded DNA 64-mer oligonucleotides (containing P1, P2, and P3 sequences) were selected based on an analysis of the open reading frame using an siRNA design software (www.ambion.com). To determine the most efficient siRNA sequences, the constructs were transiently transfected into the two tumor cells to reduce the expression of AE1. Figures 4C and 5C show that only the P2 construct had the efficiency to block the expression of AE1 in both AGS and SW1116 cells. Therefore, we selected the P2 construct to transfect the AGS and SW1116 cells. Notably, transfection with the P2 construct resulted in the accumulation of p16 in the nucleus and a remarkable decrease in the cytoplasm in the two cells (Figures 4D and 5D).
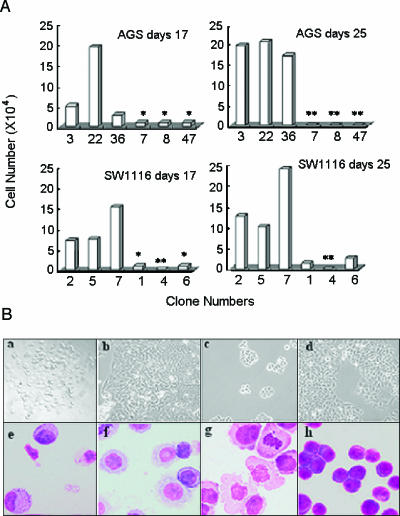
To determine whether siRNA-mediated AE1 silencing in AGS and SW1116 cells had an intrinsic effect on cell growth, stable-transfected mono cells were selected with the infinite dilution method in 96-well plates and counted on day 17 (the cells were expanded from a single clone) and day 25 (1000 cells from a single clone were continually expanded for another 8 days). The growth of P2 vector-transfected AGS and SW1116 transfectants was remarkably inhibited, compared with the scrambled vector-transfected AGS and SW1116 transfectants, as indicated in Figure 6A. After culture for another 8 days, the alive P2 vector-transfected AGS and SW1116 cells were not detected. Morphologic features were shown to be different between the P2 vector-transfected and the scrambled vector-transfected AGS and SW1116 transfectants (Figure 6B).
Figure 6.
AE1 knockdown induced cell death and growth inhibition in AGS and SW1116 cells. (A) AGS and SW1116 cells were stably transfected with P2-pSilencer or scrambled-pSilencer 3.1-H1 neo constructs, respectively. After selection by G418, 12 single clones of P2 vector or scrambled vector transfectants—numbered 3, 22, and 36 (AGS; scrambled vector transfectants); 7, 8, and 47 (AGS; P2 vector transfectants); 2, 5, and 7 (SW1116; scrambled vector transfectants); and 1, 4, and 6 (SW1116; P2 vector transfectants)—were isolated and expanded in 96-well plates. Viable cells were counted after 17 days. One thousand cells were then isolated from the same clones and expanded for another 8 days (total, 25 days), and the cells were counted. *Number of cells, < 104. **Number of cells, 0. (B) Another six clones of P2 vector or scrambled vector transfectants of AGS and SW1116 were isolated and expanded. The cell deaths of the P2 vector AGS (a) and SW1116 (c) transfectants or scrambled vector AGS (b) and SW1116 (d) transfectants were observed. The morphologic features of the P2 vector AGS (e) and SW1116 (g) transfectants or the scrambled vector AGS (f) and SW1116 (h) transfectants were examined under a microscope following Wright's staining of the cells.
Discussion
We have reported herein, for the first time, the coexpression of AE1 and AE2 in gastric and colonic tumor cells. The expressed AE1 protein did not localize to the plasma membrane, suggesting that the cells do not have the ability for AE1 membrane traffic or that AE1 lacks the ability to traffic to the plasma membrane even if it has a normal amino acid sequence. We propose that the aberrant expression and storage of AE1 in the cytoplasm interrupt the well-balanced mechanism for maintaining natural pHi in normal cells. Physiological anion exchange by AE2 usually represents chloride uptake in exchange for bicarbonate efflux [19,20]. Therefore, the alkalization of gastric and colonic cancer cells, which aberrantly express AE1, implies the dysfunction of AE2 protein. However, decreasing the pH to 6.0 in culture media without a resulting p16 return to the nucleus indicates that cellular alkalization is not responsible for the cytoplasmic distribution of p16. Co-IP of the two proteins in the tumor cells demonstrated the interaction of AE1 and p16, supporting the structural sequestration of p16 by AE1.
Two isoforms of AE proteins are found in gastrointestinal tumor cells, which could raise a question on the posteffect of aberrant AE1 through the p16 pathway. It is known that p16 protein acts as a cell-cycle suppressor in the nucleus and that its biologic function has been extensively described in the past decade; however, there are still some unresolved and controversial issues [21]. Recent studies have described the cytoplasmic localization of p16 that differs from the current view concerning p16 immunohistochemical localization. The described protein accumulation is apparently not caused by an alteration of the p16 gene [22]. Electron microscopy has also provided evidence that the cytoplasmic localization of p16 in non-small cell lung cancer is specific [23]. However, insufficient attention has been paid to the basis for immunohistochemical signals. In the present article, we demonstrated that p16 was frequently expressed in the cytoplasm and that cytoplasmic existence in most gastric and colonic cancers is closely related to aberrant cytoplasmic expression of AE1.
Gastric cancer is the fourth most common cancer worldwide [24]. The most common type of gastric cancer is adenocarcinoma, which starts in the glandular tissue of the stomach and accounts for 90% to 95% of all gastric cancers. Many genes have been analyzed to understand the molecular bases for human gastric cancers, but only a few with frequent alterations have been identified [25–27]. This genetic research uncovers a more complete understanding of gastric cancer and allows for the development of specific therapy for specifically abnormal proteins. In addition, colonic cancer results from the progressive accumulation of genetic and epigenetic alterations that lead to the transformation of normal colonic epithelial cells into colon adenocarcinoma cells [28]. Although some genes associated with colon cancer have now been identified and genetic testing is available for diagnosis [29–31], the use of genetic/molecular markers, together with new targeted therapies, remains to be investigated.
The abnormality of p16 is frequently reported in both gastric and colonic cancers or neoplasms [32]. The major mechanism for p16 dysfunction results from the hypermethylation of its promoter or gene mutations [33]. Our results provide a novel pathway for the dysfunction of p16 in gastric and colonic cancers, in which p16 is expressed but is sequestered in the cytoplasm by aberrant expression of AE1. The discovery of the cause and specific effect of the cytoplasmic distribution of p16 in gastric and colonic cancers will yield more effective chemotherapy strategies that take advantage of this unique characteristic of cancer cells and provide the potential to further improve survival.
Acknowledgements
We thank Marco F. Passeri and Jonathan T. Prigge (Center for Cell and Gene Therapy, Baylor College of Medicine, Houston, TX) and Jiankang Liu (University of California, Berkeley, CA) for critical reading of the manuscript.
Abbreviations
- AE
anion exchanger
- siRNA
small interfering RNA
Footnotes
This work was supported, in part, by the National Natural Science Foundation of China (NO30230160 and NO30570697), the National Key Program (973) for Basic Research of China (NO2002CB512805), and grants from the Science and Technology Commission of Shanghai (03XD14016).
References
- 1.Serrano M, Hannon GJ, Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993;366:704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- 2.Kamb A, Gruis NA, Weaver-Feldhaus J, Liu Q, Harshman K, Tavtigian SV, Stockert E, Day RS, III, Johnson BE, Skolnick MH. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994;264:436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- 3.Yang R, Gombart AF, Serrano M, Koeffler HP. Mutational effects on the p16INK4a tumor suppressor protein. Cancer Res. 1995;55:2503–2506. [PubMed] [Google Scholar]
- 4.Liggett WH, Jr, Sidransky D. Role of the p16 tumor suppressor gene in cancer. J Clin Oncol. 1998;16:1197–1206. doi: 10.1200/JCO.1998.16.3.1197. [DOI] [PubMed] [Google Scholar]
- 5.Liu Y, Lan Q, Siegfried JM, Luketich JD, Keohavong P. Aberrant promoter methylation of p16 and MGMT genes in lung tumors from smoking and never-smoking lung cancer patients. Neoplasia. 2006;8:46–51. doi: 10.1593/neo.05586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minami R, Muta K, Umemura T, Motomura S, Abe Y, Nishimura J, Nawata H. p16INK4a induces differentiation and apoptosis in erythroid lineage cells. Exp Hematol. 2003;31:355–362. doi: 10.1016/s0301-472x(03)00040-7. [DOI] [PubMed] [Google Scholar]
- 7.Plath T, Detjen K, Welzel M, von Marschall Z, Murphy D, Schirner M, Wiedenmann B, Rosewicz S. A novel function for the tumor suppressor p16INK4a: inhibition of anoifis via upregulation of the α5β1 fibronectin receptor. J Cell Biol. 2000;150:1467–1478. doi: 10.1083/jcb.150.6.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fahraeus R, Lane DP. The p16INK4a tumour suppressor protein inhibits alphavbeta3 integrin-mediated cell spreading on vitronectin by blocking PKC-dependent localization of alphavbeta3 to focal contacts. EMBO J. 1999;18:2106–2118. doi: 10.1093/emboj/18.8.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu GH, Wang Y, Xi YH, Shen WW, Pan XY, Shen WZ, Jiang XS, Chen GQ. Direct interaction and cooperative role of tumor suppressor p16 with band 3 (AE1) FEBS Lett. 2005;579:2105–2110. doi: 10.1016/j.febslet.2005.02.063. [DOI] [PubMed] [Google Scholar]
- 10.Casey JR, Reithmeier RA. Anion exchangers in the red cell and beyond. Biochem Cell Biol. 1998;76:709–713. doi: 10.1139/bcb-76-5-709. [DOI] [PubMed] [Google Scholar]
- 11.Alper SL. Molecular physiology of SLC4 anion exchangers. Exp Physiol. 2006;91:153–161. doi: 10.1113/expphysiol.2005.031765. [DOI] [PubMed] [Google Scholar]
- 12.Alper SL, Darman RB, Chernova MN, Dahl NK. The AE gene family of exchangers. J Nephrol. 2002;15:S41–S53. [PubMed] [Google Scholar]
- 13.Romero MF. Molecular pathophysiology of SLC4 bicarbonate transporters. Curr Opin Nephrol Hypertens. 2005;14:495–501. doi: 10.1097/01.mnh.0000168333.01831.2c. [DOI] [PubMed] [Google Scholar]
- 14.Ding Y, Casey JR, Kopito RR. The major kidney AE1 isoform does not bind ankyrin (Ank1) in vitro. An essential role for the 79 NH2-terminal amino acid residues of band 3. J Biol Chem. 1994;269:32201–32208. [PubMed] [Google Scholar]
- 15.Kollert-Jons A, Wagner S, Hubner S, Appelhans H, Drenckhahn D. Anion exchanger 1 in human kidney and oncocytoma differs from erythroid AE1 in its NH2 terminus. Am J Physiol. 1993;265:F813–F821. doi: 10.1152/ajprenal.1993.265.6.F813. [DOI] [PubMed] [Google Scholar]
- 16.Rossmann H, Alper SL, Nader M, Wang Z, Gregor M, Seidler U. Three 5′-variant mRNAs of anion exchanger AE2 in stomach and intestine of mouse, rabbit, and rat. Ann NY Acad Sci. 2000;915:81–91. doi: 10.1111/j.1749-6632.2000.tb05226.x. [DOI] [PubMed] [Google Scholar]
- 17.Lindsey AE, Schneider K, Simmons DM, Baron R, Lee BS, Kopito RR. Functional expression and subcellular localization of an anion exchanger cloned from choroid plexus. Proc Natl Acad Sci. 1990;87:5278–5282. doi: 10.1073/pnas.87.14.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kudrycki KE, Newman PR, Shull GE. cDNA cloning and tissue distribution of mRNAs for two proteins that are related to the band 3 exchanger. J Biol Chem. 1990;265:462–471. [PubMed] [Google Scholar]
- 19.Madshus IH. Regulation of intracellular pH in eukaryotic cells. Biochem J. 1988;250:1–8. doi: 10.1042/bj2500001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alper SL, Chernova MN, Stewart AK. Regulation of Na+-independent exchangers by pH. JOP J Pancreas. 2001;2:171–175. [PubMed] [Google Scholar]
- 21.Pei XH, Xiong Y. Biochemical and cellular mechanisms of mammalian CDK inhibitors: a few unresolved issues. Oncogene. 2005;24:2787–2795. doi: 10.1038/sj.onc.1208611. [DOI] [PubMed] [Google Scholar]
- 22.Emig R, Magener A, Ehemann V, Meyer A, Stilgenbauer F, Volkmann M, Wallwiener D, Sinn HP. Aberrant cytoplasmic expression of the p16 protein in breast cancer is associated with accelerated tumour proliferation. Br J Cancer. 1998;78:1661–1668. doi: 10.1038/bjc.1998.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evangelou K, Bramis J, Peros I, Zacharatos P, Dasiou-Plakida D, Kalogeropoulos N, Asimacopoulos PJ, Kittas C, Marinos E, Gorgoulis VG. Electron microscopy evidence that cytoplasmic localization of the p16INK4A “nuclear” cyclin-dependent kinase inhibitor (CKI) in tumor cells is specific and not an artifact. A study in non-small cell lung carcinomas. Biotech Histochem. 2004;79:5–10. doi: 10.1080/10520290310001659466. [DOI] [PubMed] [Google Scholar]
- 24.Ushijima T, Sasako M. Focus on the gastric cancer. Cancer Cell. 2004;5:121–125. doi: 10.1016/s1535-6108(04)00033-9. [DOI] [PubMed] [Google Scholar]
- 25.Guilford P, Hopkins J, Harraway J, McLeod M, McLeod N, Harawira P, Taite H, Scoular R, Miller A, Reeve AE. E-cadherin germline mutations in familial gastric cancer. Nature. 1998;392:402–405. doi: 10.1038/32918. [DOI] [PubMed] [Google Scholar]
- 26.Uchino S, Tsuda H, Noguchi M, Yokota J, Terada M, Saito T, Kobayashi M, Sugimura T, Hirohashi S. Frequent loss of heterozygosity at the DCC locus in gastric cancer. Cancer Res. 1992;52:3099–3102. [PubMed] [Google Scholar]
- 27.Yamada Y, Yoshida T, Hayashi K, Sekiya T, Yokota J, Hirohashi S, Nakatani K, Nakano H, Sugimura T, Terada M. p53 gene abnormalities in gastric cancer metastases and in gastric cancer cell lines derived from metastases. Cancer Res. 1991;51:5800–5805. [PubMed] [Google Scholar]
- 28.Grady WM. Genomic instability and colon cancer. Cancer Metastasis Rev. 2004;23:11–27. doi: 10.1023/a:1025861527711. [DOI] [PubMed] [Google Scholar]
- 29.Burt R, Neklason DW. Genetic testing for inherited colon cancer. Gastroenterology. 2005;128:1696–1716. doi: 10.1053/j.gastro.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 30.Aguilera O, Fraga MF, Ballestar E, Paz MF, Herranz M, Espada J, Garcia JM, Munoz A, Esteller M, Gonzalez-Sancho JM. Epigenetic inactivation of the Wnt antagonist DICKKOPF-1 (DKK-1) gene in human colorectal cancer. Oncogene. 2006;25:4116–4121. doi: 10.1038/sj.onc.1209439. [DOI] [PubMed] [Google Scholar]
- 31.Tsafrir D, Bacolod M, Selvanayagam Z, Tsafrir I, Shia J, Zeng Z, Liu H, Krier C, Stengel RF, Barany F, et al. Relationship of gene expression and chromosomal abnormalities in colorectal cancer. Cancer Res. 2006;66:2129–2137. doi: 10.1158/0008-5472.CAN-05-2569. [DOI] [PubMed] [Google Scholar]
- 32.Furth EE, Gustafson KS, Dai CY, Gibson SL, Menard-Katcher P, Chen T, Koh J, Enders GH. Induction of the tumor-suppressor p16INK4a within regenerative epithelial crypts in ulcerative colitis. Neoplasia. 2006;8:429–436. doi: 10.1593/neo.06169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim BN, Yamamoto H, Ikeda K, Damdinsuren B, Sugita Y, Ngan CY, Fujie Y, Ogawa M, Hata T, Ikeda M, et al. Methylation and expression of p16INK4 tumor suppressor gene in primary colorectal cancer tissues. Int J Oncol. 2005;26:1217–1226. [PubMed] [Google Scholar]