Abstract
Although camptothecin (CPT) has been reported to induce apoptosis in various cancer cells, the molecular details of this regulation remain largely unknown. In this study, we demonstrate that BH3-only protein Noxa is upregulated during CPT-induced apoptosis, which is independent of p53. In addition, we show that phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway is responsible for Noxa's induction. Luciferase assay and cAMP response element binding protein (CREB) knockdown experiments further demonstrate that CREB is involved in the transcriptional upregulation of Noxa. Moreover, blocking Noxa expression using specific small interfering ribonucleic acid (siRNA) significantly reduces the apoptosis in response to CPT, indicating that Noxa is an essential mediator for CPT-induced apoptosis. Interestingly, antiapoptotic Mcl-1 was also upregulated through PI3K/Akt signaling pathway upon CPT treatment. Using immunoprecipitation assay, Noxa was found to interact with Mcl-1 in the presence or absence of CPT. Knockdown of Mcl-1 expression by short hairpin ribonucleic acid (shRNA) was shown to potentiate CPT-induced apoptosis. Consistently, ectopic overexpression of Mcl-1 rescued cells from apoptosis induced by CPT. Cells coexpressing Noxa and Mcl-1 at different ratio correlates well with the extent of apoptosis, suggesting that the balance between Noxa and Mcl-1 may determine the susceptibility of HeLa cells to CPT-induced apoptosis.
Keywords: Noxa, Mcl-1, CREB, camptothecin, apoptosis
Introduction
Apoptosis, also termed as programmed cell death, is an evolutionarily conserved and genetically controlled cellular suicide mechanism [1]. It plays a pivotal role in the regulation of tissue development and homeostasis [2]. Growing evidences have shown that failure or defects in the apoptotic pathway may contribute to the generation of drug resistance [3]. The signaling events leading to apoptosis can be divided into two distinct pathways, i.e., extrinsic and intrinsic cell death pathways [4]. In the intrinsic pathway, mitochondria play a central role in the integration and execution of variety of apoptotic signals. Death signals lead to changes in mitochondrial membrane permeability and the subsequent release of proapoptotic proteins such as cytochrome c [5]. Release of cytochrome c results in the Apaf-1-mediated activation of initiator caspase-9, which, in turn, activates other effector caspases [6]. Among the components that regulate the intrinsic pathway of apoptosis, Bcl-2 family proteins have been reported to play a major role [7]. By forming heterodimeric complexes at the mitochondria, Bcl-2 family members promote or suppress apoptosis.
Bcl-2 family of proteins can be divided into three groups, i.e., multidomain antiapoptotic proteins (such as Bcl-2, Bcl-xL, and Mcl-1), multidomain proapoptotic proteins (such as Bax, BAK, and Bok), and BH3-only proapoptotic members (e.g., Puma, Noxa, Bid, and Bim) [8]. The proapoptotic BH3-only proteins are the most apical regulators of apoptosis induction. In response to apoptotic stimuli, they will be engaged in transcriptional regulations or posttranslational modifications [9], ultimately leading to the activation of Bax and BAK. It has been reported that deficiency in Bax and BAK renders cells resistant to apoptosis induced by various apoptotic stimuli [10–12], indicating that BH3-only proteins function upstream of Bax and BAK.
Despite the extensive interactions between Bcl-2 family proteins, their respective interaction appears to be selective and specific. For instance, Bim and Puma can bind all of the mutidomain antiapoptotic Bcl-2 family members, whereas Noxa only binds to Mcl-1, but not Bcl-2 and Bcl-xL [13,14]. On the contrary, Bad interacts with Bcl-2 and Bcl-xL, but not with Mcl-1 [13,15]. Strikingly, Bid and Bim are able to directly interact with BAK and Bax, resulting in their oligomerization [16–18]. Recent data showed that, in healthy cells, BAK is functionally inactive due to the formation of BAK-Mcl-1 complex; however, accumulation of Noxa leads to the release of BAK from BAK-Mcl-1 complex and its subsequent activation [19,20]. In light of these evidences, it is evident that Bcl-2 family members regulate apoptosis using two distinct models. In the first model, Bid-like BH3-only proteins activate apoptosis through direct interaction with Bax and BAK. The second is that Noxa-like BH3-only proteins sensitize apoptosis by binding to and neutralizing the antiapoptotic Bcl-2 family proteins, thereby releasing Bax and BAK.
Camptothecin (CPT) is a specific inhibitor of topoisomerase I and can bind to and stabilize the normally transient DNA-topoisomerase I cleavage complex to collide with the DNA replication fork [21,22], resulting in an irreversible double-strand break and eventually cell death [23,24]. CPT has been reported to induce apoptosis in various cancer cells and some of these cells are lacking in functional p53 [25–28], implying that CPT can induce apoptosis in a p53-independent manner.
Although some features characteristic of apoptosis induction by CPT have been already described, the underlying molecular mechanism remains to be fully elucidated. In this study, we showed that CPT was able to transcriptionally upregulate Noxa in a p53-independent manner, in which phosphatidylinositol 3-kinase (PI3K)/Akt signaling pathway is involved. In addition, we demonstrated that transcription factor cAMP response element binding protein (CREB) was responsible for the Noxa's induction. More importantly, Noxa was shown to be an essential mediator for CPT-induced and caspase-9-involved apoptosis. Unexpectedly, we found that antiapoptotic Mcl-1 was also upregulated through PI3K/Akt signaling pathway upon CPT treatment. We demonstrated that Noxa was able to interact with Mcl-1 both in CPT-treated and -untreated cells. Finally, we hypothesized that the ratio of Noxa to Mcl-1 may regulate the shift in the balance of cell fate toward cell death versus survival upon CPT treatment.
Materials and Methods
Reagents and Antibodies
The following antibodies were used in this study: anti-Mcl-1 and anti-Bax (Santa Cruz Biotechnology, Santa Cruz, CA); anti-caspase-9 (Immunotech, Marseilles, France); anti-green fluorescent protein (GFP) (BD Biosciences, Palo Alto, CA); anti-Flag (Sigma, St Louis, MO); anti-Noxa (Imgenex, San Diego, CA); anti-Puma, anti-AKT, anti-pAKT (S473), and anti-CREB (Cell Signaling Technology, Danvers, MA); and anti-β-actin (Abcam, Cambridge, United Kingdom). Cycloheximide (CHX), camptothecin (CPT), geneticin (G418), doxorubicin, and Hoechst 33342 were purchased from Sigma and LY294002 was purchased from Calbiochem (La Jolla, CA). MitoTracker Red CMXRos was purchased from Molecular Probes (Carlsbad, CA).
Cell Culture and Transfection
Cell lines HeLa and H1299 were cultured in Dulbecco's modified Eagle's medium containing 10% heat-inactivated fetal bovine serum, 1 x nonessential amino acid, 100 µg/ml penicillin, 1 x MEM sodium pyruvate, 100 µg/ml streptomycin (Invitrogen, Carlsbad, CA) at 37°C under an atmosphere of 5% CO2 in air. Transfection of cells with various mammalian expression constructs by Lipofectamine 2000 (Invitrogen) was according to the methods provided by the manufacturer.
To establish HeLa cell line stably expressing Mcl-1 or control short hairpin ribonucleic acid (shRNA), Mcl-1 or control shRNA expression plasmid (kindly provided by Dr. Gregory J. Gores) was individually transfected into HeLa cells. Forty-eight hours after transfection, clones were selected in culture medium containing 1 mg/ml geneticin (G418) for 3 weeks. The positive clones were further confirmed by Western blot analysis.
RNA Interference (RNAi)
In our study, knock out RNAi system (Clontech Laboratories, Mountain View, CA) was used to knock down CREB protein level. Nineteen-base pair target sequence for CREB was 5′-TAC AGC TGG CTA ACA ATG G-3′. To knock down the endogenous level of Noxa, small interfering ribonucleic acid (siRNA) for Noxa (sc-37305; Santa Cruz Biotechnology) was transfected into HeLa cells according to themanufacturer's instructions. The effect of RNAi on CREB or Noxa protein level was measured by Western blot analysis after 48 hours of transfection.
Western Blot Analysis and Immunoprecipitation
Western blot analyses were performed mainly as described by Mei et al. [29] with following modifications. After incubating with first and second antibodies, blots were developed by electrochemical luminescence (Lumi-Phos WB; Pierce, Rockford, IL). For immunoprecipitation, HeLa cells were lysed in a NP-40-based lysis buffer (0.2% NP-40, 150 mM NaCl, 20 mM Hepes, pH 7.5, 2 mM EDTA, and 1.5 mM MgCl2) supplemented with protease inhibitor cocktail and 20 mg/ml MG132 for 1 hour on ice. Cytosolic lysate was incubated with anti-Mcl-1 antibody bound to Protein A/G-Sepharose. After incubating at 4°C overnight, the immunoprecipitates were washed five times in lysis buffer and proteins were recovered by boiling the beads in sodium dodecyl sulfate sample buffer and analyzed by Western blot analysis using anti-Noxa antibody.
Protein Stability Assay
After incubating with or without CPT (10 µM) for 9 hours, HeLa cells were further treated with 25 µg/ml CHX for indicated periods of time and were then harvested and analyzed by Western blot analysis as described above.
Reporter Plasmids and Luciferase Assay
The reporter plasmids used in this study (pGL3-Noxa-wt, pGL3-Noxa-mtCRE, and pGL3-Noxa-mtP53) were kindly provided by Dr. C. Lallemand. To analyze the promoter activity of these reporter genes, H1299 cells were transiently transfected with these plasmids. Renilla luciferase reporter plasmid was included as an internal control. Firefly and Renilla luciferase activity were assayed (Dual-Luciferase Reporter Assay System; Promega Corporation, WI) according to the manufacturer's instructions. Tcf/Lef reporter activities were normalized relative to Renilla luciferase activities and presented as means (± SD) of three independent experiments.
Semi-Quantitative Reverse Transcription-Polymerase Chain Reaction (RT-PCR)
For semi-quantitative RT-PCR, HeLa cells were treated either with CPT (10 µM) alone or in combination with LY294002 (40 µM) for 12 hours. Total RNA was isolated using SV Total RNA Isolation System (Promega Corporation) and 0.1 µg of RNA of each sample was used as template. RT-PCR was performed by TaKaRa One-step RNA PCR Kit (TaKaRa Bio Inc., Otsu, Japan) using the following specific primer pairs: Noxa, 5′-ATG CCT GGG AAG AAG GCG CGC-3′ and 5′-TCA GGT TCC TGA GCA GAA GAG-3′; β-actin, 5′-GAC CTG ACT GAC TAC CTC ATG AAG AT-3′ and 5′-GTC ACA CTT CAT GAT GGA GTT AAG G-3′.
Apoptosis Assay
Apoptosis assay was performed mainly as described previously [30]. Briefly, cells were treated with CPT (10 µM) or in combination with other reagents for the indicated periods of time. The viability of cells was measured by counting the number of apoptotic cells characteristic of aberrant nuclei staining by Hoechst 33342, and was normalized to the number of total cells. Data were presented as means (±SD) of three independent experiments.
Results
CPT Induces Noxa Expression in a p53-Independent Manner
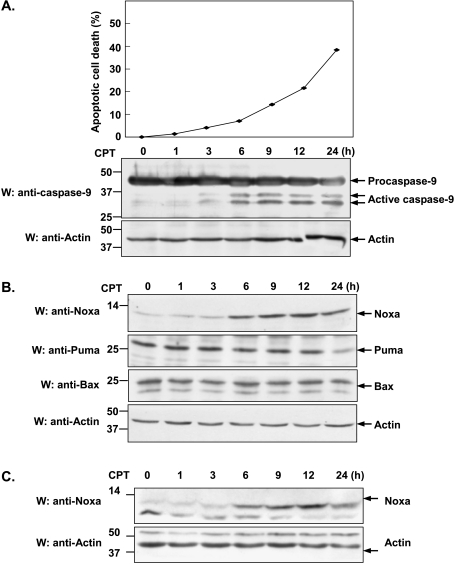
CPT, an inhibitor of topoisomerase I, is known to induce apoptosis in various cancer cells [25–28]. In accordance with these reports, we also found that CPT was able to induce apoptosis in HeLa cells. After 24 hours of exposure to CPT, HeLa cells were shown to have approximately 40% apoptotic cell death (Figure 1A, upper part). To evaluate whether caspase-9 activation is involved during CPT-induced apoptosis, Western blot analysis was performed. As shown in Figure 1A, caspase-9 activation became detectable after 3 hours of CPT treatment, indicating that mitochondria apoptotic pathway is truly involved in CPT-induced apoptosis. To further investigate the molecular mechanism underlying CPT-induced apoptosis, we compared the effect of CPT on the expression of three proapoptotic Bcl-2 family members, namely Noxa, Puma, and Bax. As shown in Figure 1B, Noxa was significantly upregulated and its induction was detectable as early as 6 hours following CPT treatment. In contrast, neither Puma nor Bax showed any induction upon CPT treatment, suggesting that Noxa is implicated in playing a specific role during CPT induced apoptosis. Because p53 was previously shown to be upregulated by CPT [31] and Noxa is a direct p53 target gene [19], we sought to determine whether p53 was required for CPT-dependent induction of Noxa. The effect of CPT on Noxa induction in p53-null H1299 cells has been examined. As shown in Figure 1C, the kinetics of Noxa upregulation by CPT in H1299 cells was similar to that in HeLa cells. Thus, we conclude that Noxa is upregulated by CPT in a p53-independent manner.
Figure 1.
Noxa is upregulated in CPT-induced apoptosis. (A) HeLa cells were treated with 10 µM CPT for the indicated periods of time (0, 1, 3, 6, 9, 12, and 24 hours). The viability of cells was measured by counting apoptotic cells characteristic of aberrant nuclei staining by Hoechst 33342 and was normalized to the number of total cells. Data were presented as means (± SD) of three independent experiments. Cell lysate harvested at different time points was used to assess the activation of caspase-9 with anti-caspase-9 antibody by Western blot analysis. Actin was used as loading control. (B) After treatment with 10 µM CPT for the indicated periods of time (0, 1, 3, 6, 9, 12, and 24 hours), HeLa cells were harvested and analyzed by Western blot analysis using anti-Noxa, anti-Bax, and anti-Puma antibody, respectively. (C) After incubating for the indicated periods of time in the presence of CPT (10 µM), H1299 cells were harvested and Western blot analysis were conducted using anti-Noxa antibody.
PI3K/Akt Signaling Pathway is Responsible for CPT-Induced Noxa Induction
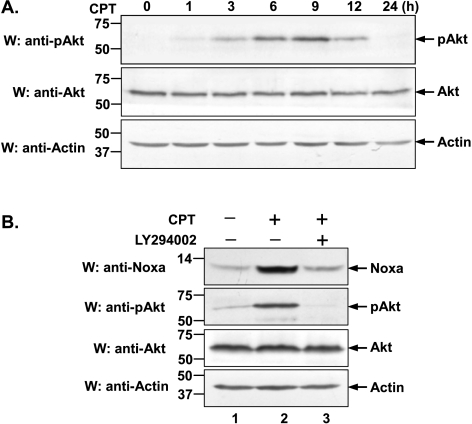
PI3K/Akt pathway has been shown to be activated by various growth factors and play a critical role in cell survival [32,33]. More specifically, previous data indicated that two types of apoptotic stimuli, i.e., staurosporine and etoposide, were capable of activating the PI3K/Akt pathway [34], which then stimulates us to inquire whether this signaling pathway can also be activated by CPT. To address this issue, we examined the PI3K-dependent activation of Akt phosphorylation after CPT treatment. HeLa cells were treated with CPT for the indicated periods of time and Akt phosphorylation on serine 473 was used as a read-out of PI3K activity [35]. As shown in Figure 2A, CPT-induced pAkt was increased in a time-dependent manner, peaking at 9 hours. To further determine that the activation of PI3K/Akt pathway indeed contributes to Noxa induction upon CPT treatment, HeLa cells were treated either with CPT alone or in combination with LY294002, a specific PI3K inhibitor, for 12 hours (Figure 2B). As was expected, Noxa was significantly induced by CPT, however, addition of LY294002 markedly abrogated CPT-induced Noxa upregulation, indicating Noxa induction is closely correlated with PI3K activity (lane 3 vs 2). Together, these data suggest that PI3K/Akt signaling pathway is responsible for CPT-induced Noxa upregulation.
Figure 2.
PI3K/Akt signaling pathway is involved in CPT-induced upregulation of Noxa. (A) After treatment with CPT (10 µM) for the indicated periods of time (0, 1, 3, 6, 9, 12, and 24 hours), whole cell lysates were prepared and immunoblotted using anti-pAkt or anti-Akt antibody. (B) HeLa cells were treated with CPT (10 µM) or with CPT (10 µM) and LY294002 (40 µM) for 12 hours. Cell lysates were then prepared and subjected to Western blot analysis using indicated antibodies. Endogenous actin was used as loading control.
Noxa is Transcriptionally Upregulated By CPT Treatment, in Which CREB is Involved
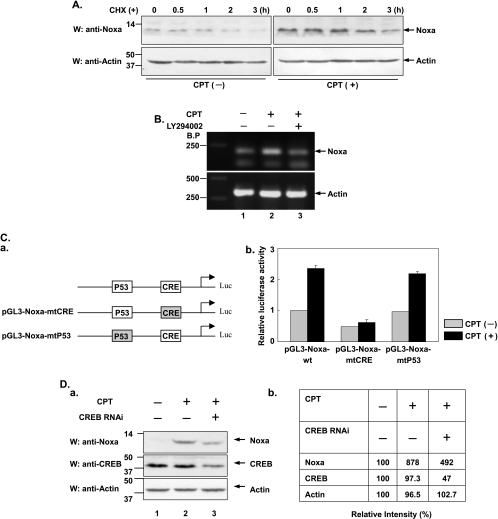
Because Noxa is an unstable protein and is subject to proteasome-mediated protein degradation [36–38], upregulated level of Noxa induced by CPT could be attributed to either less protein degradation or more RNA transcription. To distinguish between these two possibilities, the half-life of Noxa was compared in the presence and absence of CPT. As shown in Figure 3A, although the protein level of Noxa after CHX treatment was higher in the presence than in the absence of CPT, two patterns of decrease in Noxa in the presence and in the absence of CHX displayed in parallel as shown in Figure 3 (upper panels). In addition, total RNA was extracted from HeLa cells treated either with CPT alone or in combination with LY294002 and a semi-quantitative RT-PCR was performed. As shown in Figure 3B, Noxa mRNA level was increased upon CPT treatment (lane 2 vs lane 1); however, when LY294002 was additionally added, Noxa level was significantly inhibited (lane 3 vs lane 2), implying that Noxa is transcriptionally regulated by CPT through the PI3K/Akt signaling pathway.
Figure 3.
CREB, but not p53, is responsible for the transcriptional upregulation of Noxa upon CPT treatment. (A) After incubation with or without CPT (10 µM) for 9 hours, HeLa cells were further treated with CHX (20 µg/ml) for indicated times (0, 0.5, 1, 2, and 3 hours) before cell extracts were collected. The protein levels of Noxa at different time points were compared by Western blot analysis using anti-Noxa antibody and endogenous actin was used as loading control. (B) HeLa cells were treated with CPT (10 µM) alone or in combination with LY294002 (40 µM) for 12 hours. Total RNA was extracted from each of these treated samples and the equal amounts of RNA were used for amplification of Noxa cDNA fragment. β-Actin was used as internal control. (C) a. Schematic representation of the Noxa promoter luciferase reporter constructs used in this study. Mutated binding sites are indicated by shaded boxes. b. HeLa cells were transiently transfected with the various reporter plasmids shown in (a). Renilla luciferase plasmid pRL-CMV was also introduced into transfected cells as internal control. Twenty-four hours after transfection, cells were treated with or without CPT (10 µM) for another 15 hours. Luciferase activity was measured and plotted after normalizing with respect to Renilla luciferase activity (mean ± SD). (D) a. H1299 cells were individually transfected with either CREB or control shRNA expression plasmid. Forty-eight hours after transfection, cells were treated with or without CPT (10 µM) for 12 hours before cell lysates were prepared and subjected to Western blot analysis using indicated antibodies. Endogenous actin was used as loading control. b. Scanning densitometry was performed for each band in the blot indicated in D-a and an image analysis software (Scion Image; Scion, Frederick, MD) was used.
Transcription factor CREB was known as a regulatory target for the Akt kinase [39]. Recently, it has been shown that CREB is involved in the induction of Noxa by single-stranded RNA viruses by binding to cAMP-responsive element (CRE) binding site within the Noxa promoter [40]. To address whether CREB is also involved in CPT-induced Noxa induction, a reporter assay was performed. HeLa cells were individually transfected with pGL3-Noxa-wt, pGL3-Noxa-mtCRE, or pGL3-Noxa-mtp53. Twenty-four hours posttransfection, cells were treated with or without CPT for another 15 hours. As shown in Figure 3C-b, CPT was shown to activate the wt-Noxa promoter, whereas mutation in CRE binding site notably diminished the Noxa promoter activity, suggesting that CRE binding site indeed plays an essential role in the stimulation of Noxa gene transcription by CPT treatment. In contrast, mutation in the p53 binding site within the Noxa promoter has little, if any, effect on the activation of the promoter by CPT, which further supports our previous conclusion that CPT-induced Noxa upregulation is independent of p53. Next, we knocked down the expression of the CREB to further confirm its role in CPT-induced Noxa upregulation. As shown in Figure 3D, the induction of Noxa by CPT was proportionally reduced in accordance with knockdown of CREB expression using CREB RNAi (lane 3, top panel vs lane 3, middle panel). These results demonstrate that CREB is involved in the transcriptional upregulation of Noxa induced by CPT.
Noxa is Essential, But Not the Sole Determinant for CPT-Induced Apoptosis
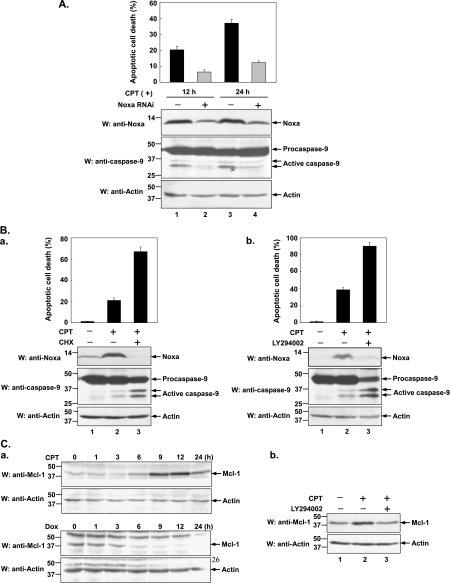
Given that Noxa is upregulated by CPT, we further investigated whether the induction of Noxa contributes to CPT-induced apoptosis. HeLa cells were transfected with control or Noxa-specific siRNA, and at 48 hours posttransfection, cells were treated with CPT for another 12 and 24 hours. As shown in Figure 4A, protein levels of Noxa were obviously lower in Noxa siRNA-transfected cells than in control siRNA-transfected cells (lower part, top panel, lane 2, 4 vs lane 1, 3). CPT-induced apoptotic cell death resulting from Noxa siRNA-transfected cells was lower than that from control siRNA-transfected cells (6% vs 21% at 12 hours and 12% vs 38% at 24 hours), suggesting that Noxa truly contributes to the CPT-induced cell death. Notably, a marked cleavage of caspase-9, as a marker of apoptosis, occurred more prominently in control siRNA-transfected cells than in Noxa siRNA-transfected cells (lower part, middle panel, lane 1, 3 vs lane 2, 4), further indicating that CPT-induced and Noxa-mediated cell death is through an intrinsic apoptotic pathway.
Figure 4.
Noxa alone cannot account for the CPT-induced apoptosis. (A) Knockdown of Noxa expression protects cells against CPT-induced apoptosis. HeLa cells were transfected with control or Noxa-specific siRNA (sc-37305; Santa Cruz Biotechnology). At 48 hours posttransfection, cells were treated with CPT (10 µM) for another 12 or 24 hours. The viability of cells was measured by counting apoptotic cells characteristic of aberrant nuclei staining by Hoechst 33342 and was normalized to the number of total cells. Data were presented as means (± SD) of three independent experiments. Cell lysates were also collected and subjected to Western blot analysis using anti-Noxa and anti-caspase-9 antibodies. Endogenous actin was detected by anti-actin antibody as internal control. (B) HeLa cells were treated with CPT (10 µM) alone or in combination with either (a) CHX (20 µg/ml) or (b) LY294002 (40 µM) for indicated periods of time. The viability of cells was measured according to the method described in (A). Noxa protein level and the activation of caspase-9 were assessed by Western blot analysis. (C) CPT-induced Mcl-1 upregulation is inhibited by LY294002. a. HeLa cells were treated with CPT (10 µM) or doxorubicin (2 µg/ml) for indicated periods of time (0, 1, 3, 6, 9, 12, and 24 hours) before cell lysates were prepared. Levels of Mcl-1 protein were assessed by Western blot analysis using anti -Mcl-1 antibody. Actin acted as a loading control. b. HeLa cells were treated with CPT (10 µM) or with CPT (10 µM) plus LY294002 (40 µM) for 12 hours. Cell lysates were then used for Western blot analysis with anti-Mcl-1 antibody and endogenous actin was used as internal control.
To examine whether additional protein(s) is (are) involved in regulating CPT-induced apoptosis, HeLa cells were treated with CPT in the absence or presence of CHX. The extent of cell death was measured by direct counting of cells with apoptotic nuclear morphology 12 hours after treatment. Caspase-9 activation was also assessed in CHX-treated or -untreated cells by its proteolytical processing. As shown in Figure 4B-a, in the absence of CHX, CPT treatment resulted in activation of caspase-9 followed by manifestation of apoptotic morphology in ∼ 22% of cells observed (lane 2 vs lane 1). However, inhibition of de nova protein synthesis by CHX significantly augmented caspase-9 activation and increased the CPT-induced apoptotic cell death from 22% to nearly 70%. We noticed that in the presence of CHX, the induction of Noxa by CPT was completely blocked and Noxa became hardly detectable (lower part, top panel, lane 3 vs lane 2). This data implies that CPT-induced apoptosis can take place when Noxa fell to an undetectable level and, logically, extra protein(s) (most likely antiapoptotic proteins) should be involved in this apoptotic induction. To further verify this assumption, HeLa cells were treated with CPT in the absence or presence of LY294002 that was shown to inhibit the Noxa expression (Figure 2B). The activation of caspase-9 was measured and apoptotic cell death was calculated 24 hours after treatment. As shown in Figure 4B-b, CPT-induced Noxa induction was almost completely inhibited by LY294002 (lower part, top panel, lane 3 vs lane 2) and additional treatment with LY294002 significantly increased CPT-induced apoptosis by 52% (upper part, lane 3 vs lane 2), which was inconsistent with the previous reports [41,42]. These results further confirm that Noxa alone cannot fully account for CPT-induced apoptosis and that determinant(s) other than Noxa should be also regulated by the PI3K/Akt signaling pathway.
During performance of this study, we found that CPT upregulated the expression of Mcl-1, one of the antiapoptotic Bcl-2 family proteins, but not Bcl-2 or Bcl-xL (data not shown). As shown in Figure 4C-a (upper part), Mcl-1 was induced by CPT in a time-dependent manner. In contrast, Mcl-1 was downregulated upon doxorubicin treatment (Figure 4C-a, lower part). To determine whether PI3K/Akt signaling pathway is involved in the upregulation of Mcl-1 by CPT, HeLa cells were treated either with CPT alone or in combination with LY294002 for 12 hours before cell lysates were harvested (CPT-untreated cells were used as a control). As a result, addition of LY294002 resulted in a significant inhibition of CPT-induced Mcl-1 upregulation (Figure 4C-b, lane 3 vs lane 2). Taken together, these results suggest that, in addition to Noxa, Mcl-1 may be another determinant that is involved in PI3K/Akt signaling in CPT-induced apoptosis.
Ratio of Noxa to Mcl-1 Regulates Cells' Susceptibility to CPT-Induced Apoptosis
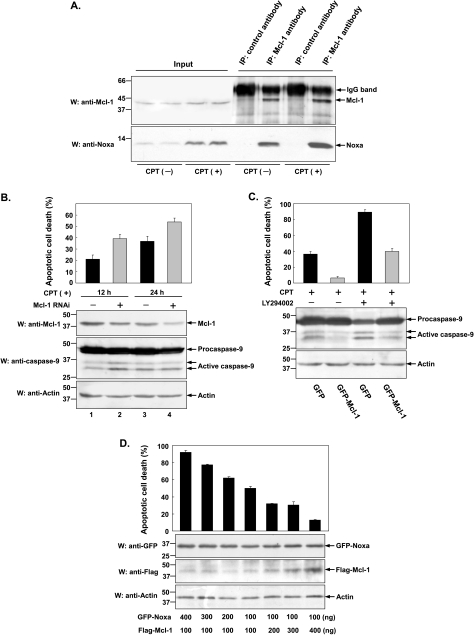
Given that both proapoptotic Noxa and antiapoptotic Mcl-1 are upregulated during CPT-induced apoptosis, it is not unreasonable to suspect that the balance between Noxa and Mcl-1 might be the underlying mechanism in regulating cell death/survival upon CPT treatment. To address this issue, we sought whether there exists an interaction between Noxa and Mcl-1 upon CPT treatment. HeLa cells were treated with or without CPT for 12 hours. Cell lysates were then incubated with either control antibody or anti-Mcl-1 antibody bound to Protein A/G-Sepharose. The immunoprecipitates were recovered and subjected to Western blot analysis using anti-Noxa antibody. As shown in Figure 5A, endogenous Mcl-1 and Noxa indeed interacts with each other both in the presence and in the absence of CPT. If the hypothesis that balance between Noxa and Mcl-1 determines cell fate after CPT treatment was true, we would expect that knockdown of Mcl-1 (or Noxa) would increase (or decrease) the susceptibility to CPT-induced apoptosis. To test this hypothesis, HeLa cells stably expressing control or Mcl-1 shRNA was individually treated with CPT for 12 or 24 hours, and the cell viability was calculated by counting apoptotic cells characteristic of aberrant nuclei. As shown in Figure 5B, effective knockdown of Mcl-1 was observed (lower part, top panel, lane 2, 4 vs lane 1, 3), and Mcl-1 knockdown resulted in a marked potentiation of CPT-induced apoptotic cell death by 19% (upper part, lane 2 vs lane 1) and 17% (upper part, lane 4 vs lane 3) at 12 and 24 hours respectively. The activation (cleavage) of caspase-9 was correlated with the extent of apoptosis. Consistently, HeLa cells stably expressing GFP-Mcl-1 showed significant resistance to apoptosis induced either by CPT alone or CPT and LY294002 compared with cells stably expressing GFP only (Figure 5C, upper part). A direct correlation was also observed between the cleavage (activation) of caspase-9 and the extent of apoptosis (lower part, top panel). As already described in Figure 4A, knockdown of Noxa is expectedly to reduce the apoptosis induced by CPT. Based on these data, it indicates that the ratio of Noxa to Mcl-1 might be involved in the regulation of cellular apoptotic response to CPT.
Figure 5.
Balance between Noxa and Mcl-1 regulates cells' susceptibility to CPT-induced apoptosis. (A) Interaction between endogenous Noxa and Mcl-1. HeLa cells were treated with or without CPT (10 µM) for 12 hours. Cell lysates were then incubated with control antibody or anti-Mcl-1 antibody bound to Protein A/G-Sepharose. The immunoprecipitates were recovered in sodium dodecyl sulfate sample buffer and subjected to Western blot analysis using anti-Noxa antibody with electrochemical luminescence-based detection. (B) Effect of Mcl-1 knockdown on the apoptosis induced by CPT. HeLa cells stably expressing control or Mcl-1 shRNA were individually treated with CPT (10 µM) for indicated periods of time. The viability of cells was measured by counting apoptotic cells characteristic of aberrant nuclei staining by Hoechst 33342 and was normalized to the number of total cells. Data were presented as means (± SD) of three independent experiments. Cell lysates were also harvested and subjected to Western blot analysis using indicated antibodies. Endogenous actin was used as internal control. (C) HeLa cells stably expressing GFP or GFP-Mcl-1 were treated with CPT (10 µM) or with CPT (10 µM) plus LY294002 (40 µM) for 24 hours. The viability of cells was measured according to the method described in (B) and Western blot analysis was performed to assess the activation of caspase-9 with anti-caspase-9 antibody. (D) HeLa cells were transiently cotransfected with GFP-Noxa and Flag-Mcl-1 expression plasmids varying in different ratios as indicated. Twenty-four hours posttransfection, the viability of cells was measured by directly counting GFP-positive apoptotic cells characteristic of aberrant nuclei staining by Hoechst 33342. Cell lysates were also harvested and subjected to Western blot analysis using indicated antibodies.
To further quantitatively validate this hypothesis, HeLa cells were transiently cotransfected with GFP-Noxa and Flag-Mcl-1 expression plasmids varying in different ratios. As shown in Figure 5D, decreasing the Noxa-to-Mcl-1 ratio from 400/100 to 100/400 concurrently reduces the apoptosis induced by CPT (upper part). Although the increased Mcl-1 level was clearly seen on the blot, the reduction of Noxa, however, is less obvious, which could be because, as the level of Noxa increases, more extensive cell death will occur. This result suggests that the ratio of Noxa to Mcl-1 may indeed regulate cell apoptosis decision to undergo apoptosis versus survival upon CPT treatment.
Discussion
Bcl-2 family proteins have been implicated in playing a major role in regulating the apoptosis pathway [7], and proapoptotic BH3-only proteins are the most apical regulators of apoptosis induction. Noxa, belonging to proapoptotic BH3-only Bcl-2 family, was reported to be upregulated by phorbol ester, p53, E2F1, hypoxia-inducible factor (HIF)-1a, UV radiation, and other DNA-damaging agents such as etoposide and adriamycin [19,43–48]. In this study, we demonstrated that CPT was able to induce Noxa expression in a p53-independent manner, despite that p53 was shown to be upregulated and activated by CPT [31]. Further results from Noxa knockdown experiments suggest that Noxa plays an important role in CPT-induced apoptosis.
PI3K/Akt pathway was shown to be activated by various growth factors and play a critical role in cell survival [32,33]. Moreover, recent study has showed that PI3K/Akt pathway can also be activated by staurosporine and etoposide [34]. Similarly, we have demonstrated that CPT was capable of activating PI3K/Akt pathway, which was involved in signaling Noxa induction. Although Noxa was reported to be an unstable protein [36–38], we have not yet found that CPT can alter Noxa protein stability. Nevertheless, we showed that Noxa was upregulated by CPT at the transcriptional level, in which CREB is involved. This result is not unconceivable, because CREB has been shown to be a regulatory target for the Akt kinase [39] and, more recently, it was shown that CREB plays a vital role in the induction of Noxa by singlestrand RNA viruses [40].
PI3K/Akt signaling pathway was known to be involved in the upregulation of Mcl-1 by interleukin-3 [49]. In this report, we demonstrated that, in addition to Noxa, Mcl-1 was also upregulated by CPT involving PI3K/Akt signaling pathway. It has already been reported that Mcl-1 protein level was upregulated in response to DNA-damaging agents other than CPT [50] and, therefore, our observation that CPT mediated Mcl-1 induction is not totally exceptional. However, upregulation of antiapoptotic Mcl-1 in response to apoptotic stimulus CPT may imply two causes. Firstly, CPT-induced Mcl-1 upregulation may represent cell's instinctive survival response to the death stimuli, or alternatively, induction of Mcl-1 may stand for the molecular basis underlying the mechanism of acquired resistance to CPT.
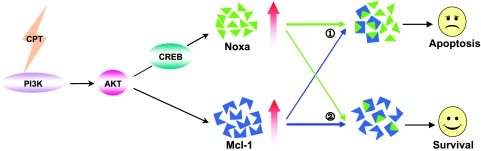
Given that both antiapoptotic protein Mcl-1 and proapoptotic protein Noxa are upregulated by CPT and, moreover, Noxa and Mcl-1 were able to interact with each other not only in CPT-untreated cells but also in CPT-treated cells, we assume that there might be a balance between Noxa and Mcl-1 to affect CPT-induced apoptosis. Knockdown of Mcl-1 is able to significantly potentiate CPT-induced apoptosis, whereas ectopic expression of Mcl-1 is capable of rescuing cells from apoptosis induced by CPT. Cells coexpressing ectopic Noxa and Mcl-1 at desired ratios result in an expected extent of apoptosis. These results indicate that the Noxa/Mcl-1 balance regulates cells' susceptibility to CPT-induced apoptosis. Alves et al. proposed recently that the Noxa/Mcl-1 axis plays an important role in glucose limitation-mediated apoptosis [51], which further supports our above conclusion. More importantly, data revealed by this study may provide useful information for clinical practice: combination treatment with CPT and Mcl-1 interfering might be therapeutically more effective and beneficial compared to monotherapy with either single agent in cancer chemotherapy. In conclusion, we propose a hypothetical model of Noxa and Mcl-1 in the regulation of CPT-induced apoptosis as shown in Figure 6.
Figure 6.
Hypothetical model of Noxa and Mcl-1 in the regulation of CPT-induced apoptosis. CPT activates PI3K/Akt signaling pathway, which, in turn, induces upregulation of both Noxa and Mcl-1. Although the upregulation of Noxa through transcriptional factor CREB has been elucidated in this study, the transcription factor responsible for CPT-induced Mcl-1 has not yet been identified. Nonetheless, based on the findings that Noxa can interact with Mcl-1 in the presence of CPT, Noxa/Mcl-1 balance model may be borrowed to explain the susceptibility of cells to CPT-induced apoptosis: when the concentration of proapoptotic protein Noxa exceeds that of antiapoptotic protein Mcl-1, CPT-induced apoptosis occurs (①); however, when Mcl-1 takes the upper hand, more free Mcl-1 will ultimately lead to CPT resistance (②).
Acknowledgements
We are grateful to C. Lallemand for pGL3-Noxa-wt, pGL3-Noxa-mtCRE, and pGL3-Noxa-mtP53 plasmids. We also thank Gregory J. Gores for providing the Mcl-1 shRNA expression vector.
Abbreviations
- CHX
cycloheximide
- CPT
camptothecin
- CREB
cAMP response element binding protein
- CRE
cAMP-responsive element
- GFP
green fluorescent protein
- PI3K
phosphatidylinositol 3-kinase
- RNAi
RNA interference
- RT-PCR
reverse transcription-polymerase chain reaction
- shRNA
short hairpin ribonucleic acid
- siRNA
small interfering ribonucleic acid
Footnotes
This research was supported by grants from the National Natural Science Foundation of China (30530200 and 30121001), grants from the Ministry of Science and Technology of China (2002CB713702 and 2006CB910300), and a grant from the Chinese Academy of Sciences (KSCX1-YW-R-57).
References
- 1.Steller H. Mechanisms and genes of cellular suicide. Science. 1995;267(5203):1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- 2.Vaux DL, Korsmeyer SJ. Cell death in development. Cell. 1999;96(2):245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 3.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108(2):153–164. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 4.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116(2):205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 5.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86(1):147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 6.Zou H, Li Y, Liu X, Wang X. An APAF-1.cytochrome c multimeric complex is a functional apoptosome that activates procaspase-9. J Biol Chem. 1999;274(17):11549–11556. doi: 10.1074/jbc.274.17.11549. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399(6735):483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 8.Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2(9):647–656. doi: 10.1038/nrc883. [DOI] [PubMed] [Google Scholar]
- 9.Puthalakath H, Strasser A. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 2002;9(5):505–512. doi: 10.1038/sj.cdd.4400998. [DOI] [PubMed] [Google Scholar]
- 10.Lindsten T, Ross AJ, King A, Zong WX, Rathmell JC, Shiels HA, Ulrich E, Waymire KG, Mahar P, Frauwirth K, et al. The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell. 2000;6(6):1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292(5517):727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zong WX, Lindsten T, Ross AJ, MacGregor GR, Thompson CB. BH3-only proteins that bind pro-survival Bcl-2 family members fail to induce apoptosis in the absence of Bax and Bak. Genes Dev. 2001;15(12):1481–1486. doi: 10.1101/gad.897601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell. 2005;17(3):393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 14.Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2(3):183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- 15.Cheng EH, Wei MC, Weiler S, Flavell RA, Mak TW, Lindsten T, Korsmeyer SJ. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell. 2001;8(3):705–711. doi: 10.1016/s1097-2765(01)00320-3. [DOI] [PubMed] [Google Scholar]
- 16.Cartron PF, Gallenne T, Bougras G, Gautier F, Manero F, Vusio P, Meflah K, Vallette FM, Juin P. The first alpha helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol Cell. 2004;16(5):807–818. doi: 10.1016/j.molcel.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 17.Harada H, Quearry B, Ruiz-Vela A, Korsmeyer SJ. Survival factor-induced extracellular signal-regulated kinase phosphorylates BIM, inhibiting its association with BAX and proapoptotic activity. Proc Natl Acad Sci USA. 2004;101(43):15313–15317. doi: 10.1073/pnas.0406837101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17(4):525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Oda E, Ohki R, Murasawa H, Nemoto J, Shibue T, Yamashita T, Tokino T, Taniguchi T, Tanaka N. Noxa, a BH3-only member of the Bcl-2 family and candidate mediator of p53-induced apoptosis. Science. 2000;288(5468):1053–1058. doi: 10.1126/science.288.5468.1053. [DOI] [PubMed] [Google Scholar]
- 20.Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19(11):1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsiang YH, Hertzberg R, Hecht S, Liu LF. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985;260(27):14873–14878. [PubMed] [Google Scholar]
- 22.Hsiang YH, Liu LF. Identification of mammalian DNA topoisomerase I as an intracellular target of the anticancer drug camptothecin. Cancer Res. 1988;48(7):1722–1726. [PubMed] [Google Scholar]
- 23.Li TK, Liu LF. Tumor cell death induced by topoisomerase-targeting drugs. Annu Rev Pharmacol Toxicol. 2001;41:53–77. doi: 10.1146/annurev.pharmtox.41.1.53. [DOI] [PubMed] [Google Scholar]
- 24.Tsao YP, Russo A, Nyamuswa G, Silber R, Liu LF. Interaction between replication forks and topoisomerase I -DNA cleavable complexes: studies in a cell-free SV40 DNA replication system. Cancer Res. 1993;53(24):5908–5914. [PubMed] [Google Scholar]
- 25.Morris EJ, Geller HM. Induction of neuronal apoptosis by camptothecin, an inhibitor of DNA topoisomerase-I: evidence for cell cycle-independent toxicity. J Cell Biol. 1996;134(3):757–770. doi: 10.1083/jcb.134.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimizu T, Pommier Y. Camptothecin-induced apoptosis in p53-null human leukemia HL60 cells and their isolated nuclei: effects of the protease inhibitors Z-VAD-fmk and dichloroisocoumarin suggest an involvement of both caspases and serine proteases. Leukemia. 1997;11(8):1238–1244. doi: 10.1038/sj.leu.2400734. [DOI] [PubMed] [Google Scholar]
- 27.Slichenmyer WJ, Nelson WG, Slebos RJ, Kastan MB. Lossof a p53-associated G1 checkpoint does not decrease cell survival following DNA damage. Cancer Res. 1993;53(18):4164–4168. [PubMed] [Google Scholar]
- 28.Uckun FM, Stewart CF, Reaman G, Chelstrom LM, Jin J, Chandan-Langlie M, Waddick KG, White J, Evans WE. In vitro and in vivo activity of topotecan against human B-lineage acute lymphoblastic leukemia cells. Blood. 1995;85(10):2817–2828. [PubMed] [Google Scholar]
- 29.Mei Y, Du W, Yang Y, Wu M. Puma(*)Mcl-1 interaction is not sufficient to prevent rapid degradation of Mcl-1. Oncogene. 2005;24(48):7224–7237. doi: 10.1038/sj.onc.1208873. [DOI] [PubMed] [Google Scholar]
- 30.Adjei PN, Kaufmann SH, Leung WY, Mao F, Gores GJ. Selective induction of apoptosis in Hep 3B cells by topoisomerase I inhibitors: evidence for a protease-dependent pathway that does not activate cysteine protease P32. J Clin Invest. 1996;98(11):2588–2596. doi: 10.1172/JCI119078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carson JP, Zhang N, Frampton GM, Gerry NP, Lenburg ME, Christman MF. Pharmacogenomic identification of targets for adjuvant therapy with the topoisomerase poison camptothecin. Cancer Res. 2004;64(6):2096–2104. doi: 10.1158/0008-5472.can-03-2029. [DOI] [PubMed] [Google Scholar]
- 32.Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22(56):8983–8998. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- 33.Marte BM, Downward J. PKB/Akt: connecting phosphoinositide 3-kinase to cell survival and beyond. Trends Biochem Sci. 1997;22(9):355–358. doi: 10.1016/s0968-0004(97)01097-9. [DOI] [PubMed] [Google Scholar]
- 34.Tang D, Okada H, Ruland J, Liu L, Stambolic V, Mak TW, Ingram AJ. Akt is activated in response to an apoptotic signal. J Biol Chem. 2001;276(32):30461–30466. doi: 10.1074/jbc.M102045200. [DOI] [PubMed] [Google Scholar]
- 35.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7(4):261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 36.Fernandez Y, Verhaegen M, Miller TP, Rush JL, Steiner P, Opipari A, Jr, Lowe SW, Soengas MS. Differential regulation of noxa in normal melanocytes and melanoma cells by proteasome inhibition: therapeutic implications. Cancer Res. 2005;65(14):6294–6304. doi: 10.1158/0008-5472.CAN-05-0686. [DOI] [PubMed] [Google Scholar]
- 37.Fribley AM, Evenchik B, Zeng Q, Park BK, Guan JY, Zhang H, Hale TJ, Soengas MS, Kaufman RJ, Wang CY. Proteasome inhibitor PS-341 induces apoptosis in cisplatin-resistant squamous cell carcinoma cells by induction of Noxa. J Biol Chem. 2006;281(42):31440–31447. doi: 10.1074/jbc.M604356200. [DOI] [PubMed] [Google Scholar]
- 38.Qin JZ, Ziffra J, Stennett L, Bodner B, Bonish BK, Chaturvedi V, Bennett F, Pollock PM, Trent JM, Hendrix MJ, et al. Proteasome inhibitors trigger NOXA-mediated apoptosis in melanoma and myeloma cells. Cancer Res. 2005;65(14):6282–6293. doi: 10.1158/0008-5472.CAN-05-0676. [DOI] [PubMed] [Google Scholar]
- 39.Du K, Montminy M. CREB is a regulatory target for the protein kinase Akt/PKB. J Biol Chem. 1998;273(49):32377–32379. doi: 10.1074/jbc.273.49.32377. [DOI] [PubMed] [Google Scholar]
- 40.Lallemand C, Blanchard B, Palmieri M, Lebon P, May E, Tovey MG. Single-stranded RNA viruses inactivate the transcriptional activity of p53 but induce NOXA-dependent apoptosis via posttranslational modifications of IRF-1, IRF-3 and CREB. Oncogene. 2007;26(3):328–338. doi: 10.1038/sj.onc.1209795. [DOI] [PubMed] [Google Scholar]
- 41.Reinhold WC, Kouros-Mehr H, Kohn KW, Maunakea AK, Lababidi S, Roschke A, Stover K, Alexander J, Pantazis P, Miller L, et al. Apoptotic susceptibility of cancer cells selected for camptothecin resistance: gene expression profiling, functional analysis, and molecular interaction mapping. Cancer Res. 2003;63(5):1000–1011. [PubMed] [Google Scholar]
- 42.Yano H, Nakanishi S, Kimura K, Hanai N, Saitoh Y, Fukui Y, Nonomura Y, Matsuda Y. Inhibition of histamine secretion by wortmannin through the blockade of phosphatidylinositol 3-kinase in RBL-2H3 cells. J Biol Chem. 1993;268(34):25846–25856. [PubMed] [Google Scholar]
- 43.Hershko T, Ginsberg D. Up-regulation of Bcl-2 homology 3 (BH3)-only proteins by E2F1 mediates apoptosis. J Biol Chem. 2004;279(10):8627–8634. doi: 10.1074/jbc.M312866200. [DOI] [PubMed] [Google Scholar]
- 44.Hijikata M, Kato N, Sato T, Kagami Y, Shimotohno K. Molecular cloning and characterization of a cDNA for a novel phorbol-12-myristate-13-acetate-responsive gene that is highly expressed in an adult T-cell leukemia cell line. J Virol. 1990;64(10):4632–4639. doi: 10.1128/jvi.64.10.4632-4639.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kim JY, Ahn HJ, Ryu JH, Suk K, Park JH. BH3-only protein Noxa is a mediator of hypoxic cell death induced by hypoxia-inducible factor 1alpha. J Exp Med. 2004;199(1):113–124. doi: 10.1084/jem.20030613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schuler M, Maurer U, Goldstein JC, Breitenbucher F, Hoffarth S, Waterhouse NJ, Green DR. p53 triggers apoptosis in oncogene-expressing fibroblasts by the induction of Noxa and mitochondrial Bax translocation. Cell Death Differ. 2003;10(4):451–460. doi: 10.1038/sj.cdd.4401180. [DOI] [PubMed] [Google Scholar]
- 47.Sesto A, Navarro M, Burslem F, Jorcano JL. Analysis of the ultraviolet B response in primary human keratinocytes using oligonucleotide microarrays. Proc Natl Acad Sci USA. 2002;99(5):2965–2970. doi: 10.1073/pnas.052678999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shibue T, Takeda K, Oda E, Tanaka H, Murasawa H, Takaoka A, Morishita Y, Akira S, Taniguchi T, Tanaka N. Integral role of Noxa in p53-mediated apoptotic response. Genes Dev. 2003;17(18):2233–2238. doi: 10.1101/gad.1103603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang JM, Chao JR, Chen W, Kuo ML, Yen JJ, Yang-Yen HF. The antiapoptotic gene mcl-1 is up-regulated by the phosphatidylinositol 3-kinase/Akt signaling pathway through a transcription factor complex containing CREB. Mol Cell Biol. 1999;19(9):6195–6206. doi: 10.1128/mcb.19.9.6195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhan Q, Bieszczad CK, Bae I, Fornace AJ, Jr, Craig RW. Induction of BCL2 family member MCL1 as an early response to DNA damage. Oncogene. 1997;14(9):1031–1039. doi: 10.1038/sj.onc.1200927. [DOI] [PubMed] [Google Scholar]
- 51.Alves NL, Derks IA, Berk E, Spijker R, van Lier RA, Eldering E. The Noxa/Mcl-1 axis regulates susceptibility to apoptosis under glucose limitation in dividing T cells. Immunity. 2006;24(6):703–716. doi: 10.1016/j.immuni.2006.03.018. [DOI] [PubMed] [Google Scholar]