Abstract
Aromatization of testosterone into estradiol plays a key role in the activation of male sexual behavior in many vertebrate species. Rapid changes in brain aromatase activity (AA) have recently been identified and the resulting changes in local estrogen bioavailability could modulate fast behavioral responses to estrogens. In quail hypothalamic homogenates, AA is down-regulated within minutes by calcium-dependent phosphorylations in the presence of ATP, MgCl2 and CaCl2 (ATP/Mg/Ca). Three kinases (Protein Kinases A and C and calmodulin kinase, PKC, PKA and CAMK) are potentially implicated in this process. If kinases decrease AA in a reversible manner, then one would expect that the enzymatic activity would increase and/or return to baseline levels in the presence of phosphatases. We showed previously that 0.1 mM vanadate (a general inhibitor of protein phosphatases) significantly decreases AA but specific protein phosphatases that could up-regulate AA have not been identified so far. The reversibility of AA inhibition by phosphorylations was investigated here with the use of alkaline and acid phosphatase (Alk and Ac PPase). Unexpectedly, Alk PPase inhibited AA in a dose-dependent manner in the presence as well as in the absence of ATP/Mg/Ca. In contrast, Ac PPase completely blocked the inhibitory effects of ATP/Mg/Ca on AA even if it moderately inhibited AA in the absence of ATP/Mg/Ca. The addition of Ac PPase was however unable to restore AA after it had been inhibited by exposure to ATP/Mg/Ca. Together these data suggest that amongst the 15 potential consensus phosphorylation sites identified on the quail aromatase sequence, some must be constitutively phosphorylated for the enzyme to be active while phosphorylation of others is involved in the rapid inhibition of AA by the competitive effects of protein kinases and phosphatases. Two out of these 15 putative phosphorylation sites occur in an environment corresponding to the consensus sites for PKC, PKA (and possibly a CAMK) and in all probability represent the sites whose phosphorylation rapidly blocks enzyme activity.
Keywords: Aromatase activity, Preoptic area, Coturnix japonica, Japanese quail, Fast steroid action
A significant number of the effects of testosterone on brain function are mediated, at the cellular level, by estrogens produced by local aromatization of the androgenic steroid (1). Brain aromatase activity thus controls the effects of testosterone on a variety of behavioral and physiological responses including the differentiation and activation of male-typical reproductive behavior and the feedback of gonadal steroids on gonadotropins secretion (1-3). Brain aromatase activity (AA) is primarily regulated by steroid-induced modifications of enzyme transcription: within a few days, treatment of castrates with testosterone or estradiol increases AA 4 to 6 times, the number of neurons expressing the enzymatic protein and the concentration of the aromatase mRNA (rats: (4, 5); ring doves: (6, 7); Japanese quail: (8)). More recently, evidence has accumulated demonstrating that preoptic AA can also be modulated very rapidly (within minutes) by non-genomic mechanisms in addition to slower transcriptional changes. Conditions that enhance protein phosphorylation, such as the presence of high concentrations of calcium, magnesium and ATP, rapidly down-regulate AA measured in preoptic homogenates, an effect blocked by specific kinase inhibitors (9, 10, 11).
The control of enzymatic activity by phosphorylation processes is a widespread phenomenon. The specific involvement of phosphorylations in the rapid control of AA was supported by experiments demonstrating that: (a) inhibitors of various protein kinases (Protein Kinases A and C and calmodulin kinase, PKA, PKC, CAMK) block the decrease in AA observed in the presence of ATP/Mg/Ca, (b) 15 putative phosphorylation sites can be identified on the predicted amino acid sequence of quail aromatase, including two consensus sites for PKC, PKA and CAMK, and (c) phosphorylated serine, threonine and tyrosine residues can be identified by Western blot on aromatase from quail preoptic areas purified by immunoprecipitation and their phosphorylation status is modulated (like the enzymatic activity) by the presence or absence of ATP/Mg/Ca (11).
The rapid changes in AA observed in homogenates of quail preoptic areas appear to be physiologically relevant since they are also observed in preoptic explants maintained in vitro. In particular, increases in intracellular calcium resulting from the mobilization of intracellular stores with thapsigargin or from the stimulation of glutamate receptors (AMPA, kainate, and to a lesser extent NMDA) depresses within min the AA measured in preoptic explants and this effect is rapidly reversible (1, 9, 10). Rapid changes in preoptic AA are also observed in vivo in male quail that have been provided visual access to a female or allowed to copulate with her. A major decrease in enzymatic activity is observed after 1 to 5 min but AA returns to baseline within 15 min (12).
The reversibility of the rapid inhibition of AA by phosphorylations could theoretically be the result of a de novo re-synthesis of non-phosphorylated active enzyme (the phosphorylated aromatase would be permanently inactivated) or of the de-phosphorylation of the regulatory sites under the influence of phosphatases. However, the time-course of the effects observed in explants or in vivo (return to baseline with 5-15 min) suggests that phosphorylations are reversible since previous work suggested that the de novo synthesis of preoptic aromatase is a slower process that takes several hours to produce detectable changes in enzymatic activity (13).
Our previous work analyzing the reversibility of aromatase inhibition by phosphorylation processes failed to detect reliable effects of several specific protein phosphatase inhibitors on AA measured in the presence of ATP/Mg/Ca (11). However sodium orthovanadate, a fairly general inhibitor of protein phosphatases, significantly decreased AA (i.e. reinforced the ATP/Mg/Ca induced inhibition) suggesting that some phosphatases must be active and compete with kinases to dephosphorylate the enzyme and restore its activity (11). We used here purified alkaline (Alk PPase; EC 3.1.3.1) and acid phosphatase (Ac PPase; EC 3.1.3.2), two general phosphatases that are commercially available, to assess further the potential role of the removal of phosphate residues on AA. Although Alk PPase is widely used in preparative biochemistry to peel phosphate groups from all types of organic molecules (proteins but also fatty acids or nucleotides) there is evidence that the action of Ac PPase on proteins may be more specific (14-17) and it was therefore decided to test these two phosphatases comparatively. It was hypothesized that they might differentially affect the enzymatic activity of aromatase.
Material and methods
Animals and preparation of brain homogenates
Experiments were carried out on adult (8-12 weeks old) sexually mature male Japanese quail (Coturnix japonica) that were obtained from a local breeder in Belgium. Birds were housed, manipulated, and sacrificed as described previously (18) in agreement with the principles of Laboratory Animal Care (NIH of the USA) and the relevant Belgian laws on the protection of animals. Birds were killed by rapid decapitation because AA is likely to be affected by anesthesia. Protocols were approved by the Ethics Committee for the Use of Animals at the University of Liège.
Birds were killed by decapitation within a minute of the removal from their home cage and their brains were immediately dissected out of the skull and frozen on dry ice. They were kept at −75° C until used for assays. The preoptic-hypothalamic block (HPOA; 60-80 mg), which contains the majority of aromatase-expressing cells in the quail brain (19), was dissected by two coronal cuts at the level of the tractus septopallio-mesencephalicus (rostral edge of the preoptic area) and of the oculomotor nerves (caudal edge of hypothalamus), two parasagittal cuts placed approximately 2 mm lateral to the brain midline and one horizontal cut about 2 mm above the floor of the brain. The HPOA was homogenized in ice-cold buffer containing 150 mM KCl, 10 mM Tris-Hepes pH 7.2 (KTH).
Effects of Alk or Ac PPase on quail preoptic aromatase activity
On an ice bath, aliquots (50 μl) of homogenate containing approximately 1 mg wet weight were added in Eppendorf tubes (1.5 ml) to 50 μl of KTH containing the Alk or Ac phosphatases to be tested at their final concentration (both obtained from Sigma-Aldrich as lyophilized dry powders; Cat. numbers : P-7640 and P-1146 respectively). Tubes were pre-incubated for 10 min at 37°C in the absence (Controls; CON) or presence of 1mM ATP, 1 mM MgCl2 and 0.5 mM CaCl2 (ATP/Mg/Ca) and then phosphorylations were blocked by chelating calcium with 1 mM EGTA (Fig. 1A: see (11) for detail of procedures).
Fig. 1.
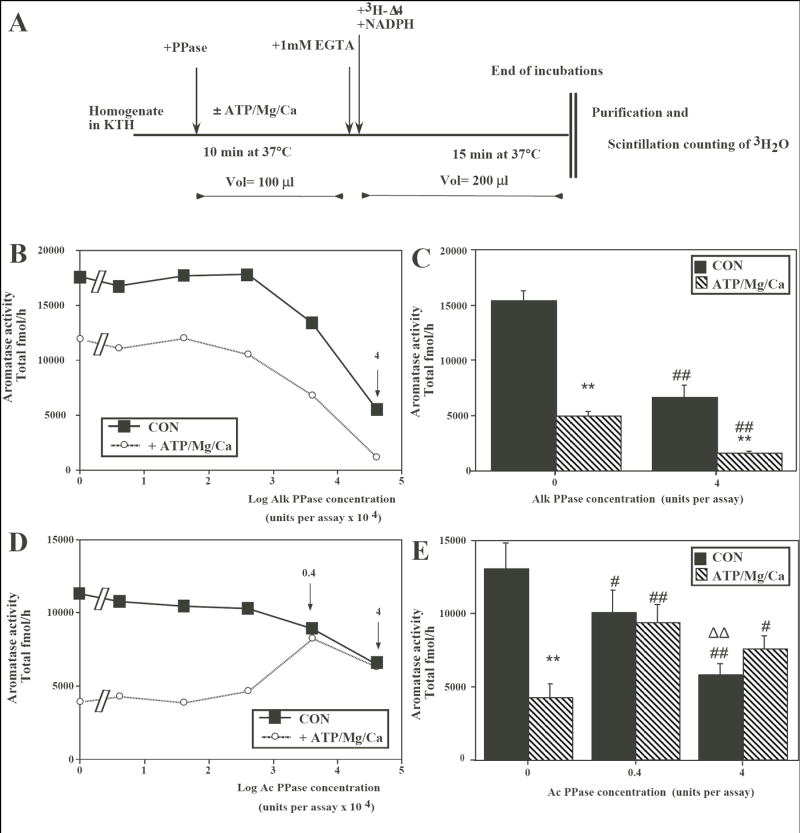
Effects of alkaline (Alk) or acid (Ac) phosphatase (PPase) on aromatase activity (AA) in preoptic-hypothalamic homogenates from male quail brain exposed or not to phosphorylating conditions (presence of ATP/Mg/Ca). (A) Schematic presentation of the experimental protocol. The sequential treatments that were applied to the homogenate aliquots are indicated by arrows and the duration of incubations and the temperatures at which they took place are listed below the horizontal line representing the time line (see also text for additional details). (B) Effect of increasing concentrations of Alk PPase on AA measured in the absence or presence of ATP/Mg/Ca. The arrow and number 4 point to the dose whose effects were studied in detail in experiments described in the next panel. (C) Effects in 4 different homogenates of a single concentration of Alk PPAse (4 units/assay) on AA measured in the absence or presence of ATP/Mg/Ca. (D) Effect of increasing concentrations of Ac PPase on AA measured in the absence or presence of ATP/Mg/Ca. The arrows and numbers (0.4 and 4) point to the dose whose effects were studied in detail in experiments described in the next panel. (E) Effects in 6 different homogenates of two concentrations of Ac PPAse on AA measured in the absence or presence of ATP/Mg/Ca. Data in C and E were analyzed by two-way ANOVA (see text) followed by Tukey HSD’s post-hoc test adapted for repeated measures whose results are indicated as follows: **: p<0.01 compared to the same concentration of PPase without ATP/Mg/Ca; # (##): p<0.05 (0.01) compared to PPase 0 units in the same condition and ΔΔ p<0.01 compared to compared to PPase 0.4 units in the same condition
AA was then quantified in the homogenates by measuring the tritiated water production from [1β-3H]-androstenedione (20) with slight modifications as previously validated for Japanese quail brain (21). Fifty μl of 100 nM [1β-3H]-androstenedione (final concentration : 25 nM) in KTH were then dispensed to all tubes. To initiate the aromatase assay 50 μl of NADPH in KTH were further added to reach a final concentration 1.2 mM. All tubes were quickly capped and incubated in a shaking bath for 15 min at 37°C. The reaction was stopped by cooling the samples in the ice bath and adding 400 μl ice-cold 10% trichloroacetic acid containing 2% activated charcoal. The tritiated water produced was purified by centrifugation and filtration on Dowex cation exchange resin columns and quantified as described previously (21). Enzyme activity was expressed in fmol of tritiated water produced after correction of the counts for quenching, recovery, blank values (obtained by addition of the aromatase inhibitor Vorozole™ in excess) and percentage of tritium in β position in the substrate. In the [1β-3H]-androstenedione commercially available (NET-926, New England Nuclear; Boston MA) only 76.8% of the tritium is in the 1β position, the rest being in 1α according to the manufacturers specification sheet. The amount of tritiated water produced during aromatization is thus underestimated and must be corrected (see (20,21) for detail).
Recovery of aromatase activity following inhibition by ATP/Mg/Ca
Additional experiments were also carried out on a total of 8 different brain homogenates to test whether endogenous brain phosphatases or exogenous Ac PPase could restore AA to its baseline level after it has been inhibited by phosphorylating conditions (addition of ATP/Mg/Ca). In these experiments, AA was first inhibited by a preincubation (5 to 30 min) at 37°C in presence or absence (control condition) of ATP/Mg/Ca. Calcium was then chelated by addition of 1 mM EGTA (to block kinases activity) and then tubes were incubated at either 25 or 37°C for various durations (5 to 90 min) after addition or not (test of endogenous phosphatases) of Ac PPase (0.4 units/assay). Aromatase activity was then measured in these homogenates by the procedure described above (incubation for 15 min at 37°C with [1β-3H]-androstenedione and NADPH followed by purification of the tritiated water produced.
Data analysis
Unless otherwise mentioned, assays on each homogenate were performed in triplicate and average of these triplicates are provided in the text without any measure of variance (coefficients of variation were usually in the range of 2-4%). Key aspects in the results were also confirmed by assaying several homogenates from different brains (n=4-6) in triplicate again and these results are then presented as means ± SEM with the number of samples considered being the number of different brains that were studied. These data were analyzed by two-way analyses of variance (ANOVA) with repeated factors (all conditions were always tested on each homogenate) followed when appropriate by post-hoc Tukey highest significant difference (HSD) tests adapted for repeated measures.
Results
Effects of phosphatases on the inhibition of AA by ATP/Mg/Ca
The effects of increasing concentrations of Alk PPase (range 0.00004 to 4 units per assay) were first assessed in one HPOA homogenate assayed in triplicate in the presence or absence of ATP/Mg/Ca. As expected based on previous results, a marked inhibition was observed in tubes containing ATP/Mg/Ca in the absence but also in the presence of Alk PPase. A clear dose-dependent drop of AA was also observed in the presence of Alk PPase. This decrease was detected both in the absence (CON) as well as in the presence of ATP/Mg/Ca (Fig. 1B) and lead to a nearly complete blockade of enzymatic activity (less than 30% remaining compared to the control condition in the absence of PPase).
The reliability of the effect of Alk PPase was then tested by measuring AA in triplicate in 4 independent HPOA homogenates exposed or not to ATP/Mg/Ca in the presence or absence of 4 units of Alk PPase. This again confirmed the inhibition of AA in the presence of ATP/Mg/Ca reflecting effects of the phosphorylation of the enzymatic protein. Alk PPase did not however counteract this effect and actually further decreased AA both in the absence and presence of ATP/Mg/Ca (Fig. 1C). Analysis of these data by two-way ANOVA with repeated factors confirmed the significant effects of ATP/Mg/Ca (F1,3= 2285,835, p<0.0001), of Alk PPase (F1,3= 64.759, p=0.0040) and the significant interaction between these two factors (F1,3= 197,956, p=0.0008). Post hoc tests confirmed the inhibition of AA by ATP/Mg/Ca both in the absence and presence of Alk PPAse as well as the inhibition of AA by Alk PPase both in presence and absence of ATP/Mg/Ca (see symbols on Fig. 1C).
A different pattern of results was obtained following the addition of Ac PPase. This “protein–specific“ PPase affected AA only moderately in the absence of ATP/Mg/Ca. The inhibition of the tritiated water production by ATP/Mg/Ca was clearly present when no or low doses of Ac PPase were added but it was completely inhibited (0.4 units/tube) or even slightly reversed (4 units/tube) at the two highest doses of PPase. To confirm the reliability of this effect, a similar experiment was repeated on six different HPOA homogenates (from different brains) that were assayed each in triplicate in the presence of the two highest doses of Ac PPase (and in control conditions) associated or not with ATP/Mg/Ca. As illustrated in Fig. 1E, Ac PPase completely inhibited the ATP/Mg/Ca-induced decrease in AA observed when the PPase was not present. Analysis of these data by two-way ANOVA with two repeated factors (presence/absence of ATP/Mg/Ca and doses of Ac PPase) confirmed the significant effect of both factors (ATP/Mg/Ca: F1,5=31.108, p=0.0026; AcPPase: F2,10=21.402, p=0.0002) as well as their interaction (F2,10=49.852, p<0.0001). Post-hoc Tukey HSD tests indicated that the inhibition induced by ATP/Mg/Ca was significant in the absence but not in the presence of Ac PPAse (see results of post-hocs on Fig. 1E). These tests, however, also demonstrated that independent of any effect on the ATP/Mg/Ca-induced inhibition, Ac PPase per se slightly but significantly inhibited AA in a dose-dependent manner.
Recovery of aromatase activity
In subsequent experiments (assays performed in duplicate, unless otherwise stated), AA was first inhibited by an incubation at 37°C with ATP/Mg/Ca for various durations (5 to 30 min). The calcium activity was blocked by addition of EGTA and then samples were incubated at 37 or 25°C (see below) for various durations in the presence of Ac PPase (0.4 units/assay) or in its absence (test of the potential activity of endogenous phosphatases) before AA was measured in the usual conditions (see Fig. 2A-D for the detail of the different experimental protocols).
Fig. 2.
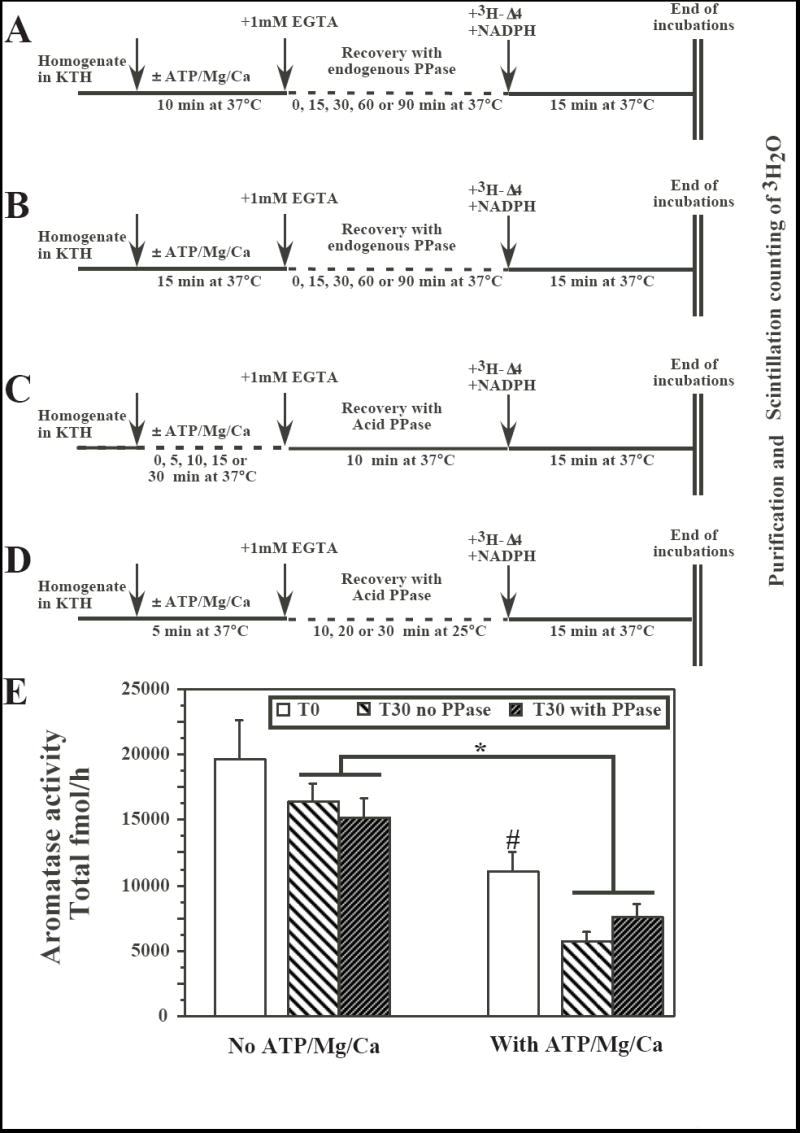
Effects of endogenous phosphatases or of exogenous acid (Ac) phosphatase (PPase; 0.4 units) on aromatase activity (AA) in preoptic-hypothalamic homogenates from male quail brain that had been previously exposed or not to phosphorylating conditions (presence of ATP/Mg/Ca). Panels A through D provide a schematic description of the different protocols that were tested. The sequential treatments that were applied to the homogenate aliquots are indicated by arrows and the duration of incubations and the temperatures at which they took place are listed below the horizontal line representing the time line (see also text for additional details). Panel E illustrates the results of a recovery experiment performed on three independent brain homogenates to further test the best conditions identified in the experiments schematized in A-D (Inhibition for 5 min with ATP/Mg/Ca, recovery in the presence of 0.4 unit Ac PPase for 30 min at 25°C). *= p<0.05 for the main effect in the ANOVA comparing at T30 samples exposed or not to ATP/Mg/Ca. This ANOVA identified no effect of the PPase and no interaction between the two factors. #= p<0.05 for the comparison of samples with or without ATP/Mg/Ca at T0.
In a first set of experiments, AA was inhibited by a 10 min (two different brain homogenates; Fig. 2A) or 15 min (one pool of two brain homogenates; Fig. 2B) incubation at 37°C with ATP/Mg/Ca. After blockade of calcium activity by EGTA, subsequent incubations for various durations up to 90 min at 37°C were not able to restore the enzymatic activity, suggesting that, in these conditions, endogenous phosphatases are not able to dephosphorylate aromatase.
In the next set of experiments (one brain homogenate), AA was inhibited by an incubation at 37°C with ATP/Mg/Ca for various durations (0-30 min) and then, after addition of EGTA and Ac PPase (0.4 units/assay), samples were incubated for 10 min at 37°C before they were assayed for AA (Fig. 2C). Addition of Ac PPase failed to restore the enzymatic activity to any significant extent (data not shown).
Finally the duration of the incubation with ATP/Mg/Ca was reduced to 5 min and after the addition of EGTA and Ac PPase (0.4 units/assay), samples were incubated for 10, 20 or 30 min at 25°C with the desired goal being that the lower temperature would prevent the slow degradation of the enzyme that was happening at 37°C (see Fig. 2D; one pool of two brain homogenates). Again these treatments did not fully restore AA to levels observed in homogenates that had not been exposed to phosphorylating conditions but in one of these conditions (30 min incubation with Ac PPase at 25°C), results suggested that a low level of recovery of AA could take place, even it this recovery was still far from significant.
These specific conditions (5 min exposure to ATP/Mg/Ca, blockade of calcium activity by EGTA, incubation with 0.4 units Ac PPase at 25°C) were thus re-tested in three separate brain homogenates, each measured in triplicate. As illustrated in Figure 2E, this experiment confirmed the major decrease of AA induced by exposure to ATP/Mg/Ca: at T0 i.e. after exposure for 5 min to these compounds, AA had decreased to 56% of its control value, an effect highly significant by one-way ANOVA with repeated measures (F1,2=35.176, p=0.0273). After inhibition of phosphorylation processes by addition of EDTA, the decrease in AA continued at a much lower rate for the next 30 min. Addition of 0.4 units of Ac PPase had however no major effect on this slow decrease and was unable to restore AA to the original level that was observed in the absence of ATP/Mg/Ca.
Analysis of these data collected at T30 by a two way ANOVA with 2 repeated factors (presence-absence of ATP/Mg/Ca and of Ac PPase) again confirmed the significant effect of ATP/Mg/Ca (F1,2=47.641, p= 0.0204) but identified no effect of the addition of Ac PPase (F1,2=0.276, p=0.6516). There was also no significant interaction between these two factors (F1,2=14.883, p=0.0611); the nearly significant trend resulting form the fact that AA was slightly lower in tubes containing Ac PPase that had not been incubated with ATP/Mg/Ca but a bit higher when such an incubation had taken place.
Discussion
Recent work demonstrated that brain aromatase in quail can be rapidly inactivated by exposure to ATP/Mg/Ca (9). It has been suggested that this enzymatic inhibition is caused by the phosphorylation of the enzymatic protein based on the observations that the inhibition is blocked in the presence of specific kinase inhibitors and that it is actually accompanied by an increased phosphorylation of amino acid residues as detected by Western blots on aromatase purified by immunoprecipitation (11). The present study provides additional evidence indicating that phosphorylation processes regulate brain AA by confirming the inhibition observed in presence of ATP/Mg/Ca and demonstrating that the addition of Ac PPAse completely blocks the effects of phosphorylating conditions on AA. These data further support the idea that brain AA is controlled by a balance of kinase and phosphatase activities that regulate the phosphorylation level of the enzymatic protein. Two types of questions are, however, raised by the present data. They concern firstly, the different reactions observed following the addition of acid versus alkaline phosphatases and secondly, the observation that the action of Ac PPase was apparently unable to promote the recovery of AA after it had been extensively inhibited by exposure to ATP/Mg/Ca, although Ac PPase could block the development of this inhibition.
Alkaline versus acid phosphatases
The addition of significant amounts of Ac PPase (0.4 or 4 units/assay) in the present experiments completely blocked the inhibiting effects of an incubation with ATP/Mg/Ca while Alk PPase at the same concentration was inactive and even further enhanced the ATP/Mg/Ca-induced inhibition of aromatase. This differential, almost opposite, action of the two phosphatases might seem puzzling at first sight. However, if one keeps in mind that Alk PPase is commonly used for removing phosphate residues from any kind of organic molecules, including proteins, fatty acids and nucleic acids while Ac PPase is known to have a greater specificity for proteins these data are not so surprising (14-17, 22). Also, our previous work showed that the predicted aromatase sequence in quail contains 15 putative consensus sites for phosphorylation but two of these only, located on threonine residues 455 and 486, represent consensus sequences corresponding to the protein kinases that were shown during pharmacological experiments to block the effects of ATP/Mg/Ca on AA (i.e. Protein kinases A, C and possibly one CAM kinase; see (11)). It is likely that some of the 13 remaining sites that are presumably not directly involved in the rapid controls of AA need nevertheless to be in a specific phosphorylation state to confer the enzyme a three-dimensional structure compatible with enzymatic activity. It is therefore conceivable that Ac PPase is able to remove relatively specifically the phosphate groups from the two threonine residues that potentially regulate AA but that a more drastic less specific action of Alk PPase removes other more stable phosphate groups from the aromatase molecule that are required for enzymatic activity to be expressed. It is even possible that Alk PPase could denaturate the enzyme. This differential action on phosphorylation sites directly or indirectly involved in the control of AA (threonine 455 and 486 versus the other 13 phosphorylation sites) may similarly explain why at high doses, Ac PPase also inhibits AA while blocking at the same time the inhibitory effects of phosphorylating conditions (see Fig. 1E).
Independent of the mechanisms involved, the present data nevertheless bring strong support to the notion that phosphorylations regulate the enzymatic activity of aromatase and thus that the activity of a given concentration of enzyme can be substantially (and rapidly) modified by the balance of kinases and phosphatases to which the enzymatic protein is exposed.
Recovery of enzymatic activity following inhibition by ATP/Mg/Ca
It was also somewhat unexpected that, although the addition of Ac PPase was able to completely block the inhibition of AA by phosphorylating conditions, Ac PPase was unable to restore the enzymatic activity of aromatase after it had been exposed to ATP/Mg/Ca. Three types of mechanisms could explain this apparent discrepancy.
It is first possible that among the multiple phosphorylation sites of aromatase some (presumably threonines 455 and 486) can be rapidly and reversibly phosphorylated (mechanism number 1 in Fig. 3) while others are phosphorylated more slowly or only under more drastic conditions but that, once several of them are phosphorylated, they modify the conformation of the enzyme and make it permanently inactive and non accessible to the action of Ac PPase. The complete phosphorylation of these sites by ATP/Mg/Ca would be prevented when Ac PPase is present but once all these sites have been phosphorylated, Ac PPase would be unable to peel the phosphate groups off these residues due to a conformational change of the protein.
Fig. 3.
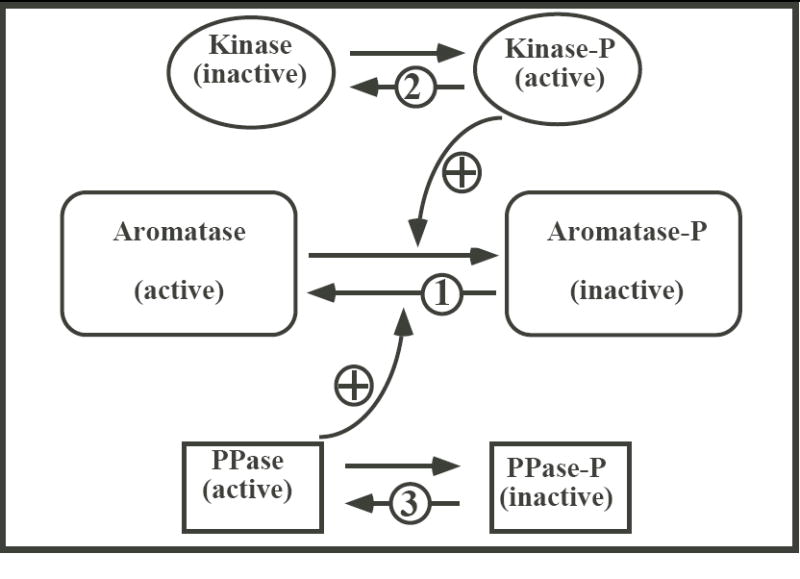
Schematic presentation illustrating three possible mechanisms (indicated by numbers in the figure) illustrating the effect of Ac PPase on AA. Ac PPase could regulate AA by removing specific phosphate groups from the aromatase protein (1), or by inactivating a kinase (2) or by activating an endogenous phosphatase (3). See text for additional explanations.
Alternatively, it is also possible that Ac PPase does not act directly on aromatase but modifies the phosphorylation status of endogenous kinases (mechanism number 2 in Fig. 3). It is indeed well known that many kinases must themselves be phosphorylated to be active (23). It is therefore possible that Ac PPase blocked the inhibition of AA by ATP/Mg/Ca not because it competed with endogenous kinases for the phosphorylation of specific residues on the aromatase protein but because it de-phosphorylated the kinases that are catalyzing the phosphorylation of aromatase. This would easily explain why Ac PPase blocks the inhibition of aromatase by phosphorylating conditions (by inactivating endogenous kinases) but is unable to restore the activity when aromatase has been inhibited (because AcPPase does not act on aromatase in this scenario).
In an analogous manner, Ac PPase could also dephophosphorylate an endogenous (unidentified) phosphatase that needs to be de-phosphorylated to be active (23); mechanism number 3 in fig. 3). This process alone would however be unable to explain why addition of Ac PPase blocks aromatase inhibition by ATP/Mg/Ca but does not restore enzymatic activity once it has been inhibited. Indeed if the action of Ac PPase is mediated by the dephosphorylation of an endogenous phosphatase which then dephosphorylates aromatase itself, there is no reason why Ac PPase would act if added before but not after ATP/Mg/Ca. Such a mechanism could only work if combined with the first scenario mentioned above involving multiple phosphorylation sites on aromatase that would be differentially regulated and would mediate a conformational change of the aromatase protein making it non accessible to the PPase action. This is obviously not the most parsimonious explanation.
To provide additional information on the potential regulation of phosphatases by phosphorylation processes in quail, we performed a few preliminary experiments on the effects of phosphorylating conditions (addition of ATP/Mg/Ca) on the activity of brain endogenous phosphatases. These experiments, performed on two brain homogenates only, demonstrated the presence of an active endogenous phosphatase activity in the quail brain that is inhibited by general inhibitors of the serine/threonine (NaF) and tyrosine (vanadate) phosphatases but, contrary to expectations based on the scenario described above, phosphorylation conditions enhanced (rather than decreased) the overall phosphatase activity (to 178 and 199% of baseline in the two homogenates). These data would however need to be confirmed and their possible generality be tested. It is clear that there are a multitude of endogenous phosphatases in animal tissues (may be more than one thousand: (15, 23, 24)) and it is expected that they are not all regulated in the same manner by phosphorylation (or other) processes: some may be activated while others are inhibited (or more precisely endogenous inhibitors of phosphatases are activated or inhibited by phosphorylation: (25)). It is therefore not a trivial task to characterize these enzymes and their patterns of regulation. Nevertheless, available evidence makes mechanism number 3 described above relatively unlikely.
In conclusion, the present studies demonstrate that phosphatases modulate in a significant manner the activity of brain aromatase in quail. These data provide additional evidence that the phosphorylation status of the enzyme is critical for its activity. The mechanism mediating the action of Ac PPase on AA is however likely to involve a de-phosphorylation of endogenous brain kinases rather than a direct action on the aromatase protein itself. Whether such a mechanism is able to explain the reversibility of the aromatase inhibition previously observed in brain explants (by altering the ratio of kinases to phosphatases activities) remains to be established.
Acknowledgments
Supported by grants from the National Institutes of Health (MH50388) GFB and JB and a grant from the Belgian FRFC (2.4562.05) to JB.
References
- 1.Balthazart J, Baillien M, Cornil CA, Ball GF. Preoptic aromatase modulates male sexual behavior: slow and fast mechanisms of action. Physiol Behav. 2004;83:247–270. doi: 10.1016/j.physbeh.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 2.Balthazart J. Steroid metabolism and the activation of social behavior. In: Balthazart J, editor. Advances in Comparative and Environmental Physiology. 1. Vol. 3. Springer Verlag; Berlin: 1989. pp. 105–159. [Google Scholar]
- 3.Panzica GC, Viglietti-Panzica C, Balthazart J. The sexually dimorphic medial preoptic nucleus of quail: a key brain area mediating steroid action on male sexual behavior. Front Neuroendocrinol. 1996;17:51–125. doi: 10.1006/frne.1996.0002. [DOI] [PubMed] [Google Scholar]
- 4.Roselli CE, Abdelgadir SE, Ronnekleiv OK, Klosterman SA. Anatomic distribution and regulation of aromatase gene expression in the rat brain. Biol Reprod. 1998;58:79–87. doi: 10.1095/biolreprod58.1.79. [DOI] [PubMed] [Google Scholar]
- 5.Roselli CE, Abdelgadir SE, Resko JA. Regulation of aromatase gene expression in the adult rat brain. Brain Res Bull. 1997;44:351–357. doi: 10.1016/s0361-9230(97)00214-1. [DOI] [PubMed] [Google Scholar]
- 6.Steimer T, Hutchison JB. Androgen increases formation of behaviourally effective oestrogen in dove brain. Nature. 1981;292:345–347. doi: 10.1038/292345a0. [DOI] [PubMed] [Google Scholar]
- 7.Hutchison JB, Steimer TH. Formation of behaviorally effective 17 beta-estradiol in the dove brain: steroid control of preoptic aromatase. Endocrinology. 1986;118:2180–2187. doi: 10.1210/endo-118-6-2180. [DOI] [PubMed] [Google Scholar]
- 8.Balthazart J, Foidart A. Brain aromatase and the control of male sexual behavior. J Steroid Biochem Mol Biol. 1993;44:521–540. doi: 10.1016/0960-0760(93)90256-v. [DOI] [PubMed] [Google Scholar]
- 9.Balthazart J, Baillien M, Ball GF. Rapid and reversible inhibition of brain aromatase activity. J Neuroendocrinol. 2001;13:61–71. doi: 10.1046/j.1365-2826.2001.00598.x. [DOI] [PubMed] [Google Scholar]
- 10.Balthazart J, Baillien M, Ball GF. Phosphorylation processes mediate rapid changes of brain aromatase activity. J Steroid Biochem Mol Biol. 2001;79:261–277. doi: 10.1016/s0960-0760(01)00143-1. [DOI] [PubMed] [Google Scholar]
- 11.Balthazart J, Baillien M, Charlier TD, Ball GF. Calcium-dependent phosphorylation processes control brain aromatase in quail. Eur J Neurosci. 2003;17:1591–1606. doi: 10.1046/j.1460-9568.2003.02598.x. [DOI] [PubMed] [Google Scholar]
- 12.Baillien M, Cornil CA, Ball GF, Balthazart J. Sexual performance and stress rapidly alter the preoptic aromatase activity in quail. Soc Neurosci Abst. 2004:Abst 88.4. [Google Scholar]
- 13.Balthazart J, Foidart A, Hendrick JC. The induction by testosterone of aromatase activity in the preoptic area and activation of copulatory behavior. Physiol Behav. 1990;47:83–94. doi: 10.1016/0031-9384(90)90045-6. [DOI] [PubMed] [Google Scholar]
- 14.Bingham EW, Farrell HM., Jr Removal of phosphate groups from casein with potato acid phosphatase. Biochim Biophys Acta. 1976;429:448–60. doi: 10.1016/0005-2744(76)90293-x. [DOI] [PubMed] [Google Scholar]
- 15.Charbonneau H, Tonks NK. 1002 protein phosphatases? Annu Rev Cell Biol. 1992;8:463–93. doi: 10.1146/annurev.cb.08.110192.002335. [DOI] [PubMed] [Google Scholar]
- 16.Fischer EH, Charbonneau H, Tonks NK. Protein tyrosine phosphatases: a diverse family of intracellular and transmembrane enzymes. Science. 1991;253:401–6. doi: 10.1126/science.1650499. [DOI] [PubMed] [Google Scholar]
- 17.Shenolikar S, Ingebritsen TS. Protein (serine and threonine) phosphate phosphatases. Methods Enzymol. 1984;107:102–29. doi: 10.1016/0076-6879(84)07007-5. [DOI] [PubMed] [Google Scholar]
- 18.Balthazart J, Schumacher M. Estradiol contributes to the postnatal demasculinization of female Japanese quail (Coturnix coturnix japonica) Horm Behav. 1984;18:287–297. doi: 10.1016/0018-506x(84)90017-5. [DOI] [PubMed] [Google Scholar]
- 19.Foidart A, Reid J, Absil P, Yoshimura N, Harada N, Balthazart J. Critical re-examination of the distribution of aromatase-immunoreactive cells in the quail forebrain using antibodies raised against human placental aromatase and against the recombinant quail, mouse or human enzyme. J Chem Neuroanat. 1995;8:267–282. doi: 10.1016/0891-0618(95)00054-b. [DOI] [PubMed] [Google Scholar]
- 20.Roselli CE, Resko JA. In vitro assay of aromatase activity in the central nervous system. In: Greenstein B, editor. Neuroendocrine research methods. 0. Vol. 2. Harwood Academic Publishers; Chur, Switzerland: 1991. pp. 937–951-0. [Google Scholar]
- 21.Baillien M, Balthazart J. A direct dopaminergic control of aromatase activity in the quail preoptic area. J Steroid Biochem Mol Biol. 1997;63:99–113. doi: 10.1016/s0960-0760(97)00080-0. [DOI] [PubMed] [Google Scholar]
- 22.Reid TW, Wilson IB. Escherichia coli alkaline phosphatase. In: Boyer PB, editor. The Enzymes. Vol. 4. Academic press; New York: 1971. pp. 373–415. [Google Scholar]
- 23.Nestler EJ, Greengard P. Serine and threonine phosphorylation. In: Siegel GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD, editors. Basic Neurochemistry Molecular, Cellular and Medical Aspects. 6. Lippincott-Raven; Philadelphia: 1999. pp. 471–495. [Google Scholar]
- 24.Cohen PT. Novel protein serine/threonine phosphatases: variety is the spice of life. Trends Biochem Science. 1997;22:245–251. doi: 10.1016/s0968-0004(97)01060-8. [DOI] [PubMed] [Google Scholar]
- 25.Oliver CJ, Shenolikar S. Physiologic importance of protein phosphatase inhibitors. Front Biosci. 1998;3:D961–72. doi: 10.2741/a336. [DOI] [PubMed] [Google Scholar]


