Abstract
The protein Doublecortin (DCX) is expressed in post-mitotic migrating and differentiating neurons in the developing vertebrate brain and, as a part of the microtubule machinery, is required for neuronal migration. DCX expression is generally maximal during embryonic and early post-natal life but decreases markedly and almost disappears in older animals in parallel with the major decrease or cessation of neurogenesis. In several seasonally breeding songbird species such as canaries, the volume of several song control nuclei in the brain varies seasonally such that the largest nuclei are observed in the late spring and early summer. This variation is based on changes in cell size, dendritic branching, and, in nucleus HVC, on the incorporation of neurons newly born in adulthood. Because songbirds maintain an active neurogenesis and neuronal incorporation in their telencephalon throughout their lives, we investigated here the distribution of DCX-immunoreactive (ir) structures in the brain of adult male canaries. Densely stained DCX-ir cells were found exclusively in parts of the telencephalon that are known to incorporate new neurons in adulthood, in particular the nidopallium. Within this brain region, the boundaries of the song control nucleus HVC could be clearly distinguished from surrounding structures by a higher density of DCX-ir structures. In most telencephalic areas, about two thirds of these cells displayed a uni- or bipolar fusiform morphology suggesting they were migrating neurons. The rest of the DCX-ir cells in the telencephalon were larger and had a round multipolar morphology. No such staining was found in the rest of the brain. The broad expression of DCX specifically in adult brain structures that exhibit the characteristic of active incorporation of new neurons suggests that DCX plays a key role in the migration of new neurons in the brain of adult songbirds as it presumably does during ontogeny.
Keywords: Doublecortin, HVC, songbirds, neuroplasticity
1. Introduction
Doublecortin (DCX) is a microtubule-associated protein expressed during development and in adulthood in post-mitotic migrating and differentiating neurons (Francis, et al., 1999; Gleeson, et al., 1999; Rao and Shetty, 2004). DCX controls the polymerization of the leading process and stabilization of the cytoskeleton during neuronal migration and thus plays a key role in the final positioning of newborn neurons (Friocourt, et al., 2003). In mammals, DCX expression is maximal during the embryonic and early post-natal life but decreases markedly in older subjects in parallel with the major decrease or cessation of neurogenesis (Couillard-Despres, et al., 2005; des Portes, et al., 1998).
The DCX mRNA and protein have also been identified in the embryonic or early post-natal brain of two avian species, the chicken and zebra finch (Capes-Davis, et al., 2005; Hannan, et al., 1999; Kim, et al., 2006). The zebra finch study (Kim et al., 2006) identified a decrease of DCX mRNA expression with age similar to what has been observed in mammals but substantial levels of this mRNA were still present in adults of this species. This study also provided some evidence that this mRNA is translated into protein in adult zebra finches and for a few select brain areas, limited immunocytochemical evidence was presented for the presence of the protein in the adult brain.
In many songbird species living in the temperate zone, vocal production varies seasonally so that high levels of song output are present in the spring when the bird has to defend a territory or court a mate (Ball, 1999; Catchpole and Slater, 1995). Seasonal changes have in parallel been detected in the volume and cytoarchitectonic organization of several brain nuclei, collectively known as the song control system, that mediate the learning and production of song (Ball, et al., 2004; Nottebohm, 1981; Smith, et al., 1997; Tramontin and Brenowitz, 2000). Most studies analyzing this seasonal brain plasticity have focused on two nuclei of the brain pathway that controls vocal production, HVC (used as a proper name; see (Reiner, et al., 2004)) and the Nucleus robustus arcopallialis (RA) as well as one nucleus of the so-called rostral forebrain pathway, area X of the medial striatum, that is involved sensorimotor learning during ontogeny and song stabilization in adulthood (Bottjer, et al., 1984; Brainard and Doupe, 2000; Nottebohm, et al., 1976; Scharff and Nottebohm, 1991). The precise cellular basis of the seasonal variation in the volume of these nuclei is still not completely understood. However, in all three nuclei it seems that the vernal growth reflects an increase of cell size, dendritic branching, glial growth and neuropil–volume (e.g., (Kafitz, et al., 1999); see (Tramontin and Brenowitz, 2000) for a review). In HVC but not in area X and RA this growth also reflects a massive incorporation of new neurons (Alvarez-Buylla, et al., 1990; Alvarez-Buylla, et al., 1988; Kirn, et al., 1994; Smith et al., 1997). In RA and area X there is good evidence that the seasonal change in volume does not involve the recruitment of new neurons (Thompson and Brenowitz, 2005; Tramontin, et al., 1998). It has been shown that large fractions of the telencephalon in songbird species are characterized by an active neurogenesis and neuronal incorporation. In adult mammals, neurogenesis appears to be more restricted in its occurrence (Gould and Gross, 2002; Taupin, 2006). The mechanisms underlying this dramatic brain plasticity in birds are still poorly understood.
Because songbirds maintain an active neurogenesis and neuronal incorporation in their telencephalon throughout their life, and DCX is implicated in the migration and positioning of newly formed neurons during ontogeny, we hypothesized that DCX should still be broadly expressed in adult songbirds. This notion was already supported by the study of zebra finches previously mentioned (Kim et al., 2006), but this species originates from the subtropical semi-arid areas of Australia (Zann, 1996) and does not display seasonal variations in song production and in the volume of song control nuclei. The brain of a seasonally breeding north temperate zone species such as the canary, which displays seasonal variation in song behavior as well as a pronounced seasonal brain plasticity, is a better model system to analyze the expression in the adult brain of DCX, a protein tightly correlated to brain development and plasticity.
We present here a detailed analysis of the distribution of DCX-immunoreactive (ir) structures in the brain of adult male canaries, a north temperate zone oscine songbird species that has been extensively used in laboratory studies on song learning and production in association with neuroanatomical, neurochemical and neurophysiological changes. We demonstrate the existence of clear anatomical relationships between the expression of this protein and the distribution of neuronal incorporation in the adult brain consistent with the notion that this protein plays a key role in the migration of new neurons in the adult avian telencephalon as it does during ontogeny.
2. Material and methods
2.1. Subjects and tissue preparation
All experiments were performed on brain sections from adult male canaries (Serinus canaria) that were bought from a local dealer in Liège (Belgium) and used previously to analyze the effects of the sex of the cage mate on the singing rate and the volume of song control nuclei (Boseret, et al., 2006). The full description of the manipulations performed on these birds can be found in this previously published paper. Briefly, these birds were castrated two weeks after their arrival in the laboratory by the method described by Sartor and collaborators (Sartor, et al., 2005). They were then allowed to recover for two weeks and transferred to a short photoperiod (6L:18D) for 8 more weeks to make sure they would be photosensitive at the beginning of the experiment (Nicholls and Storey, 1977). They were subsequently implanted with one 10 mm-long Silastic™ capsule filled with testosterone (T) (Fluka Chemika, Buchs, Switzerland, Cat. nbr: 86500) and randomly assigned to male-male (M-M; n=8) or male-female (M-F; n=4) dyads that were placed in individual cages. Photoperiod was also switched to 16L:8D at that time. Females were treated with one 10 mm-long Silastic™ capsule (Silastic Medical Adhesive Silicone type A, Coventry, UK) filled with estradiol-17β (E2; Sigma, St-Louis, USA; Cat. nbr: E8875-5G) to ensure that they would be sexually receptive (Leboucher, et al., 1994).
Singing of all males was regularly recorded during the next three weeks and the birds were then perfused. They were first tranquilized by an injection of 25 μl Medetomidin (Domitor™; Pfizer, Louvain-la-Neuve, Belgium), which was followed 10 minutes later by a complete anesthesia induced by an injection of 25 μl of a mixture of zolazepam and tiletamine both at 50 mg/ml (Zoletil™; Virbac, Louvain-la-Neuve, Belgium). The thoraco-abdominal cavity was then opened, 50 μl of heparin (20 mg/ml) was injected into the left ventricle and birds were perfused through the left ventricle with 50-75 ml saline followed by approximately 100 ml of fixative (4% paraformaldehyde with 0.1% glutaraldehyde in 0.1 M phosphate buffer [PBS], pH 7.2). The brain was removed from the skull, postfixed in the same fixative without glutaraldehyde for two additional hours and then cryo-protected in a solution of sucrose (30%) in PBS. Brains were frozen on dry ice and stored at -80°C.
All primary anatomical observations reported below were performed on the four males that had been kept in the presence of a female during the experiment. Additional observations were also performed on brain sections from males that had been kept in the presence of another male in this experiment, on a few gonadally intact sexually mature males that had been maintained for at least two weeks with a female and on a few additional castrated males similarly treated with exogenous T that had been obtained at another time. No qualitative differences in the morphology or overall distribution of DCX-ir structures could be detected between these groups of subjects.
Throughout the experiments, food, water, sand and bathing water were always available ad libitum. All experimental procedures were in agreement with the Belgian laws on “Protection and Welfare of Animals” and on the “Protection of experimental animals” and the International Guiding Principles for Biomedical Research involving Animals published by the Council for International Organizations of Medical Sciences. The protocols were approved by the Ethics Committee for the Use of Animals at the University of Liège.
2.2. Doublecortin immunocytochemistry
Brains were cut in 30 μm coronal sections on a cryostat, in a caudal to rostral direction from the level of the Locus Coeruleus (LoC) to the rostral end of Area X. Six series of alternate sections were collected. One series was Nissl-stained with toluidine blue to provide accurate anatomical localization. Immunocytochemistry was carried out on another series of sections with a polyclonal antibody (anti-DCX pep) raised in rabbit against synthetic peptides corresponding to DCX amino acids 1-10, 287-296, and 350-360 of the human sequence (GenEmbl sequence AJ003112; see Francis et al. 1999), kindly provided by Dr. Fiona Francis from the INSERM U129, Institut Cochin, Paris, France.
Floating sections were rinsed in PBS, containing 0.2% Triton X-100 (PBST), then immersed in a solution of 0.06% H2O2 to eliminate endogenous peroxidases. After 20 minutes, they were placed in a blocking solution (Normal Goat Serum, cat.no. X-0907, Dakopatts; A/S Denmark) for 30 min and then incubated at 4°C overnight with the antibody against DCX (diluted 1/200). Sections were rinsed in PBST and placed for 2 hours in a biotinylated Goat Anti-Rabbit antibody (cat.no. E-0466, Dakopatts; A/S Denmark, dilution: 1/400) and then, after a further rinse exposed for 90 min to an Avidin-Biotin Complex coupled with horseradish peroxidase (Kit VectaStain ABC Elite Standard, cat. no. PK 6100, Burlingame, CA, USA). The sections were finally reacted in a solution of 0.04% of 3,3’-diaminobenzidin tetrahydrochloride (DAB; cat no, D5637, Sigma; St-Louis, MO, USA), rinsed in PBS and mounted on microscope slides in an aqueous gelatin-based medium.
2.3. Antibody specificity
The polyclonal anti-DCX pep antibody specifically recognizes DCX in human fetal, rat and mouse brain sections and in primary neuronal cultures from fetal mouse brains. In Western blots performed with mammalian brain extracts, this antiserum stained a single band of approximately 40 kDa, the expected molecular weight of DCX (Francis et al., 1999). Preincubation of the antiserum with the synthetic peptides used as antigen completely blocked the detection of DCX on Western blot and the preimmune serum gave no signal. Additional validation tests were also performed during this study to further confirm the specificity of this antiserum on canary brain tissue.
First, we confirmed that the sequence of 3 peptides that had been used as antigens was highly conserved between birds and mammals by comparing the available sequences in man (Genbank AJ003112), mouse (Genbank NP034155), chicken (Genbank AAK15319) and zebra finch (Genbank DQ189989). The mouse and human sequences were identical and only differed by one amino acid residue out of 31 from the chicken sequences. The zebra finch sequence that was available for comparisons lacks the first and last 3 amino acid residues as compared to the human or mouse sequence. Out of the remaining 25 residues that compose the sequences of the 3 peptides used for immunization, 24 are identical suggesting that cross-reaction of the antibody with avian material should be excellent.
Secondly, we showed that the substitution of the primary antibody with buffer or the sequential omission of the various steps in the staining procedure completely suppressed all staining indicating that the detection systems used in our procedure did not generate any signal by themselves.
Thirdly, we compared based on sections derived through the same structures the immunocytochemical signal obtained with the anti-DCX pep antibody and with two other anti-DCX antibodies. The first of these antibodies had been raised either against a fusion protein corresponding to the first 110 amino acids of the N-terminal extremity of the protein (rabbit antibody Nterm, also kindly provided by Dr. F. Francis and validated in her experiments for use on mammalian brains; see (Francis et al., 1999)). The second antibody was directed against a peptide corresponding to the C-terminal part of human DCX (goat antibody Cterm; Santa Cruz Biotechnology, Heidelberg, Germany; catalog number sc-8066).
Fourthly, the polyclonal anti-DCX pep was tested during Western blot experiments on canary brain extracts (90 μg/lane). One frozen brain was homogenized in ice-cold lysis buffer (50 mM Tris HCl, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, one tablet of complete protease inhibitor; Boehringer Mannheim, Indianapolis, IN) and centrifuged at 14000 rpm for 12 min at 4°C. The protein content in the supernatant was measured by the Micro BCA Protein Assay (Pierce,Rockford, IL). Proteins were separated by electrophoresis on SDS/ polyacrylamide gels (8%) and transferred to nitrocellulose membranes (Amersham Biosciences, Uppsala, Sweden) using the Biorad miniprotean II electrophoresis system (Biorad, Hercules, CA) and a semi-dry transfer unit (Laemmli, 1970). The membranes after transfer were treated for 1 h in blocking buffer (5% nonfat dry milk in Tris-HCl 0.05M buffer at pH 7.6 containing 0.15 M NaCl (TBS) + 1% Tween 20; Merck Schuchardt, Hohenbrunn, Germany) and incubated overnight at 4°C with the primary antibody described above (dilution: 1:200). The primary antibody was visualized with Goat anti-rabbit-HRP (1:2000; Dako Cytomation, Heverlee, Belgium) and the peroxidase activity was revealed with the enhanced chemiluminescence system (SuperSignal, #34080; Pierce, Rockford, IL). Negative control immunostaining was also performed in parallel on other membranes to test the specificity of the secondary antibody by omitting the first antibody in the staining procedure.
Finally immunocytochemical staining was performed on canary brain sections with the anti-DCX pep antibody that had been preadsorbed with an excess of the three antigenic peptides as previously published (Foidart, et al., 1995). Lyophilized synthetic peptides (Eurogentec, Liège, Belgium) corresponding to the DCX sequences used as antigen were dissolved in distilled water at room temperature and subsequently diluted in PBS containing the primary antibody to reach the final concentrations of 40 or 4 μg/ml. The antibody and peptides mix was preincubated during four hours at room temperature, and the immunocytochemical staining was then performed following the procedures described above. Alternate sections were stained with normal or preadsorbed antibodies to allow a direct comparison of results.
2.4. Qualitative and quantitative analysis of doublecortin labelling
All sections were first qualitatively surveyed with a Leica DMRB microscope to identify brain areas containing doublecortin-immunoreactive (DCX-ir) cells. The anatomical nomenclature used in this paper follows two avian brain atlases (canary: (Stokes, et al., 1974); pigeon: (Karten and Hodos, 1967)) with additional anatomical information concerning the hypothalamus-preoptic areas and auditory or vocal control areas (see respectively (Balthazart, et al., 1996) and (Bottjer, et al., 2000; Bottjer, et al., 1989; Bottjer and Johnson, 1997; Mello, et al., 1998; Vates, et al., 1996). The names of brain structures follow the recommendations made by the Brain Nomenclature Forum (Reiner et al., 2004).
Drawings of the distribution of immunoreactive structures were prepared with a camera lucida in sections of the 4 T–treated castrated subjects that had been kept in a cage with a female and then checked for accuracy in 7 other birds including T-treated castrates kept with a male (n=3), T-treated castrates kept in isolation (n=3) and one gonadally intact sexually mature male kept in isolation. A summary of individual results was then constructed in the form of semi-schematic maps to illustrate the general distribution of immunoreactive cells. The density of symbols in these maps was adjusted to provide a representation of the relative density of immunoreactive structures in different brain regions (Fig. 1).
Figure 1.

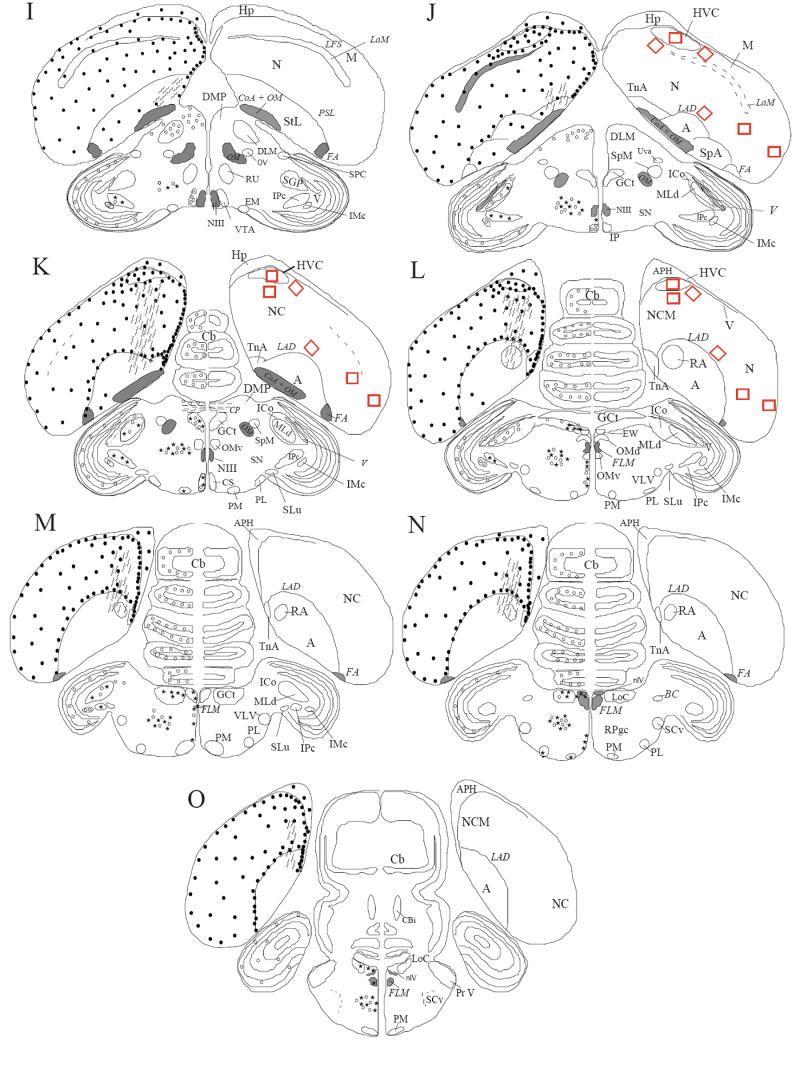
Schematic drawings of coronal sections through the canary brain illustrating the distribution of DCX-ir structures in a rostral to caudal order (from A to O). Densely stained DCX-ir perikarya are represented by dots (.) and weakly stained cells are represented by small open circles (○). Cells associated with immunoreactive DCX-ir small vesicles resembling punctate structures are represented by stars. DCX-ir fibers that are not obviously associated with immunoreactive perykarya are represented by wavy lines. The density of symbols has been adjusted within each type of DCX-ir cells to give a qualitative estimate of the number of the immunoreactive structures. The densities of symbols should, however, not be compared across cell types; the densely stained DCX-ir cells are indeed by far more numerous than the two other types of staining. The nomenclature is based on the canary brain atlas (Stokes et al 1974) but including recent modifications recommended by the avian nomenclature forum (Reiner et al., 2003) (see text for additional explanations).
Abbreviations: A: Arcopallium; APH: Area parahippocampalis; Area X: Area X; BC: Brachium conjunctivum; BSTL: Nucleus striae terminalis lateralis; Cb: Cerebellum; Cbi: Nucleus cerebellum internum; CO: Chiasma opticum; CoA: Commissura anterior; CoS: Nucleus commissuralis septi; CP: Commissura posterior; CS: Nucleus centralis superior; DLL: Nucleus dorsolateralis anterior thalami, pars lateralis; DLM: Nucleus dorsolateralis anterior thalami, pars medialis; DMP: Nucleus dorsomedialis posterior; DSV: Decussatio supraoptica ventralis; E: Entopallium; EW: Edinger-Westphal Nucleus; FA: Tractus fronto-arcopallialis; FLM: Fasciculus longitudinalis medialis; FPL: Fasciculus prosencephali lateralis; GCt: Griseum centrale; GLv: Nucleus geniculatus lateralis, pars ventralis; GP: Globus pallidus; HA: Hyperpallium apicale; HD: Hyperpallium densocellulare; Hp: Hippocampus; HVC: HVC; ICo: Nucleus Intercollicularis; IMc:Nucleus isthmi, pars magnocellularis; INP: Nucleus intrapeduncularis; IP: Nucleus Interpeduncularis; IPc: Nucleus isthmi, pars parvocellularis; IS: Nucleus Interstitialis; LAD: Lamina arcopallialis dorsalis; LaM: Lamina mesopallialis; LFM: Lamina frontalis suprema; LMAN: Nucleus lateralis magnocellularis nidopallii anterioris; LoC: Locus Coeruleus; LPS: Lamina pallio-subpallialis; LFS: Lamina frontalis superior; M: Mesopallium; MLd: Nucleus mesencephalicus lateralis, pars dorsalis; MMAN: Nucleus medialis magnocellularis nidopallii anterioris; N: Nidopallium; NC: Nidopallium caudale; NCM: Nidopallium caudomediale; NDB: Nucleus diagonalis Brocae; NIII: Nervus oculomotorius; nIV: Nucleus nervi trochlealis; OM: tractus occipito-mesencephalicus; OMd: Nucleus nervi oculomotorii, pars dorsalis; OMv: Nucleus nervi oculomotorii, pars ventralis; OV: Nucleus ovoidalis; PL: Nucleus pontis lateralis; PM: Nucleus pontis medialis; POM: Nucleus preopticus medialis; Pr V: Nucleus sensorius principalis nervi trigemini; Pt: Nucleus pretectalis; PVN: Nucleus paraventricularis; RA: Nucleus robustus arcopallialis; RPgc: Nucleus reticularis pontis caudalis, pars gigantocellularis; Rt: Nucleus rotundus; Ru: Nucleus ruber; SCv: Nucleus subcoeruleus ventralis; SGP: Substantia grisea et fibrosa periventricularis; SL: Nucleus septalis lateralis; SLu: Nucleus semi-lunaris; SM: Nucleus septalis medialis; SN: Substantia Nigra; SP: Nucleus subpretectalis; SpA: Area subpallialis amygdalae; SpL: Nucleus spiriformis lateralis; SpM: Nucleus spiriformis medialis; SRt: Nucleus subrotundus; StL: Striatum laterale; StM: Striatum mediale; TeO: Tectum opticum; TIO: Tractus isthmo-opticus; TnA: Nucleus taeniae amygdalae; TrO: Tractus opticus; TSM: tractus septopallio-mesencephalicus; Tu: Nucleus tuberis; Uva: Nucleus uvaeformis; V: Ventriculus; VLV: Nucleus ventralis lemnisci lateralis; VMN: Nucleus ventromedialis hypothalami; VTA: Area ventralis tegmenti.
Images of representative sections were digitized through the microscope equipped with a 3CCD color video camera (Sony DXC-950P) coupled to a digital still recorder (Sony DKR-700). The files were then transferred to a MacIntosh micro-computer and prepared for printing in Adobe Photoshop. No transformation of images was performed in the program except for adjustments of contrast and light intensity that were performed to equalize gray levels between different panels of a same figure.
The number of densely labeled DCX-ir cells was then counted by the experimenter (GéB) in the telencephalon of 4 castrated males treated with exogenous T that had been housed as part of a pair with a sexually receptive female. Cells were counted in multiple brain areas, that were selected based on previous studies that employed either 3H thymidine or bromo-deoxyuridine (BrdU) methods and had identified significant levels of neuronal incorporation in adulthood (e.g., DeWulf and Bottjer, 2005; Kirn et al., 1994; Kirn, et al., 1999). Two different types of DCX-ir cells (round and fusiform) were detected in the brain (see results) and these two cell types were therefore counted separately. For each area investigated, immunoreactive cells were counted in a standardized square area (200 × 200 μm or 0.04 mm2) located within the area of interest. While observing the drawing sheet through the microscope (20 × objective) and camera lucida, the area used for quantification was positioned within the structure of interest in a standard manner using clearly defined brain landmarks and all immunoreactive cells that contained a clear unstained nucleus surrounded by stained cytoplasm were marked on the drawing sheet using different symbols for round and for fusiform cells. In case of doubt, the presence of a nucleus in a cell was checked at higher magnification.
The number of DCX-ir cells was quantified in 4 T-treated castrated males that had been kept during the experiment with a female partner in 11 distinct brain areas where DCX-ir cells had been detected during a preliminary screening of the sections, with special attention being dedicated to the song control nuclei. These areas are marked by boxed areas in figure 1 and were identified as follows:
HVC and adjacent Nidopallium
DCX-ir cells were counted at 3 equally spaced different rostro-caudal levels (panels J-K-L in Fig. 1) in three separate fields at each level that were positioned in the center of HVC and in the immediately adjacent Nidopallium just lateral (lat.) or ventral (ventr.) to HVC.
Ventral Nidopallium
DCX-ir cells were also quantified at the same rostro-caudal levels (Fig. 1J-K-L) in three distinct zones of the ventral Nidopallium. The first zone was in the ventral Nidopallium adjacent to the Lamina Arcopallialis Dorsalis (LAD), which separates the Nidopallium from the Arcopallium (ventr. Nido). The counting square was placed obliquely on the dorsal edge of the LAD, at approximately equal distances from the lateral and the medial border of the brain. The second zone (lat. Nido) was placed against the lateral margin of the brain, at a level slightly more dorsal than the Tractus fronto-arcopallialis (FA). Finally, the third counting zone or intermediate Nidopallium (intermed. Nido) was placed in the medial Nidopallium halfway between the two previous areas, in the ventro-lateral part of the Nidopallium (Fig. 1 J-L). This square was placed at approximately equal distances from the first and the second square and was thus not adjacent to any lamina or discernible nucleus.
Area X and adjacent medial striatum
DCX-ir cells were counted at two rostro-caudal levels (see Fig. 1B-C) in Area X and in two adjacent regions. Area X was easily identified in the Striatum mediale (StM) just ventral to the Lamina pallio-subpalliallis (LPS) by the slightly bigger size of its cell bodies and by its round or “drop-shaped” morphology. Its location was confirmed by comparison with adjacent Nissl-stained sections. Two adjacent areas of the MSt located laterally (lat.) or ventrally (ventr.) to area X were also counted in the same sections (levels B and C) for comparison.
The lateral part of the Magnocellular Nucleus of the Anterior Nidopallium (LMAN) and adjacent area
lMan is a much smaller elongated nucleus located in the medial Nidopallium near the lateral ventricle. It was difficult to identify in sections immunostained for DCX and its precise location had thus to be determined with the help of adjacent Nissl-stained sections based on adjacent landmarks (PSL and Lamina mesopallialis, LaM) and on the larger cell size within the nucleus as compared to surrounding structures. Because this nucleus is so small, counts were performed only at one rostro-caudal level corresponding to panel C in figure 1. The counting area was centered on LMAN and thus overlapped exclusively with the LMAN core without covering the surrounding parvocellular shell (see Johnson et al., 1995; Bottjer and Johnson, 1997). DCX-ir cells were additionally counted in one square positioned laterally (lat) to lMAN.
It must be noted that the numbers of DCX-ir cells reported here are slightly overestimated because no correction for double counting was carried out. The size of the counted objects (clear nuclei surrounded by cytoplasm) is indeed much smaller (about 5 μm or less for fusiform cells) than the section thickness (30 μm) so that the overestimation due to double counting is limited (for 5 μm objects the unbiased real counts should be 85.7% (i.e. 30/[5+30]%) of the estimated numbers; see Abercrombie, 1946). Furthermore, we are only interested here in the relative numbers of cells types in different areas not in absolute numbers. This ratio is only marginally affected by problems related to double counting. Although the size of nuclei in DCX-ir fusiform cells is smaller than in round cells, even a 50 % difference (from 5 to 2.5 μm) between round and fusiform elements still result into a negligible counting bias (real numbers being 85.7 versus 92.3 % of estimated numbers respectively) far much smaller than the differences reported in this study.
3. Results
3.1. DCX-ir cell types in the canary brain
Immunostaining in all birds revealed two different types of DCX-ir cells in broad areas of the telencephalon. Approximately one third of these cells had a round multipolar morphology, while the rest of these immunoreactive cells presented a fusiform uni- or bipolar conformation (see Fig. 2).
Figure 2.
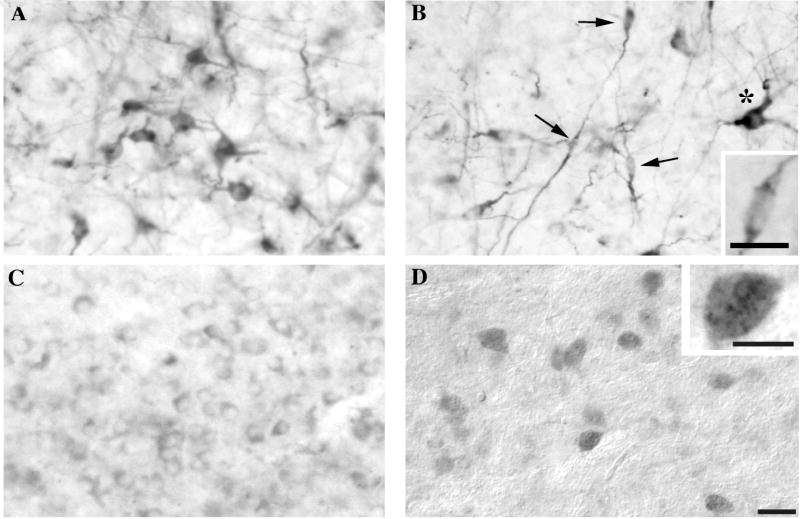
Photomicrographs illustrating the different types of DCX-immunoreactive material observed in the canary brain. A. Densely stained round multipolar cells found in the telencephalon. B. Fusiform elongated cells (arrows) found in the same telencephalic areas as round cells illustrated in A. One round imunoreactive cell (asterisk) is also seen in this field. C. Weakly stained round cells with few immunostained processes detected mainly in di- and mesencephalic areas (here in the Nucleus dorsolateralis anterior thalami, pars medialis). D. Weakly stained cells found in some di- and mesencephalic areas (here in the Substantia nigra) that are associated with small immunoreactive dots reminiscent of punctate structures. The insert illustrates a higher magnification of such a cell. Panel D was photographed with Nomarski interferential contrast. Magnification bar: 20 μm for panels A-D, 10 μm in the insert of panels B and D.
As reported in previous studies on mammals (Francis et al., 1999; Friocourt et al., 2003; Gleeson et al., 1999; Shapiro, et al., 2006), the round multipolar cells displayed a spherical or slightly pyramidal shape, with a cytoplasm densely filled with immunoreactive material. The round nucleus was not stained and clearly appeared as a white spot in the middle of the dark perikaryon (Fig. 2A). Long neurites extending from the cell bodies were also heavily labeled, conferring a typical pattern of multipolar clearly differentiated neurons.
A large fraction of the positive cells, however, were much smaller and displayed a uni- or bipolar fusiform morphology (Fig. 2B). Their cell body was elongated along one axis and only slightly wider than the thick and long neurite emerging at one end. At higher magnification, a pale elongated nucleus could be discerned at the center of the dense cytoplasm. These cells thus had the structure of migrating neurons with a long leading process extending from the neural soma. No particular orientation of the leading processes could be noticed, except along the lateral ventricles where cells adopted an orientation parallel to the ventricle walls and thus looked like tangentially migrating neurons.
Long, densely labeled processes were also observed that were apparently not associated with a cell body even when observed at high magnification. These fibers appeared longer than the length along which processes emerging from the fusiform cells could be followed on the sections. They were organized in large fascicles of parallel fibers that could be discerned in several parts of the telencephalon and were most prominent in the Striatum mediale (StM), the Nidopallium caudale (NC), the Mesopallium (M) and the optic structures (see below for detail).
Weakly stained cells could additionally be detected outside the telencephalon although they were as a whole, by far less numerous than the telencephalic fusiform or round densely stained cells. These cells were characterized by a pale weakly stained cytoplasm (see Fig. 2C) with some rare short immunoreactive processes. They were detected in a large number of brain regions that are too numerous to be presented on the maps but included among others several hypothalamic nuclei (e.g., medial preoptic and ventromedial nuclei), the dorsal thalamus, the septum, the Globus pallidus and the Substantia nigra. In most cases, the number of these weakly stained cells per unit surface was quite small but a few brain regions such as the dorsal thalamus illustrated in Figure 2C contained large numbers of these cells.
A fourth type of staining was also identified on rare occasions in some diencephalic (e.g., Nucleus spiriformis lateralis, SPL) or mesencephalic areas (e.g., Substantia nigra): round weakly labeled or unlabeled cells that were associated with high densities of darkly stained spots apparently located at their surface. Under Nomarski optics providing some three-dimensional vision, these spots appeared as little vesicles reminiscent of punctate structures potentially representing pre-synaptic boutons closely associated with the cell surface (see Fig. 2D). This will be referred to as “punctate-like” staining in the rest of this paper.
3.2. Antibody specificity
The three different antibodies raised against the 3 short peptides combined (anti-DCX pep) or against a longer sequence at the N or C terminal of the DCX molecule revealed a similar pattern of immunoreactivity in the canary brain. For example, as shown in figure 3, all antibodies identified denser populations of DCX-ir cells in HVC as compared to the surrounding tissue (Fig. 3 A-C). At higher magnifications, one could easily observe that all antibodies labeled round multipolar as well as fusiform uni- or bipolar cells (Fig. 3 D-F). At the tissue level, the three antibodies also revealed a similar overall distribution of cells and fibers. For example, the sharp transition between the dense populations of DCX-ir cells in the ventral Nidopallium and the absence of immunoreactive structures in the underlying aArcopallium was clearly visible in adjacent sections that had been stained with each of the 3 antibodies (Fig. 3 G-I).
Figure 3.
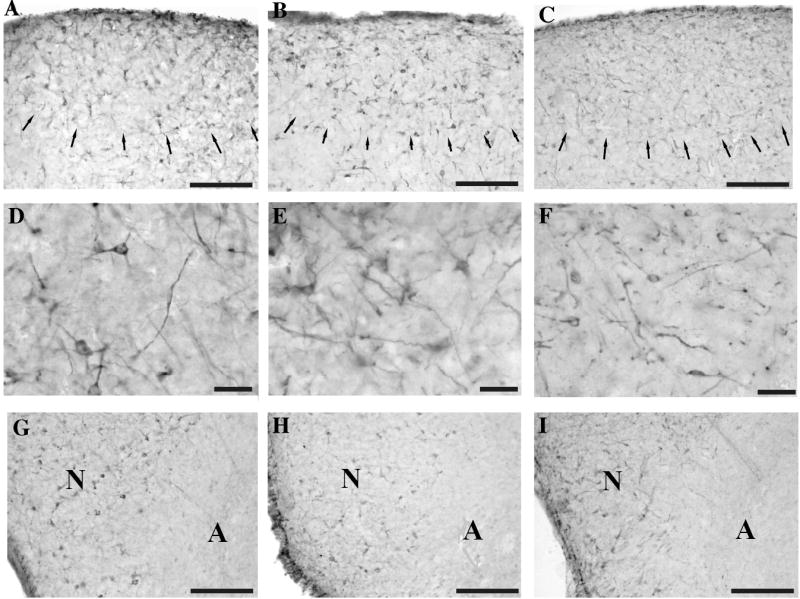
Photomicrographs of HVC at low (panels A-C) and high (panels D-F) magnification and of the border between the Nidopallium (N) and Arcopallium (A) (G-I) in sections that had been stained either with the anti-DCX pep antibody (A, D, G) or the rabbit Nterm DCX antibody (B, E, H) or the goat Cterm DCX antibody (C, F, I). Similar immunoreactive structures are identified by three different antibodies directed against different parts of the DCX protein. Arrows in panels A-C indicate the border of HVC. Panels G-I correspond to the medial border of the telencephalon at the junction between N and A at a rostro-caudal level corresponding to panel M in Figure 1. Magnification bars: 100 μm in A-C and G-I, 20 μm in D-F.
The Western blot experiment identified a prominent immunoreactive band at an apparent molecular weight slightly higher than 40 kDa entirely consistent with previous observations in mammals (Francis et al., 1999). That band was no longer observed when the membrane was directly incubated with the secondary antibody in the absence of primary antibody directed against DCX.
Preabsorbtion with synthetic peptides at both concentrations markedly decreased the immunocytochemical signal in all regions of the brain (Fig. 5). The dense staining observed in round or fusiform cells of the telencephalon was completely abolished while the much weaker staining observed in di- and mesencephalic nuclei was either completely abolished (e.g., diencephalon) or very markedly decreased (e.g. Substantia nigra) when staining was carried out with preadsorbed antibody. This suggests that both types of signal are specific, although some contamination by a non specific signal remains possible for the weakest signal observed in the di- and mesencephalon. The rest of this paper will, however, focus on the denser telencephalic staining that is more obviously specific and relates functionally to neurogenesis and neuronal plasticity.
Figure 5.
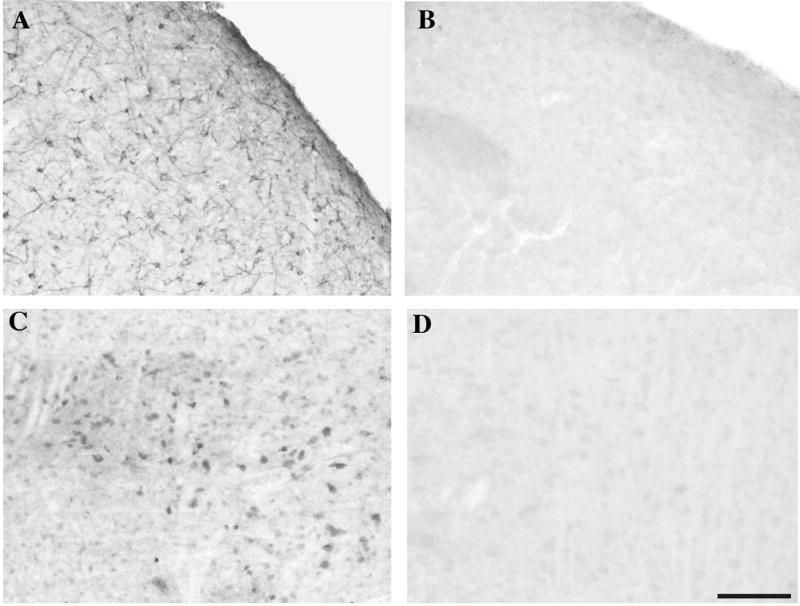
Photomicrographs of sections through HVC (A, B) or through the Substantia nigra (C, D) that were stained with the anti-DCX pep antibody (A, C) or with the antibody that had been preadsorbed with the three peptides used as antigen (B, D). Both the strong (A) and the weak (C) immunoreactive signals are completely or almost completely blocked by predsorbtion (B, D). Magnification bar: 100 μm.
Together with the fact that the sequences of the antigens used for immunization are nearly identical to the corresponding sequences in the chicken and zebra finch DCX, these experiments demonstrate as far as can be done that the immunoreactive material detected in the canary brain truly corresponds to the protein doublecortin.
3.3. Distribution of DCX-ir material throughout the forebrain
Cells
DCX-ir cells were detected in a large number of regions of the canary brain. The two types of densely stained positive cells, round and fusiform, exhibited a similar distribution among brain areas and were present in all these areas in similar relative proportions. We therefore consider both cell types simultaneously in the present description of their overall distribution in the brain, which is summarized graphically in figure 1. Photomicrographs illustrating a few key brain areas that contain a high density of immunoreactive material are presented in figure 6.
Figure 6.
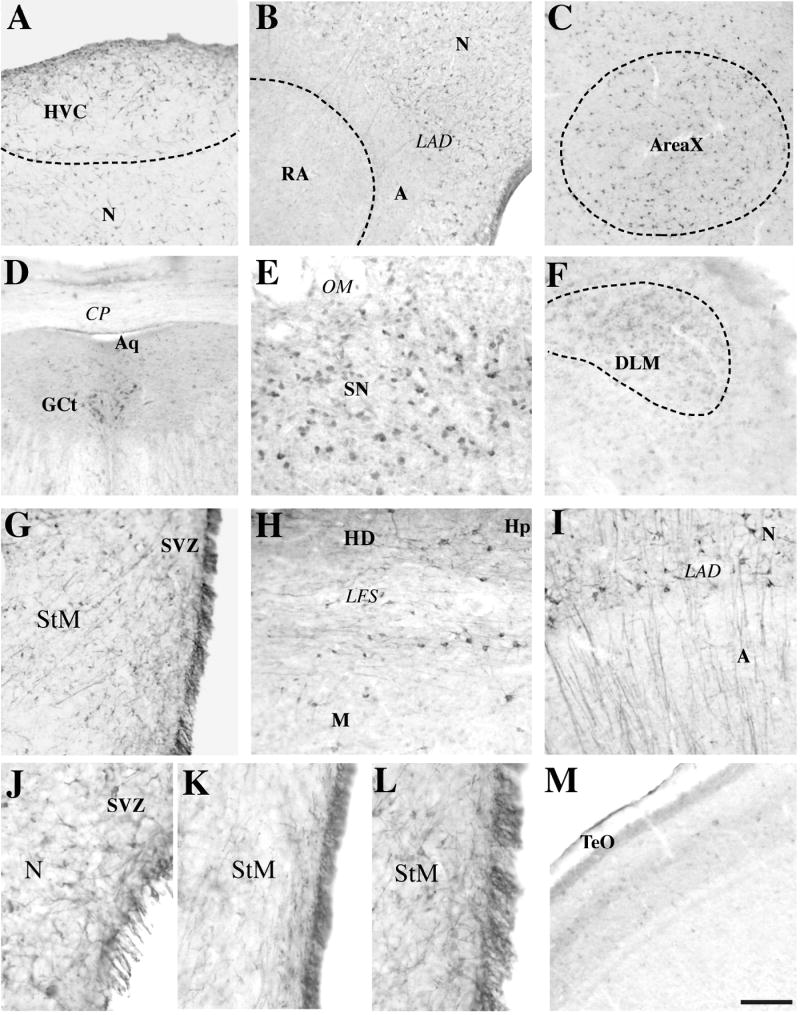
Photomicrographs illustrating the different types of DCX-ir material identified in key regions of the canary brain. A. HVC and adjacent Nidopallium (N), B. Nucleus robustus archistriatalis (RA) and adjacent Arcopallium (A) just ventral to the Lamina arcopallialis dorsalis, C. Area X and surrounding Striatum mediale, D. Weakly labeled cells in the mesencephalic Griseum centrale (GCt = periaqueductal gray) located ventrally to the Aqueductus cerebri (Aq) and the Commissura posterior (CP), E. Weakly labeled cells in the Substantia nigra (SN), F. Weakly labeled cells in the Nucleus dorsolateralis anterior thalami, pars medialis (DLM), G. Bundle of parallel fibers originating in the subventricular zone (SVZ) and crossing a large area of the Striatum mediale (MSt), H. Fibers intermingled with parallel fusiform cells in the dorsal part of the Mesopallium (M) just ventral to the Lamina frontalis superior (LFS) and the Hyperpallium densocellulare (HD), I. Parallel fibers crossing the Lamina arcopallialis dorsalis (LAD) from the Nidopallium (N) to the Arcopallium (A), J-K-L. views of the subventricular zome (SVZ) at the level of Nucleus taeniae amygdalae (level L in Fig. 1; J), of the Commissura anterior (level G in Fig. 1; K) and of the Area X (level B in Fig. 1; L), M. Low magnification of the optic lobes showing the stratified imunoreactive signal that is not generally associated with clear cellular structures. At higher magnification a few weakly labeled cells are however detected. Magnification bar shown in M represents 200 μm in A-D and F, 100 μm in E, G-I, M and 50 μm in J-L.
Pallial structures
The highest density of immunoreactive cells was observed in the pallial part of the telencephalon, although the density of positive cells varied across different pallial regions. Dense and relatively homogeneous populations of DCX-ir cells were present in the Hyperpallium densocellulare (HD) and apicale (HA), in the Mesopallium (M), and in the medial and lateral aspects of the Nidopallium (N) from the level of area X of the Striatum mediale to the caudal end of the Commissura anterior (CoA) (see Fig. 1A to H). In the most rostral part of these pallial telencephalic structures (Fig. 1A-D), DCX-ir perikarya and associated fibers were, however, present in a slightly higher density laterally than medially in these regions. A much higher density of labeled cells than in all other pallial areas was observed in the Nidopallium caudale (NC), in particular, in its most ventral aspects where a numerous population of DCX-ir cells was present along the LAD, which determines the arcopallial-nidopallial boundary (Fig. 1K-O). No or almost no DCX-ir cells were detected in the Arcopallium (A; Fig. 6B) nor in the Entopallium (E).
Throughout the telencephalon, the walls of the lateral ventricle were lined up with an extremely high density of immunoreactive material that seemed to consist of a mixture of labeled, possibly ependymal, cells intermingled with high densities of immunoreactive fibers (Fig. 1 A-G; Fig. 6J-K). At more caudal levels, this high density of immunoreactive material along the ventricle wall was in continuity with a similar high density of immunoreactive elements located along the medial edge of the brain. These immunoreactive structures were particularly abundant just dorsal to the Nucleus taeniae amygdalae (TnA), which itself contained a substantial number of positive cells.
The Hippocampus and the Area parahippocampalis also contained a high number of DCX-ir cells throughout their rostro-caudal extent but the density of these cells was clearly lower than in the caudal nidopallium.
Sub-pallial structures
The Striatum mediale and laterale (StM and StL) contained a homogeneous population of DCX-ir cells throughout their rostro-caudal extent. The density of these cells was however much higher in the area located just ventral to the Lamina pallio-subpalliallis (LPS) and in the subventricular zone (SVZ) along the lateral ventricles (Fig. 6G), as was observed in the part of the SVZ located in the pallial telencephalon. No specific accumulation of DCX-ir cells, that is similar to the so-called “hot spots” of neurogenesis as described by Alvarez-Buylla et al. (Alvarez-Buylla et al., 1990) could be detected along the ventricle wall neither in the striatum nor in the pallium. The density of immunoreactive material in the SVZ was so high that it was usually impossible to discriminate individual cells from the dense network of immunoreactive fibers.
The Globus pallidus (GP; formerly Paleostriatum primitivum; See Reiner et al., 2004) contained weakly labeled cells and contrasted markedly with the StL (formerly Paleostriatum augmentatum) where the density of labeled cells was substantially higher.
The Bed nucleus striae terminalis lateralis (BSTL; formerly Nucleus accumbens), a round nucleus positioned at the ventro-lateral tip of the lateral ventricles dorsal to the Commissura Anterior was also densely packed with DCX-ir elements (Fig. 1G-H). The density of immunostaining was in contrast much lower (fewer and more lightly stained cells) in both the Nucleus septalis medialis (SM) and lateralis (SL), which could not be distinguished based on the DCX immunostaining (Fig. 1 D-H). In contrast, the adjacent medial subventricular region contained a much denser population of darkly labeled DCX-ir cells but this density remained lower than observed along the lateral walls of the ventricles.
No staining could be identified in other basal telencephalic structures including the Nucleus intrapeduncularis (INP; Fig. 1F-H) and ventral pallidum.
Song control nuclei
The song control nucleus HVC and the surrounding Nidopallium were characterized by high numbers of DCX-ir cells. A higher density of DCX-ir cells within HVC allowed to identify this nucleus from the adjacent nidopallium (see Fig. 6A). The song control nuclei LMAN and MMAN could not be recognized in sections stained by immunocytochemistry but based on adjacent Nissl-stained sections, it could be confirmed that these nuclei contained scattered populations of positive cells that could not be distinguished from the labeling in the surrounding tissue. Nucleus RA is located in the middle of the Arcopallium, a region that appears to be devoid of DCX-ir cells and could not be distinguished in this respect from the adjacent areas (Fig. 6B). Area X like the adjacent dorsal StM contained a fairly dense population of DCX-ir cells (Fig. 6C). These cells were apparently slightly more numerous per unit surface in the song control nucleus than in the surrounding region.
Diencephalon
Some well characterized hypothalamic and preoptic nuclei were identified in sections labeled by immunocytochemistry for DCX based on Nissl-stained adjacent sections and on prominent landmarks such as fiber tracts and ventricles. No obvious pattern of DCX immunostaining could be used to differentiate specific cell groups. The nomenclature employed for these areas specifically is based on the canary brain atlas (Stokes et al., 1974) as revised based on the localization of aromatase- and vasotocin-immunoreactive structures that constitute good chemical markers for several nuclei in this region (Balthazart et al., 1996).
Very little DCX immunoreactive material was observed in this brain region. The Nucleus preopticus medialis (POM), the Nucleus ventromedialis hypothalami (VMN), and the Nucleus tuberis (Tu) contained only a few very lightly stained round cells. In contrast, these weakly stained cells were present in high number in the dorsal nuclei of the thalamus (Fig. 6F). The fibers associated with these perikarya were not, or only very weakly, stained and apparently exhibited very short processes. Contrary to what was observed in the rest of the brain, no fusiform cells could be detected in any of these nuclei.
The dorsal edge of the diencephalon, from the level of the CoA till the transition with the mesencephalon (Fig. 1 I-J) was however lined up with stained DCX-ir cells associated with clearly visible darkly stained neurites. The density of this label was however lower than in the telencephalon.
In addition, the Nucleus spiriformis lateralis (SPL) displayed the “punctate-like” immunolabeling illustrated above in Figure 2D consisting in an extremely weak cytoplasmic staining associated with a high density of immunostained dots reminiscent of punctate structures (Fig. 1H).
Mesencephalon and Rhombencephalon
Several layers of the Tectum opticum (TeO) exhibited a distinct pattern of DCX immunoreactivity that was in most cases not associated with clear cellular structures (Fig. 6M). Its specificity is therefore questionable. A few round multipolar weakly immunoreactive perikarya were also present through these layers. Weakly labeled cells were also seen in other nuclei, such as the Nucleus mesencephalicus lateralis pars dorsalis (MLd), Nucleus intercollicularis (ICo; Fig. 1 K-M), Nucleus isthmi, pars magnocellularis (IM; Fig. 1 L-M), Area ventralis tegmenti (VTA; Fig. 1I), and along the wall of the fourth ventricle or the midline of the brain (Fig. 1I-O).
In addition, some of the cells in several mesencephalic regions, such as the Substantia nigra (SN), mesencephalic Griseum centrale (GCt, know as periaqueductal gray in mammals, Fig. 6D), Nucleus isthmi, pars parvocellularis (IPC), Locus coeruleus (LoC), Nucleus pontis medialis (PM), the walls of the 4th ventricle just ventral to the Fasciculus longitudinalis medialis (FLM) and to a lesser extent the MLd, displayed a dense “punctate-like” staining (see Fig. 2D) (Fig. 1 I-O).
A very low density of immunoreactive material was also visible in Purkinje cells of the cerebellum (Fig. 1 K-N) although the specificity of this label remains also doubtful given its very weak intensity associated with the fact that Purkinje cells tend to adsorb almost any type of antibody.
Fibers
As mentioned above, most of the densely immunoreactive cells of the telencephalon were associated with labeled neurites extending often several cell body lengths away from the perikaryon. In addition, bundles of parallel long thin DCX-ir fibers that were not obviously associated with positive cells were detected in five specific brain regions.
In the StM, long oblique fibers apparently originating at the level the SVZ were observed that were crossing the entire region from a dorso-medial to ventro-lateral direction. These fibers also passed through the most ventro-medial aspects of area X. These immunoreactive fibers bundles were detectable throughout the rostro-caudal extent of StM (Fig. 1A-H) and extended more caudally in the StL (Fig 1I-J).
In the Nidopallium caudale (Fig. 1 J-O; 6I), DCX-ir fibers also apparently originating from the SVZ in or near HVC were found crossing the medial nidopallium from a dorso-medial to ventro-lateral direction. These fibers ran across the Lamina arcopallialis dorsalis (LAD) to enter the Arcopallium (A) to reach RA and TnA. Some of these fibers may represent the projection to RA of neurons recently incorporated in HVC. This would then suggest that DCX is still expressed in these neurons for quite some time after they have completed their migration and initiated their differentiation. This possibility should be investigated by combining tract-tracing with DCX immunocytochemistry.
At the level of the rostral CoA (Fig. 1G), fibers almost perpendicular to the CoA were also seen running from the tip of the lateral ventricle and the medial septum, crossing the CoA and ending in the hypothalamus more or less at the level of the POM.
The fourth much smaller but still clearly discernible bundle of immunoreactive fibers was located in the Mesopallium (fig. 1A-H; 6H), just ventrally to the Lamina frontalis superior (LFS). These fibers that were intermingled with parallel fusiform cells had an orientation parallel to the lamina and extended from the SVZ to the lateral edge of the brain.
Finally, DCX-ir fibers were also observed in high density in the Chiasma opticum (CO; Fig. 1E-F), the Tractus opticum (TrO; Fig. 1F) and to some extent the Tractus isthmo-opticum (TIO; Fig. 1G).
3.4. Quantitative evaluation of the relative abundance of small fusiform and large round cells
Given the potential importance of the densely stained DCX-ir cells in the telencephalon for the process of neuronal migration in adult songbirds, we quantified the two types of immunoreactive cells (round vs. elongated) in a large number of telencephalic brain areas in 4 males. In most brain areas but particularly in the Nidopallium in and around HVC fusiform cells were found to be much more numerous (2-3 times) than round DCX-ir cells. Such a difference was however not observed in the more ventral (striatum) or rostral (Nidopallium in and around LMAN) parts of the telencephalon (Fig. 7).
Figure 7.
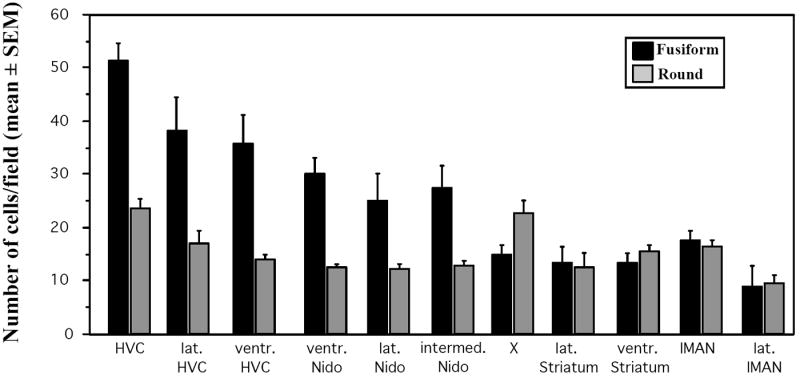
Average numbers of round or fusiform DCX-ir cells in 4 photosensitive male canaries that were castrated, treated with exogenous testosterone and exposed to a female partner. Cells were counted in 200 by 200 μm fields that were placed in standardized locations within the telencephalon, namely in the song control nucleus HVC, just lateral (lat. HVC) or ventral (ventr. HVC) to this nucleus, in the ventral (ventr.), lateral (lat.) or intermediate (intermed.) Nidopallium (Nido), in Area X (X) or in the Striatum lateral (lat.) or ventral (ventr.) to this nucleus, or finally in LMAN or just lateral to this nucleus (lat. LMAN. See methods and Fig. 1 for a more precise description of these areas).
4. Discussion
DCX is a microtubule-associated protein that plays a key role in the migration of neurons during ontogeny and probably also in the remodeling of their dendritic arborization, axonal outgrowth and synaptogenesis in adulthood (Brown, et al., 2003; Capes-Davis et al., 2005; Francis et al., 1999; Gleeson et al., 1999; Nacher, et al., 2001; Yang, et al., 2004). In mammals and birds, expression of this protein decreases sharply during ontogeny (mammals: Couillard-Despres et al., 2005; des Portes et al., 1998; in birds: Capes-Davis et al., 2005; Hannan et al., 1999; Kim et al., 2006) in parallel with the decrease or cessation of neurogenesis. Although a very active neurogenesis and incorporation of new neurons has been identified in the pallial structures of songbirds, in association with their extensive seasonal brain and behavioral plasticity (Alvarez-Buylla et al., 1990; Alvarez-Buylla et al., 1988; Kirn et al., 1994), DCX has not been studied to this date in the brain of adult songbirds with the exception of a report describing the neuroanatomical distribution of the DCX mRNA and protein in the brain of the zebra finch (Kim et al., 2006). This study indicated however that the expression of DCX sharply decreases with age in zebra finches and suggested a role for this protein related mostly to brain development during ontogeny. We show here that, in canaries, a very substantial level of expression of this protein is still found in the adult brain and may therefore relate to the substantial neurogenesis that is observed in this species and in other songbirds in relation to the seasonal plasticity of the song control system.
We describe here in detail, for the first time, the neuroanatomical distribution of DCX-ir structures in the brain of canaries, a temperate-zone songbird species well known for its extensive seasonal plasticity. We demonstrate that the presence of high densities of DCX-ir cells in the telencephalic pallial regions, in particular in the song control nucleus HVC, where active neuronal incorporation has been shown to take place and vary in a seasonal manner.
Converging evidence derived from the similarity of DCX in birds and mammals, comparisons of results obtained with 3 different antibodies, Western blotting and preadsorbtion experiments demonstrate that the immunoreactive material described here in the canary brain actually corresponds to DCX. This specificity is also supported by the similarity with the immunocytochemical results relative to the zebra finch brain obtained with yet another antibody and with the distribution of the corresponding mRNA based on in situ hybridization experiments (Kim et al., 2006).
Two separate types of DCX-ir cells were present in the canary telencephalon: round multipolar cells that apparently correspond to differentiating neurons that have reached their final destination and uni- or bipolar elongated cells that resemble migrating neurons that should presumably transform into round multiplolar cells after reaching their target. The vast majority of the immunoreactive cells thus exhibit a neuronal morphology even if we cannot exclude based on available evidence that a small fraction of these cells could be glial in nature. The neuronal nature of the DCX-ir cells that show a dense immunocytochemical staining is further supported by the distribution of brain areas that do or do not contain these cells. Densely stained DCX-ir cells were mostly observed in brain regions (pallial structures) that are known to recruit new neurons in adult canaries and other avian species (Alvarez-Buylla et al., 1990; Cao, et al., 2002; Chen, et al., 2006; DeWulf and Bottjer, 2005; Kirn et al., 1994; Kirn et al., 1999) and were rare or absent in the di-and mesencephalon, areas that do not recruit new neurons in adulthood except following lesions in the area (Cao et al., 2002; Chen et al., 2006). The DCX-ir cells that were observed in the di- and mesencephalon were weakly labeled and it is highly unlikely that DCX expression in these cells is related to neurogenesis. Expression in these brain regions of the microtubule-associated protein may relate to the cellular plasticity of adult neurons and be involved on the morphological reorganization of adult cells including changes in the dendritic tree as previously suggested based on studies of the mammalian brain (Brown et al., 2003; Nacher et al., 2001). This same explanation could also apply to the presence of weakly labeled neurons in areas such as the substantia nigra or to the Purkinje neurons of the cerebellum as previously suggested by Kim and collaborators in a study of the zebra finch brain (Kim et al., 2006)
The particularly high number of DCX-ir cells in and around HVC, a nucleus that is known to replace a large number of its neurons throughout the adult life strongly is consistent the idea that that DCX is expressed in young neurons and could potentially provide a reliable marker of new neurons in songbirds as it does in mammals (e.g. (Shapiro et al., 2006)). The formal demonstration of this notion would obviously require additional experiments in which neuron progenitors duplicating their DNA in anticipation of a cell division would be labeled with tritiated thymidine or BrdU and then double labeled a few days/weeks later with DCX. Such a BrdU-DCX time-course study would be expected to first identify a wave of fusiform BrdU positive DCX-ir cells followed closely by a wave of double-labeled multipolar cells. The anatomical evidence presented above coupled with the observation that a much larger number of presumably migrating fusiform cells were detected by comparison with the rounds cells (presumably in the process of differentiation) are fully compatible with this notion.
If DCX can reliably be used to provide quantitative estimates of new neurons in the adult brain, this method would offer substantial advantages for the study of neurogenesis and new neuron incorporation as compared to DNA labeling with thymidine of BrdU. Firstly, the quantification of the two types of DCX-ir cells (fusiform and multipolar) as done here offers an integrated view of new neurons born over an extended period of time that are currently at two different stages of their life (migration or incorporation). This is sharply different from BrdU, which labels neurons born from progenitors that were in the S (DNA synthesis) phase of their cell cycle when or soon after BrdU was injected. BrdU has a relatively short half-life when injected in an animal and can thus only label the DNA of cells during a limited period of time. In other words, BrdU only provides a snapshot of the neurogenesis (or more accurately the DNA duplication) that took place during a narrow time window following its injection while DCX is thought to visualize all neurons that have divided over a long period of time and that are engaged in the migration or differentiation process. At any given time, the number of BrdU-positive cells present in a section thus represent only a small fraction of the recently born neurons and the time when brain tissue is studied with respect to the time when BrdU was injected has a huge impact on the results. A time-course study should in principle be performed for all experimental models to describe the progressive appearance and disappearance of BrdU-positive cells after an injection of tracer. DCX in contrast has the potential to label all recently born neurons.
Secondly, from a pragmatic point of view, the analysis of neurogenesis with the BrdU technique requires two precisely timed successive manipulations of the subject (for BrdU injection and then for sacrifice and histological processing of tissue), which may be difficult to achieve in some circumstances, for example when studying free-living animals whose recapture may be difficult or in the analysis of human tissue where BrdU labeling before post-mortem tissue collection is usually impossible. DCX visualization can instead be performed directly on brain tissue without any previous manipulation. For all these reasons, DCX represents an outstanding marker of new neurons that should be more extensively exploited in combination, when possible, with the labeling of dividing neurons with BrdU or tritiated thymidine.
It must also be noted that, studies of the mammalian brain showed that DCX is not only a good marker of new neurons but, as a microtubule protein, it is also part of the cellular machinery mediating the migration of these neurons (Friocourt et al., 2003; Bai et al., 2003; Moores et al., 2004; Jin et al., 2004). DCX is thus causally involved in neuronal migration and changes in DCX expression have important consequences on where these newborn neurons will end up in the brain.
Future studies should now investigate the quantitative changes in DCX expression in the songbird brain as a function of changes in the rate of neurogenesis and try to identify the mechanisms that control DCX expression. This would then potentially provide a new method that would allow experimental manipulations of the recruitment of new neurons in specific parts of the central nervous system in order to better identify the functional significance of adult neurogenesis.
Figure 4.
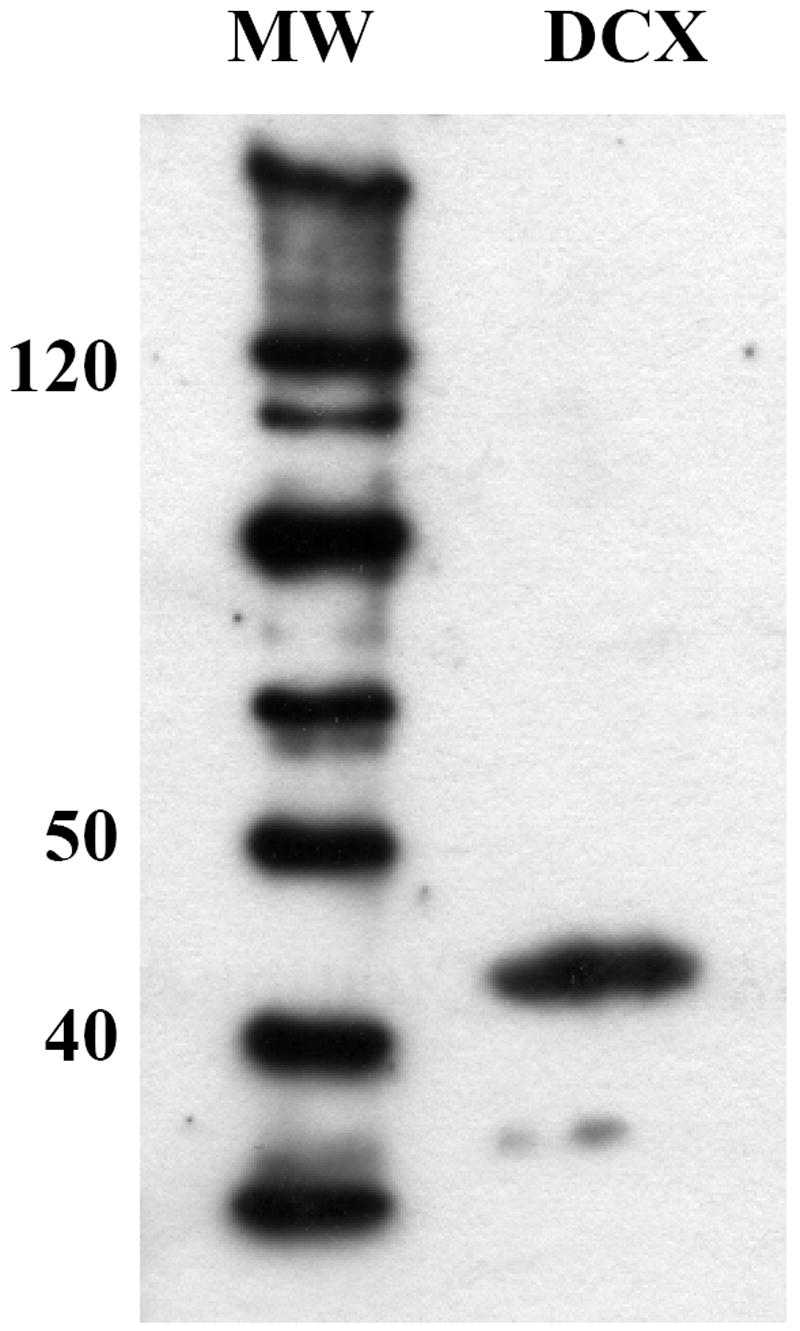
Western blot demonstrating that the anti-DCX pep antibody recognizes a protein with a molecular weight slightly higher than 40 KDa consistent with the size of DCX as identified in mammals. MW: molecular weight markers; DCX: doublecortin in a canary brain extract.
Acknowledgments
This work was supported by a grant from the NINDS (NS 35467) to GFB and JB and a grant from the Belgian FRFC (nbr. 2.4562.05) to JB. We thank Professor Fiona Francis (INSERM U129, Institut Cochin, Paris, France) for the generous gift of DCX antibodies and for very useful advice at various stages of this research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez-Buylla A, Kirn JR, Nottebohm F. Birth of projection neurons in adult avian brain may be related to perceptual or motor learning. Science. 1990;249:1444–1446. doi: 10.1126/science.1698312. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Theelen M, Nottebohm F. Birth of projection neurons in the higher vocal center of the canary forebrain before, during, and after song learning. Proc Natl Acad Sci USA. 1988;85:8722–8726. doi: 10.1073/pnas.85.22.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai J, Ramos RL, Ackman JB, Thomas AM, Lee RV, LoTurco JJ. RNAi reveals doublecortin is required for radial migration in rat neocortex. Nat Neurosci. 2003;6:1277–83. doi: 10.1038/nn1153. [DOI] [PubMed] [Google Scholar]
- Ball GF. Neuroendocrine basis of seasonal changes in vocal behavior among songbirds. In: Hauser M, Konishi M, editors. The design of animal communication. MIT Press; Cambridge,MA: 1999. pp. 213–253. [Google Scholar]
- Ball GF, Auger CJ, Bernard DJ, Charlier TD, Sartor JJ, Riters LV, Balthazart J. Seasonal Plasticity in the song control system. Multiple brain sites of steroid hormone action and the importance of variation in song behavior. Ann NY Acad Sci. 2004;1016:1–25. doi: 10.1196/annals.1298.043. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Absil P, Foidart A, Houbart M, Harada N, Ball GF. Distribution of aromatase-immunoreactive cells in the forebrain of zebra finches (Taeniopygia guttata): Implications for the neural action of steroids and nuclear definition in the avian hypothalamus. J Neurobiol. 1996;31:129–148. doi: 10.1002/(SICI)1097-4695(199610)31:2<129::AID-NEU1>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Boseret G, Carere C, Ball GF, Balthazart J. Social context affects testosterone-induced singing and the volume of song control nuclei in male canaries (Serinus canaria) J Neurobiol. 2006;66(10):1044–60. doi: 10.1002/neu.20268. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Brady JD, Cribbs B. Connections of a motor cortical region in zebra finches: relation to pathways for vocal learning. J Comp Neurol. 2000;420(2):244–60. [PubMed] [Google Scholar]
- Bottjer SW, Halsema KA, Brown SA, Miesner EA. Axonal connections of a forebrain nucleus involved with vocal learning in zebra finches. J Comp Neurol. 1989;279:312–326. doi: 10.1002/cne.902790211. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Johnson F. Circuits, hormones, and learning: Vocal behavior in songbirds. J Neurobiol. 1997;33:602–618. doi: 10.1002/(sici)1097-4695(19971105)33:5<602::aid-neu8>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Miesner EA, Arnold AP. Forebrain lesions disrupt development but not maintenance of song in passerine birds. Science. 1984;224:901–903. doi: 10.1126/science.6719123. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. Auditory feedback in learning and maintenance of vocal behaviour. Nat Rev Neurosci. 2000;1(1):31–40. doi: 10.1038/35036205. [DOI] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467(1):1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Cao J, Wenberg K, Cheng MF. Lesion induced new neuron incorporation in the adult hypothalamus of the avian brain. Brain Res. 2002;943(1):80–92. doi: 10.1016/s0006-8993(02)02537-4. [DOI] [PubMed] [Google Scholar]
- Capes-Davis A, Tolhurst O, Dunn JM, Jeffrey PL. Expression of doublecortin (DCX) and doublecortin-like kinase (DCLK) within the developing chick brain. Dev Dyn. 2005;232(2):457–67. doi: 10.1002/dvdy.20240. [DOI] [PubMed] [Google Scholar]
- Catchpole CK, Slater PJB. Bird song: Biological themes and variations. 1. Cambridge University Press; Cambridge and New York: 1995. [Google Scholar]
- Chen G, Bonder EM, Cheng MF. Lesion-induced neurogenesis in the hypothalamus is involved in behavioral recovery in adult ring doves. J Neurobiol. 2006;66(6):537–51. doi: 10.1002/neu.20247. [DOI] [PubMed] [Google Scholar]
- Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21(1):1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- des Portes V, Francis F, Pinard JM, Desguerre I, Moutard ML, Snoeck I, Meiners LC, Capron F, Cusmai R, Ricci S, Motte J, Echenne B, Ponsot G, Dulac O, Chelly J, Beldjord C. doublecortin is the major gene causing X-linked subcortical laminar heterotopia (SCLH) Hum Mol Genet. 1998;7(7):1063–70. doi: 10.1093/hmg/7.7.1063. [DOI] [PubMed] [Google Scholar]
- DeWulf V, Bottjer SW. Neurogenesis within the juvenile zebra finch telencephalic ventricular zone: A map of proliferative activity. J Comp Neurol. 2005;481(1):70–83. doi: 10.1002/cne.20352. [DOI] [PubMed] [Google Scholar]
- Foidart A, Reid J, Absil P, Yoshimura N, Harada N, Balthazart J. Critical re-examination of the distribution of aromatase-immunoreactive cells in the quail forebrain using antibodies raised against human placental aromatase and against the recombinant quail, mouse or human enzyme. J Chem Neuroanat. 1995;8:267–282. doi: 10.1016/0891-0618(95)00054-b. [DOI] [PubMed] [Google Scholar]
- Francis F, Koulakoff A, Boucher D, Chafey P, Schaar B, Vinet MC, Friocourt G, McDonnell N, Reiner O, Kahn A, McConnell SK, Berwald-Netter Y, Denoulet P, Chelly J. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23(2):247–56. doi: 10.1016/s0896-6273(00)80777-1. [DOI] [PubMed] [Google Scholar]
- Friocourt G, Koulakoff A, Chafey P, Boucher D, Fauchereau F, Chelly J, Francis F. Doublecortin functions at the extremities of growing neuronal processes. Cereb Cortex. 2003;13(6):620–6. doi: 10.1093/cercor/13.6.620. [DOI] [PubMed] [Google Scholar]
- Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23:257–71. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- Gould E, Gross CG. Neurogenesis in adult mammals: Some progress and problems. J Neurosci. 2002;22(3):619–623. doi: 10.1523/JNEUROSCI.22-03-00619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan AJ, Henke RC, Seeto GS, Capes-Davis A, Dunn J, Jeffrey PL. Expression of doublecortin correlates with neuronal migration and pattern formation in diverse regions of the developing chick brain. J Neurosci Res. 1999;55:650–7. doi: 10.1002/(SICI)1097-4547(19990301)55:5<650::AID-JNR12>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Jin K, Mao XO, Cottrell B, Schilling B, Xie L, Row RH, Sun Y, Peel A, Childs J, Gendeh G, Gibson BW, Greenberg DA. Proteomic and immunochemical characterization of a role for stathmin in adult neurogenesis. Faseb J. 2004;18:287–99. doi: 10.1096/fj.03-0973com. [DOI] [PubMed] [Google Scholar]
- Johnson F, Sablan MM, Bottjer SW. Topographic organization of a forebrain pathway involved with vocal learning in zebra finches. J Comp Neurol. 1995;358:260–278. doi: 10.1002/cne.903580208. [DOI] [PubMed] [Google Scholar]
- Kafitz KW, Güttinger HR, Müller CM. Seasonal changes in astrocytes parallel neuronal plasticity in the song control area HVc of the canary. Glia. 1999;27:88–100. [PubMed] [Google Scholar]
- Karten HJ, Hodos W. A stereotaxic atlas of the brain of the pigeon (Columba livia) Johns Hopkins; Baltimore: 1967. [Google Scholar]
- Kim YH, Peregrine J, Arnold AP. The distribution of expression of doublecortin (DCX) mRNA and protein in the zebra finch brain. Brain Res. 2006;1106(1):189–96. doi: 10.1016/j.brainres.2006.05.080. [DOI] [PubMed] [Google Scholar]
- Kirn J, O’Loughlin B, Kasparian S, Nottebohm F. Cell death and neuronal recruitment in the high vocal center of adult male canaries are temporally related to changes in song. Proc Natl Acad Sci USA. 1994;91:7844–7848. doi: 10.1073/pnas.91.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirn JR, Fishman Y, Sasportas K, Alvarez-Buylla A, Nottebohm F. Fate of new neurons in adult canary high vocal center during the first 30 days after their formation. J Comp Neurol. 1999;411:487–94. [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Leboucher G, Kreutzer M, Dittami J. Copulation-solicitation displays in female canaries (Serinus canaria): are oestradiol implants necessary? Ethology. 1994;97:190–197. [Google Scholar]
- Mello CV, Vates GE, Okuhata S, Nottebohm F. Descending auditory pathways in the adult male zebra finch (Taeniopygia guttata) J Comp Neurol. 1998;395:137–160. [PubMed] [Google Scholar]
- Moores CA, Perderiset M, Francis F, Chelly J, Houdusse A, Milligan RA. Mechanism of microtubule stabilization by doublecortin. Mol Cell. 2004;14:833–9. doi: 10.1016/j.molcel.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Nacher J, Crespo C, McEwen BS. Doublecortin expression in the adult rat telencephalon. Eur J Neurosci. 2001;14(4):629–44. doi: 10.1046/j.0953-816x.2001.01683.x. [DOI] [PubMed] [Google Scholar]
- Nicholls TJ, Storey CR. The effect of duration of the daily photoperiod on recovery of photosensitivity in photorefractory canaries (Serinus canarius) Gen Comp Endocrinol. 1977;31:72–74. doi: 10.1016/0016-6480(77)90192-7. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. A brain for all seasons: Cyclical anatomical changes in song-control nuclei of the canary brain. Science. 1981;214:1368–1370. doi: 10.1126/science.7313697. [DOI] [PubMed] [Google Scholar]
- Nottebohm F, Stokes TM, Leonard CM. Central control of song in the canary, Serinus canarius. J Comp Neurol. 1976;165:457–486. doi: 10.1002/cne.901650405. [DOI] [PubMed] [Google Scholar]
- Rao MS, Shetty AK. Efficacy of doublecortin as a marker to analyse the absolute number and dendritic growth of newly generated neurons in the adult dentate gyrus. Eur J Neurosci. 2004;19(2):234–46. doi: 10.1111/j.0953-816x.2003.03123.x. [DOI] [PubMed] [Google Scholar]
- Reiner AD, Perkel J, Bruce L, Butler A, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Güntürkün O, Lee DW, Mello CV, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, S C, J ED. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol. 2004;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor JJ, Balthazart J, Ball GF. Coordinated and dissociated effects of testosterone on singing behavior and song control nuclei in canaries (Serinus canaria) Horm Behav. 2005;47(4):467–76. doi: 10.1016/j.yhbeh.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Scharff C, Nottebohm F. A comparative study of the behavioral deficits following lesions of various parts of the zebra finch song system: Implications for vocal learning. J Neurosci. 1991;11:2896–2913. doi: 10.1523/JNEUROSCI.11-09-02896.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro LA, Upadhyaya P, Ribak CE. Spatiotemporal profile of dendritic outgrowth from newly born granule cells in the adult rat dentate gyrus. Brain Res. 2006 doi: 10.1016/j.brainres.2006.07.032. [DOI] [PubMed] [Google Scholar]
- Smith GT, Brenowitz EA, Beecher MD, Wingfield JC. Seasonal changes in testosterone, neural attributes of song control nuclei, and song structure in wild songbirds. J Neurosci. 1997;17:6001–6010. doi: 10.1523/JNEUROSCI.17-15-06001.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes TM, Leonard CM, Nottebohm F. The telencephalon, diencephalon, and mesencephalon of the canary, Serinus canaria, in stereotaxic coordinates. J Comp Neurol. 1974;156:337–374. doi: 10.1002/cne.901560305. [DOI] [PubMed] [Google Scholar]
- Taupin P. Adult neurogenesis in mammals. Curr Opin Mol Ther. 2006;8(4):345–51. [PubMed] [Google Scholar]
- Thompson CK, Brenowitz EA. Seasonal change in neuron size and spacing but not neuronal recruitment in a basal ganglia nucleus in the avian song control system. J Comp Neurol. 2005;481(3):276–283. doi: 10.1002/cne.20381. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Brenowitz EA. Seasonal plasticity in the adult brain. TINS. 2000;23:251–258. doi: 10.1016/s0166-2236(00)01558-7. [DOI] [PubMed] [Google Scholar]
- Tramontin AD, Smith GT, Breuner CW, Brenowitz EA. Seasonal plasticity and sexual dimorphism in the avian song control system: Stereological measurement of neuron density and number. J Comp Neurol. 1998;396:186–192. doi: 10.1002/(sici)1096-9861(19980629)396:2<186::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Vates GE, Broome BM, Mello CV, Nottebohm F. Auditory pathways of caudal telencephalon and their relation to the song system of adult male zebra finches (Taenopygia guttata) J Comp Neurol. 1996;366:613–642. doi: 10.1002/(SICI)1096-9861(19960318)366:4<613::AID-CNE5>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Yang HK, Sundholm-Peters NL, Goings GE, Walker AS, Hyland K, Szele FG. Distribution of doublecortin expressing cells near the lateral ventricles in the adult mouse brain. J Neurosci Res. 2004;76(3):282–95. doi: 10.1002/jnr.20071. [DOI] [PubMed] [Google Scholar]
- Zann RA. The zebra finch: a synthesis of field and laboratory studies. 0. Oxford. Univ. Press; Oxford: 1996. [Google Scholar]


