Abstract
We examined the distribution of adult cell proliferation throughout the brain of an anuran amphibian using 5-bromo-2′-deoxyuridine (BrdU). BrdU, a thymidine analog, is a commonly used cellular marker that is incorporated into actively dividing progenitor cells. Adult green treefrogs, Hyla cinerea, received injections of BrdU and were sacrificed 2 hours, 2 days, 2 weeks, or 30 days later. Immunohistochemistry revealed BrdU-immunopositive (BrdU+) cells to be distributed in ventricular zones throughout the brain. The heaviest concentrations of cells were located in the telencephalon, primarily in the ventrolateral region of the lateral ventricles, and the ventricles of olfactory bulbs. Numerous BrdU+ cells were located around the preoptic and hypothalamic recesses and few around the third ventricle in the diencephalon. Proceeding caudally towards the midbrain, there was a marked decrease in BrdU-labeling and few BrdU+ cells were found in the hindbrain. Consistent with previous studies in ectothermic vertebrates, BrdU+ cells were found predominantly in the ventricular zone (VZ) and immediately adjacent to the VZ; at later time points (i.e., 30 days), the cells appeared to have migrated into parenchymal regions. The extent of cellular proliferation in anurans is similar to that of fishes and reptiles and thus is more widespread compared to mammals.
Keywords: amphibian, BrdU, cell proliferation, cell migration, ventricular zone
1. Introduction
Documentation of postnatal neurogenesis has generated interest in patterns of cell proliferation in brains of adult vertebrates. Adult neurogenesis is defined as the birth and maturation of new neurons that add to or replace neurons in existing circuitry (Lindsey & Tropepe, 2006), encompassing four processes: proliferation, migration, differentiation and survival. Patterns of cellular proliferation have been mapped in the developing anuran brain (Schmidt & Roth, 1993; Chapman et al., 2005; Simmons et al., 2006), but comprehensive studies of cell proliferation in adults are not well-documented. Adult cell proliferation has been demonstrated in anurans (Raucci et al., 2006, and references therein); specifically, in Rana temporaria via tritiated thymidine (i.e., radioactive thymidine; Bernocchi et al., 1990, Chetverukhin & Polenov, 1993; Polenov & Chetverukhin, 1993), in R. esculenta via proliferating cell nuclear antigen (PCNA; Raucci et al., 2006), and in R. catesbeiana via BrdU-labeling (Saijo et al., 2006).
Although there are a few studies detailing some aspect (i.e., proliferation or differentiation) of adult neurogenesis in anuran amphibians, the story is not yet complete. With the exception of the work of Saijo et al. (2006), the ranid studies focus on specific brain regions (e.g., the preoptic area in Chetverukhin & Polenov, 1993), include only one time point of cell proliferation (Bernocchi et al., 1990, Chetverukhin & Polenov, 1993; Polenov & Chetverukhin, 1993), or use alternative cell proliferation markers (tritiated thymidine in Bernocchi et al., 1990; PCNA in Raucci et al., 2006). The amount and potential sites of cell proliferation should be interpreted cautiously as cell proliferation markers differ in their method of labeling (such as labeling molecules only during specific parts of the cell cycle; e.g., PCNA, Wullimann & Puelles, 1999). BrdU is currently the most utilized technique to assess cell proliferation (due to the ease of double-labeling with cell phenotypic markers; Taupin, 2007); hence, a description of the distribution of BrdU labeling is necessary in anurans if the results are to be compared to the current literature on the distribution of cell proliferation across vertebrates. To our knowledge, this report is the first comprehensive study of the regional distribution of cell proliferation via BrdU-labeling in adult Hyla cinerea incorporating the time course and migration of new cells. A preliminary report of this work has been presented (Almli & Wilczynski, 2004).
2. Results
Distribution
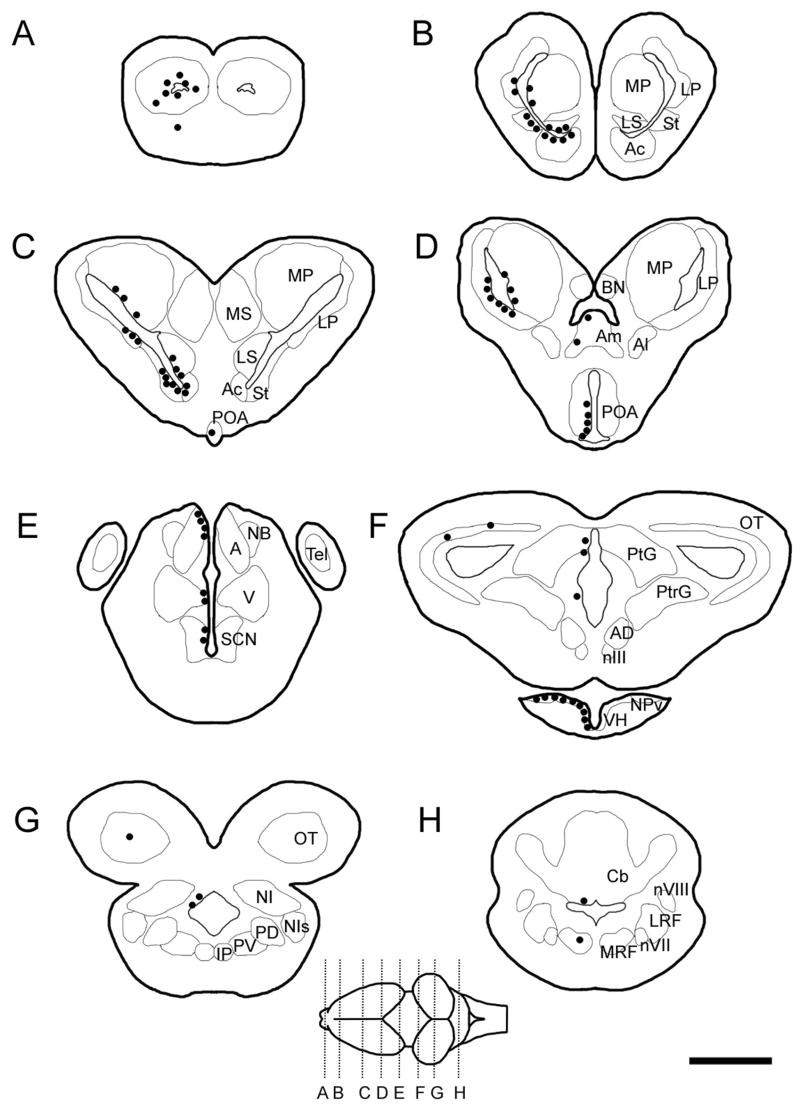
Proliferating cells were regionally distributed in the adult amphibian brain as seen in Figure 1. Within 48 hours, proliferating cells (i.e., labeled with BrdU) mainly occurred in the VZ (i.e., ependymal layer or first layer of cells) especially in forebrain regions and often appeared in clusters or pairs of labeled cells. Cells within the first layer of cells may be ependymal in nature as some were elongated, but the type of cell is undetermined as of yet. The BrdU+ cells in the parenchyma were not elongated and resembled the surrounding Nissl-labeled cells. The heaviest concentrations of BrdU+ cells were located in the telencephalon, primarily around the lateral ventricles and the ventricles of olfactory bulbs (Table 1). Proliferating cells were scattered throughout the olfactory bulbs, including the accessory olfactory bulb, and were uniformly distributed (Fig. 1A). In the middle to caudal telencephalon, a greater number of progenitor cells were distributed in the ventrolateral region of the lateral ventricles (adjacent to the striatum laterally and septal nuclei medially) as compared to the dorsal half of the ventricle (Fig. 1B, C). Further, the lateral and ventral regions, specifically the lateral pallium and subpallial regions, contained more BrdU+ cells than the medial pallium (Table 1). An exception to the mainly ventricular location of proliferating cells in the forebrain was the amygdala, in which there were occasional labeled cells not immediately adjacent to a ventricle (Fig. 1D). There were many proliferating cells in the preoptic recess, especially in the ventral extent of the preoptic area (POA; Fig 1D). Proceeding caudally in the POA, the proliferating cells extended to more dorsal regions (in addition to the ventral region), though never reached the dorsal extent of the POA. Proliferating cells in the thalamic regions were lesser in number considering the greater regional area included in this sample (Fig. 1E), and BrdU+ cells appeared more abundant in the dorsal extent of the anterior thalamus near the habenula. Occasional proliferating cells were located in the habenula (not shown) and suprachiasmatic nucleus (Fig. 1E). Moving ventrally, numerous BrdU+ cells were located around the hypothalamic recess (mostly on the ventral edge) and became more abundant caudally, while a few appeared around the third ventricle in the diencephalon throughout its rostral-caudal extent (Fig. 1F).
Figure 1.
Schematics of brain sections showing the distribution of BrdU+ cells (black circles) at 2–48 hours. (ABBREVIATIONS: A, anterior thalamic nucleus; Ac, nucleus accumbens; AD, anterodorsal tegmentum; Al, lateral amygdala; Am, medial amygdala; BN, bed nucleus of the pallial commissure; Cb, cerebellum; Ip, interpeduncular nucleus; LP, lateral pallium; LRF, lateral reticular formation; LS, lateral septal nucleus; MP, medial pallium; MRF, medial reticular formation; MS, medial septal nucleus; NB, nucleus of Bellonci; NI, nucleus isthmi; NIs, secondary isthmal nucleu; NPv, nucleus of the periventicular organ; nIII, oculomotor nucleus, nVII, vestibular nucleus; nVIII, facial motor nucleus; OT, optic tectum; PD, posterodorsal tegmentum; PV, posteroventral tegmentum; PO, preoptic area; PtG, pretectal gray; PtrG, pretoral gray; SCN, suprachiasmatic nucleus; St, striatum; V, ventral thalamus.)
Table 1.
BrdU-labeled cells in each region of interest (N=3/time point). Numbers in parentheses correspond to the ratio of the number of parenchymal cells to the total number of cells (cells in the ventricular zone plus cells in the parenchyma).
| Time point | MP | LP | St/Ac | Septum | Amyg | POA | Thal | VH | Tectum | Torus | Cb | RTF |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2 hours | ++ (0.00) | +++ (0.00) | +++ (0.00) | +++ (0.01) | + (0.36) | + (0.00) | ++ (0.04) | +++ (0.00) | + (0.44) | + (0.38) | + (0.25) | + (0.56) |
| 48 hours | ++ (0.02) | +++ (0.00) | +++ (0.00) | +++ (0.01) | + (0.50) | + (0.00) | ++ (0.04) | +++ (0.00) | + (0.25) | + (0.00) | + (0.17) | + (0.85) |
| 14 days | ++ (0.03) | +++ (0.00) | +++ (0.00) | +++ (0.10) | + (0.18) | ++ (0.04) | ++ (0.21) | +++ (0.15) | + (0.70) | + (0.53) | + (0.63) | + (0.64) |
| 30 days | ++ (0.16) | +++ (0.17) | +++ (0.31) | +++ (0.27) | + (0.82) | ++ (0.33) | + (0.35) | +++ (0.27) | + (0.59) | + (0.54) | + (0.41) | + (0.74) |
[Note: + = 0–50 cells, ++ = 51–100 cells, +++ > 101 cells. Abbreviations: MP, medial pallium; LP, lateral pallium; St/Ac, striatum/nucleus accumbens; amyg, amygdala; POA, preoptic area; Thal, thalamus; VH, ventral hypothalamus; Torus, torus semicircularis; Cb, cerebellum; RTF, reticular formation.]
Proceeding caudally into the brainstem, there was a significant decrease in BrdU+ cells. Proliferating cells were distributed with lesser density along the cerebral aquaduct (Fig. 1G), with cells predominantly in the dorsal extent of the aquaduct. The VZ of the tectal ventricles contained few cells and appeared to lack a rostral-caudal gradient (Fig. 1F, G). Of note were occasional BrdU+ cells in the nucleus isthmi (Fig. 1G). Few BrdU+ cells were found in the hindbrain; these included rare non-ventricular cells in the cerebellum and reticular formation (Fig. 1H).
Survival and migration
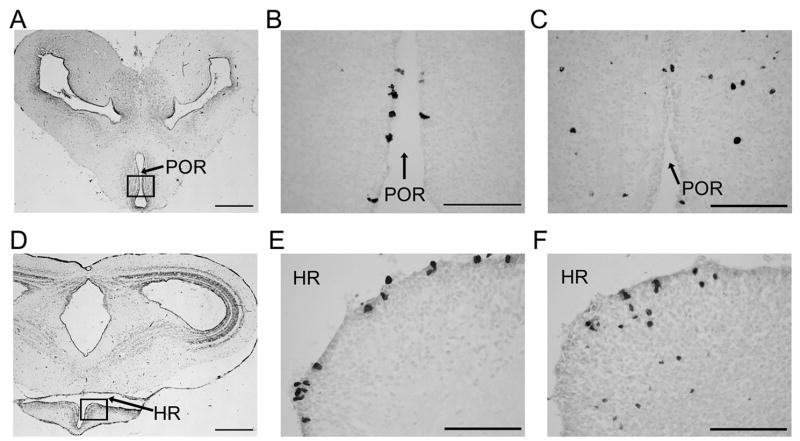
Although the number of BrdU+ cells varied in the adult amphibian brain depending on the region sampled, there was a relative stability in the numbers of labeled cells per ROI over time (2 hours – 30 days; Table 1). However, when the location of the BrdU+ cells was noted (Table 1), the percentage of parenchymal cells was different depending on the time point sampled (specifically in the forebrain at 2 hours compared with 30 days). BrdU+ cells in forebrain regions (excluding the amygdala) were mostly located in the VZ at time points up to 2 weeks, but at 30 days ca. 25% of cells appeared in the parenchyma (Table 1). This change in cell location is depicted in Figure 2, which shows most BrdU+ cells inside the VZ of the POA and ventral hypothalamus (VH) at 48 hours compared to outside the VZ at 30 days. Midbrain and hindbrain regions also showed an increase in the percentage of cells in the parenchyma over time, although not as dramatically because parenchymal cells were also seen at the 2 hour time point in these regions.
Figure 2.
Photomicrographs of the anuran amphibian telencephalon: Nissl-stained sections (A, D) with squared regions containing BrdU-labeled cells (B, C, E, F). Preoptic area depicted in top row and ventral hypothalamus in bottom row (preoptic recess, POR; hypothalamic recess, HR). BrdU+ cells are in the ependymal layer at 48 hrs (B, E) and in the parenchyma at 30 days (C, F). (Scale bar in A, D = 500 μm; scale bar in B, C, E, F = 100 μm).
3. Discussion
Amphibians have been central to studies of proliferation and regeneration for decades (reviewed in Raucci et al. 2006), yet the lack of consistency or completeness in these studies has made taxonomic comparisons with other vertebrates difficult. We viewed proliferating cells using BrdU staining, the most commonly used procedure in other vertebrates. Consistent with previous research in vertebrates, we found BrdU+ cells in adult amphibians in the VZ and immediately adjacent to the VZ after short-term survival, primarily in the ventrolateral extent of the telencephalic ventricles. There were regional differences in the numbers of proliferating cells with forebrain regions having the highest numbers of BrdU+ cells (pallial, subpallial regions, and the VH being the most noteworthy). This is not to imply that all of the newly formed cells migrate to and/or remain in those regions, but they are born or proliferate there. Despite regional differences in the number of BrdU+ cells, there is a temporal stability in all sampled brain regions between 2 hours and 30 days; however, in most brain regions, more notably in the forebrain, cells appear to migrate away from the VZ and into the parenchyma.
Proliferation
Different techniques make comparing between species and treatments very challenging (Rakic, 2002; Lindsey and Tropepe, 2006). Nevertheless, results in anurans using different cell proliferation markers were largely comparable to those described in this study, although we find some differences in the amount and location of proliferating brain cells. The medial pallium in our study had much lower numbers of proliferating cells than the lateral pallium; the opposite is reported in other amphibian studies using different markers of cell proliferation (Bernocchi et al., 1990; Raucci et al., 2006). Compared to the forebrain, fewer proliferating cells were found in the midbrain (e.g., tectum) and hindbrain regions (e.g., cerebellum) in all anuran studies. However, the medulla was noted to have a moderately high labeling index (percent of labeled cells with respect to the total number of cells per brain region) in Bernocchi et al. (2000) but had very few labeled cells in our study. Future work using density counts instead of total number of labeled cells may reconcile this discrepancy.
The taxonomic variation in adult cell proliferation is significant. However, in general, the occurrence of adult cell proliferation in amniotic vertebrates (reptiles, birds, and mammals) is mostly restricted to the forebrain (Nottebohm, 2002; Garcia-Verdugo et al., 2002) unlike that seen in anamniotes (e.g., fishes and anurans). In reptiles (see Font et al., 2001) and birds (see Alvarez-Buylla, 1990), adult cell proliferation has been described in many forebrain regions, but proliferating cells are rarely seen in the walls of the third, tectal, and fourth ventricles. Mammals have the most limited distribution of adult generated neurons: the hippocampus, the olfactory bulb (reviewed in Ming and Song, 2005), and perhaps the cerebellum and neocortex (e.g., Magavi et al., 2000). In fish, 75% of proliferating cells are generated in the cerebellum, but many other areas exhibit adult cell proliferation (reviewed in Zupanc, 2006). Anuran amphibians exhibit a cell proliferation pattern intermediate between fish and reptiles.
Although differences exist in the destination of proliferating cells in vertebrates, there appears to be a common neurogenic region in the telencephalic ventricles (usually the lateral ventricles; Lindsey and Tropepe, 2006; Doetsch and Scharff, 2001) that can be considered a “hot spot,” or concentrated region of proliferating cells (Alvarez-Buylla et al., 1990). Teleost fishes exhibit the greatest number of proliferation zones/neurogenic regions and mammals have only two, the subventricular zone and the subgranular zone of the dentate gyrus (reviewed in Lindsey and Tropepe, 2006). In frogs, the ventrolateral wall of the lateral ventricles is a proliferation hot spot as it is in birds (Alvarez-Buylla et al., 1990). However, many regions of the anuran telencephalic VZ appear to be proliferation zones, a pattern similar to that generally seen in reptiles (for exceptions cf. Font, 2001).
Survival and migration
In the adult anuran, the number of BrdU+ cells in each ROI between 2 hours and 30 days remained constant. This suggests that newly proliferating cells survived for at least 30 days. Alternatively, there was a balance between dividing and dying cells; e.g., newly proliferating cells died before 30 days and the population of labeled cells at 30 days consisted of daughter cells from initially labeled cells. BrdU studies such as ours cannot distinguish this.
The temporal stability of proliferating cells in anurans is similar to that seen in fish (specifically the fish cerebellum) and reptiles, in contrast to birds and mammals. In birds, there seems to be a significant amount of turnover in the number of surviving cells (Alvarez-Buylla and Nottebohm, 1988). There are regional differences reported in the stability of proliferating cells in mammals as well: most of the new cells die upon reaching the olfactory bulb (Biebl et al., 2000; Kempermann and Gage, 2002), though most are integrated into the neuronal circuitry in the hippocampus (Kempermann et al., 2003). Because little or no degenerating cells have been detected, the majority of cells produced in the adult fish and reptile brains seem to complete their development and become part of existing neuronal populations (Zupanc et al., 1996; Font et al., 2001). The present study suggests that frogs are similar due to comparable numbers of BrdU+ cells labeled over time.
Migration of newly proliferated cells (ca. 25%) away from the ventricle was evident at the 30 day time point in most brain regions sampled. The forebrain regions had few if any parenchymal cells at early time points (less than 2 weeks) and a greater percentage after 30 days. Most forebrain areas (with the exception of the amygdala) largely surround the ventricles; whereas brainstem regions have more of their cellular areas distant from ventricles. The greater amount of non-ventricular area in the brainstem could be the reason why many BrdU+ cells were found in the parenchyma at 2 hours. These cells were most likely born in situ.
The time course of cellular migration in anurans is consistent with that of fishes, reptiles, and birds, where most new cells take ca. 30 days to reach their final destination (Zupanc et al., 1996; Perez-Canellas and Garcia-Verdugo, 1996; Nottebohm, 2002). The phenomenon in mammals is unique with cells migrating in under 2 weeks (for review see Ming and Song, 2005). Further, the spatial restriction of neuronal migration in mammals is unlike that seen in other vertebrates that exhibit widespread migration into parenchymal regions (Goldman, 1998). Equally important to the migration of proliferating cells in the adult brain is the differentiation of cells into neurons and glia. It would be informative to address, in future studies, whether the new cells in amphibians are of neuronal or glial origin.
Mechanism and functional significance
Ectothermic vertebrates, in general, tend to generate new neurons in many brain regions as compared to the limited distribution in mammals (Zupanc et al, 2001; Font et al., 2001; Raucci et al., 2006), a phenomenon which may be incidental to the continual brain growth of non-mammalian vertebrates (Kaslin et al., 2007). One mechanism that may suppress adult neurogenesis in mammals appears to be the absence of permissive signals for proliferation and further development of neuronal precursors (Kaslin et al., 2007; Zupanc, 2006; Emsley et al., 2005). By comparison, such neurogenesis-permissive factors are present in fish and reptilian brains (Zupanc, 2006), and are likely to be present in amphibians in light of their ability for organ and limb regeneration (Brockes and Kumar, 2002).
Although the phenomenon of adult neurogenesis is now widely accepted, it is difficult to determine the functional significance of the new cells. The degree to which new cells are incorporated into existing circuitry or are able to replace injured or dying cells varies widely in non-mammalian vertebrates (Font et al., 2001; Zupanc et al., 2001, Doetsch and Scharff, 2001) and is quite limited in mammals (reviewed in Cayre et al., 2002; Emsley et al., 2005; Lindsey and Tropepe, 2006). Undoubtedly, more work needs to be conducted on adult neurogenesis in frogs; anurans have such widespread and high rates of cell proliferation as adults and thus are excellent models in which to pursue questions surrounding the interplay between behavior, changing environment, and cell proliferation.
4. Experimental Procedure, Acknowledgements, References
Animals
Adult male Hyla cinerea were purchased from a commercial supplier (Charles Sullivan, Inc., Nashville, TN), and group-housed in glass aquaria containing water bowls, plastic plants, and rocks. The frogs had free access to water and were fed crickets (Acheta domestica, ca.12 mm long) twice/week. The animal room was maintained at ca. 24°C on a 14:10 L:D cycle. Animal procedures were approved by the University of Texas Institutional Animal Care and Use Committee (IACUC) and conformed to NIH guidelines.
Frogs (N=12; mean snout-vent length = 50 mm, range = 46–52 mm) received two consecutive injections intraperitoneally at 12:00h (Day 1) with BrdU (100 mg/kg; Sigma, St. Louis, MO) and sacrificed at 2 hours, 48 hours, 14 or 30 days later (n=3 per group). Brains were immersion-fixed in 4% paraformaldehyde and cryoprotected in 20% sucrose. Using a cryostat (Leica CM 1900), brains were sectioned coronally at a thickness of 20 μm. Sections were collected on gelatinized subbed slides and four series of adjacent sections were created per animal. Sections were stored at −20°C until immunohistochemical staining was carried out.
Immunohistochemistry
One series of sections for each animal was processed for BrdU using 3, 3′-diaminobenzidine tetrahydrocloride (DAB) as the chromogen; the other three series were reserved for future studies. Slide edges were marked with a PAP pen (Research Products International Corp, Mount Prospect, IL). For detection of BrdU-labeled nuclei, the DNA was denatured with 2 N HCl at 37°C for 30 minutes and neutralized with 0.1 M boric acid buffer (pH 8.5) for 15 minutes. Endogenous peroxidases were quenched with 1% (v/v) H202 in 0.1 M PBS with 1% Triton X-100 (v/v; Sigma) for 10 minutes at room temperature. Nonspecific antigen binding sites were blocked by preincubation with 10% (v/v) normal rabbit serum in blocking solution [0.1 M PBS, 0.3% (v/v) Triton X-100, 0.2% (w/v) bovine serum albumin (Sigma)] that included avidin (1 drop/ml; Vector Laboratories, Burlingame, CA) for 60 minutes at room temperature. Slides were then incubated with anti-BrdU antibody (1:100; Accurate Chemicals, Westbury, NY) in blocking solution containing biotin (2 drops/ml; Vector Laboratories) for 48 hours at 4°C. Avidin/biotin blocking was used in previous steps to reduce nonspecific biotin binding. After washing four times with PBS-T [0.3% (v/v) Triton X-100], sections were incubated in biotinylated secondary antibody (rabbit anti-rat, 1:200; Vector Laboratories) for 60 minutes. Signal amplification was achieved by exposing the tissue to horseradish peroxidase conjugated Avidin-Biotin Complex (Vector Laboratories) for 60 minutes at room temperature. After washing, sections were immersed in a DAB solution [0.25% (w/v) DAB with 0.006% (v/v) H202] for 15 minutes at room temperature to yield brown nuclei, and the cell bodies were counter-stained with toluidine blue [0.25% (w/v)] to visualize regional boundaries. (The photomicrographs shown in Fig. 2B, C, E, F were taken of alternate sections that were not counterstained by toluidine blue.) Slides were coverslipped using Permount (Fisher Scientific, Pittsburgh, PA) after dehydration with graded ethanols and clearance in xylenes. Omission of the primary antibody eliminated all staining and served as a negative control. Control slides from an animal that did not receive a BrdU injection revealed no staining.
Quantification
Every section in the stained series (i.e. first series) was counted to determine the numbers of labeled cells in each region of interest (ROI), the boundaries of which were determined by toluidine blue counterstaining. Slides were coded so that cell counts were obtained blind to time point condition, and sections were observed with a light microscope (Nikon Eclipse 80i, Melville, NY) attached to a digital camera (Nikon Digital Sight, DS-2Mv). DAB-positive cells (i.e., BrdU+ cells) were visualized and counted using a 20x objective (unless multiple cells overlapped and then a 40x objective was used to confirm the number of labeled cells). All BrdU+ cells were counted within the ROI. Because anuran cells in the ROI are only ca. 10 μm in diameter and sections counted were 80 μm apart, there is little possibility that labeled nuclei would be split between sections and be counted twice, causing an overestimation of proliferating cells. Furthermore, a positive DAB-labeled cell was counted when the cell appeared of the same general size/shape of a Nissl-labeled cell (with the exception of elongated cells in the ependymal layer, see below), thereby excluding partial cells (which were rarely identifiable). In addition, the BrdU label becomes diluted with subsequent divisions and may appear punctate (though not apoptotic; Miller & Nowakowski, 1988); punctate deposits were not counted. Therefore, we might have underestimated the number of proliferating cells at latter time points. For this reason, we only reported qualitative indications of BrdU cell density and no statistical comparisons were made between time points.
We counted the total number of BrdU+ cells in the VZ in addition to the BrdU+ cells outside the VZ (i.e., parenchyma) using the same criteria as above. To assess cellular migration, we calculated the percentage of parenchymal cells to the total number of BrdU+ cells and compared this ratio for early and late time points (e.g., 2 hours and 30 days). An increase in the ratio after the 2 hour time point suggests migration into the parenchymal layer.
Acknowledgments
This work was funded by NIH R01 MH057066. We thank Alex Baugh, Brian Dias, Tomoko Hattori, Kim Hoke, Jason Miranda, Alison Tannenbaum, and several anonymous reviewers for comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almli L, Wilczynski W. Regional distribution of progenitor cell populations in the brain of the adult treefrog (Hyla cinerea). Society for Neuroscience Annual Meeting; San Diego, CA. 2004. [Google Scholar]
- Alvarez-Buylla A, Nottebohm F. Migration of young neurons in adult avian brain. Nature. 1988;335:353–4. doi: 10.1038/335353a0. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, et al. Proliferation "hot spots" in adult avian ventricular zone reveal radial cell division. Neuron. 1990;5:101–9. doi: 10.1016/0896-6273(90)90038-h. [DOI] [PubMed] [Google Scholar]
- Bernocchi G, et al. Premitotic DNA synthesis in the brain of the adult frog (Rana esculenta L.): an autoradiographic 3H-thymidine study. Anat Rec. 1990;228:461–70. doi: 10.1002/ar.1092280413. [DOI] [PubMed] [Google Scholar]
- Biebl M, et al. Analysis of neurogenesis and programmed cell death reveals a self-renewing capacity in the adult rat brain. Neurosci Lett. 2000;291:17–20. doi: 10.1016/s0304-3940(00)01368-9. [DOI] [PubMed] [Google Scholar]
- Brockes JP, Kumar A. Plasticity and reprogramming of differentiated cells in amphibian regeneration. Nat Rev Mol Cell Biol. 2002;3:566–74. doi: 10.1038/nrm881. [DOI] [PubMed] [Google Scholar]
- Cayre M, et al. The common properties of neurogenesis in the adult brain: from invertebrates to vertebrates. Comp Biochem Physiol B Biochem Mol Biol. 2002;132:1–15. doi: 10.1016/s1096-4959(01)00525-5. [DOI] [PubMed] [Google Scholar]
- Chapman JA, et al. Cell proliferation in the Rana catesbeiana auditory medulla over metamorphic development. J Neurobiol. 2006;66:115–33. doi: 10.1002/neu.20209. [DOI] [PubMed] [Google Scholar]
- Chetverukhin VK, Polenov AL. Ultrastructural radioautographic analysis of neurogenesis in the hypothalamus of the adult frog, Rana temporaria, with special reference to physiological regeneration of the preoptic nucleus. I. Ventricular zone cell proliferation. Cell Tissue Res. 1993;271:341–50. doi: 10.1007/BF00318621. [DOI] [PubMed] [Google Scholar]
- Doetsch F, Scharff C. Challenges for brain repair: insights from adult neurogenesis in birds and mammals. Brain Behav Evol. 2001;58:306–22. doi: 10.1159/000057572. [DOI] [PubMed] [Google Scholar]
- Emsley JG, et al. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75:321–41. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Font E, et al. Neurogenesis and neuronal regeneration in the adult reptilian brain. Brain Behav Evol. 2001;58:276–95. doi: 10.1159/000057570. [DOI] [PubMed] [Google Scholar]
- Garcia-Verdugo JM, et al. The proliferative ventricular zone in adult vertebrates: a comparative study using reptiles, birds, and mammals. Brain Res Bull. 2002;57:765–75. doi: 10.1016/s0361-9230(01)00769-9. [DOI] [PubMed] [Google Scholar]
- Goldman SA. Adult neurogenesis: from canaries to the clinic. J Neurobiol. 1998;36:267–86. [PubMed] [Google Scholar]
- Kaslin J, et al. Proliferation, neurogenesis and regeneration in the non-mammalian vertebrate brain. Philos Trans R Soc Lond B Biol Sci. 2007 doi: 10.1098/rstb.2006.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempermann G, Gage FH. Genetic influence on phenotypic differentiation in adult hippocampal neurogenesis. Brain Res Dev Brain Res. 2002;134:1–12. doi: 10.1016/s0165-3806(01)00224-3. [DOI] [PubMed] [Google Scholar]
- Kempermann G, et al. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–9. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Lindsey BW, Tropepe V. A comparative framework for understanding the biological principles of adult neurogenesis. Prog Neurobiol. 2006;80:281–307. doi: 10.1016/j.pneurobio.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Magavi SS, et al. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–5. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- Miller MW, Nowakowski RS. Use of bromodeoxyuridine-immunohistochemistry to examine the proliferation, migration and time of origin of cells in the central nervous system. Brain Res. 1988;457:44–52. doi: 10.1016/0006-8993(88)90055-8. [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian central nervous system. Annu Rev Neurosci. 2005;28:223–50. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- Nottebohm F. Neuronal replacement in adult brain. Brain Res Bull. 2002;57:737–49. doi: 10.1016/s0361-9230(02)00750-5. [DOI] [PubMed] [Google Scholar]
- Perez-Canellas MM, Garcia-Verdugo JM. Adult neurogenesis in the telencephalon of a lizard: a [3H]thymidine autoradiographic and bromodeoxyuridine immunocytochemical study. Brain Res Dev Brain Res. 1996;93:49–61. doi: 10.1016/0165-3806(96)00014-4. [DOI] [PubMed] [Google Scholar]
- Polenov AL, Chetverukhin VK. Ultrastructural radioautographic analysis of neurogenesis in the hypothalamus of the adult frog, Rana temporaria, with special reference to physiological regeneration of the preoptic nucleus. II. Types of neuronal cells produced. Cell Tissue Res. 1993;271:351–62. doi: 10.1007/BF00318622. [DOI] [PubMed] [Google Scholar]
- Rakic P. Adult neurogenesis in mammals: an identity crisis. J Neurosci. 2002;22:614–8. doi: 10.1523/JNEUROSCI.22-03-00614.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raucci F, et al. Proliferative activity in the frog brain: a PCNA-immunohistochemistry analysis. J Chem Neuroanat. 2006;32:127–42. doi: 10.1016/j.jchemneu.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Saijo E, et al. Region-dependent Pattern of Newborn Cell Proliferation and Survival in an Adult Amphibian Brain. Society for Neuroscience Annual Meeting; Atlanta, GA. 2006. [Google Scholar]
- Schmidt A, Roth G. Patterns of cellular proliferation and migration in the developing tectum mesencephali of the frog Rana temporaria and the salamander Pleurodeles waltl. Cell and Tissue Research. 1993;272:273–287. doi: 10.1007/BF00302734. [DOI] [PubMed] [Google Scholar]
- Simmons AM, et al. Developmental changes in cell proliferation in the auditory midbrain of the bullfrog, Rana catesbeiana. J Neurobiol. 2006;66:1212–24. doi: 10.1002/neu.20301. [DOI] [PubMed] [Google Scholar]
- Taupin P. BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Brain Res Rev. 2007;53:198–214. doi: 10.1016/j.brainresrev.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Wullimann MF, et al. Secondary neurogenesis in the brain of the African clawed frog, Xenopus laevis, as revealed by PCNA, Delta-1, Neurogenin-related-1, and NeuroD expression. J Comp Neurol. 2005;489:387–402. doi: 10.1002/cne.20634. [DOI] [PubMed] [Google Scholar]
- Zupanc GK. A comparative approach towards the understanding of adult neurogenesis. Brain Behav Evol. 2001;58:246–9. doi: 10.1159/000057568. [DOI] [PubMed] [Google Scholar]
- Zupanc GK. Neurogenesis and neuronal regeneration in the adult fish brain. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2006:1–22. doi: 10.1007/s00359-006-0104-y. [DOI] [PubMed] [Google Scholar]
- Zupanc GK, et al. Postembryonic development of the cerebellum in gymnotiform fish. J Comp Neurol. 1996;370:443–64. doi: 10.1002/(SICI)1096-9861(19960708)370:4<443::AID-CNE3>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]